Back to Journals » Clinical Interventions in Aging » Volume 13
Benign paroxysmal positional vertigo in the elderly: current insights
Authors Balatsouras DG, Koukoutsis G, Fassolis A, Moukos A, Apris A
Received 8 April 2018
Accepted for publication 11 July 2018
Published 5 November 2018 Volume 2018:13 Pages 2251—2266
DOI https://doi.org/10.2147/CIA.S144134
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Walker
DG Balatsouras,1 G Koukoutsis,1 A Fassolis,1 A Moukos,1 A Apris2
1Department of ENT, Tzanion General Hospital of Piraeus, Piraeus, Greece; 2Department of ENT, Nicosia General Hospital, Nicosia, Cyprus
Abstract: Balance disorders, unsteadiness, dizziness and vertigo in the elderly are a significant health problem, needing appropriate treatment. One third of elderly patients with vertigo were diagnosed with benign paroxysmal positional vertigo (BPPV), the most common cause of dizziness in both primary care specialist Neurology and Ear Nose Throat settings. BPPV presents a specific paroxysmal positional nystagmus which can be obtained using the appropriate diagnostic positional test and can be treated effectively using specific therapeutic maneuvers. This review presents current insights into the diagnostic, pathogenetic and therapeutic aspects of BPPV in the elderly. BPPV in older patients does not differ significantly from BPPV in younger patients, with regard to pathogenesis, diagnosis and treatment. However, in older patients, its prevalence is higher and it responds less effectively to treatment, having a tendency for recurrence. Specific issues which should be considered in the elderly are: 1) difficulty in obtaining an accurate history; 2) difficulty in performing the diagnostic and therapeutic maneuvers, which should be executed with slow and gentle movements and extremely cautiously to avoid any vascular or orthopedic complications; and 3) the relation between BPPV and falls.
Keywords: dizziness, vestibular, benign paroxysmal positional vertigo, balance, elderly
Prevalence
Vestibular symptoms and dizziness are a usual and significant problem in the elderly, where their prevalence has been estimated to be 30% in persons older than 60 years,1,2 and approaching 50% after the age of 85 years.1 The presence of dizziness constitutes a major predictor of falls in the elderly, which are the leading cause of accidental death after the age of 65 years.3 Dizziness has increased prevalence in women4 and is a major factor for disability in persons beyond 65 years of age.5 Common causes of vertigo in the elderly may manifest differently, with a more confusing set of symptoms, as patients tend to report rotatory vertigo less and nonspecific dizziness and instability more than younger patients with the same condition.6
A specific feature of balance disorders in elderly is that older patients complain less of rotatory vertigo and more often of nonspecific unsteadiness and dizziness than younger persons with a similar disease.6 The main feature of dizziness in the elderly is the gradual multisensory deterioration of balance. Vestibular and proprioceptive functions are affected and the impairment of their central integration along with various other sensory inputs that are related to aging, including vision and hearing, constitute the phenomenon of presbyequilibrium, occasionally called multisensory dizziness.7 Accordingly, dizziness in the elderly is actually a multifactorial geriatric syndrome manifesting in multiple ways and involving several organic systems, such as sensory, neural and cardiovascular.8 Although most types of dizziness in the elderly are benign, in a few patients however, there underlies a serious and probably life-threatening cause, such as a stroke, which occurs more commonly in older patients.9
Barin and Dodson2 divided the underlying causes of disequilibrium and dizziness in the elderly into three broadly defined types:
- Age-related deterioration of acuity in sensori-motor pathways in conjunction with decline of integration processes within the central nervous system. These types of impairment, which are common in older adults, may be considered a normal part of aging.
- Pathologies that may evoke dizziness in persons of any age but are more common in older persons, either due to increased susceptibility because of age-related processes or due to increased probability of exposure to these factors with the passage of time.
- Finally, an assortment of environmental and lifestyle factors, which may be responsible for increased frequency of balance disorders and dizziness in older persons. These may include limited mobility, absence of activities and frequent use of medications, having the common adverse reaction of dizziness.
Benign paroxysmal positional vertigo (BPPV) may be included in the second category, because although it may manifest at any age, it is much more common in the elderly, owing to the continuous degradation of the maculae of the sensory otolith organs of the vestibule. In the National Health Interview Survey conducted in 2008 in the USA, more than 7 million Americans exceeding 65 years of age complained of balance disorders. The most common causes that were reported included adverse reactions to medication (11.3%), infections of the inner ear (11.0%), heart disease (8.6%) and loose otoliths (7.9%), which probably indicates a diagnosis of BPPV.10 Regarding patients referred to otolaryngologists or Neurotology clinics, BPPV is by far the most common diagnosis in patients with dizziness, especially in the elderly.11
It has been reported that a diagnosis of BPPV is established in 17%–42%12 of patients with vertigo. In a registry with data from 4,105 vertigo patients collected during a 28-month period from 618 centers in 13 countries presented 26.9% were diagnosed with BPPV.13 The 1 year prevalence of BPPV in patients older than 60 years rises abruptly with age, being almost seven times higher in comparison with that of patients 18–39 years old.11 Sogebi et al in a study of vestibular disorders in the elderly reported positional vertigo in 33.3% of the study group, although some of the patients did not fit the stringent criteria of BPPV.14 In another study, almost 40% of patients with age exceeding 70 years received diagnosis of BPPV.15 The prevalence of unrecognized geriatric BPPV is also high. Consecutive examinations in a geriatric population revealed 9% of those examined having unrecognized BPPV.16 The authors found that patients with undiagnosed BPPV were more likely characterized by depression, occurrence of fall during the previous 3 months and reduced activities of daily living chores.
Pathogenesis
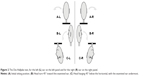
Pathophysiology of BPPV may be explained by two cardinal theories, cupulolithiasis and canalolithiasis (Figure 1). Shuknecht reported in 196917 that BPPV was the result of “cupulolithiasis” of the posterior semicircular canal (SCC).
This theory supports that otoconia from the utricle and saccule may be detached and adhere to the cupula of the posterior SCC. Since otoconia has a relative density about three times greater than that of the endolymph, cupula becomes sensitive to gravity. Consequently, when the head takes a hanging position, a pathologic vestibulo-ocular reflex occurs. Specific head movements can cause inappropriate cupula displacement and resulting endolymph movement, causing nystagmus and vertigo. Moreover, Shucknecht documented the presence of basophilic deposits on the cupula of the posterior SCC in three patients who had BPPV during their lives, supporting his theory of cupulolithiasis. The latent period of time observed before the beginning of nystagmus may be explained as the time needed for the mass of otoconia to be displaced because of inertia, and the phenomenon of fatigability is explained by the dissolution of the otoconial debris in the endolymph.17 However, this theory does not explain convincingly the fatigability on repetitive diagnostic positional tests and the absence of spontaneous nystagmus, which should be more or less persistent and should be provoked not only by specific diagnostic maneuvers, but also from any vertical, oblique or horizontal linear acceleration of the head.18
Another, more plausible, explanation of the BPPV pathogenesis was proposed in 1979 by Hall et al.19 The authors proposed that BPPV was caused by floating otoconia of the otolithic membranes moving freely within the posterior SCC, and named their theory “canalolithiasis”. Canalolithiasis may explain more convincingly the finding of fatigability, which occurs when the otoconia gets dispersed in the endolympatic fluid.19 All other features of BPPV may be sufficiently explained as well, and moreover, the success of the therapeutic maneuvers that aim to remove the floating otoconia from the endolymph of the posterior SCC is the best argument supporting the validity of this theory.20 Howeverthe cupulolithiasis theory (heavy cupula)21 of the posterior SCC is a plausible pathogenetic mechanism in patients with persistent torsional/vertical positional nystagmus and specifically in cases resistant to standard treatment maneuvers.21,22
In both theories, the initial pathogenetic factor is dislodgement of otoconia. This phenomenon is more common in the elderly, because during lifetime the number and volume of otoliths are progressively reduced and the interconnecting fibers between the otoliths may weaken from age-related reduction of calcium carbonate crystals in the process of demineralization. The result is the separation of the otoconia from the otolithic membrane and free movement within the endolymph.20 Altered endolymphatic pH and calcium concentration are age-related processes that may contribute to the pathogenesis of BPPV and aggravate the symptoms.
Loose otoliths are found very frequently in the lumens of all SCC, and especially in the posterior canal, but are quite common in other SCC as well. Their presence is, however, mostly asymptomatic, because a critical mass is necessary for the manifestation of the clinical presentation.23
Etiology
BPPV may be distinguished from idiopathic and secondary BPPV induced by the detachment of otoconia due to various causes. According to Riga et al, for a causative association to be valid, BPPV should occur on the same side as the causative disease and the clinical symptoms should appear either concurrently or soon after the manifestation of the primary disease.24 Occasionally, it is not evident if there is a real causative association or rather an accidental relation. It seems that most patients with BPPV suffer from idiopathic BPPV and only in a minority of them a specific etiological factor can be detected. In various studies, different rates of secondary BPPV have been found, ranging from 3% to 66%.25–31 It should be noted that in two large studies conducted by Caldas et al29 (1,271 patients) and Karlberg et al27 (2,847 patients), the incidence of secondary BPPV varied significantly between 25.2% and 3% correspondingly. This may be attributed to different diagnostic criteria, differences in the patient groups studied and variance in referral patterns. The main causes of secondary BPPV include Meniere disease (0.5%–30%), head injury (8.5%–27%), vestibular neuritis (0.8%–20%) and sensorineural hearing loss (0.2%–5%).25–31 Less frequent causes of BPPV such as recent myocardial infarction, sarcoidosis, active ulcerative colitis, carcinomas treated by chemotherapy and/or radiotherapy and leukemia have been reported.32
Various medical conditions may possibly induce BPPV. Older people commonly have various comorbidities that can negatively affect the quality of life and autonomy. Several neurotological conditions commonly associated with dizziness and balance disorders such as tinnitus, hearing loss and vestibular hypofunction occur frequently in the elderly.33–36 Common pathologies in advanced ages, such as hypertension and diabetes mellitus, are related to progressive hearing deterioration or even to sudden sensorineural hearing loss and furthermore, a relation between psychiatric disorders and vestibular diseases has been found. An influence of comorbidities of the elderly on the vestibular system is also possible. Such comorbidities include arthritis, hypertension, diabetes and various metabolic disorders, osteoporosis and depression. Santos and Bittar37 evaluated the metabolic features of 325 patients with vertigo and compared them with those found in the general population. They concluded that patients with vertigo had more metabolic disorders, such as increased or decreased levels of thyroid hormones, increased level of low-density lipoprotein and increased frequency of diabetes.
In a cross-sectional, multicentre study of 1,092 BPPV patients collected from 11 referring Centers,33 the role of the most common comorbidities affecting the older people (hypertension, diabetes, osteoarthritis, osteoporosis and depression) in the recurrence of BPPV was studied. The authors found the presence of at least one comorbid disorder in 19.8% and two or more in 37.4% of the patients. There was also a positive statistical relation between the presence of comorbidities and the recurrence of the disease. Among the various comorbidities hypertension was the more frequent (15%) compared with diabetes, osteoarthritis, osteoporosis and depression, with approximate frequencies between 1% and 1.5% each. Additionally, it was reported that hypertension or diabetes mellitus presented a significant risk of BPPV recurrence. This may be attributed to the known effect of hypertension and vascular disease on the inner ear, causing reduced blood flow to the labyrinth and resulting in dislodgement of otoliths and manifestation of BPPV.38 Α similar mechanism may be hypothesized in cases with migraine, since vasospasm of the inner ear arteries may cause detachment of otoconia from the otolithic membrane.
The relationship between BPPV and diabetes mellitus is not quite clear. In a case–control histopathologic study of temporal bones in humans,39 the authors compared the prevalence of cupular and free-floating otoconia in the SCCs between the temporal bones of patients with type 1 diabetes and normal controls. They reported that the presence of otoconia in the horizontal and posterior SCC was significantly higher in the patient group than in the control group. However, the increased prevalence of the otoconia was related to the duration of the disorder and not to the age of the patients, which may be attributed to the fact that type 1 diabetes affects younger persons and commonly begins before the age of 40.
In a retrospective paper on the data of 3,933 patients,38 it was reported that the occurrence of BPPV was higher in people with type 2 diabetes (46%) compared to those without the metabolic disease (37%). Furthermore, it was found that hypertension provided the interconnecting pathway for the effect of diabetes on the vestibular system, since the prevalence of arterial hypertension was 40%–60% higher in 45–70 years old patients with type 2 diabetes mellitus, as compared to subjects without diabetes. In a recent report, Jáuregui-Renaud et al40 studied the function of horizontal SCCs and utricle in patients with type 2 diabetes, who did not have any history of dizziness or other vestibular symptoms. The authors found that the patients had reduced responses to unilateral centrifugation compared to healthy volunteers, but their responses to horizontal SCC stimulation were similar and they had also a larger sway area and a lengthier sway path in the test of posturography. They concluded that utricular function may be defective even in the absence of horizontal SCC involvement or a history of falls. Accordingly, this utricular hypofunction may predispose diabetic patients, after a long duration of the disease, to manifestation of BPPV.
Besides diabetes mellitus, osteoporosis has raised much interest and recent research has revealed that this is a risk factor for BPPV, probably because abnormal calcium metabolism may underlie BPPV.32,41 A large population-based study including 6,649 patients with osteoporosis and 26,596 matched controls demonstrated that the risk of manifesting BPPV in patients with osteoporosis was a 1.82-fold higher than in the controls.42 A usual cause of disorders in the metabolism of calcium is the deficiency of vitamin D. Yang et al43 evaluated the relationship between bone mineral density and 25-hydroxyvitamin D with the presence and recurrence of BPPV. The authors found that low bone mineral density in women and low serum 25-hydroxyvitamin D levels in men were significantly associated with the recurrence of BPPV, whereas age was an independent predictor of recurrence. The pathogenetic role of osteoporosis has been confirmed in several other studies as well.44–46
Types of BPPV
The posterior SCC is the more frequently involved SCC in the pathogenesis of BPPV. The explanation for this is the anatomical location of the SCC at the most dependent position in the vestibular apparatus in a standing person.23 If otoconia gets detached, it moves to the back and the base of the system, at the location of the posterior canal. The less frequent BPPV of the horizontal SCC was first reported from McClure47 and Cipparrone et al48 in 1985 independently, hypothesizing a similar pathogenesis. The horizontal SCC BPPV has a prevalence exceeding 20%49 of all cases in various reports, although according to other authors and in our own experience, its prevalence is quite lower, with a rate approaching 10%.23,50,51 The frequency of anterior SCC involvement has been found, in some series, to be as high as 12%,52 but according to our data and most of the relevant reports, its occurrence is much lower.23 Anagnostou et al53 in a systematic review estimated an occurrence of anterior SCC BPPV among patients with BPPV of 3% (range 1%–17.1%). Bilateral involvement and involvement of two SCCs, found at a rate of 9.3% in our previous report, is quite common as well.51
It seems that anterior SCC BPPV is not very common in older people. Soto-Varela et al54 who studied the demographic, pathogenetic and therapeutic differences between patients with BPPV according to the SCC involved, found that patients with anterior SCC BPPV had a mean age of 52.75, whereas the mean age in BPPV of posterior and horizontal SCC was 58.73 and 57.79 years, respectively. The differences in age were statistically significant. However, Yeo et al49 in a recent study in which the authors compared two groups with idiopathic BPPV divided by age (study group age 65 years or more and control group with age less than 64 years), found similar rates of affected semicircular canals. According to our experience, however, anterior SCC BPPV very rarely occurs in older people.
Diagnosis
The involvement of the posterior SCC comprises the majority of the cases which a clinician comes into contact with and thus we are going to discuss this in more detail. The diagnostic work in patients suffering from vertigo starts with a thorough evaluation of their medical history. A detailed recording of their associated symptoms and duration, previous surgical interventions, history of infections or trauma and medications should be performed followed by a general physical and neurological examination. Otoscopy should be performed in order to exclude obvious pathology in the external or middle ear and confusing symptoms, in the context of a complete otolaryngologic examination. The examiner should be able, at this point, to define the possible cause of the disease or, at least, to distinguish between peripheral and central vertigo.55–58
History taking is very helpful in the diagnosis of BPPV. Patients typically describe rotation of the environment during an abrupt change of position, as when rising from a supine position, getting an object from above and in other circumstances of hyperextension of the neck. Many cases with idiopathic BPPV are probably related to sleeping59 and typically patients describe the appearance of vertigo when they lie on the bed, upon rolling from side to side, or when they rise from the bed in the morning. Vertigo is usually intense but has a brief duration of a few seconds only, followed by a longer period with postural unsteadiness.
Older adults, however, tend to present ess typical features of BPPV,60 a longer duration of the disease and increased recurrences. Batuecas-Caletrio et al61 in their report on the features of elderly patients with BPPV found that patients with age exceeding 70 years delayed asking for medical consultation, and when compared to younger patients they presented balance disorders and unsteadiness without typical vertigo more frequently.
Diagnosis of BPPV is established by the Dix-Hallpike maneuver. The test is performed by bringing down the patient rapidly from a sitting position to a position with the head hanging 20°–40° below horizontal over the edge of the bed with the test ear undermost (Figure 2). The examiner should test the least suspected ear first, but in cases with no evidence of laterality it does not matter which ear is tested first. The response is considered positive when the patient complains of vertigo and an accompanying nystagmus is observed. The nystagmus presents several typical features characteristic of excitation of the posterior SCC: 1) short latency of a few seconds; 2) rotatory-vertical nystagmus with a rotatory component beating counterclockwise on testing of the right ear and clockwise on testing of the left ear, as seen by the examiner, and a superimposed upbeating vertical component; 3) reversal of the nystagmus upon return to the sitting position; 4) transience; and 5) fatigability.55,56
Although the standard and most effective test to diagnose BPPV of posterior SCC is the Dix-Hallpike maneuver, there are several contraindications and protective measures which should be occasionally taken.62 The risk of vascular injury and stroke should be always considered in patients with vascular disease. Care should also be taken in patients with various comorbidities, such as kyphoscoliosis, limited cervical motion, cervical spinal stenosis, cervical radiculopathy, advanced rheumatoid arthritis, lower back pain and dysfunction and morbid obesity, which may exist more frequently in older people. However, specifically designed adjustable examination tables permit the safe performance of the Dix-Hallpike test in patients with physical limitations.
The side-lying maneuver is an alternative diagnostic test which can be used on patients unable to undergo the Dix-Hallpike maneuver, but has much lower sensitivity of only 65%.63,64 It should be noted that since older adults do not always describe rotation but only unsteadiness or dizziness, the Dix-Hallpike maneuver should be performed ubiquitously when the disease is suspected, even in the absence of vertigo with positional changes.45,61,65 Furman et al65 state that the physical examination of the elderly with dizziness is similar to that of the younger subjects, with the following exceptions: “definitely obtain orthostatic signs, perform a mini mental status examination, check for extremity and postural tremor, and perform a Dix-Hallpike maneuver in all cases”.
In the instances where positional testing is not feasible because of disorders of mobility or other comorbidities, diagnostic and treatment problems may exist. A test for BPPV has been proposed, based on the detection of the presence of otoconia proteins in the patient serum, but further research is needed to corroborate this approach.66 An alternative solution, not available worldwide and rather expensive, could be the use of specific equipment; biaxial rotational chairs that can treat patients regardless of other mobility difficulties.67 Patients are tied to the chair and wear infrared video goggles in order to qualitatively and quantitatively enable the study of the provoked nystagmus in various positions. There are two such devices, the Epley Omniax System developed by Epley,68 an automated, electronically managed, multi-axial device that can move and position the patient appropriately to treat the involved canal, and the TRV chair. The latter was invented by Thomas Richard-Vitton in Marseille,69 has a horizontal and vertical axis of rotation and can be locked in certain positions. It is manually handled and can revolve between two axes in all the respective planes of the SCCs for up to 360°, while velocity of rotation can be adjusted freely.
Nystagmography is not useful in diagnosis of posterior SCC BPPV because recording of the rotatory element of the positional nystagmus is not possible by standard equipment. However, abnormal nystagmographic findings are common in patients with the disease, but not specific and diagnostic of BPPV.70 The role of imaging in BPPV patients has been extensively discussed but is controversial. History and physical examination may provide diagnosis in the majority of patients and neuroimaging should not be used as a routine diagnostic means in patients with BPPV.
According to the 2008 and 2017 guidelines from the American Academy of Otolaryngology – Head and Neck Surgery Foundation,71,72 computed tomography scans and MRI of the central nervous system should be performed only in patients with possible BPPV confounded by atypical additional neurologic symptoms. Neuroimaging may also be performed in patients with a positive history of BPPV and inconclusive positional testing or in patients with neurologic signs and findings atypical of BPPV, such as visual disturbances, severe headaches or cranial nerve abnormalities.
A similar explanation has been given for the pathogenesis of BPPV of the horizontal canal.56,73 When a patient with this condition, lying in the supine position turns the head from side to side in a rolling movement in a test called the supine roll test, he experiences severe vertigo. However, the patient can bend or hyperextend the head and rise from the bed or lie down with minimal symptoms. The vertigo is accompanied by a purely horizontal or torsional-horizontal paroxysmal positional nystagmus. Horizontal SCC BPPV owing to canalolithiasis, which is the most common type, manifests usually with bilateral geotropic (beating toward the ground) horizontal or torsional-horizontal nystagmus, which is more intense on the involved side.74–77 Frequently, a reversal of the nystagmus without changing the position of the head may appear, characterized by smaller frequency and amplitude and an apogeotropic direction of the fast phase (beating away from the ground).78 The nystagmus resulting from the excitation of the horizontal SCC BPPV has an abrupt onset, very short latency, and longer duration in comparison with the corresponding nystagmus resulting from the excitation of the posterior SCC. Additionally, the vertigo is usually more intense and accompanied by severe manifestations from the autonomic system. However, except for this common horizontal SCC BPPV with transient direction changing geotropic nystagmus, there is a minority of cases with persistent geotropic nystagmus, which lasts for more than 1 minute and is thought to be due to light cupula of the horizontal canal.79,80
A less frequent type of horizontal SCC BPPV derives from cupulolithiasis (heavy cupula) and occurs in 1/3 of the cases with horizontal SCC BPPV. This differs from the canalolithiasis type in the direction of the fast phase of the horizontal nystagmus, which is apogeotropic. The nystagmus also is always persistent (duration of more than 1 minute) and more intense on the noninvolved side.75,81,82 Occasionally, the same findings may appear in canalolithiasis within the anterior arm of the horizontal SCC near the ampulla, presenting bilateral apogeotropic nystagmus also.57 Contrary to the involvement of the posterior and anterior SCC, in patients with BPPV of the horizontal type, nystagmographic recording may be performed to show the typical features of the horizontal SCC BPPV nystagmus.70
Finally, anterior SCC BPPV has a similar pathogenetic mechanism and may be diagnosed using the Dix-Hallpike maneuver,53,83–85 but will not be discussed here in detail, because it is quite rare in older people. Table 1 shows the diagnosis of the involved semicircular canal and the side of disease, according to the type of the resulting nystagmus after performing the appropriate diagnostic test. Table 2 shows the specific features of the main BPPV types.
  | Table 2 Diagnostic test, characteristics and treatment of BPPV types |
In several circumstances, a patient may present a typical history of BPPV, but the diagnostic maneuver does not produce any positional nystagmus. Absence of symptoms and typical positional paroxysmal nystagmus during the Dix-Hallpike maneuver may usually be attributed to fatigue due to previous exercise, remission of the disease or another etiology.86 Occasionally, some patients complain of vertigo and manifestations from the autonomic system without presence of any observable nystagmus. This clinical entity has been called “subjective BPPV” and occurs quite frequently.87 We studied this clinical entity in a previous report,88 in which we compared a group of patients with subjective BPPV and a group of controls with typical BPPV. Both groups of patients had similar demographic and clinical characteristics, with the exception of the somewhat older mean age of patients with subjective BPPV (55.4 vs 53.4, a difference not statistically significant). We concluded that subjective BPPV is quite frequent, occurring in more than one-fourth of patients with BPPV and has common findings with typical BPPV, without any difference in treatment methods and outcomes.
Several theories attempt to explain the absence of positional nystagmus in subjective BPPV. Weider et al89 attributed the absence of nystagmus to the effect of ocular fixation. However, the theory of limited otoconia offers a more likely explanation.86 It has been reported that free otoconia is found quite frequently within the endolymph of all the SCCs but occurs most commonly in the posterior SCC. In most cases, its presence is asymptomatic, but occasionally vertigo may be provoked depending on the composition, the gravity, the mass and the number of the free-floating particles, which may vary in each SCC and in different patients.23 Thus, SCC involvement may be asymptomatic and the patient may show clinical symptomatology only after the otoconial mass has exceeded a critical quantity. Initially, vertigo may be the only symptom, but when the free-floating otoliths increase, the complete picture of BPPV, including the typical paroxysmal positional nystagmus, will appear. Finally, a mild nystagmus may not be observable with the naked eye, but Frenzel lenses and nystagmography may assist in its observation and recording.87,88
Many peripheral and central disorders may produce positional nystagmus and differential diagnosis of such clinical entities from BPPV should be done.32,90 The most commonly found central disorders that can cause positional vertigo include transient vertebrobasilar ischemia and central positional vertigo. Various types of central positional vertigo exist, which present long-lasting downbeating nystagmus, vertigo and vomiting. In all patients who have atypical findings and in whom treatment outcome is unsuccessful, MRI should be ordered. Several disorders are associated with manifestation of BPPV as a sequel, such as vestibular neuritis, vestibular migraine, Meniere’s disease, neurolabyrinthitis and sudden sensorineural hearing loss. Uptake of medications, including antidepressants, anticonvulsants, tranquilizers, anxiolytics, sedatives, muscle relaxants, strong analgesics, and antiarrythmics, may cause positional vertigo as well.90
Treatment
In general, vestibular suppressants and antiemetic medications are not recommended in the treatment of BPPV. The guidelines of the American Academy of Otolaryngology – Head and Neck Surgery Foundation (2008 and 2017),71,72 recommend against routine treatment of BPPV with vestibular suppressant drugs such as antihistamines, benzodiazepines or anticholinergics. However, their use may have a role in the short-term control of symptoms from the autonomic nervous system, such as nausea, pallor or vomiting, in patients with severe clinical picture.32,91 Typical examples of brief treatment with vestibular suppressants include patients who present severe symptoms after a therapeutic maneuver, and patients who decline treatment although their symptoms are severe. Vestibular suppressants may also be used as a prophylactic in patients with a history of severe autonomic symptoms accompanying the performance of the Dix-Hallpike tests and in whom a therapeutic maneuver is programmed. In cases of prescription for these specific indications, physicians should warn their patients that use of this category of drugs may potentially cause falls, drug interactions, and affect machinery handling and driving. The use of vestibular suppressants in the elderly is potentially inappropriate because their adverse reactions include falls, urinary retention and confusion.92 Accordingly, medications should be considered in the elderly with BPPV only in the context of patients’ comorbidities.
Historically, the treatment of BPPV had a long journey from being neglected in the first half of the past century to aggressive surgical procedures in the latter half. Substantial progress was achieved in the 1980s, when several therapeutic maneuvers were invented for the therapy of BPPV, as an alternative to conservative treatment (observation and avoidance of abrupt head movements provoking vertigo) and surgical interventions. Such maneuvers are intended to return the dislodged otoliths to the utricle so that the altered gravity sensitivity no longer affects the vestibulo-ocular reflex during head movements. Brandt and Daroff93 induced the first effective therapy for BPPV in 1980, consisting of a set of vestibular habituation exercises repeated several times a day for 3 weeks. Semont et al94 were the first to propose in 1988 a single physical liberatory maneuver as an alternative, based on the same pathogenetic mechanism theory. However, the major evolution in the treatment of BPPV was the “canalith repositioning procedure” (CRP) induced by Epley in 1992,95 based on the mechanism of canalolithiasis.
CRP begins with the patient sitting on the examination table. The physician turns the head 45° to the side involved (Figure 3). Then the patient is asked to lie down backward, so that the head is just off the edge of the table and slightly extended 20°–30° below the horizontal, with the head turned to the affected side. The next stage involves moving of the head slowly toward the opposite healthy ear that is now under. Then the body of the patient is rolled to a side-lying position in line with the head, which is then turned 45° more toward the same healthy ear and downward to the ground. Then, the patient is brought upright to the sitting position, a movement which must be done slowly and cautiously because during this stage, the patient may experience intense vertigo and a tendency to fall. The result of this procedure is that the detached otoliths move through the common crus of the posterior and anterior SCC and enter the cavity of the utricle, causing no more symptoms. After the therapeutic maneuver, the patient is advised not to bend over or hyperextend the head for the next 2–3 days. An additional prophylactic measure is sleeping in a slightly elevated position without turning the head toward the diseased ear.
If the maneuver fails to treat the patient, it must be repeated at least once or twice and occasionally several times, but if the symptoms continue, Semont’s “liberatory” maneuver may be performed. This maneuver may be useful in both cupulolithiasis and canalolithiasis and intends detaching the otoliths from the cupula, moving them out of the posterior SCC into the utricle. The patient sits on the edge of the examination table with the head turned horizontally 45° to the healthy ear (Figure 4). The examiner lays the patient down to the side-lying position on the affected side with a fast movement, without changing the direction of the head that touches the bed behind the affected ear. After 1–3 minutes, the patient is brought swiftly, without stopping in the initial upright position, to the contralateral side-lying position, with the head still turned 45° to the healthy ear and with the nose down. After 1–3 minutes the patient sits, up slowly. The same posttreatment instructions are given to the patients, as previously described for the Epley CRP. However, according to the authors’ experience, performance of Semont’s maneuver is very difficult in older adults because a sufficient level of mobility is required which is frequently missing in the elderly. Rapid body movements involved in Semont’s maneuver may not be appropriately obtained in obese or frail patients,96 although it is easier to use this maneuver in patients with decreased neck mobility, because it does not involve any head reclination.
In many older patients with BPPV, however, performance of CRPs may be difficult because of various orthopedic and vascular problems, including limited range of motion in the cervical spine, kyphosis, or a history of vertebrobasilar insufficiency or stroke.65 Accordingly, it would be appropriate in these cases to use slow mild movements and avoid sudden fast movements and hyperextension of the head. Caution should also be exercised in performing extension of the neck in patients with a history of vascular disorders since vessel compromise may frequently occur in the elderly. Another issue which must be seen is that the presence of downbeating nystagmus may be frequently and mistakenly attributed to BPPV.97 So care should be exercised to ascertain that the patient’s nystagmus has the typical features of fatigability and short onset latency as soon as the head is brought to the provoking position. A helpful means of performing the CRPs in the elderly is putting the examination bed in the Trendelenburg position, because the key to a successful CRP is the position of the head relative to gravity rather than its position with regard to the body.65
Anterior SCC BPPV may be treated either by the same CRPs that are currently in use for the much more frequent posterior SCC BPPV or by the specific maneuvers,83 which will not be described herein, due to the rare occurrence of this type of BPPV in older patients.
The effectiveness of the CRPs is widely recognized in literatures. In a Cochrane review,98 based on a systematic review of all relevant randomized controlled trials, it was reported that the Epley maneuver was quite effective in the treatment of posterior SCC BPPV without any complications. Another Cochrane review has further examined postmaneuver postural instructions after performance of the Epley CRP,99 such as using a neck collar, avoiding bending and hyperextension of the head and sleeping in a semi-recumbent position. The authors reported that a successful treatment outcome was obtained for 89% of the experimental group and 78% of the control group, and they concluded that there was a significant advantage of posttreatment restrictions after the Epley maneuver. However, it is difficult for older patients to comply with sleeping restrictions, because it has been reported that poor sleep in the elderly may result in increased risk of falls.100
In a Brazilian review of the effectiveness of CRPs in the treatment of older adults, Ribeiro et al101 confirmed the improvement in symptomatology in a group of 300 patients with an average age that ranged between 67.2 and 74.5 years. Prokopakis et al102 studied 965 individuals with BPPV, and found that CRPs resulted in successful treatment of BPPV, with 85% effectiveness after the first session. In 14% of the patients BPPV recurred, but most of them were older or had a positive history of vestibular neuropathy or head trauma. The authors proposed that because of the higher recurrence rate, additional education and instructions were necessary for the elderly to minimize the potential morbidity of their falls.
In cases of horizontal SCC BPPV, various CRPs have been introduced, but we consider the “forced prolonged position” recommended by Vannucchi et al103 as the most simple and effective. The patients are instructed to lie on their beds on the unaffected side and to stay there for ~12 hours. In this position, the affected ear is up and the horizontal SCC assumes a vertical position. This results in gradual removal of loose otoliths from within the endolymph of the nonampullary arm of the SCC out of the canal into the utricle, by gravitational pull. In case the older patients cannot stay for the entire time interval in the forced prolonged position, they can sleep for more than one night in the same position, or even perform another maneuver, such as the barbecue or the Gufoni maneuver, which is effective for both geotropic and apogeotropic nystagmus varieties of horizontal SCC BPPV.104–106
Recently, researchers proposed that chronic persistent dizziness in the elderly may be attributed to low intensity, horizontal, direction-changing apogeotropic positional nystagmus, which often manifests in cases with horizontal SCC BPPV.107 The authors reported that 49% of a total of 200 elderly subjects with chronic dizziness studied had this type of nystagmus, and suggested that this mild form of BPPV occured quite frequently in the elderly. These cases should be treated according to the previously mentioned principles.
In terms of treatment of BPPV in the elderly, difficulty in treatment of older adults with BPPV is admitted in most papers108,109 and can be explained in various ways: 1) difficulty in performing the CRP appropriately due to decreased neck mobility and lower back dysfunction; 2) a continuous process of degenerative demineralization of otoliths that causes detachment of otoliths from the otoconial membrane and disintegration;110 3) extended bed rest and inactivity which contributes to excessive dislodgement of otoconia, its separation from the otoconial membrane and dissolution within the endolymph of the SCCs. However, a few researchers state that rates of successful treatment outcome of CRPs in the elderly are similar to those with younger adults.49,111 Also, according to most authors, older adults present increased risk of recurrence49 which may be attributed to age-related detachment of otoconia.108,112 The recurrence rate in the elderly is estimated to be 23.5%–50% of the cases.108,113 However, Yeo et al49 did not notice any difference between older and younger patients in 1- and 5-year recurrence rates. According to our own experience, recurrences are more frequent in older patients, probably because of an on-going process of utricular degeneration.
Surgical management
With deeper knowledge of the pathophysiology of BPPV and development of effective repositioning maneuvers, surgical intervention for this benign disease has become rarer. However, a small percentage of patients have intractable and multiply recurrent BPPV which can be disabling and for whom surgical management should be considered.32 In one study it was reported that, out of 5,364 individuals treated in a vestibular unit, less than 1% needed surgical treatment.114 Surgical procedures for persistent posterior SCC BPPV include plugging of the posterior SCC and singular neurectomy.115 Canal plugging can be applied to horizontal and anterior SCC as well. Surgical complications include sensorineural or conductive hearing loss and protracted presence of unsteadiness and dizziness.116
BPPV and falls
In the last section of this review, we shall deal with the interesting topic of the relation of BPPV in the elderly to falls. Several reports correlate the presence of BPPV and increased risk of falls in older adults. Falls are the sixth most common cause of death in people older than 65 years, 30% of whom experience a fall at least once a year.117,118 Additionally, falls are the cause of 70% of accidental death in people older than 75 years.
Falls are significantly correlated with a decline in physical abilities which accompanies the aging process, and is functionally represented by a decrease or complete loss of the ability to perform daily tasks and functions when confronting environmental challenges.117 According to Walther et al,96 falls may be due to inactivity attributed to various causes such as laziness, lack of motivation, anxiety, fear, distress, psychogenic dizziness resulting from vestibular hypesthesia, vision disorders, or cardiac, orthopedic and neurologic morbidities. This can further provoke fear of one more fall, which leads to a greater extent of inactivity resulting in a positive recycling feedback. It has been reported that there is significant correlation between the rate of falls and reduction of activities after the last fall. Older patients with vestibular disease who experienced at least two falls present more significant decrease of activity in comparison with older adults who underwent only one fall.119 The limitation of activities may originate from fear of falls, trauma, medical instructions and various coexisting conditions. For older patients with chronic vestibular involvement, the environment demands greater postural control, making the tasks almost impossible to perform. In the outdoor environment, visual target stabilization, movement of the head and neck in coordination with the trunk, and dynamic balance to confront any obstacle, are probably much more difficult to achieve.117
In a neurotology clinic, falls can occur during the acute phase of peripheral vestibular disease, such as vestibular neuritis, in case of vestibular migraine, during attacks of Meniere’s disease, and especially during otolithic crises in the advanced stages of a Meniere’s disease, in case of defective vestibular compensation after bilateral vestibular failure and as a result of inactivity.96 A significant risk factor for falls in the elderly, however, is the presence of BPPV. Dynamic and static balance tests confirm that BPPV changes postural control of patients who did not receive treatment and increases unsteadiness, occurrence of falls and restriction of daily activities.120 After treatment with CRPs of BPPV in the elderly, a significant decrease in the number of falls has been reported in various publications.117,118 Significant functional improvement is obtained, and body instability and risk of falls is reduced.117
However, after successful treatment outcome and disappearance of vertigo and the accompanying positional nystagmus, older people continue to fall for several months after the performed CRPs and some aspects of postural balance are not improved.121 This may be attributed to disorders of other systems associated with body control which must be evaluated and treated. Thus, more extensive work is necessary in older patients with BPPV after a fall, including balance function tests and posturography. Another factor that contributes to the continuation of falls in the elderly, even after successful treatment of BPPV with CRPs, is residual dizziness, which may be present at a rate reported to approach 61% and lasting more than 2 weeks, and which may increase unsteadiness and risk of falls.122 Teggi et al123 reported that in patients older than 72 years, duration of vertigo and presence of anxiety are correlated with higher risk of residual dizziness after effective treatment of BPPV. The treatment of falls in the older population needs an approach based on prophylaxis, disease control and management of medical conditions which can predispose to falls. Additionally, extrinsic factors, such as environmental hazards, should be dealt with, besides the particular treatment of balance disorders diagnosed in each patient.65,124
Vestibular rehabilitation has been proven useful in patients with unsteadiness of aging.125 In a recent study of 240 elderly patients, Jung et al126 reported that vestibular rehabilitation treatment was useful in decreasing dizziness even in the absence of a specific diagnosis. The American Geriatric Society and British Geriatrics Society have published a clinical practice guideline on fall risk screening, assessment, and management, in which it is suggested that exercises for balance in standing are beneficial to older patients at risk of falling.127 Several authors112,128 reported that balance vestibular rehabilitation in conjunction with CRPs in elderly patients with BPPV improved dynamic balance measures in comparison with patients who received CRPs only. This could imply that addition of balance vestibular rehabilitation in older adults with BPPV could potentially reduce the risk of adverse sequelae of BPPV, such as falls and orthopedic injuries.
Conclusion
In conclusion, BPPV in the elderly does not differ significantly from BPPV in younger patients, with regard to pathogenesis, diagnosis and treatment. However, its prevalence is higher in older adults and it responds less effectively to treatment, having a tendency for recurrence. Specific issues which should be considered in the elderly are the relation to falls, difficulties in obtaining an accurate history, and some difficulty in performing the diagnostic and therapeutic maneuvers, which should be executed with slower movements and cautiously to avoid any vascular or orthopedic complications.
Author contributions
All authors of this manuscript have: substantially contributed to conception and design, acquisition of data, and analysis and interpretation of data; drafted the article and revised it critically for important intellectual content; approve the final version to be published; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy and integrity of any part of the work are appropriately investigated and resolved.
Disclosure
The authors report no conflicts of interest in this work.
References
Jonsson R, Sixt E, Landahl S, Rosenhall U. Prevalence of dizziness and vertigo in an urban elderly population. J Vestib Res. 2004;14:47–52. | ||
Barin K, Dodson EE. Dizziness in the elderly. Otolaryngol Clin North Am. 2011;44(2):437–454. | ||
Kannus P. Fall-induced injuries and deaths among older adults. JAMA. 1999;281(20):1895–1899. | ||
Maarsingh OR, Dros J, Schellevis FG, van Weert HC, Bindels PJ, Horst HE. Dizziness reported by elderly patients in family practice: prevalence, incidence, and clinical characteristics. BMC Fam Pract. 2010;11(1):2. | ||
Mueller M, Strobl R, Jahn K, Linkohr B, Peters A, Grill E. Burden of disability attributable to vertigo and dizziness in the aged: results from the KORA-Age study. Eur J Public Health. 2014;24(5):802–807. | ||
Piker EG, Jacobson GP. Self-report symptoms differ between younger and older dizzy patients. Otol Neurotol. 2014;35(5):873–879. | ||
Tuunainen E, Jäntti P, Poe D, Rasku J, Toppila E, Pyykkö I. Characterization of presbyequilibrium among institutionalized elderly persons. Auris Nasus Larynx. 2012;39(6):577–582. | ||
Tinetti ME, Williams CS, Gill TM. Dizziness among Older adults: a possible geriatric syndrome. Ann Intern Med. 2000;132(5):337–344. | ||
Saber Tehrani AS, Kattah JC, Mantokoudis G, et al. Small strokes causing severe vertigo: frequency of false-negative MRIs and nonlacunar mechanisms. Neurology. 2014;83(2):169–173. | ||
Jönsson R, Sixt E, Landahl S, Rosenhall U. Prevalence of dizziness and vertigo in an urban elderly population. J Vestib Res. 2004;14:47–52. | ||
Neuhauser HK, von Brevern M, Radtke A, et al. Epidemiology of vestibular vertigo: a neurotologic survey of the general population. Neurology. 2005;65(6):898–904. | ||
Hanley K, O’Dowd T, Considine N. A systematic review of vertigo in primary care. Br J Gen Pract. 2001;51:666–671. | ||
Benecke H, Agus S, Kuessner D, Goodall G, Strupp M. The burden and impact of vertigo: findings from the REVERT patient registry. Front Neurol. 2013;2(4):136. | ||
Sogebi OA, Ariba AJ, Otulana TO, Osalusi BS. Vestibular disorders in elderly patients: characteristics, causes and consequences. Pan Afr Med J. 2014;19:146. | ||
Katsarkas A. Dizziness in aging: the clinical experience. Geriatrics. 2008;63:18–20. | ||
Oghalai JS, Manolidis S, Barth JL, Stewart MG, Jenkins HA. Unrecognized benign paroxysmal positional vertigo in elderly patients. Otolaryngol Head Neck Surg. 2000;122(5):630–634. | ||
Cupulolithiasis SHF. Arch Otolaryngol. 1969;90:765–768. | ||
Brandt T. Vertigo: Its Multisensory Syndromes. 2nd ed. London: Springer-Verlag; 2003:251–283. | ||
Hall SF, Ruby RF, Mcclure JA. The mechanics of benign paroxysmal vertigo. J Otolaryngol. 1979;8:151–158. | ||
Andrade LR, Lins U, Farina M, Kachar B, Thalmann R. Immunogold TEM of otoconin 90 and otolin: relevance to mineralization ofotoconia, and pathogenesis of benign positional vertigo. Hear Res. 2012;292(1–2):14–25. | ||
Imai T, Takeda N, Ito M, et al. 3D analysis of benign positional nystagmus due to cupulolithiasis in posterior semicircular canal. Acta Otolaryngol. 2009;129(10):1044–1049. | ||
Ichijo H. Cupulolithiasis of the posterior semicircular canal. Am J Otolaryngol. 2013;34(5):458–463. | ||
Korres S, Balatsouras DG, Kaberos A, Economou C, Kandiloros D, Ferekidis E. Occurrence of semicircular canal involvement in benign paroxysmal positional vertigo. Otol Neurotol. 2002;23(6):926–932. | ||
Riga M, Bibas A, Xenellis J, Korres S. Inner ear disease and benign paroxysmal positional vertigo: a critical review of incidence, clinical characteristics, and management. Int J Otolaryngol. 2011;2011(11, Part 1):709469. | ||
Baloh RW, Honrubia V, Jacobson K. Benign positional vertigo: clinical and oculographic features in 240 cases. Neurology. 1987;37(3):371–378. | ||
Hughes CA, Proctor L. Benign paroxysmal positional vertigo. Laryngoscope. 1997;107(5):607–613. | ||
Karlberg M, Hall K, Quickert N, Hinson J, Halmagyi GM. What inner ear diseases cause benign paroxysmal positional vertigo? Acta Otolaryngol. 2000;120:380–385. | ||
Lee N-H, Ban J-H, Lee K-C, Kim SM. Benign paroxysmal positional vertigo secondary to inner ear disease. Otolaryngol Head Neck Surg. 2010;143(3):413–417. | ||
Caldas MA, Ganança CF, Ganança FF, Ganança MM, Caovilla HH. Clinical features of benign paroxysmal positional vertigo. Braz J Otorhinolaryngol. 2009;75(4):502–506. | ||
Katsarkas A. Benign paroxysmal positional vertigo (BPPV): idiopathic versus post-traumatic. Acta Otolaryngol. 1999;119(7):745–749. | ||
Gordon CR, Levite R, Joffe V, Gadoth N. Is posttraumatic benign paroxysmal positional vertigo different from the idiopathic form? Arch Neurol. 2004;61(10):1590–1593. | ||
Parham K, Kuchel GA. A geriatric perspective on benign paroxysmal positional vertigo. J Am Geriatr Soc. 2016;64(2):378–385. | ||
de Stefano A, Dispenza F, Suarez H, et al. A multicenter observational study on the role of comorbidities in the recurrent episodes of benign paroxysmal positional vertigo. Auris Nasus Larynx. 2014;41(1):31–36. | ||
Ganança FF, Gazzola JM, Ganança CF, Caovilla HH, Ganança MM, Cruz OL. Elderly falls associated with benign paroxysmal positional vertigo. Braz J Otorhinolaryngol. 2010;76:113–120. | ||
Aimoni C, Bianchini C, Borin M, et al. Diabetes, cardiovascular risk factors and idiopathic sudden sensorineural hearing loss: a case-control study. Audiol Neurotol. 2010;15(2):111–115. | ||
Chang TY, Liu CS, Huang KH, Chen RY, Lai JS, Bao BY. High-frequency hearing loss, occupational noise exposure and hypertension: a cross-sectional study in male workers. Environ Health. 2011;25:35. | ||
Santos MD, Bittar RS. Vertigo and metabolic disorders. Int Tinnitus J. 2012;17:16–20. | ||
D’Silva LJ, Staecker H, Lin J, et al. Retrospective data suggests that the higher prevalence of benign paroxysmal positional vertigo in individuals with type 2 diabetes is mediated by hypertension. J Vestib Res. 2016;25(5–6):233–239. | ||
Yoda S, Cureoglu S, Yildirim-Baylan M, et al. Association between type 1 diabetes mellitus and deposits in the semicircular canals. Otolaryngol Head Neck Surg. 2011;145(3):458–462. | ||
Jáuregui-Renaud K, Aranda-Moreno C, Herrera-Rangel A. Utricular hypofunction in patients with type 2 diabetes mellitus. Acta Otorhinolaryngol Ital. 2017;37:430–435. | ||
Kahraman SS, Ozcan O, Arli C, et al. Calcium homeostasis during attack and remission in patients With idiopathic benign paroxysmal positional vertigo. Otol Neurotol. 2016;37(9):1388–1392. | ||
Chan K-C, Tsai Y-T, Yang Y-H, Chen P-C, Chang P-H. Osteoporosis is associated with increased risk for benign paroxysmal positional vertigo: a nationwide population-based study. Arch Osteoporos. 2017;12(1):106. | ||
Yang CJ, Kim Y, Lee HS, Park HJ. Bone mineral density and serum 25-hydroxyvitamin D in patients with idiopathic benign paroxysmal positional vertigo. J Vestib Res. 2018;27(5–6):287–294. | ||
Yamanaka T, Shirota S, Sawai Y, Murai T, Fujita N, Hosoi H. Osteoporosis as a risk factor for the recurrence of benign paroxysmal positional vertigo. Laryngoscope. 2013;123(11):2813–2816. | ||
Parham K, Leonard G, Feinn RS, Lafreniere D, Kenny AM. Prospective clinical investigation of the relationship between idiopathic benign paroxysmal positional vertigo and bone turnover. Laryngoscope. 2013;123(11):2834–2839. | ||
Talaat HS, Abuhadied G, Talaat AS, Abdelaal MSS. Low bone mineral density and vitamin D deficiency in patients with benign positional paroxysmal vertigo. Eur Arch Oto-Rhino-Laryngol. 2015;272(9):2249–2253. | ||
Mcclure JA. Horizontal canal BPV. J Otolaryngol. 1985;14(1):30–35. | ||
Cipparrone L, Corridi G, Pagnini P. Cupulolitiasi. In: Dufour A, editor. V Giornata Italiana di Nistagmografia Clinica Nistagmografia e Patologia Vestibolare Periferica. Milano: CSS Boots-Formenti; 1985:36–53. | ||
Yeo S-C, Ahn S-K, Lee HJ, et al. Idiopathic benign paroxysmal positional vertigo in the elderly: a long-term follow-up study. Aging Clin Exp Res. 2018;30(2):153–159. | ||
Balatsouras DG, Koukoutsis G, Aspris A, et al. Benign paroxysmal positional vertigo secondary to mild head trauma. Ann Otol, Rhinol Laryngol. 2017;126(1):54–60. | ||
Balatsouras DG. Benign paroxysmal positional vertigo with multiple canal involvement. Am J Otolaryngol. 2012;33(2):250–258. | ||
Herdman SJ, Tusa RJ, Clendaniel RA. Eye movement signs in vertical canal benign paroxysmal positional vertigo. In: Fuchs AF, Brandt T, Buttner U, Zee D, editors. Contemporary Ocular Motor and Vestibular Research: A Tribute to David A. Robinson. Stuttgart: Georg Thieme Verlag; 1994:385–387. | ||
Anagnostou E, Kouzi I, Spengos K. Diagnosis and Treatment of anterior-canal benign paroxysmal positional vertigo: a systematic review. J Clin Neurol. 2015;11(3):262–267. | ||
Soto-Varela A, Santos-Perez S, Rossi-Izquierdo M, Sanchez-Sellero I. Are the three canals equally susceptible to benign paroxysmal positional vertigo? Audiol Neurotol. 2013;18(5):327–334. | ||
Korres SG, Balatsouras DG. Diagnostic, Pathophysiologic, and therapeutic aspects of benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg. 2004;131(4):438–444. | ||
Korres SG, Balatsouras DG, Papouliakos S, Ferekidis E. Benign paroxysmal positional vertigo and its management. Med Sci Monit. 2007;13:CR275–CR282. | ||
Nuti D, Masini M, Mandal M. Benign paroxysmal positional vertigo and its variants. Handb Clin Neurol. 2016;137:241–256. | ||
Kim JS, Zee DS. Clinical practice. Benign paroxysmal positional vertigo. N Engl J Med. 2014;370:1138–1147. | ||
Ichijo H. Onset time of benign paroxysmal positional vertigo. Acta Otolaryngol. 2017;137(2):144–148. | ||
Fernández L, Breinbauer HA, Delano PH. Vertigo and dizziness in the elderly. Front Neurol. 2015;6:144. | ||
Batuecas-Caletrio A, Trinidad-Ruiz G, Zschaeck C, et al. Benign paroxysmal positional vertigo in the elderly. Gerontology. 2013;59(5):408–412. | ||
Humphriss RL, Baguley DM, Sparkes V, Peerman SE, Moffat DA. Contraindications to the Dix-Hallpike manoeuvre: a multidisciplinary review. Int J Audiol. 2003;42:166–173. | ||
Cohen HS. Side-lying as an alternative to the Dix-Hallpike test of the posterior canal. Otol Neurotol. 2004;25(2):130–134. | ||
Dros J, Maarsingh OR, van der Horst HE, Bindels PJ, Ter Riet G, van Weert HC. Tests used to evaluate dizziness in primary care. Can Med Assoc J. 2010;182(13):E621–E631. | ||
Furman JM, Raz Y, Whitney SL. Geriatric vestibulopathy assessment and management. Curr Opin Otolaryngol Head Neck Surg. 2010;18(5):386–391. | ||
Parham K, Sacks D, Bixby C, Fall P. Inner ear protein as a biomarker in circulation? Otolaryngol Head Neck Surg. 2014;151(6):1038–1040. | ||
West N, Hansen S, Møller MN, Bloch SL, Klokker M. Repositioning chairs in benign paroxysmal positional vertigo: implications and clinical outcome. Eur Arch Oto-Rhino-Laryngol. 2016;273(3):573–580. | ||
Nakayama M, Epley JM. BPPV and variants: improved treatment results with automated, nystagmus-based repositioning. Otolaryngol Head Neck Surg. 2005;133(1):107–112. | ||
Richard-Vitton T, Seidermann L, Fraget P, Mouillet J, Astier P, Chays A. Benign positional vertigo, an armchair for diagnosis and for treatment: description and significance. Rev Laryngol Otol Rhinol. 2005;126:249–251. | ||
Korres SG, Balatsouras DG, Ferekidis E. Electronystagmographic findings in benign paroxysmal positional vertigo. Ann Otol, Rhinol Laryngol. 2004;113(4):313–318. | ||
Bhattacharyya N, Baugh RF, Orvidas L, et al. American Academy of Otolaryngology-Head and Neck Surgery Foundation. Clinical practice guideline: benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg. 2008;139(5 Suppl 4):S47–S81. | ||
Bhattacharyya N, Gubbels SP, Schwartz SR, et al. Clinical Practice Guideline: benign paroxysmal positional vertigo (update). Otolaryngol Head Neck Surg. 2017;156(3 Suppl):S1–S47. | ||
Oron Y, Cohen-Atsmoni S, Len A, Roth Y. Treatment of horizontal canal BPPV: pathophysiology, available maneuvers, and recommended treatment. Laryngoscope. 2015;125(8):1959–1964. | ||
Bisdorff AR, Debatisse D. Localizing signs in positional vertigo due to lateral canal cupulolithiasis. Neurology. 2001;57(6):1085–1088. | ||
Ichijo H. Cupulolithiasis of the horizontal semicircular canal. Eur Arch Oto-Rhino-Laryngol. 2012;269(1):53–56. | ||
Ichijo H. Positional nystagmus of horizontal canalolithiasis. Acta Otolaryngol. 2011;131(1):46–51. | ||
Hiruma K, Numata T. Positional nystagmus showing neutral points. ORL. 2004;66(1):46–50. | ||
Lee S-H, Kim M-K, Cho K-H, Kim JS. Reversal of initial positioning nystagmus in benign paroxysmal positional vertigo involving the horizontal Canal. Ann N Y Acad Sci. 2009;1164(1):406–408. | ||
Ichijo H. Persistent direction-changing geotropic positional nystagmus. Eur Arch Oto-Rhino-Laryngol. 2012;269(3):747–751. | ||
Kim M-B, Hong SM, Choi H, et al. The light cupula: an emerging new concept for positional vertigo. J Audiol Otol. 2018;22(1):1–5. | ||
Balatsouras DG, Koukoutsis G, Ganelis P, Korres GS, Kaberos A. Diagnosis of single- or multiple-canal benign paroxysmal positional vertigo according to the type of nystagmus. Int J Otolaryngol. 2011;2011(7):483965. | ||
Riga M, Korres S, Korres G, Danielides V. Apogeotropic variant of lateral semicircular canal benign paroxysmal positional vertigo: is there a correlation between clinical findings, underlying pathophysiologic mechanisms and the effectiveness of repositioning maneuvers? Otol Neurotol. 2013;34:1155–1164. | ||
Korres S, Riga M, Sandris V, Danielides V, Sismanis A. Canalithiasis of the anterior semicircular canal (ASC): treatment options based on the possible underlying pathogenetic mechanisms. Int J Audiol. 2010;49(8):606–612. | ||
Korres S, Riga M, Balatsouras D, Sandris V. Benign paroxysmal positional vertigo of the anterior semicircular canal: atypical clinical findings and possible underlying mechanisms. Int J Audiol. 2008;47(5):276–282. | ||
Bertholon P, Tringali S, Faye MB, Antoine JC, Martin C. Prospective study of positional nystagmus in 100 consecutive patients. Annal Otol Rhinol Laryngol. 2006;115(8):587–594. | ||
Haynes DS, Resser JR, Labadie RF, et al. Treatment of benign positional vertigo using the semont maneuver: efficacy in patients presenting without nystagmus. Laryngoscope. 2002;112(5):796–801. | ||
Balatsouras DG. Subjective benign paroxysmal positional vertigo. In: De Stefano A, Dispenza F, editors. Understanding Benign Paroxysmal Positional Vertigo. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd; 2017:157–170. | ||
Balatsouras DG, Korres SG. Subjective benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg. 2012;146(1):98–103. | ||
Weider DJ, Ryder CJ, Stram JR. Benign paroxysmal positional vertigo: analysis of 44 cases treated by the canalith repositioning procedure of Epley. Am J Otol. 1994;15:321–326. | ||
De Stefano A, Dispenza F. Malignant paroxysmal positional vertigo. In: Dispenza F, De Stefano A, editors. Textbook of Vertigo: Diagnosis and Management. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd; 2013:145–150. | ||
Tusa RJ, Gore R. Dizziness and vertigo: emergencies and management. Neurol Clin. 2012;30(1):61–74. | ||
American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616–631. | ||
Th B, Daroff RB. Physical therapy for benign paroxysmal positional vertigo. Arch Otolaryngol. 1980;106:484–485. | ||
Semont A, Freyss E, Vitte P. Curing the BPPV with a liberatory maneuver. Adv Otorhinolaryngol. 1988;4:290–293. | ||
Epley JM. The canalith repositioning procedure: for treatment of benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg. 1992;107(3):399–404. | ||
Walther LE, Rogowski M, Schaaf H, Hörmann K, Löhler J. Falls and dizziness in the elderly. Otolaryngol Polska. 2010;64(6):354–357. | ||
Cho EI, White JA. Positional vertigo: as occurs across all age groups. Otolaryngol Clin North Am. 2011;44(2):347–360. | ||
Hilton MP, Pinder DK. The Epley (canalith repositioning) manoeuvre for benign paroxysmal positional vertigo. Cochrane Database Syst Rev. 2014;12:CD003162. | ||
Hunt WT, Zimmermann EF, Hilton MP. Modifications of the Epley (canalith repositioning) manoeuvre for posterior canal benign paroxysmal positional vertigo (BPPV). Cochrane Database Syst Rev. 2012;4:CD008675. | ||
Blatt P, Georgakakis G, Herdman S, Clendaniel R, Tusa R. The effect of the canalith repositioning maneuver on resolving postural instability in patients with benign paroxysmal positional vertigo. Am J Otolaryngol. 2000;21(3):356–363. | ||
In press. Ribeiro KF, Oliveira BS, Freitas RV, Ferreira LM, Deshpande N, Guerra RO. Effectiveness of otolith repositioning maneuvers and vestibular rehabilitation exercises in elderly people with benign paroxysmal positional vertigo: a systematic review. Braz J Otorhinolaryngol. In press 2017. | ||
Prokopakis E, Vlastos IM, Tsagournisakis M, Christodoulou P, Kawauchi H, Velegrakis G. Canalith repositioning procedures among 965 patients with benign paroxysmal positional vertigo. Audiol Neurotol. 2013;18(2):83–88. | ||
Vannucchi P, Giannoni B, Giuffreda P. The therapy of benign paroxysmal positional vertigo of the horizontal semicircular canal. In: Versino M, Zambarbieri D, editors. In: Proceedings of the International Workshop on Eye Movements. Pavia: Fondazione IRCCS; 1994:211–220. | ||
Lempert T. Horizontal benign positional vertigo. Neurology. 1994;44(11):2213–2214. | ||
Gufoni M, Mastrosimone L, di Nasso F. Repositioning maneuver in benign paroxysmal vertigo of horizontal semicircular canal. Acta Otorhinolaryngol Ital. 1998;18:363–367. | ||
Vannucchi P, Libonati GA, Gufoni M, et al. The physical treatment of lateral semicircular canal canalolithiasis. Audiol Med. 2005;3(1):52–56. | ||
Johkura K, Momoo T, Kuroiwa Y. Positional nystagmus in patients with chronic dizziness. J Neurol Neurosurg Psychiatry. 2008;79(12):1324–1326. | ||
von Brevern M, Radtke A, Lezius F, et al. Epidemiology of benign paroxysmal positional vertigo: a population based study. J Neurol Neurosurg Psychiatry. 2007;78(7):710–715. | ||
Soto-Varela A, Rossi-Izquierdo M, Martínez-Capoccioni G, Labella-Caballero T, Santos-Pérez S. Benign paroxysmal positional vertigo of the posterior semicircular canal: efficacy of Santiago treatment protocol, long-term follow up and analysis of recurrence. J Laryngol Otol. 2012;126(4):363–371. | ||
Walther LE, Wenzel A, Buder J, et al. Detection of human utricular otoconia degeneration in vital specimen and implications for benign paroxysmal positional vertigo. Eur Arch Otorhinolaryngol. 2014;271(12):3133–3138. | ||
Sato G, Sekine K, Matsuda K, Takeda N. Risk factors for poor outcome of a single Epley maneuver and residual positional vertigo in patients with benign paroxysmal positional vertigo. Acta Otolaryngol. 2013;133(11):1124–1127. | ||
Ribeiro KM, Freitas RV, Ferreira LM, Deshpande N, Guerra RO. Effects of balance Vestibular Rehabilitation Therapy in elderly with benign paroxysmal positional vertigo: a randomized controlled trial. Disabil Rehabil. 2017;39(12):1198–1206. | ||
Plodpai Y, Atchariyasathian V, Khaimook W. The characteristic differences of benign paroxysmal positional vertigo among the elderly and the younger patients: a 10-year retrospective review. J Med Assoc Thai. 2014;97(8):850–855. | ||
Ahmed RM, Pohl DV, Macdougall HG, Makeham T, Halmagyi GM. Posterior semicircular canal occlusion for intractable benign positional vertigo: outcome in 55 ears in 53 patients operated upon over 20 years. J Laryngol Otol. 2012;126(7):677–682. | ||
Leveque M, Labrousse M, Seidermann L, Chays A. Surgical therapy in intractable benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg. 2007;136(5):693–698. | ||
Sismanis A. Surgical management of common peripheral vestibular diseases. Curr Opin Otolaryngol Head Neck Surg. 2010;18(5):431–435. | ||
Ganança FF, Gazzola JM, Ganança CF, Caovilla HH, Ganança MM, Cruz OL. Elderly falls associated with benign paroxysmal positional vertigo. Braz J Otorhinolaryngol. 2010;76(1):113–120. | ||
Jumani K, Powell J. Benign paroxysmal positional vertigo: management and its impact on falls. Ann Otol Rhinol Laryngol. 2017;126(8):602–605. | ||
Gazzola JM, Ganança FF, Aratani MC, Perracini MR, Ganança MM. Circumstances and consequences of falls in elderly people with vestibular disorder. Braz J Otorhinolaryngol. 2006;72(3):388–392. | ||
Vaz DP, Gazzola JM, Lança SM, Dorigueto RS, Kasse CA. Clinical and functional aspects of body balance in elderly subjects with benign paroxysmal positional vertigo. Braz J Otorhinolaryngol. 2013;79(2):150–157. | ||
Silva CN, Ribeiro KM, Freitas RV, Ferreira LM, Guerra RO. Vertiginous symptoms and objective measures of postural balance in elderly people with benign paroxysmal positional vertigo submitted to the Epley maneuver. Int Arch Otorhinolaryngol. 2016;20(1):61–68. | ||
Seok JI, Lee HM, Yoo JH, Lee DK. Residual dizziness after successful repositioning treatment in patients with benign paroxysmal positional vertigo. J Clin Neurol. 2008;4(3):107–110. | ||
Teggi R, Giordano L, Bondi S, Fabiano B, Bussi M. Residual dizziness after successful repositioning maneuvers for idiopathic benign paroxysmal positional vertigo in the elderly. Eur Arch Otorhinolaryngol. 2011;268(4):507–511. | ||
Hansson EE, Månsson NO, Ringsberg KA, Håkansson A. Falls among dizzy patients in primary healthcare: an intervention study with control group. Int J Rehabil Res. 2008;31(1):51–57. | ||
McPherson D, Whitaker S, Wrobel B. DDX: disequilibrium of aging. In: Goebel JA, editor. Practical Management of the Dizzy Patient. 2nd ed. Philadelphia: Lippincott Williams and Wilkins; 2001:297–344. | ||
Jung JY, Kim JS, Chung PS, Woo SH, Rhee CK. Effect of vestibular rehabilitation on dizziness in the elderly. Am J Otolaryngol. 2009;30(5):295–299. | ||
Panel on Prevention of Falls in Older Persons, American Geriatrics Society and British Geriatrics Society. Summary of the updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc. 2011;59(1):148–157. | ||
Angeli SI, Hawley R, Gomez O. Systematic approach to benign paroxysmal positional vertigo in the elderly. Otolaryngol Head Neck Surg. 2003;128(5):719–725. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.