Back to Journals » Clinical Ophthalmology » Volume 13
Baseline Predictive Factors of Visual Outcome and Persistence of Subretinal Fluid Based on Morphologic Changes in Spectral Domain Optical Coherence Tomography in Patients with Idiopathic Central Serous Chorioretinopathy
Authors Suwal B , Khadka D , Shrestha A , Shrestha S, Shrestha N, Khatri B
Received 2 October 2019
Accepted for publication 29 November 2019
Published 9 December 2019 Volume 2019:13 Pages 2439—2444
DOI https://doi.org/10.2147/OPTH.S233273
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Barsha Suwal,1 Deepak Khadka,1 Arjun Shrestha,1 Sangeeta Shrestha,1 Nirsara Shrestha,1 Bijay Khatri2
1Department of Ophthalmology, BP Eye Foundation, Hospital for Children, Eye, ENT and Rehabilitation Services (CHEERS), Lokanthali, Bhaktapur, Nepal; 2Department of Academics and Research, Hospital for Children, Eye, ENT, Rehabilitation Services (CHEERS), Lokanthali, Bhaktapur, Nepal
Correspondence: Barsha Suwal
Department of Ophthalmology, BP Eye Foundation, Hospital for Children, Eye, ENT and Rehabilitation Services (CHEERS), Lokanthali, Bhaktapur, Nepal
Tel +977-9803056313
Fax +977-16639735 Email [email protected]
Background: To determine the influence of spectral domain optical coherence tomography (OCT) changes on visual outcome and persistence of subretinal fluid (SRF) in patients with idiopathic central serous chorioretinopathy (CSCR).
Materials and methods: In a retrospective study done in 48 eyes of 45 patients diagnosed as CSCR, all eyes were subjected to fundus photography, spectral domain OCT, and fluorescein angiography (FA) in selected cases.
Results: Retinal pigment epithelium detachment was present in 22.91% of the cases at presentation. The logMar best corrected visual acuity improved from 0.46±0.29 at presentation to 0.18±0.22 at 3 months (P-value<0.01). The mean foveal thickness was 486.81±146.06 μm at presentation and 259±94.5 μm at 3 months (P-value<0.01) (paired T-test). OCT factors that were associated with poor visual outcome (BCVA>0.3 logMar) were disruption in the inner segment (IS)/outer segment (OS) junction or external limiting membrane (ELM) line and hyper-reflective dots in the intra/subretinal layer (P-value<0.05) (Fischer’s Exact test). Out of the total 48 eyes, 26 had persistent SRF at 3 months. The presence of discontinuation in IS/OS junction and hyper-reflective dots in the intra/subretinal layer were the only two OCT factors that were associated with the persistence of SRF (P-value<0.01) (Pearson’s Chi-square test).
Conclusion: Visual outcome and persistence of subretinal fluid at 3 months can be predicted on the basis of early morphologic changes in OCT. This will aid in counseling patients regarding its course and may guide us in its management.
Keywords: central serous chorioretinopathy, optical coherence tomography, visual outcome, persistent subretinal fluid
Introduction
Central serous chorioretinopathy (CSCR) is defined as the presence of serous detachment of the neurosensory retina involving the macula. It is characterized by choroidal vascular hyper- permeability, which is demonstrated by indocyanine green angiography (ICG) and focal leakage at the level of retinal pigment epithelium (RPE), visualized by fluorescein angiogram (FA).1 As both the above tests are invasive, Optical Coherence Tomography (OCT) has now become an important non- invasive tool to visualize the changes in microstructure of the different layers of the retina.
Various OCT changes reported in CSCR are foveal serous detachment, pigment epithelial detachment (PED), retinal pigment epithelial (RPE) bumps, thickened outer retina layer, fibrinous exudates in the sub-retinal space, hyper-reflective dots in the intra-retinal and/or sub-retinal layer, loss of the outer photoreceptor layer (OPRL), and discontinuity of the outer segment and inner segment of the photoreceptor layer. Some of these can be used to stage and prognosticate the entity.1,,3
This study aims to determine whether the baseline morphologic changes in OCT can help us predict visual prognosis and clinical course in Nepalese patients with CSCR.
Materials and Methods
This retrospective observational cross-sectional study was carried out in the Hospital for Children, Eye, ENT and Rehabilitation Services from January 2016 to December 2018. Ethical clearance was obtained from the Nepal Health Research Council and it also conformed to the provisions of the Declaration of Helsinki 1995.
All patients who presented with acute CSCR were included. CSCR was characterized by the presence of serous detachment of the neurosensory retina involving the macula that was confirmed using spectral domain OCT in all cases. Acute CSCR was defined as the presence of symptoms such as diminution of vision or metamorphopsia for the last 6 weeks.
A thorough ocular examination was done with a slit lamp and +90 Diopter lens after dilatation of the pupil using 1% phenylephrine, following which all patients were subjected to OCT imaging using spectral domain OCT (Topcon 3D, version 8.20.003.04).
Spectral Domain Optical Coherence Tomography Measurement and Its Analysis
A 6 mm X 6 mm area of the macular region was examined by spectral domain OCT; volume scans of 25 sections were centered on the fovea; and nine B-scan images at each section were averaged. All of the examinations were performed by a well-trained optometrist. The eye-tracking system of the device was used to ensure the correct position during the scanning process. Retinal thickness was measured at the fovea automatically by the OCT software using the macular thickness map as the distance between the inner limiting membrane (ILM) and either the RPE or Bruch’s membrane. These OCT images were further analyzed by three retinal physicians (BS, DK, AS) to reach a common consensus using the following five parameters: Pigment Epithelial Detachment (PED), Retinal Pigment Epithelial (RPE) bumps, discontinuation in Inner segment/outer segment (IS/OS) junction or external limiting membrane (ELM), fibrinous exudates in the sub-retinal space, and hyper-reflective dots in the intraretinal and/or subretinal layer. Schematic representations are shown in Figures 1–3.
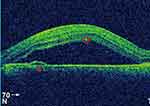 |
Figure 1 OCT showing hyper-reflective deposits in the intra-retinal layer (circle), PED (Triangle), and neurosensory retinal detachment. |
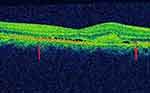 |
Figure 2 The disrupted IS/OS junction is demonstrated by a thin arrow. |
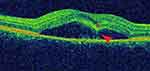 |
Figure 3 Locally thickened neurosensory retina (“retinal dipping”, star), connected by a hyper-reflective flow, suggesting high fibrin/protein content (arrow). |
PED was identified when a dome-shaped elevated space formed between the RPE and Bruch’s membrane. The junction of the IS and OS of the photoreceptors was visualized as a hyper-reflective layer distinct from the adjacent RPE reflectivity, and ELM was identified as a hyper-reflective layer parallel to the IS/OS line. Disruption of the IS/OS line or ELM was defined as the loss of hyper-reflective line at the said anatomical location.
A detailed proforma was filled which included age, sex, duration of symptoms, laterality, best corrected visual acuity (BCVA), fundus photo, and spectral domain OCT. Cases were followed up at 1 month and 3 months. CSCR was considered non-resolving when there was persistence of SRF on OCT even after 3 months of onset of symptoms, characteristic “hot spots” of leakage, diffuse leakage, and RPE alterations visualized on FA. In cases of complete resolution of SRF before 3 months, the data at the last follow-up was taken.
Patients with chronic CSCR, history of previous CSCR in the same eye, high myopia (>-6 Diopter), idiopathic choroidal neovascularization, polypoidal choroidal vasculopathy, CSCR secondary to neovascular maculopathy such as secondary to retinal vascular disease, age related macular degeneration, intraocular inflammation, epiretinal membrane, or posterior segment tumors were excluded.
Statistical Analysis
The best corrected visual acuity (BCVA) was converted to a logarithm of the minimum angle of resolution (logMar) equivalent. Statistical analysis was performed using Statistical Package for the Social Science version 19 for windows (SPSS Inc, Chicago, IL). We used the paired t-test for test of significance of initial and final foveal thickness. Fischer’s Exact test was used to analyze the association of the final BCVA with OCT factors (because the cells had expected counts less than 5). The association of resolution of CSCR with OCT factors was analyzed using the Chi-square test and Fischer’s exact test where applicable. A P-value less than 0.05 was considered to be statistically significant.
Results
The study was done in 48 eyes of 45 patients, of which 40 patients (83.3%) were males and 8 (16.7%) were females, with a ratio of 5:1, which highlights male preponderance. The mean age of presentation was 40.13±7.45 years, which is also the common age group to be affected. Mean duration of symptoms from the onset of symptoms to the time of presentation was 3.3 weeks±2.5 weeks and the mean duration of follow-up was 9.67 weeks.
Average log Mar BCVA improved from 0.46±0.29 at presentation to 0.18±0.22 at 3 months (P-value<0.01). The mean foveal thickness decreased from 486.81±146.06 µm at presentation to 259±94.5 µm at 3 months (P-value<0.01) (paired t-test).
All 48 eyes had focal neurosensory retinal detachment at presentation. Retinal pigment epithelium detachment was present in 22.91% of the cases at presentation. The subretinal fluid resolved completely without treatment in 22 eyes (45.83%), but persisted in 26 eyes (54.17%), even after 3 months of presentation. We found that OCT factors that were associated with persistent SRF were hyper-reflective dots in intra or subretinal layers and discontinuity in IS/OS or ELM line. Table 1 summarizes the correlation of OCT changes with the non-resolution of CSCR.
 |
Table 1 Comparision of OCT Features in Resolved and Non-Resolved CSCR |
At the final follow-up, 42 eyes (87.5%) had good visual outcome (BCVA<0.3 logMar) and only six eyes (12.5%) had poor visual outcome (BCVA>0.3 logMar). Likewise, the factors that were associated with poor visual outcome were hyper-reflective dots in intra or subretinal layers and discontinuity in IS/OS or ELM line. Table 2 demonstrates the correlation of OCT findings with the outcome of vision.
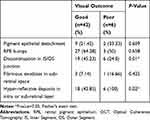 |
Table 2 OCT Findings in Relation with Visual Outcome |
Discussion
In our study, we investigated the baseline OCT factors that were associated with the persistence of SRF, which helped us identify cases at risk for persistence, before the development of photoreceptor and RPE damage due to long lasting subretinal detachment.
We found that the presence of discontinuation in IS/OS junction or ELM line and hyper-reflective dots in intra/subretinal layer were the only two OCT factors that were associated with persistence of SRF at 3 months (P-value<0.01) (Pearson Chi-square test) and poor visual outcome (BCVA>0.3 logMar) (P-value<0.05) (Fischer’s Exact test). Despite resolution of SRF, some patients may still complain of poor vision because of structural damage to photoreceptors and RPE.
IS/OS Line/ELM Line
It is suggested that the IS/OS line corresponds to the interface between photoreceptor inner segments and outer segments, whereas ELM consists of the line between the inner segments and the nuclei of photoreceptors.4 The importance of the photoreceptor IS/OS junction line integrity has been emphasized in some reports.5,6 In recent years, the IS/OS line has been used to indicate photoreceptor integrity and its disruption has been associated with poor final BCVA.5,7,13
However, some opinions have been put forward regarding the misinterpretation of the IS/OS line, especially in the presence of neurosensory retinal detachment. Some authors have suggested that the altered incident angle of the light striking the detached retina leads to an optical error. This phenomenon is said to obscure the actual IS/OS line and evoke a false perception of IS/OS line disruption.14 Also, it is considered that the disruption of ELM mostly reflects a combined damage to the photoreceptor outer segments and inner cell bodies.3 Hence, we either looked for discontinuation in IS/OS line or for the ELM line in cases were the IS/OS line was difficult to appreciate.
Likewise, we found that the presence of hyper-reflective dots in the intra or subretinal layer was also associated with the persistence of SRF and poor visual outcome, which was statistically significant (P-value<0.01, Pearson Chi square test and P<0.05, Fischer Exact test).
Hyper-Reflective Dots
Evidence in the literature suggests that hyper-reflective dots, when present in the intraretinal layer may be composed of macrophages and remnants of disintegrated outer photoreceptors and, when present in the subretinal layer, they might be an aggregate of plasma lipoprotein exudation that is induced by increased choroidal hyperpermeability.15 It is hypothesized that the intraretinal dots may accumulate secondary to the inability of the RPE cells to pump the highly proteinaceous SRF from the intraretinal and subretinal spaces.16
Intraretinal hyper-reflective dots at the level of outer plexiform layer (OPL), outer nuclear layer (ONL), ELM, IS/OS band have been reported in 21–100% of all CSCR forms.17 Hyper-reflective dots when present in intraretinal or subretinal layer have been correlated with lower final BCVA.3,16,18,19 Also, they were found to be the only OCT parameter associated with the non-resolution of SRF.20
The other OCT factors studied, such as the presence of PED, RPE bumps, and fibrinous exudates in the sub-retinal space did not have a statistically significant positive correlation with the persistent SRF or with visual outcome.
Limitations
The limitations of this study include the relatively small number of cases and retrospective nature of the study. Also, in this study, visual acuity alone has been considered to evaluate the visual outcome of CSCR. However, it is known that BCVA represents the foveal function and does not reflect structural changes of the neurosensory retina outside the foveal region.21 Therefore, macular function tests such as microperimetry along with BCVA might have been more appropriate to determine the functional prognosis in CSCR.
Conclusion
In our clinics, we have seen that both anatomical/visual recovery and recurrence tendency vary for each patient. Thus, this study provides an insight to predict the course of the disease based on patient’s own OCT morphologic changes. In addition, the OCT parameters help us decide early treatment prior to the occurrence of irreversible damage to photoreceptor and retinal pigment epithelial (RPE) cells.
Abbreviations
OCT, Optical Coherence Tomography; SRF, Sub-Retinal Fluid; CSCR, Central Serous Chorioretinopathy; FA, Fluorescein Angiogram; µm, Microns; IS, Inner Segment; OS, Outer Segment; ELM, External Limiting Membrane; ICG, Indocyanine Green; RPE, Retinal Pigment Epithelium; PED, Pigment Epithelial Detachment; OPRL, Outer Photoreceptor Layer; SRF, Sub-Retinal Fluid; BCVA, Best Corrected Visual Acuity; logMar, logarithm of the Minimum angle of resolution; OPL, Outer Plexiform Layer; ONL, Outer Nuclear Layer.
Ethics Approval and Consent for Publication
This study is approved by the institution and ethical review committee of Nepal Health Research Council and conformed to the Declaration of Helsinki. The reference number of ethics committee approval was 215/2019. Patients’ consent to review their medical records was waived by the Nepal Health Research Council because the study did not have any risk to the participants and the waiver did not adversely affect the rights and welfare of the participants. Confidentiality and anonymity of the participants were strictly maintained while extracting and analyzing data.
Data Sharing Statement
The datasets generated and/or analyzed during the current study are not publicly available because the data are strictly confidential and are the property of institution and the Nepal Health Research Council, but are available from the author on reasonable request.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no competing interests.
References
1. Song IS, Shin YU, Lee BR. Time-periodic characteristics in the morphology of idiopathic central serous chorioretinopathy evaluated by volume scan using spectral-domain optical coherence tomography. Am J Ophthalmol. 2012;154:366–375. doi:10.1016/j.ajo.2012.02.031
2. Lee H, Lee J, Chung H, et al. Baseline spectral domain optical coherence tomographic hyperreflective foci as a predictor of visual outcome and recurrence for central serous chorioretinopathy. Retina. 2016;36:1–9. doi:10.1097/IAE.0000000000000929
3. Yalcinbayir O, Gelisken O, Akova-Budak B, et al. Correlation of spectral domain optical coherence tomography findings and visual acuity in central serous chorioretinopathy. Retina. 2014;34:705–712. doi:10.1097/IAE.0000000000000001
4. Coscas G. Optical coherence tomography in age-related macular degeneration (OCT in AMD). In: Chapter 7: OCT Interpretation. G. Coscas, F. Coscas, S.Vismara, A. Zourdani, C. I. Li Calzi Heidelberg, Germany: Springer Medizin Verlag; 2009:159–166.
5. Matsumoto H, Sato T, Kishi S. Outer nuclear layer thickness at the fovea determines visual outcomes in resolved central serous chorioretinopathy. Am J Ophthalmol. 2009;148:105–110. doi:10.1016/j.ajo.2009.01.018
6. Kim YY, Flaxel CJ. Factors influencing the visual acuity of chronic central serous chorioretinopathy. Korean J Ophthalmol. 2011;25:90–97. doi:10.3341/kjo.2011.25.2.90
7. Aggio FB, Roisman L, Melo GB, et al. Clinical factors related to visual outcome in central serous chorioretinopathy. Retina. 2010;30:1128–1134. doi:10.1097/IAE.0b013e3181cdf381
8. Eandi CM, Chung JE, Cardillo-Piccolino F, et al. Optical coherence tomography in unilateral resolved central serous chorioretinopathy. Retina. 2005;25:417. doi:10.1097/00006982-200506000-00004
9. Moon JW, Yu HG, Kim TW, et al. Prognostic factors related to photodynamic therapy for central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2009;247:1315–1323. doi:10.1007/s00417-009-1104-8
10. Ooto S, Hangai M, Sakamoto A, et al. High-resolution imaging of resolved central serous chorioretinopathy using adaptive optics scanning laser ophthalmoscopy. Ophthalmology. 2010;117:1800–1809. doi:10.1016/j.ophtha.2010.01.042
11. Piccolino FC, de la Longrais RR, Ravera G, et al. The foveal photoreceptor layer and visual acuity loss in central serous chorioretinopathy. Am J Ophthalmol. 2005;139:87–99. doi:10.1016/j.ajo.2004.08.037
12. Wang MS, Sander B, Larsen M. Retinal atrophy in idiopathic central serous chorioretinopathy. Am J Ophthalmol. 2002;133:787–793.
13. Furuta M, Iida T, Kishi S. Foveal thickness can predict visual outcome in patients with persistent central serous chorioretinopathy. Ophthalmologica. 2009;223:28–31. doi:10.1159/000161880
14. Ko TH, Fujimoto JG, Duker JS, et al. Comparison of ultrahigh and standard-resolution optical coherence tomography for imaging macular hole pathology and repair. Ophthalmology. 2004;111:2033–2043. doi:10.1016/j.ophtha.2004.05.021
15. Shinojima A, Hirose T, Mori R, et al. Morphologic findings in acute central serous chorioretinopathy using spectral domain-optical coherence tomography with simultaneous angiography. Retina. 2010;30:193–202. doi:10.1097/IAE.0b013e3181c70203
16. Plateroti AM, Witmer MT, Kiss S, Amico DJD. Characteristics of intraretinal deposits in acute central serous chorioretinopathy. Clinical Ophthalmol. 2014;8:673–676.
17. Ahlers C, Geitzenauer W, Stock G, Golbaz I, Schmidt-Erfurth U, Prünte C. Alterations of intraretinal layers in acute central serous chorioretinopathy. Acta Ophthalmol. 2009;87:511–516. doi:10.1111/j.1755-3768.2008.01468.x
18. Kon Y, Iida T, Maruko I, Saito M. The optical coherence tomographyophthalmoscope for examination of central serous chorioretinopathy with precipitates. Retina (Phila Pa). 2008;28:864–869. doi:10.1097/IAE.0b013e3181669795
19. Landa G, Barnett JA, Garcia PMT, Tai KW, Rosen RB. Quantitative and qualitative spectral domain optical coherence tomography analysis of subretinal deposits in patients with acute central serous retinopathy. Ophthalmologica. 2013;230:62–68. doi:10.1159/000350231
20. Lai W-Y, Tseng C-L, Lin H-S, Sheu S-J. Correlation between baseline retinal microstructures in spectral-domain optic coherence tomography and need for early intervention in central serous chorioretinopathy. BMJ Open Ophth. 2017;2:e000054. doi:10.1136/bmjophth-2016-000054
21. Reibaldi M, Boscia F, Avitabile T, et al. Functional retinal changes measured by microperimetry in standard-fluence vs low-fluence photodynamic therapy in chronic central serous chorioretinopathy. Am J Ophthalmol. 2011;151:953–960. doi:10.1016/j.ajo.2010.12.007
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
