Back to Journals » Journal of Asthma and Allergy » Volume 13
Association Between FeNO, Total Blood IgE, Peripheral Blood Eosinophil and Inflammatory Cytokines in Partly Controlled Asthma
Authors Badar A , Salem AM , Bamosa AO , Qutub HO, Gupta RK, Siddiqui IA
Received 26 July 2020
Accepted for publication 28 September 2020
Published 29 October 2020 Volume 2020:13 Pages 533—543
DOI https://doi.org/10.2147/JAA.S274022
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Amrita Dosanjh
Ahmed Badar,1 Ayad Mohammed Salem,1 Abdullah Omar Bamosa,1 Hatem Othman Qutub,2 Rakesh Kumar Gupta,2 Intisar Ahmad Siddiqui3
1Department of Physiology, College of Medicine, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia; 2Department of Internal Medicine, College of Medicine & King Fahd Hospital of the University, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia; 3Department of Dental Education, College of Dentistry, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
Correspondence: Abdullah Omar Bamosa
Department of Physiology, College of Medicine, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
Email [email protected]
Background: Fractional exhaled nitric oxide (FeNO) is a convenient to use biomarker of airway inflammation. However, the mutual relationship between FeNO, peripheral blood eosinophil, total immunoglobulin E (IgE) and inflammatory cytokines showed some controversy.
Objective: This study was carried out to determine the accuracy of peripheral blood eosinophil and total IgE to detect eosinophilic airway inflammation as determined by two FeNO cutoff points. The correlation between FeNO, peripheral blood eosinophil, total IgE and certain inflammatory cytokines was also examined.
Methods: Seventy-six patients with partly controlled asthma performed the following tests on the same day: FeNO, pulmonary function tests (PFTs), peripheral blood eosinophils, total IgE, and inflammatory cytokine assay. The correlation between these markers was investigated and the diagnostic accuracy of peripheral blood eosinophils and total IgE to identify eosinophilic asthma phenotype was calculated using receiver operating characteristics area under the curve (ROC AUC).
Results: FeNO was positively correlated with percentage of blood eosinophils (r=0.276, p=0.017) and total blood IgE (r=0.3647; p=0.0013). No relationship between FeNO and serum inflammatory cytokines was detected. AUC of blood eosinophils and total IgE were 57% and 64% at FeNO ≥ 25 ppb and were 67% and 64% at FeNO > 50, respectively. The higher predictive ability was detected at FeNO > 50 ppb where the best cutoff point for blood eosinophil % was ≥ 4.0% (sensitivity 66.7%, specificity 60.0%) and the best cutoff point for total IgE was ≥ 350 (sensitivity 66.7%, specificity 63.6%).
Conclusion: In patients with partly controlled asthma, peripheral blood eosinophil and total IgE showed equal useful accuracy in predicting eosinophilic airways. However, higher predictive values were reported at FeNO level > 50 ppb. FeNO was positively correlated with peripheral blood eosinophil, total IgE but not with any of the studied cytokines.
Keywords: partly controlled asthma, FeNO, eosinophils, IgE, cytokines
Introduction
Asthma is a heterogeneous pathological condition that in its most typical form presents as expiratory airflow limitation associated with chronic inflammation of the airway.1 Traditionally the diagnosis and monitoring of asthma has depended mainly on pulmonary function tests (PFTs), bronchodilator reversibility assessment and bronchial challenge tests to support the history and physical examination.2 As asthma consists of various phenotypes, therefore the diagnosis of asthma becomes challenging due to two main reasons. Firstly the clinical presentation might not be very specific for the disease in most cases, and secondly, it might be very challenging to demonstrate airflow limitation using the available lung function tests (ie, spirometry, bronchodilation test, bronchial challenges, peak expiratory flow (PEF) measurements) especially in children.3 As none of these tests could directly measure the airway inflammation, therefore in the last two decades fractional exhaled nitric oxide (FeNO) has slowly made its way up in the battery of tests for asthma as a noninvasive, convenient and reasonably sensitive biomarker for atopy and eosinophilic lower airway inflammation.3,4
A little amount of nitric oxide (NO) is found in the exhaled air of normal people. For the first time it was reported in 1991 that it was possible to measure endogenous pulmonary nitric oxide production in the exhaled air in man.5 Later on it was discovered that during inflammation the respiratory epithelium produces more NO by upregulation of enzyme nitric oxide synthase (NOS)1. Since then a lot of researchers have worked on the relationship of FeNO with different aspects of presentation, diagnosis, and management of asthma.
The amount of FeNO is known to increase in direct proportion to bronchial wall inflammation, induced-sputum eosinophilia, and airway hyperresponsiveness.1 In addition, an increase in FeNO is confirmed to be associated with a deteriorating asthma control and a fall in FeNO occurs with anti-inflammatory treatment.6 In line with the guidelines of the American Thoracic Society (ATS), FeNO was previously thought to be just a specific marker for eosinophilic airway inflammation. However, recent studies have led to reclassification of FeNO as a broader marker of T helper cell type 2–mediated allergic inflammation.2,7 The inflammation in asthma is stimulated by T helper type 2 (Th-2) cells that secrete cytokines interleukin IL-4, IL-5, and IL-13 leading to type 2 immunity characterized by high IgE antibody levels and eosinophilia.8
The major goal in the management of asthma is improvement in the symptoms and decrease in frequency of exacerbations at minimum possible dose of inhaled corticosteroids (ICS).2 It is recognized that FeNO along with the Global Initiative for Asthma guidelines (GINA) has a beneficial impact on correct determination of ICS in asthma patients.9
Several studies have tried to establish relationships between new and old diagnostic and prognostic markers of asthma. Despite the entire advance, the diagnostic value of FeNO in patients with asthma remains controversial.10 Until very recently studies have shown skepticism over the actual role of FeNO in the diagnosis and management of asthma.11 In addition, early studies have found a correlation between circulating Th2 cytokines with airway inflammation biomarkers (FeNO and sputum eosinophil counts),12 however, recent evidence has refined the idea of adaptive immune response predominated by Th2 cytokines in asthma, the role of innate immune cells and newly discovered cytokines have been described in the new model of type 2-low asthma with distinct clinical features and inflammatory markers.13
The current study was designed to determine the accuracy of peripheral blood eosinophils and total IgE as indicators of eosinophilic airway inflammatory phenotypes in patients with partly controlled asthma using FeNO as the gold standard. Two different cutoff values (25 and 50 ppb) recommended by different asthma task forces14 were used. In addition, as secondary outcome, the correlation between FeNO, peripheral blood eosinophils percentages, total IgE and selected inflammatory cytokines was also investigated.
Patients and Methods
We made a retrospective analysis on the baseline data of 76 patients with partly controlled asthma who participated in our previous study.15 According to the GINA, partly controlled asthma patients were identified when one or two of the following are present during the last four weeks: daytime asthma symptoms or reliever usage more than twice per week, or the presence of any waking episode during the night or activity limitation because of asthma.2
We included patients with partly controlled asthma aged 18–65 years. The exclusion criteria were: smokers, patients with chronic diseases (eg COPD, pulmonary infections, diabetes mellitus, renal disease, rhinosinusitis), asthma exacerbation during the previous one month, usage of leukotriene modifiers, anti-cholinergic drugs, oral steroids, non-steroidal anti-inflammatory drugs (NSAIDs), and antihistamines within one month prior to the study period or use rescue inhaled steroid within 72 hours from day of measurements. Pregnancy and lactation were also excluded.
An ethical approval was obtained from the Institutional Review Board—Imam Abdulrahman Bin Faisal University (IRB‐2019‐01‐100) and the study has followed the principle of the Declaration of Helsinki.16 Each patient has been informed about the purpose of the study and gave a written consent before enrolment.
Procedures and Assessments
The following assessments and tests were done on the same day in the following order: clinical assessment, FeNO, spirometry, blood collection for blood investigations. During the week following the assessment day, additional PEF measurements were done twice a day at home and PEF variability was calculated accordingly.
Clinical Assessment
History about duration of the disease, the ongoing maintenance medications, other medications, or illnesses, was taken. Any exacerbation, rescue treatment, or emergency visit was noted and recorded for each patient. The degree of asthma control was further verified using asthma control test questionnaire (ACT).17
FeNO Measurements
Fractional exhaled nitric oxide (FeNO) was measured according to the American Thoracic Society (ATS) recommendations18 using small portable nitric oxide analyzer (Niox Mino®). The patients were asked to avoid strenuous exercise and nitrate rich diet (eg sausage, spinach, broccoli) within three hours of measurement and to stop their maintenance medications, if any, for at least 72 hours prior to the test. To avoid any possible effect of spirometry technique on nitric oxide results, FeNO measurement was done first. Based on ATS proposed cutoff points for FeNO to detect eosinophilic airway inflammation,14 we put two cutoff points as positive for significant increase in FeNO (≥25 ppb, and >50 ppb).
PFTs Measurements
Spirometry was done according to the ATS criteria,19 the absolute and predicted values of forced expiratory volume in first second (FEV1), forced vital capacity (FVC) and FEV1/FVC ratios were recorded. Three valid maneuvers were recorded, and the best value was used for analysis. In addition, the peak expiratory flow (PEF) was measured at home twice daily (early morning and at night) by all patients using Mini Wright® peak flow meter during the week after enrolment. All patients made three measurements every time and reported the highest one for analysis. Then PEF variability was calculated by dividing the lowest morning PEF value over the highest PEF reading during the week. The higher index indicates good control for asthma and vice versa.2
Blood Investigations
Eight milliliters of peripheral venous blood were collected from every patient. Two milliliters of the blood were used for complete blood count (CBCs) using automated flow cytometry analyzer (Beckman Coulter counter USA), and 6 mL were collected into plain tubes, after clotting at room temperature, samples were centrifuged at 3000 rpm for 15 min, then the serum was isolated and stored at (–80°C) for future analysis. A fluoroenzyme immunoassay kits (Immuno-CAP®, Phadia AB, Sweden) was used to measure the total IgE. The inflammatory serum cytokines (INF-γ, Eotaxin, IL-4, IL-10, and IL-17) were quantified in single using ELISA kits (IBL— Germany), with inter and intra-assay coefficient of variation of less than 10%.
Statistical Analysis
Statistical data were analyzed by using SPSS v.20.0, (IBM Corporation, Armonk, NY, USA). Numeric data based on demographic features and biomarkers’ results were presented as mean, standard deviation following most of these were normally distributed variables. Unpaired t-test or Wilcoxon Mann–Whitney U-test for continuous normally and non-normally distributed data respectively were applied to compare between groups based on baseline FeNO biomarker cutoff values. The categorical variables like gender and stratified variables like age, body mass index (BMI), duration of disease and baseline FeNO categories based on cutoff values were presented into frequencies and percentages.
The receiver operating curve (ROC) was used to illustrate predictive validity of blood eosinophil percentage and total IgE for two cutoff points of FeNO level following positive for ≥25 and >50 as gold-standard criteria separately. Area under the curve (AUC) with 95%CI was presented to compare the predictive validity of biomarkers. Comparative tables of sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) on various cutoff points of blood eosinophil percentage and total IgE for baseline FeNO positive for ≥25 and >50 as gold-standard criteria were presented to identify the best cutoff points with a higher sensitivity (true positive rate) and specificity (true negative rate). The relationship between FeNO, blood eosinophil percentage and total IgE with demographic variables and other biomarkers were assessed using the Pearson’s correlation coefficient metrics. Scatter plot was made to illustrate linear relationship between FeNO, blood eosinophil percentage and total IgE. A p-value ≤0.05 was considered statistically significant result.
Results
Total 76 of patients with asthma were evaluated for predictive validity of biomarkers. Out of these 76 patients, (65.8%) were females and 26 (34.2%) were males. All demographic and clinical characteristics of the patients were listed in (Table 1).
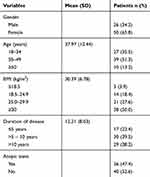 |
Table 1 Demographic and Clinical Characteristics of the Patients |
Differences According the Two FeNO Cutoff Points
FeNO on a cutoff point <25 was seen in 46 (60.5%) and ≥25 in 30 (39.5%) patients. While shifting the cutoff point of FeNO on 50, a majority (88.2%) had FeNO ≤50 while only nine (11.8%) had FeNO>50. While comparing the demographic features including age, weight, height, BMI and duration of disease between FeNO<25 and FeNO ≥25 have shown nonsignificant difference of mean (SD) and the similar pattern was observed when compared the same between FeNO ≤50 and FeNO>50 cutoff points (Table 2). Whereas comparing the mean SD of other biomarkers between FeNO ≤25 vs FeNO>25 showed significant decrease in FEV1/FVC ratio and FEF25-75 in FeNO ≥25 cutoff group: FEV1/FVC ratio=78.5 (10.9) vs 73.1 (9.74), p=0.031; FEF25–75=2.63 (1.17) vs 2.0 (1.13), p=0.021. In contrast, total IgE was significantly higher in FeNO ≥25 cutoff group than FeNO <25 cutoff group: 660.9 (801.7) vs 373.3 (549.1), respectively. However, the eosinophil (%) in peripheral blood was the only factor among the all biomarkers that was found significant (p=0.039) when comparing between the FeNO ≤50 vs FeNO>50 cutoff groups, respectively: 3.87 (2.48) vs 5.81 (3.40). Other variables showed no significant differences between groups as detailed in (Table 2).
 |
Table 2 Comparisons of Demographic Characteristics and Other Biomarkers According to Two Different FeNO Cutoff Points |
Peripheral Blood Eosinophil and Total IgE Predictive Ability
ROC analysis for the predictive validity of eosinophil (%) in peripheral blood and total IgE biomarkers in comparison with baseline FeNO level as gold-standard criteria following positive for ≥25 and negative for <25 cutoff points. Area under the curve (AUC, 95%CI) for peripheral blood eosinophil (%) was 0.57 (95%CI: 0.44–0.71) and of total IgE was 0.64 (0.52–0.77), that reveals higher predictive validity of total IgE than blood eosinophil (%) for FeNO ≥25 cutoff point to be positive (Figure 1). The best cutoff point for blood eosinophil -(%) based on optimal sensitivity and specificity was ≥3.5 where sensitivity and specificity were 56.7% and 54.3%, respectively. While the best cutoff point for total IgE for the similar criterion was ≥250 (sensitivity 63.3% and specificity 64.4%) (Table 3).
 |
Table 3 Predictive Validity of Peripheral Blood Eosinophil (%) and Total IgE Following FeNO as Gold-Standard (Positive for FeNO ≥25) |
ROC analysis on another cutoff point of FeNO level following positive for >50 and negative for ≤50 cutoff points as gold standard was also performed to evaluate the predictive validity of blood eosinophil (%) and total IgE biomarkers. Area under the curve (AUC, 95%I) for blood eosinophil -(%) was found 0.67 (0.48–0.87), and for IgE was 0.64 (0.46–0.83), that reveals higher predictive validity of blood eosinophil (%) than total IgE biomarker for FeNO >50 cutoff point to be positive (Figure 2). The best cutoff point for blood eosinophil (%) based on optimal sensitivity and specificity was ≥4.0 where sensitivity and specificity were 66.7% and 60.0% respectively. While the best cutoff point for total IgE for the similar criterion was ≥350 (sensitivity 66.7% and specificity 63.6%) (Table 4).
 |
Table 4 Predictive Validity of Peripheral Blood Eosinophil (%) and Total IgE Following FeNO as Gold-Standard (Positive for FeNO >50) |
Associations Between FeNO, Blood Eosinophil, Total IgE, PFT and Inflammatory Cytokines
There was found significant positive relationship between baseline FeNO and peripheral blood eosinophil (%) (r=0.276, p=0.017), also significant positive correlation between baseline FeNO and total IgE (r=0.390, p=0.000) (Figure 3A and B). A significant negative correlation was found between peripheral blood eosinophil (%) with FEV1, FEV1% pred and FEF25–75% pred (r=−0.28, p=0.014; r=−0.25, p=0.030 and r=−0.24, p=0.039 respectively). There was nonsignificant correlation of FeNO, blood eosinophil, and total IgE with other PFT measurements and inflammatory cytokines as shown in correlation metrics (Table 5).
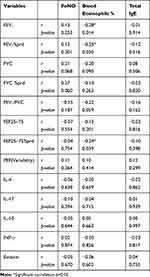 |
Table 5 Correlation of FeNO, Blood Eosinophils (%), and Total IgE with PFT Measurements and Inflammatory Cytokines |
Discussion
This study was designed to determine the best cutoff points of peripheral blood eosinophils and total IgE for detecting eosinophilic airway inflammatory phenotypes in patients with partly controlled asthma using FeNO as the gold standard. The higher predictive ability was found at FeNO >50 ppb, where the best cutoff point for blood eosinophil % was ≥4.0% (sensitivity 66.7%, specificity 60.0%) and the best cutoff point for total IgE was ≥350 (sensitivity 66.7%, specificity 63.6%). In addition, FeNO ≥25 was associated with a significant decrease in FEV1/FVC ratio and FEF25–75 and a significant increase in the total IgE. However, only eosinophils in the peripheral blood were significantly more in the patients with FeNO ≥50. FeNO was also significantly correlated with peripheral blood eosinophils and total IgE but not with any of the serum inflammatory cytokines.
Inflammation is regarded as the major pathophysiological factor in asthma, therefore, its direct measurement is logically the best method to diagnose and monitor asthma. FeNO that is an established marker of airway inflammation associated with atopic or allergic asthma has established its place in the early part of this century.1,3 However, GINA2 and likewise the British Thoracic Society/Scottish Intercollegiate Guidelines (BTS/SIGN)20 do not recommend adopting FeNO alone for monitoring of asthma patients. One of the reasons for this is the controversy about appropriate cutoff values for help in diagnosis or monitoring of asthma.
The ATS and International Classification of Diseases (ICD) rank a FeNO of less than 25 ppb as low, more than 25 but less than 50 ppb as intermediate, and more than 50 ppb as high.21 However, studies indicate that low FeNO cannot be relied upon to exclude asthma. BTS/SIGN management of asthma guidelines suggest >35 for children and >50 for adults,20 while GINA recommends >50 ppb as elevated both for adults as well as for children (11–16 years of age). However, GINA is of the opinion that different values from 20–50 might help in stratification of treatment at asthma clinics.2 This was supported by a meta-analysis of 26 studies which concluded that determining definitive cutoff values for FeNO is apparently impossible just on the basis of the sum of highest sensitivity and specificity, therefore, positive predictive value (PPV) must be included in calculations for determining the cutoff for FeNO%.22 Another study pointed out variation in FeNO values in asthma patients with different phenotypes and proposed that cutoff value of FeNO should be different for each subpopulation of asthma patients.10
In this study 30 (39.5%) of the subjects had FeNO 25 or more, but only nine (11.8%) of them had FeNO >50. The majority, that is, 46 (60.5%) had FeNO <25. A significant decrease in FEV1/FVC ratio and FEF25–75 is seen in this study at FeNO ≥25. FeNO may be influenced by a decrease in the airway caliber, therefore, FeNO cannot be considered a standalone biomarker. In reality it integrates very well with the airway inflammation as well as changes in the lung functions.23 The combination of spirometry and FeNO is definitely highly sensitive as well as specific for the diagnosis of asthma.24 A study specifically pointed out that multiple FeNO measurements along with FEF25%–75% were better determinants to monitor control of asthma than others.25
He et al, evaluated associations of potential determinants of FeNO in asthma patients and reported a negative correlation of FEV1 with FeNO (r=−0.197, p<0.001).10 Malinovschi et al, investigated additive information of two inflammatory biomarkers, FeNO and blood eosinophils in relation to FEV1 in 410 subjects on treatment for asthma. Subjects with both elevated FeNO (>25 ppb) and blood eosinophils (>0.3×109/L) had lower FEV1% (p<0.001) than subjects with normal FeNO and blood eosinophils.26
A study by Qian et al reported that FeNO 25 ppb significantly correlated with FEV1/FVC ratio (p=0.032)27 while a recent study carried out on 160 Korean school children with asthma being monitored a FeNO value ≥35 ppb synchronized with asthma exacerbation. FEV1/FVC ratio as well as FEF25%–75% was significantly less than baseline in these children.28 Furthermore, a study looking for the relationship of asthma control test (ACT) score, FeNO and respiratory function tests in adult asthmatics concluded although FeNO levels had a negative correlation with ACT scores, their relation with lung function was not significant.29
In this study eosinophil (%) in peripheral blood is significantly more in FeNO >50 patients. In addition, the peripheral blood eosinophils (%) had a significant positive correlation with FeNO. Although induced sputum remains a “gold standard” for airway inflammatory phenotypes, yet FeNO is now extensively accepted in routine clinical practice because of its being readily available and ease of performance. FeNO values below a reference cutoff value (≤25 ppb) reflects an absence of eosinophilic inflammation, however, used alone this is not diagnostic of asthma.14 The cutoff point of FeNO for support in diagnosis of asthma recommended by the asthma guidelines in China is 32 ppb since 2016, as this cutoff value was reported to be linked with sputum eosinophils ≥2.5%.30 In a recent study, a FeNO cutoff of 25 ppb showed a significant correlation with eosinophils between >5% and <5%.27 BTS/SIGN British guidelines approve use of FeNO as an evidence of eosinophilic inflammation.20 The ATS has recommended using FeNO for monitoring eosinophilic airway inflammation and the control of asthma.14 A study that related FeNO to the “inflammatory phenotype” of asthma concluded that FeNO was capable of identifying eosinophilic asthma more accurately compared with the other phenotypes.31
In our study total IgE was significantly higher in FeNO ≥25 cutoff group than FeNO <25 cutoff group. In addition, there is a significant positive correlation between FeNO and total IgE. AUC of total IgE was 64% at both FeNO ≥25 ppb, and FeNO >50. The higher predictive ability was detected at FeNO >50 ppb where the best cutoff point for total IgE was ≥350 (sensitivity 66.7%, specificity 63.6%). A recent study by Qian et al reported that FeNO 25 ppb had a significant correlation with serum IgE between 200 and <200 IU/mL level l.27
The current study showed no relationship between FeNO and serum inflammatory cytokines. This was not unexpected due to phenotype heterogeneity in any given patient population. Different phenotypes of asthma show wide heterogeneity in clinical manifestations, response to treatment, and underlying pathogenesis. Some of these heterogeneous inflammatory responses associated with asthma may not be assessed by the existing biomarkers (FeNO, blood eosinophil, IgE), hence requiring novel biomarkers.32 This could explain the controversial results regarding the correlation of blood cytokine profiles and the biomarkers of airway inflammation.
The cytokines are secreted by a number of immune system cells, among them Th-2 cells. FeNO values more than 35–50 ppb have been determined by various studies to reflect inflammation due to Th-2 cells as well as exacerbations in patients undergoing treatment for asthma.33 Some researchers think that biomarkers including FeNO can be used to personalize asthma therapy and it is a dependable Th-2 biomarker for determining status of airway caliber and status of asthma exacerbation.34 At the moment along with history, lung function test, and eosinophils, FeNO is the only noninvasive, quick and readily available test to diagnose type 2 inflammation in patients with asthma.35
In the real patient scenarios FeNO can be part of overall evaluation in diagnosis as well as monitoring the treatment. However, currently FeNO is not a stand-alone test it must be combined with others. A systematic review of 32 studies (24 adults, eight children) reported that the diagnostic accuracy of “stand-alone” FeNO, blood eosinophils, and IgE is just moderate. If any of those is used as a single marker it would generate a number of false results.36 Now the question is what other tests are the most appropriate to accompany FeNO? We feel that the time-tested blood eosinophils and IgE are two such tests that must form part of the diagnostic and prognostic testing along with FeNO.
A strength of this work is the full numerical ROC curve report, which is in line with the recommendation of Karrasch et al after their meta-analysis of 26 studies. They suggested that the FeNO studies for diagnostic accuracy must report full ROC curve numerically in order to facilitate meta-analysis.22 However, our study had some limitations. One limitation was the difficulty in obtaining a bronchoalveolar lavage or induced sputum samples for inflammatory cells and marker analysis. Because both procedures are uncomfortable and have a significant risk of bronchospasm, most of the patients refuse to do it, furthermore the processing techniques for these sample is very sophisticated with a limited success rate even in highly qualified centers.37 We compensated this limitation by substituting these procedures with the noninvasive novel FeNO measurement, which has a comparable reliability and specificity to those invasive techniques.31,38 In addition, owing to the cross-sectional nature of this study, we cannot determine the causal relationship between FeNO and other studied variables.
In conclusion, our data indicate that blood eosinophils and total IgE represent useful biomarkers that can be used to evaluate airway inflammation and to guide asthma treatment together with FeNO. However, cutoff reference values of FeNO level critically tangled the accuracy of both markers. Serum cytokine levels were found to be poor surrogates for FeNO. More studies by taking a balanced cohort sizes based on FeNO cutoff value are suggested to validate the results.
Acknowledgment
This study was carried out with the support from King Abdul Aziz City for Science & Technology (KACST), grant number (10-med-1338-46).
Disclosure
Authors have declared no conflict of interest.
References
1. Ricciardolo FL, Sorbello V, Ciprandi G. A pathophysiological approach for FeNO: a biomarker for asthma. Allergol Immunopathol. 2015;43(6):609–616. doi:10.1016/j.aller.2014.11.004
2. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2020 [cited 2020 23 June]; Available from: https://ginasthma.org/gina-reports/.
3. Heffler E, Carpagnano GE, Favero E, et al. Fractional Exhaled Nitric Oxide (FENO) in the management of asthma: a position paper of the Italian Respiratory Society (SIP/IRS) and Italian Society of Allergy, Asthma and Clinical Immunology (SIAAIC). Multidiscip Respir Med. 2020;15(1):28. doi:10.4081/mrm.2020.36
4. Roos AB, Mori M, Grönneberg R, et al. Elevated exhaled nitric oxide in allergen-provoked asthma is associated with airway epithelial iNOS. PLoS One. 2014;9:2. doi:10.1371/journal.pone.0090018
5. Gustafsson LE, Leone AM, Persson MG, Wiklund NP, Moncada S. Endogenous nitric oxide is present in the exhaled air of rabbits, guinea pigs and humans. Biochem Biophys Res Commun. 1991;181(2):852–857. doi:10.1016/0006-291X(91)91268-H
6. Neelamegan R, Saka V, Tamilarasu K, Rajaram M, Selvarajan S, Chandrasekaran A. Clinical utility of fractional exhaled Nitric Oxide (FeNO) as a biomarker to predict severity of disease and response to Inhaled Corticosteroid (ICS) in asthma patients. J Clin Diagn Res. 2016;10(12):1.
7. Schneider A, Linde K, Reitsma JB, Steinhauser S, Rücker G. A novel statistical model for analyzing data of a systematic review generates optimal cutoff values for fractional exhaled nitric oxide for asthma diagnosis. J Clin Epidemiol. 2017;92:69–78. doi:10.1016/j.jclinepi.2017.09.001
8. Zeiger RS, Schatz M, Yang SJ, Chen W. Fractional exhaled nitric oxide-assisted management of uncontrolled persistent asthma: a Real-World Prospective Observational Study. Perm J. 2019;23:18–109.
9. Truong-Thanh T, Vo-Thi-Kim A, Vu-Minh T, Truong-Viet D, Tran-Van H, Duong-Quy S. The beneficial role of FeNO in association with GINA guidelines for titration of inhaled corticosteroids in adult asthma: a randomized study. Adv Med Sci. 2020;65(2):244–251. doi:10.1016/j.advms.2020.03.001
10. He L, Wei M, Luo J, Du W, Zhang L, Liu C. Re-evaluation of the diagnostic value of fractional exhaled nitric oxide & its impact in patients with asthma. Indian J Med Res. 2018;148(4):441–448. doi:10.4103/ijmr.IJMR_1478_16
11. Garg Y, Kakria N, Katoch CDS, Bhattacharyya D. Exhaled nitric oxide as a guiding tool for bronchial asthma: a randomised controlled trial. Med J Armed Forces India. 2020;76(1):17–22. doi:10.1016/j.mjafi.2018.02.001
12. Shirai T, Inui N, Suda T, Chida K. Correlation between peripheral blood T-cell profiles and airway inflammation in atopic asthma. J Allergy Clin Immunol. 2006;118(3):622–626. doi:10.1016/j.jaci.2006.05.005
13. Lambrecht BN, Hammad H, Fahy JV. The cytokines of asthma. Immunity. 2019;50(4):975–991. doi:10.1016/j.immuni.2019.03.018
14. Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–615. doi:10.1164/rccm.9120-11ST
15. Salem AM, Bamosa AO, Qutub HO, et al. Effect of Nigella sativa supplementation on lung function and inflammatory mediators in partly controlled asthma: a randomized controlled trial. Ann Saudi Med. 2017;37(1):64–71. doi:10.5144/0256-4947.2017.64
16. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi:10.1001/jama.2013.281053
17. Al-Moamary MS, Alhaider SA, Alangari AA, et al. The Saudi initiative for asthma - 2019 update: guidelines for the diagnosis and management of asthma in adults and children. Ann Thorac Med. 2019;14(1):3–48. doi:10.4103/atm.ATM_327_18
18. Exhaled NO. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171(8):912–930. doi:10.1164/rccm.200406-710ST
19. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi:10.1183/09031936.05.00034805
20. British Thoracic Society and Scottish Intercollegiate Guidelines Network. BTS/SIGN British guideline on the management of asthma. 2019 [cited 2020 July 11]; Available from: https://www.brit-thoracic.org.uk/quality-improvement/guidelines/asthma/.
21. Donohue JF, Herje N, Crater G, Rickard K. Characterization of airway inflammation in patients with COPD using fractional exhaled nitric oxide levels: a pilot study. Int J Chron Obstruct Pulmon Dis. 2014;9:745–751. doi:10.2147/COPD.S44552
22. Karrasch S, Linde K, Rücker G, et al. Accuracy of FENO for diagnosing asthma: a systematic review. Thorax. 2017;72(2):109–116. doi:10.1136/thoraxjnl-2016-208704
23. Haccuria A, Michils A, Michiels S, Van Muylem A. Exhaled nitric oxide: a biomarker integrating both lung function and airway inflammation changes. J Allergy Clin Immunol. 2014;134(3):554–559. doi:10.1016/j.jaci.2013.12.1070
24. Szefler SJ, Mitchell H, Sorkness CA, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet. 2008;372(9643):1065–1072. doi:10.1016/S0140-6736(08)61448-8
25. Yoon JY, Woo SI, Kim H, Sun YH, Hahn YS. Fractional exhaled nitric oxide and forced expiratory flow between 25% and 75% of vital capacity in children with controlled asthma. Korean J Pediatr. 2012;55(9):330–336. doi:10.3345/kjp.2012.55.9.330
26. Malinovschi A, Nordvall L, Magnus B, Christer J, Alving K. The combination of elevated FeNO and blood eosinophils relate to reduced lung function and increased bronchial responsiveness in young asthmatics. Eur Respir J. 2014;44(Suppl 58):3409.
27. Qian L, Pan S, Shi J, Du Y, Huang Q, Jie Z. Association between fractional exhaled nitric oxide (FeNO) cutoff values (25 ppb) and risk factors of cough. Clin Respir J. 2018;12(1):193–199. doi:10.1111/crj.12512
28. Kang MG, Yoon SA, Sim JH, Woo SI. Fractional exhaled nitric oxide and forced expiratory volume in 1 second/forced vital capacity have predictive value of asthma exacerbation in Korean school children. Asia Pac Allergy. 2020;10(1). doi:10.5415/apallergy.2020.10.e7
29. Habib SS, Alzoghaibi MA, Abba AA, Hasan M. Relationship of the Arabic version of the asthma control test with ventilatory function tests and levels of exhaled nitric oxide in adult asthmatics. Saudi Med J. 2014;35(4):397–402.
30. Asthma Workgroup of Chinese Society of Respiratory Diseases (CSRD), Chinese Medical Association. The Chinese national guidelines on diagnosis and management of asthma. Chin J Tuberc Respir Dis. 2016;39:675–697.
31. Gao J, Wu F. Association between fractional exhaled nitric oxide, sputum induction and peripheral blood eosinophil in uncontrolled asthma. Allergy Asthma Clin Immunol. 2018;14(1):21. doi:10.1186/s13223-018-0248-7
32. Bhakta NR, Woodruff PG. Human asthma phenotypes: from the clinic, to cytokines, and back again. Immunol Rev. 2011;242(1):220–232. doi:10.1111/j.1600-065X.2011.01032.x
33. Bjermer L, Alving K, Diamant Z, et al. Current evidence and future research needs for FeNO measurement in respiratory diseases. Respir Med. 2014;108(6):830–841. doi:10.1016/j.rmed.2014.02.005
34. Hanania NA, Wenzel S, Rosén K, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187(8):804–811. doi:10.1164/rccm.201208-1414OC
35. Kuo CR, Spears M, Haughney J, et al. Scottish consensus statement on the role of FeNO in adult asthma. Respir Med. 2019;155:54–57. doi:10.1016/j.rmed.2019.07.010
36. Korevaar DA, Westerhof GA, Wang J, et al. Diagnostic accuracy of minimally invasive markers for detection of airway eosinophilia in asthma: a systematic review and meta-analysis. Lancet Respir Med. 2015;3(4):290–300. doi:10.1016/S2213-2600(15)00050-8
37. Guiot J, Demarche S, Henket M, et al. Methodology for sputum induction and laboratory processing. J Vis Exp. 2017;17(130):56612.
38. Gao J, Chen Z, Jie X, Ye R, Wu F. Both fractional exhaled nitric oxide and sputum eosinophil were associated with uncontrolled asthma. J Asthma Allergy. 2018;11:73–79. doi:10.2147/JAA.S155379
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



