Back to Journals » Journal of Asthma and Allergy » Volume 14
Are Volatile Organic Compounds Able to Identify Airflow Decline in Asthma?
Authors Graff S , Zanella D, Stefanuto PH, Henket M, Paulus V, Guissard F, Moermans C , Van Steen K, Louis R, Schleich F
Received 30 October 2020
Accepted for publication 23 December 2020
Published 25 January 2021 Volume 2021:14 Pages 67—70
DOI https://doi.org/10.2147/JAA.S289278
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Amrita Dosanjh
Sophie Graff,1 Delphine Zanella,2 Pierre-Hugues Stefanuto,2 Monique Henket,1 Virginie Paulus,1 Francoise Guissard,1 Catherine Moermans,1 Kristel Van Steen,3 Renaud Louis,1 Florence Schleich1
1Department of Respiratory Medicine, GIGA I3, CHU Sart-Tilman, Liege, Belgium; 2Organic and Biological Analytical Chemistry Group, University of Liege, Liege, Belgium; 3BIO3-Systems Genetics, GIGA-R, University of Liege, Liege, Belgium
Correspondence: Sophie Graff
Department of Respiratory Medicine, CHU Sart-Tilman, GIGA +4, CHU - B34, Avenue de l’Hôpital, 11, Liège 4000, Belgium
Tel +32 4366 45 92
Email [email protected]
Asthma is a chronic inflammatory disease of the airways. Asthmatics are at risk of developing structural changes resulting in persistent airflow limitations and remodeling.1 On average, asthma patients have lower lung function than healthy individuals and their lung function (FEV1) decline can be greater over time.1 Nevertheless, not all asthma patients decline over time; some show stable lung function while others can outgrow their asthma disease.2 Previous studies have revealed risk factors for accelerated FEV1 decline in asthma including airway inflammation. Indeed, high eosinophil sputum numbers,2 as well as higher blood eosinophil numbers3 have been linked to the accelerated rate of lung function decline in asthma. Analysis of exhaled breath for endogenously generated volatile organic compounds (VOCs) has previously been found to be able to noninvasively differentiate eosinophilic from neutrophilic asthma.4 In a previous study, we were also able to identify in vitro VOCs discriminating between eosinophil and neutrophil cultures, regardless the activation status.5 As VOCs are able to reflect inflammation,4 we were interested to evaluate if VOCs are able to quantify airflow decline in asthma. VOC measurements could be an easy and non-invasive way to identify ongoing airflow decline in asthmatic for whom no prior measurement of lung function was available. This could have an impact on the follow-up visit schedule and treatment regimen.
We conducted a prospective study on a population of unselected asthmatics recruited from the University Asthma Clinic of Liege after gaining fully informed written consent and with approval from the ethics committee of CHU Liège (2005/181) in accordance with the Helsinki Declaration. Patients underwent measurements of fraction of exhaled nitric oxide (FeNO), spirometry, sputum induction, and gave a blood sample at two time points (baseline and 5 years later). VOC measurements were collected once at the 5-year-follow-up visit. Subjects with asthma were allocated in two groups (airflow decliners and non-decliners) retrospectively according to their lung function (post-BD FEV1) decline per year calculated as by subtracting the follow-up measured value from the baseline post-BD FEV1, subdivided by the number of months separating the two measurements, and multiplied by 12. Decline was defined as a loss of FEV1 (% predicted)/year greater than zero. The thermal desorption tubes, containing the exhaled breath of the patients were desorbed onto a Pegasus 4D HRT (LECO Corporation, St. Joseph, MI, USA) GC × GC-HR ToF MS instrument with an Agilent 7890 GC equipped with a TD100-xr thermal desorber (Markes International Ltd.). The data were acquired and analyzed using ChromaTOF HRT software, version 5.20 (LECO Corporation) and GC Image software version 2.5HRMS®. Analysis of the data output files was performed in successive steps as previously described.6 The identification of the VOCs was performed following high analytical standards (bi-dimensional chromatographic retention times and mass spectral matching factors). To estimate the discrimination importance of each VOC, conditional inference forest algorithm7 along with variable importance measure were used to assess the discriminatory power of each VOC in the different scenarios. Receiver operating characteristic (ROC) curves were constructed to assess the discriminatory power of the biomarkers for lung function decline.
Exhaled breath of 45 asthmatics was analyzed. Patient characteristics and baseline lung function parameters are presented in Table 1. Twenty-five patients (56%) were airflow decliners over a 5-year period and 20 patients (44%) were non-decliners. Looking at baseline characteristics, we did not find any significant difference between groups regarding gender, age, age of onset, BMI, smoking history, baseline FEV1, FeNO, sputum eosinophil and neutrophil counts, Asthma Control Questionnaire or blood eosinophils and neutrophils, exacerbations, hospitalizations in the last 12 months, or treatment regimen. Fifty-four percent of airflow decliners were receiving high ICS daily doses, as compared to 25% in the non-decliners. OCS maintenance treatment was present in 16% of airflow decliners versus 10% in non-decliners. Sixty percent of decliners and 75% of non-decliners were treated with long-acting β2 agonists. Twenty percent of decliners versus 5% of non-decliners were receiving long-acting anti-muscarinic agent therapy.
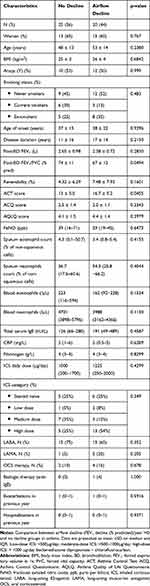 |
Table 1 Demographic, Clinical and Inflammatory Characteristics at Baseline of Asthmatics with VOC Measurements (n=45) |
According to ERS/ATS criteria,8 40% of these patients were severe asthmatics. Eight of them were started on biologic therapy during the study. Three of them were still part of the airflow decliners group. Sputum inflammatory phenotypes and inhaled corticosteroid doses were quite stable over time.
Based on a set of 640 VOCs, the comparison of these two groups revealed no difference between airflow decliners and non-decliners. Moreover, no difference between groups was observed based on the 25 VOCs with the highest classification performance. With a Random Forest estimated prediction error of 48.6%, no significant discrimination could neither be shown between the two groups. ROC curves for the prediction of FEV1 decline using 5, 10, 15, 25, 50, 100 VOCs had AUC of 0.529, 0.533, 0.545, 0.538, 0.547, 0.544, respectively (Figure 1).
Although Lazar et al9 previously found that bronchoconstriction after methacholine challenge did not affect Breathprint using eNose, we hypothesized that VOCs could discriminate between airflow decliners and non-decliners as several cytokines and inflammatory cells may play a role in airflow decline. Our study is the first attempt to relate exhaled VOC profiles to lung function decline in asthmatic patients over 5 years. According to our results, VOCs are not able to discriminate between airflow decliners and non-decliners. In our study, airflow decliners and non-decliners had similar levels of FeNO, blood and sputum eosinophils at baseline. It has already been shown that VOCs reflect airway inflammation.4 Our observations bring out that exhaled VOCs are possibly more to be considered as a reflection of the inflammatory mechanism rather than an airway caliber. Our results should be confirmed in a larger cohort using multiple VOC measurements over a longer period of time to see if the change in VOC levels over time can reflect FEV1 decline and if other variables may affect the relationship between VOC measurements and lung function.
Disclosure
Prof. Dr Renaud Louis reports grants and/or personal fees from GSK, AZ, Novartis, Chiesi, and Sanofi, outside the submitted work. The authors report no other conflict of interest with this work.
References
1. Lange P, Parner J, Vestbo J, et al. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med. 1998;339(17):1194–1200. doi:10.1056/NEJM199810223391703
2. Broekema M, Volbeda F, Timens W, et al. Airway eosinophilia in remission and progression of asthma: accumulation with a fast decline of FEV(1). Respir Med. 2010;104:1254–1262. doi:10.1016/j.rmed.2010.03.030
3. Graff S, Demarche S, Henket M, et al. Increase in blood eosinophils during follow-up is associated with lung function decline in adult asthma. Respir Med. 2019;152:60–66. doi:10.1016/j.rmed.2019.04.020
4. Schleich FN, Zanella D, Stefanuto P-H, et al. Exhaled volatile organic compounds are able to discriminate between neutrophilic and eosinophilic asthma. Am J Respir Crit Care Med. 2019;200(4):444–453. doi:10.1164/rccm.201811-2210OC
5. Schleich FN, Dallinga JW, Henket M, et al. Volatile organic compounds discriminate between eosinophilic and neutrophilic inflammation in vitro. J Breath Res. 2016;10(1):016006. doi:10.1088/1752-7155/10/1/016006
6. Garcia‐Marcos L, Edwards J, Kennington E, et al. Priorities for future research into asthma diagnostic tools: A PAN-EU consensus exercise from the European asthma research innovation partnership (EARIP). Clin Exp Allergy. 2018;48(2):104–120. doi:10.1111/cea.13080
7. Hothorn LA. How to deal with multiple treatment or dose groups in randomized clinical trials? Fundam Clin Pharmacol. 2007;21(2):137–154. doi:10.1111/j.1472-8206.2007.00469.x
8. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi:10.1183/09031936.00202013
9. Lazar Z, Fens N, van der Maten J, et al. Electronic nose breathprints are independent of acute changes in airway caliber in asthma. Sensors (Basel). 2010;10:9127–9138. doi:10.3390/s101009127
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

