Back to Journals » Clinical Ophthalmology » Volume 8
Bimatoprost/timolol fixed combination versus latanoprost in treatment-naïve glaucoma patients at high risk of progression: a pilot study
Authors Gutierrez-Diaz E, Silva Cotta J, Muñoz-Negrete FJ , Gutierrez-Ortiz C, Morgan-Warren R, Maltman J
Received 29 October 2013
Accepted for publication 15 January 2014
Published 10 April 2014 Volume 2014:8 Pages 725—732
DOI https://doi.org/10.2147/OPTH.S56735
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Esperanza Gutierrez-Diaz,1 Jose Silva Cotta,2 Francisco J Muñoz-Negrete,3 Consuelo Gutierrez-Ortiz,4 Robert J Morgan-Warren,5 John Maltman5
On behalf of the GIFT study group
1Department of Ophthalmology, Hospital Doce de Octubre, Universidad Complutense, Madrid, Spain; 2Department of Ophthalmology, Hospital de São João, Porto, Portugal; 3Department of Ophthalmology, Hospital Ramón y Cajal, IRYCIS, Universidad Alcalá, Madrid, Spain; 4Department of Ophthalmology, Hospital Universitario Príncipe de Asturias, Madrid, Spain; 5Medical Affairs, Allergan Holdings Ltd, Marlow, UK
Objective: To compare a fixed combination of 0.03% bimatoprost and 0.5% timolol (BTFC) with latanoprost monotherapy (LM) in treatment-naïve patients with open-angle glaucoma (OAG) and risk factors for glaucomatous progression.
Methods: Patients were enrolled at 15 sites in Spain and Portugal, and were randomized 1:1 to BTFC or LM. Patients instilled one drop of medication once per day at 8 pm for 12 weeks. The primary outcome was change in intraocular pressure (IOP) at 12 weeks.
Results: Of 81 patients enrolled, 43 were randomized to BTFC and 38 to LM. Mean (SD) change in IOP from baseline to 12 weeks was significantly greater for BTFC than for LM: -13.5 mmHg (4.48) versus -11.4 mmHg (3.19), respectively (P=0.003). Similarly, at 12 weeks, significantly more BTFC patients than LM patients had IOP reductions of ≥40% (74.4% versus 47.4%, P=0.015) or ≥50% (46.5% versus 15.8%, P=0.003). Adverse events were more frequent with BTFC than with LM (33 versus 13 events), but most were mild in severity. The only serious adverse event (colon cancer) was adjudged unrelated to the study medication.
Conclusion: BTFC was effective and well tolerated in treatment-naïve patients with OAG at high risk of progression.
Keywords: open-angle, fixed combinations
Introduction
European Glaucoma Society (EGS) guidelines on the treatment of primary open-angle glaucoma (POAG) recommend an individualized approach to management, setting a target intraocular pressure (IOP) on the basis of risk factors such as family history, IOP at presentation, visual field defects or the presence of pseudoexfoliation, with the goal of preventing significant visual disability in the patient’s lifetime.1 In particular, data from the Early Manifest Glaucoma Trial (EMGT) show that the more advanced the glaucoma is at presentation, with greater loss of visual field, the greater the long-term risk of further glaucomatous progression.2 It therefore follows that in patients with advanced glaucoma, risk factors for glaucomatous progression and a reasonable life expectancy, early and aggressive initial IOP lowering may offer the best chance of preventing visual impairment.
While the EGS guidelines recommend that initial therapy for glaucoma is usually with a single agent,1 they also note that many patients require additional agents to reach their target IOP, and that in such cases, a fixed combination medication may offer advantages over concurrent therapy in terms of convenience, adherence, and tolerability.1 Prostaglandin analogs are now well established as the most effective monotherapies in terms of IOP lowering,3 and a meta-analysis suggests that bimatoprost has a greater overall IOP-lowering effect than latanoprost or travoprost.4 Similarly, a recent meta-analysis suggested that a fixed combination of 0.03% bimatoprost and 0.5% timolol (BTFC) was more effective at lowering IOP than either latanoprost/timolol or travoprost/timolol fixed combinations;5 no tafluprost/timolol fixed combination is currently available. BTFC therefore seems a rational choice for maximal IOP lowering in patients for whom this is the overriding therapeutic consideration.
This study investigated the use of BTFC compared with latanoprost monotherapy (LM) in treatment-naïve subjects with risk factors for glaucomatous progression. Our hypothesis was that BTFC would result in better IOP control and quicker attainment of an individual target IOP than LM.
Methods
This was a Phase IV, randomized, multicenter, investigator-masked study, registered with ClinicalTrials.gov (NCT012435676) and the EU Clinical Trials Register (2009-012799-28). Patients were eligible for inclusion if they fulfilled all of the following criteria: i) aged ≥18 and ≤85 years (male or female); ii) diagnosed with open-angle glaucoma (POAG or pseudoexfoliative glaucoma), with baseline IOP ≥27 mmHg and ≤34 mmHg in one or both eyes; iii) no previous ocular hypotensive medications; iv) at least one of the following risk factors for rapid progression: pseudoexfoliation, family history of glaucoma, pigment dispersion, optic disc hemorrhage, visual field mean deviation worse than −6 dB, IOP ≥27 mmHg in both eyes; v) best corrected visual acuity (BCVA) of Snellen equivalent 20/60 or better in each eye. To be included, patients also needed to give informed consent to participate in the study, and to have two reliable visual field tests: one performed within 6 months prior to baseline (day 0) visit and another at baseline prior to randomization.
Patients were excluded from participation if they had any of: i) a history of refractive surgery; ii) intraocular surgery within 3 months prior to baseline; iii) a visual field defect requiring medical intervention; iv) any contraindication to β-adrenoceptor antagonist therapy; v) any other known allergy or sensitivity to the study medications or their components; vi) any ocular inflammation or infection within 3 months prior to baseline, apart from mild blepharitis, or any history of uveitis; vii) any corneal abnormalities in either eye that would preclude accurate IOP readings with an applanation tonometer; viii) an ocular trauma in either eye within 6 months prior to baseline; ix) a requirement for chronic use during the study of ocular medications other than the study medications, in either eye; x) intermittent use of oral, injectable, or topical ophthalmic steroids within 21 days prior to baseline, or anticipated use during the study; xi) any other condition or situation that might put the subject at significant risk, confound the study results, or significantly impede participation. Female patients who were pregnant, nursing, or planning a pregnancy, or who were of childbearing potential and not using a reliable means of contraception, were also excluded.
This trial was conducted in accordance with the principles of GCP CPMP/ICH/135/95.7 All subjects gave informed consent to participate, and the study was approved by the University Hospital Ramon y Cajal Ethics Committee for Clinical Research (Spain) and the National Ethics Committee for Clinical Investigation (Portugal). The randomization list and envelopes were generated by an independent statistician with no connection to the study. Prior to initiation of study treatment, each subject qualifying for entry was assigned a unique randomization number sequentially at the recruitment site.
Study protocol
Patients were enrolled at 15 sites in Spain (10 sites) and Portugal (5 sites) (see ‘Acknowledgments’ section for list), and were randomized 1:1 to either BTFC or LM. The fixed-combination formulation of bimatoprost and timolol means that the beta-blocker and the prostaglandin analog are combined into a single drop and therefore they cannot be separated. Patients were aware of their treatment allocation, but were instructed not to reveal it to assessing investigators. They were instructed to instill one drop of study medication in each eye requiring treatment, once a day at 8 pm, for 12 weeks. Evening dosing was used because 24-hour IOP monitoring suggests it may provide slightly better IOP control than morning dosing.8,9 Whilst BTFC can be administered in the morning or evening, evening dosing is usually recommended and matched the requirement for LM dosing. If both eyes were treated, the eye with the highest IOP was the study eye and was used for analysis; if both eyes had the same IOP, then the right eye was the study eye.
There were five scheduled visits: i) visit 0 (pre-study, day −14 to day 0), 2 weeks prior to baseline; ii) visit 1 (baseline, day 0), with IOP measurements at 8 am, 12 pm, and 4 pm; iii) visit 2 (week 2), with an IOP measurement at 8 am; iv) visit 3 (week 6), with IOP measurements at 8 am, 12 pm, and 4 pm; v) visit 4 (week 12), with IOP measurements at 8 am, 12 pm, and 4 pm. The first dose of study medication was administered on the evening of the baseline visit (day 0), and the last dose on the evening prior to the last visit at week 12.
Study outcomes
The primary efficacy endpoint of the study was the change in IOP (mean of 8 am, 12 pm, and 4 pm measurements) from baseline to 12 weeks. The secondary efficacy endpoints were: i) specific changes in IOP at 8 am, 12 pm, and 4 pm from baseline to 12 weeks; ii) the percentages of patients reaching predefined target pressure thresholds (ranging from 13 to 18 mmHg) and reductions (of 20% to 50%) at 12 weeks; iii) the absolute difference between patients’ highest IOP at baseline and at 12 weeks; iv) the absolute difference between patients’ lowest IOP at baseline and at 12 weeks.
The safety endpoints were the incidence of adverse events (AEs) throughout the study, and BCVA, which was measured at baseline, 6 weeks, and 12 weeks. Diagnosis of hyperemia was in the investigator’s clinical judgment.
Statistical methods
Unless otherwise specified, analyses were by intention to treat (ITT), and included all patients who were randomized. For ITT analyses, missing values of IOP were imputed using the method of last observation carried forward (LOCF): data from a patient’s last observed visit were carried forward in the analysis to the same hour of the subsequent visit. All secondary endpoint efficacy analyses were by ITT only.
The primary efficacy endpoint was analyzed by ITT, but also per protocol (PP) for confirmation purposes; these analyses included all patients who were randomized, received at least one dose of study medication, had at least one follow-up visit, and who did not fulfill any of a predefined list of protocol violation criteria. PP analyses were based on observed cases only, with no missing values imputed.
Baseline and safety analyses included all patients who were randomized and treated with at least one dose of study medication.
Treatment groups were compared using analysis of covariance (ANCOVA) with pooled investigator site as a factor for continuous variables, or the Cochran–Mantel–Haenszel (CMH) test with stratification by pooled investigator site for categorical variables, unless otherwise stated. All analyses used SAS software (v9.1.3; SAS Institute, Cary, NC, USA). No power calculation was performed, as this was a pilot study, part of the function of which was to provide an estimate of effect for larger studies.
Results
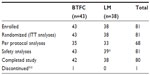
A total of 81 patients were randomized, 43 to BTFC and 38 to LM, and ITT analyses were based on this group. Of these, 13 were classified as major protocol violators, thus PP analyses were based on 68 patients. Table 1 shows the overall flow of patients through the study. Patients were enrolled from June 3, 2010 to February 14, 2012.
Baseline characteristics
Of the 81 patients enrolled, 43 (53.1%) were male and 38 (46.9%) were female. Almost all were of Caucasian ethnicity (98.8%); the remainder were Afro-Caribbean. Their mean (SD) age was 64.6 years (12.0).
With regard to risk factors for progression, 23 (28.4%) had pseudoexfoliation, 27 (33.3%) a family history of glaucoma, seven (8.6%) pigment dispersion, one (1.2%) optic disc hemorrhage, 35 (43.2%) a visual field mean deviation worse than −6 dB, and 45 (55.6%) an IOP ≥27 mmHg in both eyes. Some patients had more than one risk factor, hence the total exceeds 100%.
Overall IOP change from baseline
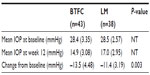
Analyzed by ITT, the primary endpoint of mean (SD) change in IOP from baseline to 12 weeks was significantly greater in the BTFC group than in the LM group: −13.5 mmHg (4.48) for BTFC versus (vs) −11.4 mmHg (3.19) for LM; least squares mean (LSM) difference −2.04 mmHg, 95% confidence interval (CI): −3.35 to −0.73, P=0.003 (Table 2). PP analyses gave similar results, with an LSM difference of −1.96 mmHg (95% CI: −3.46 to −0.46, P=0.011). Both ITT and PP analyses found no significant interaction between pooled site and this outcome, indicating that the effect of treatment did not differ between pooled sites. Individual results are shown as a scattergram in Figure 1.
IOP change from baseline by time of day
At 12 weeks, the secondary endpoint of mean (SD) changes from baseline IOP by time of day were: at 8 am, –14.6 mmHg (4.04) for BTFC vs –12.3 mmHg (3.64) for LM (P=0.006); at 12 pm, –13.6 mmHg (5.50) for BTFC vs –11.8 mmHg (3.14) for LM (P=0.008); and at 4 pm, −12.4 mmHg (5.16) for BTFC vs −10.3 mmHg (4.24) for LM (P=0.003). The difference between the two treatments was thus not greatly affected by time of day, being about 2 mmHg at all measured times.
Attainment of IOP targets
At 12 weeks, the secondary endpoints of predefined pressure reductions were reached by significantly more patients in the BTFC group than in the LM group, for IOP reductions of at least 40% (74.4% vs 47.4%, P=0.015) and at least 50% (46.5% vs 15.8%, P=0.003); other differences were not statistically significant (Figure 2A).
Similarly, at 12 weeks, significantly more patients had attained notional IOP targets of ≤14, ≤15, and ≤16 mmHg in the BTFC group than in the LM group (P=0.001, 0.006, and 0.008, respectively), whereas differences with respect to higher and lower targets were not statistically significant (Figure 2B).
Differences from baseline in highest and lowest IOP recorded
At 12 weeks, the mean change from baseline for the highest and lowest IOP was greater in both respects for the BTFC group than for the LM group: highest IOP (mmHg) −15.3 (SD 4.44) for BTFC vs −12.9 (3.82) for LM, P=0.007; lowest −11.8 (5.23) for BTFC vs −9.9 (3.71) for LM, P=0.007. The difference between BTFC and LM was again approximately 2 mmHg for both measurements, suggesting that the greater overall IOP reduction with BTFC is not associated with greater variability in IOP, compared to LM.
Safety and tolerability
All 81 patients randomized also received at least one dose of medication, and were thus included in safety analyses; however, one patient received both BTFC (up to visit 3) and LM (thereafter) and was therefore counted in both treatment groups for the safety analyses.
Adverse events
A total of 46 AEs were reported by 27 patients (33.3%) during the study. More AEs were reported in the BTFC group (33 events in 19 patients, 44.2% of the group) than in the LM group (13 events in eight patients, 20.5% of the group). The majority of AEs were mild in severity (28/46) and assessed as related to the study drug (30/46). Ocular hyperemia, blepharitis, conjunctival hyperemia, and eye irritation were the most frequently reported AEs in both treatment groups (Table 3).
  | Table 3 Ocular adverse events reported during the study |
At the last study visit, 27 AEs were ongoing (17 in the BTFC group and ten in the LM group), while 18 had resolved without sequelae (15 in the BTFC group and three in the LM group). Only one AE (colon cancer) resolved with sequelae (in the BTFC group), as detailed in the next paragraph.
Two patients in the BTFC group reported one severe AE each. The first was reduced visual acuity (from 20/20 pre-study and at baseline to 20/40 at weeks 6 and 12), reported as ongoing at the last study visit; this was assessed as not related to the study drug, and was not considered a serious AE (SAE). The second was colon cancer, which was moderate at baseline and became severe in intensity during the study, leading to the patient’s withdrawal from the study; this was the only SAE reported during the study, resolved with sequelae, and was assessed as not related to the study drug. This was also the only patient who discontinued treatment because of adverse events. No deaths were reported during the study.
Discussion
While fixed combination treatments may have advantages over the same medications prescribed concurrently in terms of compliance, lack of washout, and reduced exposure to preservatives, the current EGS guidelines recommend their use only when monotherapy has failed to achieve sufficient IOP reduction.1 The basis of this recommendation is essentially two-fold: the general principle of using the minimal therapeutic approach that is effective, and the specific tactical consideration that if a combination treatment is given to treatment-naïve patients and is poorly tolerated or not as effective as expected, it is sometimes difficult to be sure which component of the treatment is causing the problem.
However, if a patient appears to be at high risk of visual disability from glaucoma, it may be clinically justifiable to prescribe a fixed combination immediately for greater IOP lowering and more rapid disease control. The rationale for this is that by the time it becomes clinically apparent that monotherapy is not achieving sufficient disease control, the glaucoma damage may already have progressed significantly towards the onset of visual disability. Similarly, although there is a theoretical problem with poor tolerability, in practice, the common adverse effects of such treatments are mild, manageable, and well known based on long-term use, so long as expectations are correctly set, and patients are unlikely to discontinue provided the treatment is effective. The principal clinical factors prompting this approach are likely to be advanced glaucoma damage, very high IOP, or the presence of exfoliation.
In this group of treatment-naïve patients with POAG or pseudoexfoliative glaucoma at high risk of glaucomatous progression, BTFC and LM both lowered IOP effectively with an acceptable safety profile. These patients had quite wide variation in their risk factors for progression, making it unlikely that this result is specifically related to any one risk factor. However, while patients receiving BTFC and those receiving LM both reported IOP reductions at 12 weeks, greater overall IOP reductions were seen in patients receiving BTFC.
Few previous studies have specifically assessed the use of fixed-combination therapies in treatment-naïve patients with glaucoma or ocular hypertension, and only one has compared BTFC and LM in this clinical setting, to our knowledge.
A 2013 randomized, observer-masked study (Konstas et al) compared BTFC to LM as initial therapy in 41 patients with newly diagnosed, previously untreated exfoliation syndrome or exfoliative glaucoma, and baseline morning IOP >29 mmHg.10 Twenty-four-hour IOP was measured at baseline and at 3 and 6 months, with treatment groups crossed over at 3 months to the alternate therapy. Mean 24-hour IOP was significantly lower with BTFC vs LM (18.9 vs 21.2 mmHg, P<0.001), and BTFC reduced IOP significantly more than latanoprost at every time point, for the mean peak and trough 24-hour IOP (P<0.001). While the Konstas study was wholly in patients with exfoliative disease (vs only 28.4% in the current study) and their baseline IOP was somewhat higher, the difference reported in mean IOP between treatments was quite similar (2.3 mmHg in Konstas et al vs 2.04 mmHg in this study).
A 2007 randomized, double-masked study (Hommer et al) compared BTFC with either an unfixed combination of bimatoprost and timolol, or bimatoprost monotherapy, in 445 treatment-naïve patients with ocular hypertension or glaucoma and IOP of 24–34 mmHg.11 At 3 weeks, BTFC was non-inferior to the unfixed combination, reduced mean diurnal IOP by 8.8 mmHg from baseline, and was well tolerated, with fewer adverse events than the other two regimens. The smaller effect of BTFC on IOP in the Hommer study compared with the present data may reflect both the shorter study duration and the inclusion of patients with lower IOP (24–34 vs 27–34 mmHg).
A 2003 randomized study compared dorzolamide/timolol fixed combination (DTFC) with LM in 65 newly diagnosed patients with exfoliative glaucoma.12 At 2 months, among the 54 patients completing the study, IOP was reduced by 13.1 mmHg in the DTFC group, and by 12.3 mmHg in the LM group. However, eight patients discontinued therapy because of lack of IOP control, implying that an analysis by ITT would yield lower results. It is also unclear how far results in exfoliative glaucoma patients, who usually also have very high IOP, can be applied to the general glaucoma population, although this subgroup is certainly at high risk of progression, as exfoliation may carry an increased risk of progression even beyond the high IOP that generally accompanies it. Only 28.4% of the patients in the present study had exfoliative glaucoma.
Similarly, a 2008 randomized study compared the effect of DTFC with LM in 27 newly diagnosed and previously untreated patients with POAG, using a crossover design of two 6-week periods. However, the effects reported were relatively small, with observed 24-hour IOP reductions of 7.4 mmHg with DTFC vs 6.1 mmHg with LM (P<0.0001), perhaps partly because baseline IOP was relatively low at 22.7 mmHg after washout.13
A small, uncontrolled 2009 study assessed the use of latanoprost/timolol fixed combination (LTFC) in 28 previously untreated glaucoma patients with IOP ≥30 mmHg (21 POAG, six pseudoexfoliative glaucoma, and one pigmentary glaucoma).14 At 1 month, IOP was reduced by 14.6 mmHg, but from a higher baseline IOP than in this study and over a shorter time period.
A 2010 open-label, unmasked study assessed the effectiveness of DTFC, both alone and in combination with latanoprost, in 164 patients with untreated open-angle glaucoma or ocular hypertension.15 Over 12 weeks, DTFC lowered IOP by 12.2 mmHg, and DTFC plus latanoprost by 13.4 mmHg. By comparison, in the present study, BTFC lowered IOP by 13.5 mmHg over the same time period, suggesting that BTFC may be more effective than DTFC in treatment-naïve patients, and perhaps as effective as DTFC plus latanoprost. However, the two studies had slightly different inclusion criteria (all patients in the present study had glaucoma) and the patient groups thus may not be directly comparable.
A larger effect of DTFC was reported in a small 2005 open-label, unmasked study in 18 patients with IOP >30 mmHg (mean 37.5 mmHg) who had been untreated for 1 month, rather than being entirely treatment-naïve.16 At 2 months, IOP reduction was reported as 19.9 mmHg at peak and 16.4 mmHg at trough, but the average baseline IOP was nearly 10 mmHg higher than in this study.
A long-term, open-label, unmasked 2010 study compared DTFC with LTFC in 178 previously untreated patients.17 Over 4 years, DTFC lowered IOP by 8.8 mmHg, and LTFC by 7.6 mmHg, with some evidence of reduced glaucomatous progression in the DTFC group. Given the different time frame, it is difficult to assess how these results compare with those of the present study, but clearly fixed combination treatment is a reasonable option in patients at sufficient risk of progression.
Despite some variation in the findings, all these studies suggest that treatment with a fixed combination is generally well tolerated and effective in treatment-naïve patients, with no serious safety concerns. Similarly, the present study also reveals no new safety concerns for BTFC, with the adverse events reported consistent with those observed in previous studies. Both treatments were generally well tolerated, with only one discontinuation during the study, adjudged not related to study treatment. The incidence of ocular hyperemia was comparable between drugs (18.6% of patients with BTFC vs 15.4% with LM), consistent with existing evidence that adding timolol to bimatoprost reduces the likelihood of this effect.18
In conclusion, BTFC appears to be effective and well tolerated in treatment-naïve patients with POAG or pseudoexfoliative glaucoma who are at high risk of progression, in line with the existing data supporting its use in patients who are insufficiently responsive to monotherapy.
Acknowledgments
The GIFT (Ganfort as Initial Fixed-combination Therapy in high risk patients) study group consists of:
Writing group: Esperanza Gutierrez-Diaz, Jose Silva Cotta, Francisco J Muñoz-Negrete, Consuelo Gutierrez-Ortiz, Robert Morgan-Warren, and John Maltman.
Coordinating investigators: (Spain) Dr Pedro Corsino Fernández-Vila, Hospital Provincial de Pontevedra, Pontevedra; Dr Manuel Cordido, Complejo Hospitalario Universitario A Coruña (CHUAC), A Coruña; Dra Elena Millá, Hospital Clínic de Barcelona, Barcelona; Dr Andrés Suárez, Complejo Universitario de Santiago, Santiago de Compostela; Dr José Luis Urcelay Segura, Hospital Gregorio Marañon Instituto Oftálmico, Madrid; Dra Ana Fernández Granda, Hospital del Tajo, Madrid; Dr David Antolín, Hospital Universitario La Paz, Madrid; (Portugal) Dr Pedro Miguel Faria, AIBILI, Coimbra; Dr António Castanheira Faria, Instituto de Oftalmologia Dr Gama Pinto, Lisboa; Dr Antonio Rodrigues Figueiredo, Hospital de Santa Maria, Lisboa; Dr Maria João Fernandes dos Santos Meneres, Hospital de Santo Antonio, Porto.
This study was funded by Allergan Pharmaceuticals Ireland. The authors thank Darwin Healthcare Communications for writing and editing support, funded by Allergan Pharmaceuticals Ireland.
Disclosure
Esperanza Gutierrez-Diaz has received grants from Allergan and Sylentis, consultancy fees from Merck, and lecture fees from Alcon and Merck. Robert Morgan-Warren and John Maltman are employees of Allergan Holdings Ltd. The other authors report no conflicts of interest in this work.
References
European Glaucoma Society. Terminology and Guidelines for Glaucoma. 3rd ed. Savona, Italy: Editrice Dogma; 2008. | |
Leske MC, Heijl A, Hyman L, et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114:1965–1972. | |
van der Valk R, Webers CA, Lumley T, et al. A network meta-analysis combined direct and indirect comparisons between glaucoma drugs to rank effectiveness in lowering intraocular pressure. J Clin Epidemiol. 2009;62:1279–1283. | |
Aptel F, Cucherat M, Denis P. Efficacy and tolerability of prostaglandin analogs: a meta-analysis of randomized controlled clinical trials. J Glaucoma. 2008;17:667–673. | |
Aptel F, Cucherat M, Denis P. Efficacy and tolerability of prostaglandin-timolol fixed combinations: a meta-analysis of randomized clinical trials. Eur J Ophthalmol. 2012;22:5–18. | |
Allergan. Safety and Efficacy of Bimatoprost/Timolol Fixed Combination Versus Latanoprost in Patients With Open-Angle Glaucoma Who Have Never Been Treated. Available from: http://clinicaltrials.gov/show/NCT01243567. NLM identifier:NCT01243567. Accessed 3 February, 2014. | |
[No authors listed]. Good Clinical Practice Guideline 1997. Available from: http://ec.europa.eu/health/files/eudralex/vol-10/3cc1aen_en.pdf. Accessed 7 February 2014. | |
Konstas AG, Lake S, Economou AI, et al. 24-Hour control with a latanoprost-timolol fixed combination vs timolol alone. Arch Ophthalmol. 2006;124:1553–1557. | |
Konstas AG, Hollo G, Mikropoulos D, et al. Twenty-four-hour intraocular pressure control with bimatoprost and the bimatoprost/timolol fixed combination administered in the morning, or evening in exfoliative glaucoma. Br J Ophthalmol. 2010;94:209–213. | |
Konstas AG, Hollo G, Mikropoulos DG, et al. 24-hour efficacy of the bimatoprost-timolol fixed combination versus latanoprost as first choice therapy in subjects with high-pressure exfoliation syndrome and glaucoma. Br J Ophthalmol. 2013;97:857–861. | |
Hommer A. A double-masked, randomized, parallel comparison of a fixed combination of bimatoprost 0.03%/timolol 0.5% with non-fixed combination use in patients with glaucoma or ocular hypertension. Eur J Ophthalmol. 2007;17:53–62. | |
Konstas AG, Kozobolis VP, Tersis I, et al. The efficacy and safety of the timolol/dorzolamide fixed combination vs latanoprost in exfoliation glaucoma. Eye. 2003;17:41–46. | |
Quaranta L, Miglior S, Floriani I, et al. Effects of the timolol-dorzolamide fixed combination and latanoprost on circadian diastolic ocular perfusion pressure in glaucoma. Invest Ophthalmol Vis Sci. 2008;49:4226–4231. | |
Ozkurt YB, Sengor T, Evciman T, et al. Administration of the fixed combination of latanoprost 0.005% and timolol 0.5% in glaucoma patients with an intraocular pressure over 30 mmHg. Clin Ophthalmol. 2009;3:337–339. | |
Crichton AC, Harasymowycz P, Hutnik CM, et al. Effectiveness of dorzolamide-timolol (COSOPT) in patients who were treatment naive for open-angle glaucoma or ocular hypertension: the COSOPT first-line study. J Ocul Pharmacol Ther. 2010;26:503–511. | |
Henderer JD, Wilson RP, Moster MR, et al. Timolol/dorzolamide combination therapy as initial treatment for intraocular pressure over 30 mm Hg. J Glaucoma. 2005;14:267–270. | |
Pajic B, Pajic-Eggspuehler B, Hafliger IO. Comparison of the effects of dorzolamide/timolol and latanoprost/timolol fixed combinations upon intraocular pressure and progression of visual field damage in primary open-angle glaucoma. Curr Med Res Opin. 2010;26:2213–2219. | |
Brandt JD, Cantor LB, Katz LJ, et al. Bimatoprost/timolol fixed combination: a 3-month double-masked, randomized parallel comparison to its individual components in patients with glaucoma or ocular hypertension. J Glaucoma. 2008;17:211–216. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.