Back to Journals » Clinical Interventions in Aging » Volume 12
Assessment of effects of differences in trunk posture during Fowler’s position on hemodynamics and cardiovascular regulation in older and younger subjects
Authors Kubota S, Endo Y, Kubota M, Shigemasa T
Received 14 January 2017
Accepted for publication 23 February 2017
Published 29 March 2017 Volume 2017:12 Pages 603—610
DOI https://doi.org/10.2147/CIA.S132399
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Walker
Satoshi Kubota,1 Yutaka Endo,1 Mitsue Kubota,1 Tomohiko Shigemasa2
1School of Nursing and Rehabilitation Sciences at Odawara, International University of Health and Welfare, Odawara, Kanagawa, Japan; 2Department of Cardiology, International University of Health and Welfare Atami Hospital, Atami, Shizuoka, Japan
Background: Downward shifts in blood volume with changing position generally cause tachycardic responses. Age-related decreases in vagal nerve activity could contribute to orthostatic hypotension in older individuals. Fowler’s position is a reclined position with the back between 30° and 60°, used to facilitate breathing, eating, and other routine daily activities in frail and elderly patients.
Objective: This study examined whether stroke volume (SV) was higher and heart rate (HR) lower in Fowler’s position with an upright upper trunk than in Fowler’s position with the whole trunk upright in both older and younger subjects, based on the assumption that lower HR would result from reduced sympathetic activation in older individuals.
Methods: We assessed hemodynamics and HR variability from electrocardiography, noninvasive arterial pressure and impedance cardiography in 11 younger male subjects (age range, 20–22 years) and 11 older male subjects (age range, 64–79 years), using three positions: supine, or Fowler’s positions with either 30° of lower trunk inclination and 60° of upper trunk inclination (UT60) or 60° of whole trunk inclination (WT60). Comparisons were then made between age groups and between positions.
Results: Reductions in SV and tachycardic response were smaller with UT60 than with WT60, in both younger and older subjects. In addition, reduced tachycardic response with upright upper trunk appeared attributable to decreased vagal withdrawal in younger subjects and to reduced sympathetic activation in older subjects.
Conclusion: Our findings indicate that an upright upper trunk during Fowler’s position allowed maintenance of SV and inhibited tachycardic response compared to an upright whole trunk regardless of age, although the autonomic mechanisms underlying tachycardic responses differed between younger and older adults. An upright upper trunk in Fowler’s position might help to reduce orthostatic stress and facilitate routine activities and conversation in frail patients.
Keywords: aging, cardiovascular regulation, hemodynamics, Fowler’s position, stroke volume
Introduction
Bed rest, heart failure, depletion of intravascular volume, and aging often induce hypotension when the body or trunk is upright.1–5 Generally, a tachycardic response occurs due to vagal withdrawal according to the downward blood shift that occurs under orthostatic stress in healthy humans,6 although orthostatic hypotension presents as an excessive drop in cardiac output (Q) and lower tachycardic response in frail and elderly patients as a consequence of inadequate vascular contraction or autonomic dysfunction.7,8 Numerous studies have suggested that advancing age is associated with decreases in vagal nerve activity.9–12 Age-related decreases in vagal nerve activity have been suggested as contributing factors for orthostatic hypotension in elderly individuals.7,13
Orthostatic hypotension has been reported in more than 60% of frail older patients in an acute geriatric ward.5 Previous studies have observed orthostatic hypotension in 54% of seated older inpatients resting on a bed for more than 12 h.14 In light of these facts, methods of preventing hypovolemia and hypotension during orthostatic stress need to be investigated. Gorelik et al. pointed out that studies of orthostatic hypotension in frail patients, such as those with decompensated heart failure, are scarce and preventive interventions remain insufficient.2
Clinically, frail patients are placed in Fowler’s position or a semiseated position on the bed. These positions are often clinically used without distinction between acute- or chronic-phase illness. The patient is placed in a reclined position with the backrest between 30° and 60° and with flexed or straight knees in Fowler’s position.15,16 Breathing, eating, and other routine daily activities are considered to be facilitated in Fowler’s position.15,17–20 Some studies have suggested a relationship between trunk angle and hemodynamics during Fowler’s position.21–24 Cicolini et al. reported that blood pressure in Fowler’s position was intermediate between the seated and supine positions in both young healthy individuals and hypertensive patients.23,24 Those studies indicated that the effects of orthostatic stress on hemodynamics in Fowler’s position may be substantial in frail patients. How posture can prevent the hypovolemia and hypotension caused by orthostatic stress during Fowler’s position is important and should be addressed. Our previous study indicated that Fowler’s position with an upright upper trunk is effective for maintaining circulatory volume with lower tachycardia via vagal withdrawal compared with having the whole trunk upright in younger subjects.25 However, whether an upright upper trunk in Fowler’s position allows easy maintenance of circulatory volume in cases with autonomic hypofunction associated with aging remains unclear.
We postulated that stroke volume (SV) would be higher and heart rate (HR) would be lower with the upper trunk upright than with the whole trunk upright in Fowler’s position in both older and younger subjects. In addition, we presumed that the lower HR would be caused by reduced sympathetic activation in older individuals but by reduced vagal withdrawal in younger individuals. The present study was performed as a cross-sectional study to assess our hypothesis with a fundamental study of the effects of the two Fowler’s positions on hemodynamics and HR variability parameters in healthy older and younger subjects.
Methods
Subjects
We assessed hemodynamics and HR variability in 11 male younger subjects (mean age ± standard error of the mean [SEM]: 20.5±0.2 years; range: 20–22 years; weight: 60.6±1.8 kg; height: 170.2±1.8 cm) and 11 male community-dwelling older subjects (mean age ± SEM: 69.5±1.2 years; range: 64–79 years; weight: 62.0±2.1 kg; height: 165.4±1.8 cm). All subjects were interviewed regarding medical history, and blood pressure was checked after a period of rest. Final decisions on subject selections were made by consultations between a medical doctor and researchers. Potential subjects were excluded when they had a history of cardiovascular disease, respiratory disease or other chronic medical disease, or were not normotensive (systolic bold pressure below 140 mmHg and diastolic blood pressure below 90 mmHg). All subjects in the final analysis were healthy and had no history of chronic or acute cardiovascular or respiratory disease or other chronic diseases.
Subjects were instructed to refrain from beverages containing caffeine or alcohol for 24 h before starting experiments and to refrain from eating and drinking after 2,200 h on the day prior to the experiments that started in the morning or to consume a light breakfast before experiments that started in the afternoon.
All experiments were implemented between 1,100 and 1,400 h, because blood pressure fluctuates according to a circadian pattern stabilizing at approximately noon. The Ethics Commission of the International University of Health and Welfare approved the study, and all participants provided voluntary written consent to participate after being fully informed about the procedure, risks, and protocol.
Procedure
We collected physiological data with the patient in the supine position and two Fowler’s positions. Prior to experiments, subjects rested in a thermoneutral room at 28°C for 15 min and were set up for electrocardiography (ECG), noninvasive arterial pressure monitoring, and impedance cardiography (ICG). Measurements in the supine position were made with sufficient time between measurements in the two Fowler’s positions. Between measurements in each Fowler’s position, subjects rested for more than 3 min. All measurements in the two Fowler’s positions were repeated at random. Data were recorded for 5 min in each of the supine position and the two Fowler’s positions.
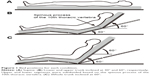
Subjects were placed in the Fowler’s positions on a bed at 30° of lower trunk inclination and 60° of upper trunk inclination (UT60) and 60° of whole trunk inclination (WT60) (Figure 1). We defined the upper and lower segments at UT60 around the spinous process of the 10th thoracic vertebra. The height of the bottom of the upper and lower trunk was adjusted according to individual trunk size. All Fowler’s positions allowed slight hip and knee joint flexion.
All experiments were performed in our laboratory (Odawara, Kanagawa, Japan) under controlled thermoneutral temperature.
Measurements
HR was estimated from type II ECG (ECG 100C; BIOPAC Systems, Goleta, CA, USA) in all experiments. ICG (NICO 100C; BIOPAC Systems) was performed using eight spot electrodes (Vitrode M; Nihon Kohden, Tokyo, Japan) attached to the neck and lower thorax of each subject for ICG based on a previous study.26 SV was estimated from transthoracic bioimpedance using Bernstein’s method that has been validated as offering high reliability comparable to the technique of thermodilution.26 ICG allows continuous noninvasive evaluation of SV. Q was calculated as the product of SV and HR. Noninvasive continuous arterial pressure was measured in the radial artery by tonometry using an arterial pressure monitor (BP-608EV; Omron Colin, Tokyo, Japan). Continuous arterial pressure was calibrated using oscillometric sphygmomanometry to measure intermittent cuff blood pressure. The tonometry sensor was maintained at the level of the heart during all experiments. Mean arterial pressure (MAP) was estimated as one third pulse pressure plus diastolic pressure. ECG, thoracic bioimpedance, and continuous arterial pressure signals were recorded on a personal computer using the MP150 data acquisition system (BIOPAC Systems) at a sampling rate of 1 kHz. Five-min means of all hemodynamics values were calculated.
We performed frequency domain and time domain analysis of ECG for the assessment of vagal function. We calculated logarithm-transformed respiratory sinus arrhythmia (ln RSA) from the power spectrum of the RR intervals (0.15–0.4 Hz). The maximum entropy method was used for power spectrum analysis of RR intervals. We thus calculated the square root of the mean of the sum of the squares of differences between adjacent RR intervals (RMSSD), number of pairs of adjacent RR intervals differing by more than 50 ms (NN50) and the proportion derived by dividing NN50 by the total number of RR intervals (pNN50) for 5 min. All parameters of HR variability reflect vagal modulation.27,28
Statistical analysis
Changes in hemodynamic variables from supine and HR variability parameters in the three positions in subjects from the two age groups were compared by two-way mixed-design analysis of variance (ANOVA). When three positions × two age group interactions were significant, one-way repeated-measures ANOVA of the three positions in each subject and one-way ANOVA of subjects from the two age groups in each position were used to evaluate simple main effects. Subsequent post hoc analysis to determine differences between the three positions in each age group were evaluated using a paired t-test with Shaffer’s multiple comparison procedure in each age group.29 The level of statistical significance was set at P<0.05. All values are shown as mean and SEM. Data were statistically analyzed using R for Windows version 3.3.0 statistical software (www.r-project.org) and the car 2.1–4 package.
Results
Hemodynamics
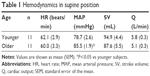
Table 1 shows the results of hemodynamic variables while supine. No differences in hemodynamic variables other than MAP were seen between groups. MAP was significantly higher in older subjects than in younger subjects (P<0.05).
Figure 2 shows the results of changes in hemodynamic variables from supine at all positions. Change in HR (ΔHR) increased and change in SV (ΔSV) decreased with the change from supine to an upright trunk in younger and older subjects. SV at UT60 was maintained compared with WT60. Two-way mixed-design ANOVA of ΔHR and ΔSV revealed significant main effects of the three positions (ΔHR, F[2,40] =31.73; ΔSV, F[2,40] =15.73; P<0.05), but no significant main effects of the two age groups (P>0.05) and no significant three positions × two age groups interactions (P>0.05). Multiple comparisons for each age group showed that ΔHR (UT60 and WT60 in younger, 2.9±1.2 beats/min and 6.6±1.4 beats/min, respectively; UT60 and WT60 in older, 2.8±0.7 beats/min and 5.0±0.9 beats/min, respectively) was significantly lower at UT60 than at WT60 and at supine than at UT60 and WT60. In addition, ΔSV (UT60 and WT60 in younger, −4.4±2.8 mL and −13.7±2.9 mL, respectively; UT60 and WT60 in older, −0.3±3.3 mL and −7.4±3.1 mL, respectively) was significantly higher at UT60 and supine than at WT60 in older and younger subjects (P<0.05).
ANOVA for changes in Q (ΔQ: UT60 and WT60 in younger, 0.1±0.2 L and −0.3±0.2 L, respectively; UT60 and WT60 in older, 0.2±0.2 L and 0.0±0.2 L, respectively) revealed a significant main effect of the three positions (ΔQ, F[2,40] =3.55, P<0.05) but no significant main effect of the two age groups (P>0.05) and no significant interaction of three positions × two age groups (P>0.05). Multiple comparisons among each age group showed no significant differences between the three positions (P>0.05). ANOVA of changes in MAP (ΔMAP: UT60 and WT60 in younger, −1.2±1.9 mmHg and 4.2±4.0 mmHg, respectively; UT60 and WT60 in older, −0.5±2.8 mmHg and −2.7±2.6 mmHg, respectively) revealed no significant main effects for the three positions (P>0.05) or two age groups (P>0.05) and no significant interaction of the three positions × two age groups (P>0.05).
HR variability
Figure 3 shows the results for ln RSA and RMSSD in all positions. Although all parameters decreased with the change from supine to upright trunk in younger subjects, significant differences were not seen between all positions in older subjects. These parameters in older subjects were lower compared with younger subjects for each position (Table 2). Two-way mixed-design ANOVA of ln RSA, RMSSD, NN50, and pNN50 revealed significant main effects of the three positions (P<0.05) and two age groups (P<0.05) but significant three positions × two age groups interactions (P<0.05). Post hoc analysis revealed significant simple main effects of the three positions in younger subjects (ln RSA in younger, F[2,20] =8.67; RMSSD in younger, F[2,20] =6.71; NN50 in younger, F[2,20] =5.95; pNN50 in younger, F[2,20] =7.12; P<0.05) but no significant simple main effect of the three positions in older subjects (P>0.05) and significant simple main effects of the two age groups among each position (ln RSA of supine, F[1,20] =34.6; RMSSD of supine, F[1,20] =25.18; NN50 of supine, F[1,20] =27.45; pNN50 of supine, F[1,20] =29.77; ln RSA of UT60, F[1,20] =24.72; RMSSD of UT60, F[1,20] =19.87; NN50 of UT60, F[1,20] =20.43; pNN50 of UT60, F[1,20] =19.46; ln RSA of WT60, F[1,20] =14.36; RMSSD of WT60, F[1,20] =4.95; NN50 of WT60, F[1,20] =6.59; pNN50 of WT60, F[1,20] =6.12; P<0.05). Multiple comparisons among the three positions showed that all HR variability parameters were significantly higher at UT60 and supine position than at WT60 in younger subjects (P<0.05).
Discussion
The major findings of the present study were as follows: 1) the reductions in SV and tachycardic response were smaller with the upper trunk upright compared with the whole trunk upright, in healthy young and older individuals, and 2) the reduced tachycardic response with the upright upper trunk would be caused by decreased vagal withdrawal in younger subjects and reduced sympathetic activation in older subjects.
Decreases in SV were smaller at UT60 than at WT60 in both subject groups. Orthostatic stress reportedly occurs with the shift in blood volume to the abdomen and legs in healthy individuals.30,31 At UT60, subdivision of the trunk into upper and lower segments was defined by the spinous process of the 10th thoracic vertebra, under the presumption that since the heart is located close to the center of rotation of the bottom of the dorsal thorax, even if the upper trunk is upright, the level of the heart is lower with UT60 than with WT60. In addition, the abdomen and pelvic segments at UT60 were not upright compared with WT60. Orthostatic stress might therefore be smaller at UT60 than at WT60, suggesting differences in the distribution of the blood volume shift to lower parts of the body at UT60 compared to WT60 in both subject groups. Moreover, these results were observed to be similar to the findings of our previous study in healthy young subjects.25
Some previous studies have reported the effects of advancing age on SV under orthostatic stress induced by lower body negative pressure (LBNP). These previous studies revealed that the reduction of SV was significantly lower among older individuals than among younger individuals under higher LBNP, although changes in SV did not differ between age groups under lower level LBNP.32,33 Tsutsui et al. indicated that hypovolemia was less among older subjects than among the younger during higher level LBNP because leg compliance in the older subjects was reduced according to decreased capacitance function with age.33 With the present findings, significant differences in ΔSV between age groups with the two Fowler’s positions were not observed. These results appear similar to previous findings during lower level LBNP but appear to differ from previous findings for higher-level LBNP.33 In Fowler’s position, since only the trunk and head were upright and the lower extremities were similar to the level in the supine position, orthostatic stress would be limited. Differences in leg compliance with aging were thus indicated to have no effect on differences in ΔSV between younger and older subjects, because the shift in blood volume to the lower extremities was regarded as too small. In this study, ΔSV was less than approximately 14 mL during the two Fowler’s positions in both age groups.
Positive ΔHR occurred with the change from supine to upright trunk and ΔHR was lower at UT60 than at WT60 in both age groups. These tachycardic responses were suggested to be the result of the negative ΔSV being lower at UT60 than at WT60. However, since all HR variability parameters in older subjects differed from those of younger subjects in the three positions, the mechanisms underlying the tachycardic responses reflected in the present results were suggested to differ between younger and older subjects. Generally, tachycardic response less than 100 beats/min is attributed to vagal withdrawal.34 We therefore concluded that the tachycardic responses in the present results were caused by vagal withdrawal in younger subjects. In addition, vagal withdrawal would be lower at UT60 than at WT60 because ln RSA, RMSSD, NN50, and pNN50 were higher at UT60 compared with WT60 in younger subjects. However, no significant differences were seen among all positions and ln RSA, RMSSD, NN50, and pNN50 were lower in older subjects than in younger subjects in each position. Vagal function is well known to reduce with age.9–12 These results suggested that vagal function was reduced in older subjects and tachycardic response was caused by mechanisms other than vagal withdrawal. Nevertheless, the tachycardic response was significantly different between the two Fowler’s positions in older subjects. Some previous studies have indicated that sympathetic nerve function and sympathetic baroreflex function are maintained with age.10,32,35–37 The tachycardic response of older subjects with an upright trunk might thus be suggested to result from sympathetic activation in the present study, rather than vagal withdrawal. From the above, compared with WT60, UT60 might inhibit decreases in SV and inhibit tachycardic response by reducing vagal withdrawal in younger subjects and by reducing sympathetic activation in older subjects.
Although ΔSV was higher at UT60 than at WT60 in both age groups, ΔQ and ΔMAP did not differ between the two Fowler’s positions in either age group or between the two age groups in either Fowler’s position. These results suggest that the tachycardic response and vasomotor contraction response maintained ΔQ and ΔMAP to compensate for reduced ΔSV under orthostatic stress regardless of age. Such findings may be attributed to the regulation of tachycardic response and vasomotor contraction via vagal withdrawal in younger individuals and via sympathetic activation in older individuals.
Our findings indicate that an upright upper trunk during Fowler’s position allowed maintenance of SV and inhibited tachycardic response compared to an upright whole trunk regardless of age, although the autonomic mechanisms underlying tachycardic responses differed between younger and older adults. An upright upper trunk in Fowler’s position might help to reduce orthostatic stress and facilitate routine activities and conversation in frail patients. Some studies have suggested that leg compression bandaging could prevent orthostatic hypotension in older patients.2,38 Although that method might indeed be effective, that approach requires a more time-consuming procedure and the patient may feel a sense of uncomfortable restriction from the bandaging. Our proposed method offers the advantages that the procedure to tilt the upper trunk is very simple and patients do not require compression bandaging or use of stockings to maintain circulatory volume when the patient wants to raise the body on the bed.
Limitations
The present results suggest that reduced tachycardic response with an upright upper trunk may be regulated by reduced sympathetic activation compared with having the whole trunk upright during Fowler’s position in older subjects. However, the present study did not directly assess sympathetic nerve activity in the two Fowler’s positions or while supine. To corroborate our findings, confirmation by direct measurement of sympathetic nerve activity or venous plasma norepinephrine concentration in Fowler’s positions in younger and older subjects may be necessary. In consideration of using a Fowler’s position such as UT60 at clinical sites, validation in patients with orthostatic intolerance is needed because the influences of an upright upper trunk on hemodynamics and cardiovascular regulation in such patients have yet to be completely clarified.
Conclusion
We demonstrated that an upright upper trunk during Fowler’s position allowed maintenance of SV and inhibited tachycardic responses compared to having the whole trunk upright in both younger and older subjects. Such inhibition of tachycardic responses might be caused by reduced vagal withdrawal in younger subjects and by reduced sympathetic activation in older subjects. An upright upper trunk during Fowler’s position might be useful to maintain circulatory volume for reduced orthostatic stress and to facilitate daily activities in frail patients at clinical sites.
Acknowledgments
We are grateful to Yusuke Ishizuka and Professor Yoko Sugawara for their invaluable assistance. This work was supported by JSPS KAKENHI Grant Number 24593244.
Disclosure
The authors report no conflicts of interest in this work.
References
Cowie DA, Shoemaker JK, Gelb AW. Orthostatic hypotension occurs frequently in the first hour after anesthesia. Anesth Analg. 2004;98(1):40–45. | ||
Gorelik O, Almoznino-Sarafian D, Litvinov V, et al. Seating-induced postural hypotension is common in older patients with decompensated heart failure and may be prevented by lower limb compression bandaging. Gerontology. 2009;55(2):138–144. | ||
Hiitola P, Enlund H, Kettunen R, Sulkava R, Hartikainen S. Postural changes in blood pressure and the prevalence of orthostatic hypotension among home-dwelling elderly aged 75 years or older. J Hum Hypertens. 2009;23(1):33–39. | ||
Ooi WL, Hossain M, Lipsitz LA. The association between orthostatic hypotension and recurrent falls in nursing home residents. Am J Med. 2000;108(2):106–111. | ||
Weiss A, Grossman E, Beloosesky Y, Grinblat J. Orthostatic hypotension in acute geriatric ward: is it a consistent finding? Arch Intern Med. 2002;162(20):2369–2374. | ||
Rowell LB. Reflex Control During Orthostasis. In: Human Cardiovascular Control. New York: Oxford University Press USA; 1993:37–80. | ||
Gupta V, Lipsitz LA. Orthostatic hypotension in the elderly: diagnosis and treatment. Am J Med. 2007;120(10):841–847. | ||
Lanier JB, Mote MB, Clay EC. Evaluation and management of orthostatic hypotension. Am Fam Physician. 2011;84(5):527–536. | ||
Bonnemeier H, Richardt G, Potratz J, et al. Circadian profile of cardiac autonomic nervous modulation in healthy subjects: differing effects of aging and gender on heart rate variability. J Cardiovasc Electrophysiol. 2003;14(8):791–799. | ||
Monahan KD. Effect of aging on baroreflex function in humans. Am J Physiol Regul Integr Comp Physiol. 2007;293(1):R3–R12. | ||
De Meersman RE, Stein PK. Vagal modulation and aging. Biol Psychol. 2007;74(2):165–173. | ||
Shannon DC, Carley DW, Benson H. Aging of modulation of heart rate. Am J Physiol. 1987;253(4 Pt 2):H874–H877. | ||
Shi X, Wray DW, Formes KJ, et al. Orthostatic hypotension in aging humans. Am J Physiol Heart Circ Physiol. 2000;279(4):H1548–H1554. | ||
Cohen N, Gorelik O, Fishlev G, et al. Seated postural hypotension iscommon among older inpatients. Clin Auton Res. 2003;13(6):447–449. | ||
Carol T, Carol L, Pamela L, Priscilla L. Activity. In: Fundamentals of Nursing: The Art and Science of Nursing Care.8th ed. Philadelphia: Wolters Kluwer; 2015:1037–1117. | ||
Judith AM. Immobility. In: Potter PA, editor. Fundamentals of Nursing.9th ed. St. Louis: Elsevier; 2017:407–441. | ||
Her C, Frost EA. Assessment of right ventricular function by right ventricular systolic time intervals in acute respiratory failure. Crit Care Med. 1999;27(12):2703–2706. | ||
Son JT, Lee E. Comparison of postprandial blood pressure reduction in the elderly by different body position. Geriatr Nurs. 2013;34(4):282–288. | ||
Potter PA. Immobility; Hygiene. In: Potter PA, editor. Fundamentals of Nursing.9th ed. St. Louis: Elsevier; 2017:407–870. | ||
Rauen CA, Makic MB, Bridges E. Evidence-based practice habits: transforming research into bedside practice. Crit Care Nurse. 2009;29(2):46–59. | ||
Shih FJ. Patient positioning and the accuracy of pulmonary artery pressure measurements (180f). Int J Nurs Stud. 1999;36(6):497–505. | ||
Driscoll A, Shanahan A, Crommy L, Foong S, Gleeson A. The effect of patient position on the reproducibility of cardiac output measurements. Heart Lung. 1995;24(1):38–44. | ||
Cicolini G, Pizzi C, Palma E, et al. Differences in blood pressure by body position (supine, Fowler’s, and sitting) in hypertensive subjects. Am J Hypertens. 2011;24(10):1073–1079. | ||
Cicolini G, Gagliardi G, Ballone E. Effect of Fowler’s body position on blood pressure measurement. J Clin Nurs. 2010;19(23–24):3581–3583. | ||
Kubota S, Endo Y, Kubota M, Ishizuka Y, Furudate T. Effects of trunk posture in Fowler’s position on hemodynamics. Auton Neurosci. 2015;189:56–59. | ||
Bernstein DP, Lemmens HJ. Stroke volume equation for impedance cardiography. Med Biol Eng Comput. 2005;43(4):443–450. | ||
Berntson GG, Cacioppo JT, Quigley KS. Respiratory sinus arrhythmia: autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology. 1993;30(2):183–196. | ||
Task Force of the European Society of Cardiology; North American Society of Pacing and Electrophysiology, Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93(5):1043–1065. | ||
Shaffer JP. Modified sequentially rejective multiple test procedures. J Am Stat Assoc. 1986;81(395):826–831. | ||
Self DA, White CD, Shaffstall RM, Mtinangi BL, Croft JS, Hainsworth R. Differences between syncope resulting from rapid onset acceleration and orthostatic stress. Aviat Space Environ Med. 1996;67(6):547–554. | ||
Smit AA, Halliwill JR, Low PA, Wieling W. Pathophysiological basis of orthostatic hypotension in autonomic failure. J Physiol. 1999;519 Pt 1:1–10. | ||
Taylor JA, Hand GA, Johnson DG, Seals DR. Sympathoadrenal-circulatory regulation of arterial pressure during orthostatic stress in young and older men. Am J Physiol. 1992;263(5 Pt 2):R1147–R1155. | ||
Tsutsui Y, Sagawa S, Yamauchi K, Endo Y, Yamazaki F, Shiraki K. Cardiovascular responses to lower body negative pressure in the elderly: role of reduced leg compliance. Gerontology. 2002;48(3):133–139. | ||
Rowell LB. Central Circulatory Adjustments to Dynamic Exercise. In: Human Cardiovascular Control. New York: Oxford University Press USA; 1993:162–203. | ||
Ebert TJ, Morgan BJ, Barney JA, Denahan T, Smith JJ. Effects of aging on baroreflex regulation of sympathetic activity in humans. Am J Physiol. 1992;263(3 Pt 2):H798–H803. | ||
Matsukawa T, Sugiyama Y, Iwase S, Mano T. Effects of aging on the arterial baroreflex control of muscle sympathetic nerve activity in healthy subjects. Environ Med. 1994;38(1):81–84. | ||
Matsukawa T, Sugiyama Y, Mano T. Age-related changes in baroreflex control of heart rate and sympathetic nerve activity in healthy humans. J Auton Nerv Syst. 1996;60(3):209–212. | ||
Podoleanu C, Maggi R, Brignole M, et al. Lower limb and abdominal compression bandages prevent progressive orthostatic hypotension in elderly persons: a randomized single-blind controlled study. J Am Coll Cardiol. 2006;48(7):1425–1432. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.