Back to Journals » International Journal of Nanomedicine » Volume 15
Antibacterial Activity of Chitosan Nanoparticles Against Pathogenic N. gonorrhoea
Authors Alqahtani F , Aleanizy F , El Tahir E , Alhabib H, Alsaif R, Shazly G, AlQahtani H , Alsarra I , Mahdavi J
Received 23 July 2020
Accepted for publication 24 September 2020
Published 13 October 2020 Volume 2020:15 Pages 7877—7887
DOI https://doi.org/10.2147/IJN.S272736
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Thomas Webster
Fulwah Alqahtani,1,* Fadilah Aleanizy,1,* Eram El Tahir,1 Hiba Alhabib,1 Raghad Alsaif,1 Gamal Shazly,1,2 Hajar AlQahtani,3 Ibrahim Alsarra,1 Jafar Mahdavi4
1Department of Pharmaceutics, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia; 2Department of Industrial Pharmacy, Faculty of Pharmacy, Assiut University, Assiut, Egypt; 3Department of Pharmacy Services, King Abdul-Aziz Medical City, Ministry of National Guard, Health Affairs, Riyadh, Saudi Arabia; 4School of Life Sciences, Centre for Biomolecular Sciences, University of Nottingham, University Park, Nottingham, UK
*These authors contributed equally to this work
Correspondence: Fulwah Alqahtani
Department of Pharmaceutics, College of Pharmacy, King Saud University, P.O. Box: 22452, Riyadh 11495, Saudi Arabia
Tel +966-11-8051478
Email [email protected]
Purpose: The emergence of Neisseria gonorrhoeae strains that are resistant to the most commonly used antibiotics represents a great concern for global public health. This challenges the effectiveness of clinical treatment regimens and demands the development of alternative antigonococcal agent. In this regard, chitosan nanoparticles (CNPs) are known to have antimicrobial activity against a wide range of pathogens. Thus, they have become a potential candidate for combatting this era of multi-drug resistance. This study aims to formulate CNPs, characterize their physicochemical properties, and examine their antimicrobial activity against gonococcus.
Materials and Methods: The ionic gelation method was used to prepare CNPs of different concentrations. Characterization for their particle size (PZ), polydispersity index (PDI), and zeta potential (ZP) was performed. The anti-microbial activity of CNPs was investigated against 13 WHO N. gonorrhoeae reference strains, using the broth dilution method. Cytotoxicity of CNPs and their effect on bacterial adhesion to HeLa cells were investigated.
Results: The average PZ and ZP of the prepared NPs were increased when the concentration of chitosan was increased from 1 to 5 mg/mL and found to be in the range of 193 nm ± 1.9 to 530 nm ± 13.3, and 14 mV ± 0.5 to 20 mV ± 1, respectively. Transmission electron microscopes (TEM) images revealed spherical NPs, and the NPs had a low PDI value of ≤ 0.27. The formed CNPs produced antibacterial activity against all tested strains, including those resistant to multiple antibiotics, with a minimum inhibitory concentration (MIC90) of 0.16 to 0.31 mg/mL and a minimum bactericidal concentration (MBC) of 0.31 to 0.61 mg/mL. Of note, at all MIC90 and MBC, the CNPs had no significant cytotoxic effect on HeLa cells and reduced bacterial adhesion to these cells at MBC doses.
Conclusion: The present work findings suggest the potential of the CNPs for the treatment of gonorrhoea.
Keywords: N. gonorrhoeae, chitosan nanoparticles, antimicrobial, adhesion
Introduction
N. gonorrhoeae, the gonococcus (GC), is a Gram-negative bacterium which strictly infects humans. N. gonorrhoeae causes gonorrhea which is considered one of the most common sexually transmitted infections (STI), with an estimated number of 106 million new infections annually worldwide.1 It colonizes the mucosal tissues of the endocervix, pharynx, urethra, rectum, and conjunctiva. It can be transferred to the upper genital tract, leading to pelvic inflammatory disease (PID) in women.2 As a complication of PID, in rare cases, the gonococcal infection can cause sterility in men and infertility in women.2 Approximately 80% of gonococcal infections in women are asymptomatic3 which is seen to a lesser extent in men.3,4 For the past 70 to 80 years, gonorrhea has been treated successfully through the use of antimicrobials. However, the high prevalence of resistant gonococcal strains, to both the majority of previously used antimicrobials and current treatments such as sulfonamides, penicillins, earlier cephalosporins, tetracyclines, macrolides, and fluoroquinolones was detected internationally.5–8 The emergence of gonococcal strains that exhibit a high level of antibiotic resistance has raised great concern globally, leading to the development of global, regional, and national action/response plans9–12 and an increased number of publications.8,9,13–15 In the United States,16 the United Kingdom,17 and all over Europe,18 recommendations to use dual-antimicrobial therapy, ie mainly ceftriaxone and azithromycin, to overcome the development of resistance against monotherapy have been introduced.19 However, rather alarmingly, initial reports have identified strains that are resistant to both ceftriaxone and azithromycin.20 This rapid increase in antibiotic resistance emphasises the necessity of identifying new antimicrobials that work against N. gonorrhoeae.
Chitosan (Cs) has demonstrated a wide spectrum of antimicrobial activity against Gram-positive and Gram-negative bacteria.21,22 Chitosan is a linear polysaccharide polymer composed of β-(1–4)-linked N-acetyl-D-glucosamine.23 It is derived from partial or total deacetylation of chitin.21,23 The US Food and Drug Administration has recognized shrimp-derived chitosan as being generally safe24 for general use in foods.25 Chitosan has also been approved as a food additive in Japan and South Korea since 1983 and 1995, respectively.26
The antimicrobial activity of chitosan has been explained by different theories; however, the exact mechanisms are still unknown.21,22,27 Intracellular leakage, in which the positively charged chitosan binds to negatively charged bacterial surfaces such as lipopolysaccharides (LPS), is among these theories.21,22,27 This binding has led to the alteration of the bacterial membrane permeability, causing leakage of intracellular constituents and cell death.21,22,27 Chitosan nanoparticles (CNPs) produced higher antimicrobial activity against a wide range of fungi, Gram-positive, and Gram-negative bacteria when compared with chitosan.28–31 This suggests that there is potential for CNPs to act as an antimicrobial against pathogens such as gonococcus. Therefore, this study aims to formulate chitosan nanoparticles (CNPs), characterise their physicochemical properties, and examine their antimicrobial activity against N. gonorrhoeae.
Materials and Methods
Materials
Low molecular weight chitosan (LMW, 50–190 kDa, Degree of deacetylation; DD = 75–85%), tripolyphosphate (TPP), and acetic acid were purchased from Sigma-Aldrich (St Louis, USA). Gonococcus medium (GC) agar, supplemented with 1% IsoVitale X and gonococcus broth medium (GCP) were obtained from (Difco). All the other solvents and chemicals were of analytical grade. Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and antibiotic-antimycotic solution were obtained from Gibco (NY, USA).
Preparation of the CNPs
The CNPs were prepared using the ionic gelation method as described previously, with minor modifications.32,33 Solutions of LMW chitosan were prepared at concentrations of 1, 2.5 and 5 mg/mL by dissolving 0.01, 0.025 and 0.05 g, respectively, of chitosan in 10 mL of 1% v/v acetic acid. A volume of 1 mL of 0.1% w/v TPP solution was added to 4 mL of chitosan solution under continuous magnetic stirring at 800 rpm for 1 hr and the nanoparticles were formed spontaneously. Then, the nanoparticle solution was centrifuged at 10,000 × g for 20 min and the retrieved nanoparticles were suspended in distilled water and subsequently centrifuged to wash twice. Eventually, the CNPs were reconstituted in deionized distilled water for further characterization and experiments and stored at 4°C.
Characterization of Particle Size
The particle size, polydispersity index (PDI), and zeta potential were measured using dynamic light scattering with a Zeta sizer (Particle Sizing Systems, Port Richey, FL, USA). All measurements were performed in triplicate and reported as the means ± standard deviation (SD). The NPs morphology was examined using the Tecnai transmission electron microscope (TEM) (OR, USA).
Bacterial Strains and Growth Conditions
A control strain of N. gonorrhoeae NCTC 12,700 and 12 WHO N. gonorrhoeae reference strains described in Table 1, were obtained from Public Health England (PHE) and grown overnight in GC agar or GCB broth at 37°C in 5% CO2 and subsequently used in the antimicrobial testing.
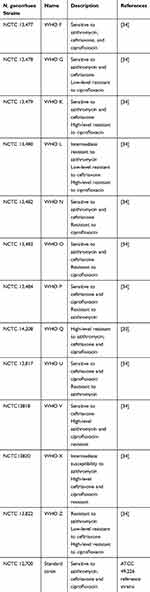 |
Table 1 Gonococcal Isolates Used in the Current Study |
Antimicrobial Assay
Broth microdilution assays were used to determine the minimal inhibitory concentration of 90 (MIC90), and the minimal bactericidal concentration (MBC) of the CNPs. The CNPs were briefly diluted in GCB broth medium in which the pH of the broth was adjusted to <6 in standard Bioscreen C 100 well microtiter plates (Labsystems Oy, Helsinki, Finland). Microtiter plates were prepared using serial twofold dilutions of the CNPs (concentration ranging from 2.5mg/mL to 0.016 mg/mL with a total volume of 100 µL) in the respective media. To prepare the inoculum, all of the bacterial cell suspensions were adjusted to McFarland 0.5 (1–2 × 108 cfu/mL). The suspension was further diluted, to provide a final inoculum density of 5105 cfu/mL in the wells of the microdilution panels. The plates were incubated for 16 hours at 37°C and 5% CO2. After 18 hours of incubation, the optical density (OD) of the bacterial growth was recorded and the MIC90 was calculated. Sub-culturing of the incubated bacteria on the GC agar was performed to calculate MBC. The OD reading of each concentration of tested NPs and GCB medium alone were subtracted from OD of the wells inoculated with bacteria to eliminate the background. The MIC90 was defined as the minimal inhibitory concentration of the CNPs that reduces bacterial growth by 90%, and the MBC was defined as the lowest concentration of the CNPs that inhibited bacterial growth.
Cell Viability Analysis
The cytotoxicity of the CNPs was investigated using Alamar Blue as previously described.34 Briefly, HeLa (human cervical cancer epithelial cells) were obtained from American Type Culture Collection (ATCC CCL-2) and seeded in 24 wells in DMEM media supplemented with 10% FBS, and 1% antibiotic-antimycotic overnight, until 70% to 90% confluency had been reached. Then, the cells were treated with the CNPs (2.5–0.016 mg/mL, for 24 and 48 hours). After experimental treatment, the cell media was replaced and Alamar blue was added to the cells at 10% for each well and the cells were incubated for 2–4 hours at 37° C, 5% CO2. Then, fluorescence was recorded at 550 excitations and 590 emission wavelengths using SpectraMax M5 (California, United States). The viability of the cells was expressed in comparison to non-treated cells as follows:
Adhesion Assay
The adhesion assay was carried out using non-confluent (40%) HELA cells as reported from the previous study.35 Previously tested gonococcal strains were resuspended in DMEM and added to the cells at a multiplicity of infection (M.O.I.) of 100. Then, the bacteria were allowed to adhere for 2 hr at 37°C and 5% CO2. The cells were later washed three times with PBS and treated with 1% saponin for 5 minutes and subsequently serially diluted, spread on GC plates and incubated overnight at 37°C and 5% CO2. The next day, any colony-forming units were counted.
Statistical Analysis
The data were presented as the mean ± standard deviation (SD). Analysis of the data was performed using (GraphPad Prism version 8.0.0 for macOS Software, Inc) with two-tailed Student’s t-test and one-way analysis of variance (ANOVA) where appropriate. A p-value of less than 0.05 was considered significant.
Results
Characterization and Morphology of the Prepared CNPs
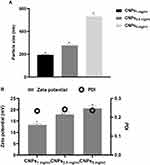
In the current study, the ionic gelation method was used to prepare various CNP formulations using LMW chitosan in different concentrations to investigate their antimicrobial activity against N. gonorrhoea. As shown in Figure 1, the average particle size and zeta potential of the prepared NPs increased significantly when chitosan the concentration increased from 1 to 5 mg/mL (p< 0.0036). The Nanoparticles prepared from Cs at concentration 1 mg/mL (CNPs1 mg/mL) exhibited the smallest particle size (192.8 nm ± 1.86) and zeta potential (13.6 mV ± 0.52). When the Cs concentration was increased to 2 mg/mL, the particle size and zeta potential of the prepared CNPs2.5 mg/mL increased to 276.2 nm ± 9.14 and 17.9 mV ± 1.14, respectively. The CNPs prepared from 5 mg/mL Cs concentration CNPs5 mg/mL produced the largest particle size (530 nm ± 13.13) and the highest zeta potential (20.6 mV ± 1.04). All of the NPs displayed a narrow size distribution with low PDI values (≤0.27) revealing the high homogeneity (Figure 1). The graphs for the particle size distribution of these NPs are demonstrated in Figure 2. TEM images of the CNPs are presented in Figure 2 reveals a spherical shape, smooth surface, and size range of approximately 193 to 530 nm.
 |
Figure 2 TEM images and particle size distribution of formulated CNPs. Images represent CNPs1 mg/mL (A), CNPs2.5 mg/mL (B), and CNPs5 mg/mL (C). |
The Anti-Gonococcal Activity of the CNPs
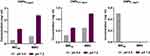
We know that the antimicrobial activity of CNPs requires a pH of less than 6. Thus, the growth of control strain N. gonorrhoeae at GCB adjusted to pH 5.5 using hydrochloric acid (HCl) and acetic acid (AA) was investigated and compared with bacterial growth at pH 7 (Figure 3). A similar growth pattern was observed in pH 5.5 adjusted by HCl and pH 7. Thus, HCl was utilized for modification of GCB pH for all subsequent experiments.
 |
Figure 3 Growth of N. gonorrhoeae NCTC 12,700 at different pH. The pH of the GCB broth was adjusted using HCl and AA to 5.5. |
The antibacterial activity of the prepared CNPs was tested against both WHO reference and standard gonococcal strains using the broth dilution method using GCP medium at pH 5.5. As shown in Table 2, the MIC90 and MBC of CNPs5 mg/mL were 0.16 mg/mL and 0.31 mg/mL, respectively, against WHO U, V, F, and O. Using the same nanoparticles, the concentration of MIC90 and MBC was increased to 0.31 mg/mL and 0.63 mg/mL, respectively, when tested against WHO X, Z, G, K, L, N, P, Q, and standard strain. While with CNPs2.5 mg/mL the MIC90 and MBC were equal to 0.16 mg/mL and 0.31 mg/mL, respectively, for the majority of strains except WHO Q and standard strain, in which the MIC90 and MBC were increased to 0.31 mg/mL and 0.63 mg/mL correspondingly (Table 2). Other nanoparticles were prepared from C concentrations of 1 mg/mL, CNPs1 mg/mL, exhibited MIC90 of 5 mg/mL against six of the tested strains and the MBC was not detected. Strains including WHO V, X, F, L, N, and O were more sensitive to CNPs1 mg/mL and exhibited MIC90 of 0.25 mg/mL and MBC of 0.5 mg/mL (Table 2). It is to be noted that azithromycin was used in the experiment as a positive control. As shown in Table 2, isolates resistant to azithromycin, including WHO Z, U, and P (MIC 1 and 4 µg/mL) displayed 1000- and 250-fold higher MICs at pH 5.5. While isolates WHO V and Q which are highly resistant to azithromycin (MIC ˃ 256 µg/mL) exhibited a 4-fold increase in their MIC at pH 5.5. The MICs of sensitive isolates (WHO F, G, K, N, O, and control strain) and isolates with intermediate susceptibility to azithromycin (WHO X and L) increased from 0.25 to 0.5 µg/mL at neutral pH to 1 mg/mL at pH 5.5 (Table 2).
When the pH of the GCB media was not modified and kept at pH 7.2, the MIC90 of CNPs5 mg/mL and CNPs2.5 mg/mL against a standard strain of N. gonorrhoeae increased to 1.25 and 0.625 mg/mL, respectively. Similarly, the MBC of both formula against standard strain at neutral pH increased to 2.5 and 1.25 mg/mL, respectively (Figure 4). Notably, neutral pH abolished the anti-gonococcal activity of CNPs1 mg/mL (Figure 4). This effect was also confirmed through the bacterial growth curve that was in the presence of CNPs at pH 7.2 (data not shown). As expected, the azithromycin MIC was 0.25 µg/mL at pH 7.2 (data not shown).
Growth Inhibition of N. gonorrhoeae Strains Treated with CNPs
The growth of gonococcal strains in the presence of CNPs was studied and the results were presented in Figure 5 and S1. Four strains, including WHO F, O, U and V were more sensitive to CNPs5 mg/mL, in which 90% of their growth was inhibited at 0.16 mg/mL and prevented at 0.31 mg/mL of these NPs. While in other strains, 0.31 mg/mL of CNPs5 mg/mL decreased bacterial growth by 90% and inhibited growth at 0.63 mg/mL (Figure 5). In gonococcal strains treated with CNPs2.5 mg/mL, the growth of all strains except standard strain and WHO Q declined by 90% at 0.16 mg/mL concentration and inhibited at 0.31 mg/mL. The CNPs1 mg/mL at a concentration of 0.25 mg/mL reduced gonococcal growth by 90% of WHO F, L, N, O, V and X and inhibited their growth at 0.5 mg/mL. The growth of N. gonorrhoeae strains involving G, K, P, Q, U, Z, and standard strain require a higher concentration of CNPs1 mg/mL equal to 0.5 mg/mL to reduce their growth by 90% and their growth inhibition was not detected by this formula (Figure 5 and S1). The data confirmed MIC90 and MBC illustrated in Table 2.
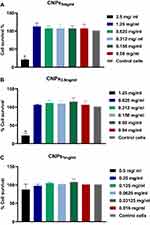
Cytotoxicity Assay of CNPs
The cytotoxicity of prepared CNPs on HELA cells was evaluated. The CNPs5 mg/mL and CNPs2.5 mg/mL decreased HELA cells viability significantly (p < 0.05) at 2.5 mg/mL and 1.25 mg/mL concentrations only, respectively, as shown in Figure 6A and B, while CNPs1mg/mL at all used concentrations did not cause any significant cytotoxicity on HeLa cells (Figure 6C). Notably, all formulated NPs were not toxic at any of the MIC and MBC concentrations used.
The Effects of CNPs on Gonococcal Adhesion to HELA Cells
Adhesion of gonococcal St. strain and WHO Q to HeLa cells in the presence of CNPs at MBCs were studied and the results are presented in Figure 7. The number of N. gonorrhoea NCTC 12,700 adhered to HeLa cells was reduced significantly in the presence of CNPs5 mg/mL and CNPs2.5 mg/mL at a minimum bactericidal concentration (0.63 mg/mL) when compared with untreated control cells (Figure 7A). Although bacterial adhesion appears to be reduced in cells pre-treated with CNPs1 mg/mL, it was not found to be statically significant. Furthermore, the incubation of HeLa cells with MBC of all the CNPs prepared has shown significantly reduced bacterial adhesion (p < 0.05) (Figure 7B).
Discussion
The rise in the antimicrobial resistance of N. gonorrhoeae has limited successful treatment and highlights the necessity for the development of a new antimicrobial. In our current study, we found that the CNPs exhibited antimicrobial activity against N. gonorrhoeae, including those resistant to multiple antibiotics. The CNPs exhibited higher antimicrobial activity than chitosan against a wide spectrum of pathogens including fungi and both Gram-positive and Gram-negative bacteria.28–31 To the best of our knowledge, no previous study to date has investigated the antimicrobial efficacy of CNPs against gonococci.
In agreement with previous reports,28,33,36 the current study showed us that the particle size and zeta potential of formulated CNPs increased significantly when the concentration of chitosan was increased. The MIC90 and MBC of CNP5 mg/mL and CNP2.5 mg/mL were found to have a range of 0.16 to 0.31 mg/mL and 0.31 to 0.63 mg/mL, respectively, against gonococcal isolates used. While in case of using CNPs prepared from 1 mg/mL concentration of Cs solution, CNP1 mg/mL, the MIC90 ranged from 0.25 to 0.5 mg/mL, and MBC was 0.5 mg/mL for only six of the strains tested. Among gonococcal strains tested, WHO Q is a strain that is highly resistant to azithromycin, ceftriaxone, and ciprofloxacin.20 Interestingly, both st. strain and WHO Q showed similar MIC90 and MBC values for all prepared CNPs tested. Other isolates were more sensitive to CNP5 mg/mL, CNP2.5 mg/mL, and CNP1 mg/mL. This antibacterial effect of CNPs are consistent with previous studies reported the antibacterial activity of CNPs against a wide range of Gram-negative bacteria.28,30,33 Such effect is proposed to be mediated by the polycationic nature of CNPs in acidic medium.28,30
The antibacterial screening tests performed in our present work were conducted using a medium with a pH of 5.5 as it has been experimentally demonstrated by previous reports to enhance the antimicrobial activity of CNPs.28,33 This was confirmed when we found that the MIC90 and MBC values of CNP5 mg/mL and CNP2.5 mg/mL increased four-fold and two-fold, respectively, when the pH of GCM medium increased to 7.2. In this regard, neutral pH also abolished the antibacterial effect of CNP1 mg/mL against gonococcus. The observed increase in the MIC90 and MBC values as well as the absence of antibacterial activity of CNPs at neutral pH was due to the positive charge on the surface of CNPs that decreased at neutral pH. This occurs because once the pH of the medium is under the pKa of the chitosan, which is 6.5, amine groups in the chitosan backbone become protonated into positive ions, enhancing chitosan interaction with negatively charged bacteria. In contrast, when the pH of the medium is close to neutral or alkaline, the protonation of primary amines is decreased; thus, the binding between CNPs and a negatively charged bacterial surface will decrease.28,37 Although at neutral pH, CNPs still produce anti-gonococcal effects, a higher concentration of Cs is needed. These results revealed the inverse relationship between pH and antimicrobial activity of CNPs, which corroborates the findings of other studies.37–39
In a prior study conducted by Mallegol et al,40 the effect of pH on the activity of azithromycin against N. gonorrhoeae at pH 5 was investigated. The study was carried out at an acidic pH to cover the conditions that the antibiotic could be exposed to during gonococcal infection, intracellularly, and in the extracellular milieu.40 Their study showed that azithromycin was less stable at an acidic pH level, leading to a loss of cladinose sugar resulting in the increase in the azithromycin MICs values against gonococcal isolates tested.40 Their findings explained the increased MIC of azithromycin observed in our study when the experiment was performed at a pH of 5.5.
In our current study, the cytotoxicity of CNPs in HELA cells was analysed and the results revealed that only higher concentrations of CNP5 mg/mL and CNP2.5 mg/mL were cytotoxic. This is due to the high zeta potential at those concentrations as observed in previous studies.41 It is noteworthy that all prepared CNPs were not cytotoxic at the MIC90 and MBC concentrations after 24 hours, indicating the cytocompatibility of CNPs.
The bacterial adhesion studies conducted in our present work showed that CNPs reduced adhesion of the st strain and multidrug-resistant strain (WHO Q) to HELA cells at MBC dose relative to the cells not exposed to NPs. These findings indicate the anti-gonococcal activity of CNPs and their capability to interfere with bacterial attachment to host cells.
Conclusion
Together, the data of this study show that the formulated CNPs exhibited anti-gonococcal activity against the tested strains. The anti-gonococcal activity took place at specific concentrations, at which NPs were cytocompatible and reduced bacterial adhesion to cells. Thus, the current work highlights the therapeutic potential of CNPs as an antimicrobial against N. gonorrhoeae.
Acknowledgments
This research project was supported by a grant from the “Research Centre of the Female Scientific and Medical Colleges”, Deanship of Scientific Research, King Saud University.
Disclosure
The authors report no conflicts of interest in this work.
References
1. WHO. Global Action Plan to Control the Spread and Impact of Antimicrobial Resistance in Neisseria Gonorrhoeae. Geneva: World Health Organization; 2012.
2. Burnett AM, Anderson CP, Zwank MD. Laboratory-confirmed gonorrhea and/or chlamydia rates in clinically diagnosed pelvic inflammatory disease and cervicitis. Am J Emerg Med. 2012;30(7):1114–1117. doi:10.1016/j.ajem.2011.07.014
3. WHO. Prevalence and Incidence of Selected Sexually Transmitted Infections, Chlamydia Trachomatis, Neisseria Gonorrhoeae, Syphilis and Trichomonas Vaginalis: Methods and Results Used by WHO to Generate 2005 Estimates. Geneva: World Health Organisation; 2011:38.
4. Farley TA, Cohen DA, Elkins W. Asymptomatic sexually transmitted diseases: the case for screening. Prev Med. 2003;36(4):502–509. doi:10.1016/S0091-7435(02)00058-0
5. Camara J, Serra J, Ayats J, et al. Molecular characterization of two high-level ceftriaxone-resistant Neisseria gonorrhoeae isolates detected in Catalonia, Spain. J Antimicrob Chemother. 2012;67(8):1858–1860.
6. Ohnishi M, Golparian D, Shimuta K, et al. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob Agents Chemother. 2011;55(7):3538–3545. doi:10.1128/AAC.00325-11
7. Unemo M, Golparian D, Nicholas R, Ohnishi M, Gallay A, Sednaoui P. High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob Agents Chemother. 2012;56(3):1273–1280. doi:10.1128/AAC.05760-11
8. Unemo M, Nicholas RA. Emergence of multidrug-resistant, extensively drug-resistant and untreatable gonorrhea. Future Microbiol. 2012;7(12):1401–1422. doi:10.2217/fmb.12.117
9. (CDC) CfDCaP. Cephalosporin-Resistant Neisseria Gonorrhoeae Public Health Response Plan. Atlanta, GA; 2012.
10. (ECDC) ECfDPaC. Response Plan to Control and Manage the Threat of Multidrug-Resistant Gonorrhoea in Europe. Stockholm, Sweden; 2012.
11. F L-nm N, Unemo M. The serious threat of multidrug-resistant and untreatable gonorrhoea: the pressing need for global action to control the spread of antimicrobial resistance, and mitigate the impact on sexual and reproductive health. Sex Transm Infect. 2012;88:317–318. doi:10.1136/sextrans-2012-050674
12. WHO. Global Action Plan to Control the Spread and Impact of Antimicrobial Resistance in Neisseria Gonorrhoeae. Department of Reproductive Health and Research; 2012.
13. Bolan GA, Sparling PF, Wasserheit JN. The emerging threat of untreatable gonococcal infection. N Engl J Med. 2012;366(6):485–487. doi:10.1056/NEJMp1112456
14. Ison CA. Antimicrobial resistance in sexually transmitted infections in the developed world: implications for rational treatment. Curr Opin Infect Dis. 2012;25(1):73–78. doi:10.1097/QCO.0b013e32834e9a6a
15. Whiley DM, Goire N, Lahra MM, et al. The ticking time bomb: escalating antibiotic resistance in Neisseria gonorrhoeae is a public health disaster in waiting. J Antimicrob Chemother. 2012;67(9):2059–2061. doi:10.1093/jac/dks188
16. (CDC) CfDCaP. Update to CDC’s sexually transmitted diseases treatment guidelines, 2010: oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb Mortal Wkly Rep. 2012;61:590–594.
17. Bignell C, Fitzgerald M. UK national guideline for the management of gonorrhoea in adults, 2011. Int J STD AIDS. 2011;22(10):541–547. doi:10.1258/ijsa.2011.011267
18. Bignell C, Unemo M, Radcliffe K. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS. 2013;24(2):85–92. doi:10.1177/0956462412472837
19. Unemo M, Shafer WM. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev. 2014;27(3):587–613.
20. Eyre DW, Sanderson ND, Lord E, et al. Gonorrhoea treatment failure caused by a Neisseria gonorrhoeae strain with combined ceftriaxone and high-level azithromycin resistance, England, February 2018. Euro Surveill. 2018;23(27):1800323.
21. Kong M, Chen XG, Xing K, Park HJ. Antimicrobial properties of chitosan and mode of action: a state of the art review. Int J Food Microbiol. 2010;144(1):51–63. doi:10.1016/j.ijfoodmicro.2010.09.012
22. Rabea EI, Badawy ME, Stevens CV, Smagghe G, Steurbaut W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules. 2003;4(6):1457–1465. doi:10.1021/bm034130m
23. Tikhonova VE, Stepnovaa EA, Babaka VG, et al. Bactericidal and antifungal activities of a low molecular weight chitosan and its N-/2(3)-(dodec-2-enyl)succinoyl/-derivatives. Carbohydr Polym. 2006;64(1):66–72. doi:10.1016/j.carbpol.2005.10.021
24. Hjerde RJN, Varum KM, Grasdalen H, Tokura S, Smidsrød O. Chemical composition of O-(carboxymethyl)-chitins in relation to lysozyme degradation rates. Carbohydr Polym. 1997;34(3):131–139. doi:10.1016/S0144-8617(97)00113-6
25. Administration UFaD. Shrimp-derived chitosan GRAS notification. 2012. Available from: www.accessdata.fda.gov/scripts/fcn/gras_notices/GRN000443pdf.
26. Mahae N, Chalat C, Muhamud P. Antioxidant and antimicrobial properties of chitosan-sugar complex. Int Food Res J. 2011;18(4):1543–1551.
27. Goy RC, de Britto DD, Assis OBG OBG. A review of the antimicrobial activity of chitosan. Ciência e Tecnologia. 2009;19(3):241–247. doi:10.1590/S0104-14282009000300013
28. Qi L, Xu Z, Jiang X, Hu C, Zou X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr Res. 2004;339(16):2693–2700.
29. de Campos AM, Diebold Y, Carvalho EL, Sanchez A, Alonso MJ. Chitosan nanoparticles as new ocular drug delivery systems: in vitro stability, in vivo fate, and cellular toxicity. Pharm Res. 2004;21(5):803–810. doi:10.1023/B:PHAM.0000026432.75781.cb
30. Divya K, Vijayan S, George TK, Jisha MS. Antimicrobial properties of chitosan nanoparticles: mode of action and factors affecting activity. Fibers Polym. 2017;18(2):221–230. doi:10.1007/s12221-017-6690-1
31. Wardani G, Mahmiah SSA. In vitro antibacterial activity of chitosan nanoparticles against mycobacterium tuberculosis. Pharmacogn J. 2018;10(1):162–166. doi:10.5530/pj.2018.1.27
32. Calvo P, Remunan-Lopez C, Vila-Jato JL, Alonso MJ. Chitosan and chitosan/ethylene oxide-propylene oxide block copolymer nanoparticles as novel carriers for proteins and vaccines. Pharm Res. 1997;14(10):1431–1436. doi:10.1023/A:1012128907225
33. Aleanizy FS, Alqahtani FY, Shazly G, et al. Measurement and evaluation of the effects of pH gradients on the antimicrobial and antivirulence activities of chitosan nanoparticles in Pseudomonas aeruginosa. Saudi Pharm J. 2018;26(1):79–83. doi:10.1016/j.jsps.2017.10.009
34. AbuMousa RA, Baig U, Gondal MA, et al. Photo-catalytic killing of HeLa cancer cells using facile synthesized pure and Ag loaded WO3 nanoparticles. Sci Rep. 2018;8(1):15224. doi:10.1038/s41598-018-33434-7
35. Jones A, Jonsson AB, Aro H. Neisseria gonorrhoeae infection causes a G1 arrest in human epithelial cells. FASEB J. 2007;21(2):345–355. doi:10.1096/fj.06-6675com
36. Ing LY, Zin NM, Sarwar A, Katas H. Antifungal activity of chitosan nanoparticles and correlation with their physical properties. Int J Biomater. 2012;2012:632698.
37. Kravanja G, Primozic M, Knez Z, Chitosan-based LM. (Nano)materials for novel biomedical applications. Molecules. 2019;24(10):1960. doi:10.3390/molecules24101960
38. Gomes LP, Andrade CT, Del Aguila EM, Alexander C, Paschoalin VMF. Assessing the antimicrobial activity of chitosan nanoparticles by fluorescence-labeling. Int J Biotechnol Bioeng. 2018;12(4):112–117.
39. Jeon SJ, Oh M, Yeo W-S, Galvao KN, Jeong KC, Virolle M-J. Underlying mechanism of antimicrobial activity of chitosan microparticles and implications for the treatment of infectious diseases. PLoS One. 2014;9(3):e92723. doi:10.1371/journal.pone.0092723
40. Mallegol J, Fernandes P, Seah C, Guyard C, Melano RG. Determination of in vitro activities of solithromycin at different pHs and its intracellular activity against clinical isolates of Neisseria gonorrhoeae from a laboratory collection. Antimicrob Agents Chemother. 2013;57(9):4322–4328.
41. Omar Zaki SS, Ibrahim MN, Katas H. Particle size affects concentration-dependent cytotoxicity of chitosan nanoparticles towards mouse hematopoietic stem cells. J Nanotechnol. 2015;2015:919658. doi:10.1155/2015/919658
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.