Back to Journals » ClinicoEconomics and Outcomes Research » Volume 7
Annual biologic treatment cost for new and existing patients with moderate to severe plaque psoriasis in Greece
Authors Fragoulakis V, Raptis E, Vitsou E, Maniadakis N
Received 1 October 2014
Accepted for publication 11 November 2014
Published 8 January 2015 Volume 2015:7 Pages 73—83
DOI https://doi.org/10.2147/CEOR.S75263
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Giorgio L Colombo
Vassilis Fragoulakis,1 Efklidis Raptis,2 Elli Vitsou,2 Nikolaos Maniadakis1
1Health Services Organization and Management, National School of Public Health, 2Pfizer Hellas, Athens, Greece
Aim: The aim of the present study was to estimate the annual per-patient cost of treatment with adalimumab, etanercept, infliximab, and ustekinumab by response status for new and existing patients with moderate to severe psoriasis in Greece.
Methods: An economic analysis was developed from a national health care perspective to estimate the direct cost of treatment alternatives for new and existing patients within a 1-year time horizon. The model included drug acquisition and administration costs for responders and nonresponders. Real-world treatment pattern and resource use data were extracted through nationwide field research using telephone-based interviews with a representative sample of dermatologists. Unit costs were collected from official sources in the public domain.
Results: The mean annual cost of treatment for new patients who responded (or did not respond) to treatment was as follows: adalimumab €10,686 (€3,821), etanercept €10,415 (€3,224), infliximab €14,738 (€7,582), and ustekinumab €17,155 (€9,806). For existing patients the mean annual cost was €9,916, €9,462, €12,949, and €17,149, respectively. Results did not change significantly under several one-way sensitivity and scenario analyses.
Conclusion: Under the base-case scenario, the cost of treatment with etanercept is lower than that of the other biological agents licensed for moderate to severe plaque psoriasis in Greece, for both new and existing patients, irrespective of response status.
Keywords: adalimumab, etanercept, infliximab, ustekinumab, economic evaluation, biologics
Corrigendum for this article has been published
Introduction
Psoriasis is a chronic, relapsing, immune-mediated inflammatory disorder, whose most common form, plaque psoriasis (PP), accounts for up to 85%–90% of cases, and is characterized by erythematous scaly patches.1,2 Psoriasis affects approximately 2%–3% of the total population on a worldwide scale.3 Nonetheless, its prevalence varies among different populations; a number of factors have been proposed to account for this variability, including climate, genetic susceptibility, and environmental antigen exposure.4 The latest data indicate that the prevalence of psoriasis in adults ranges from 0.91% (US) to 8.5% (Norway), while the reported incidence varies from 78.9/100,000 person-years (US) to 230/100,000 person-years (Italy).5 The estimated prevalence of the disease in Greece is 2.0%–2.8%.6–9
The severity of PP may vary from a few small, localized patches to coverage of most of the skin and is often associated with cosmetic problems as well as a number of comorbidities, altogether impacting the health-related quality of life of sufferers.1,2 While considered a nonlife-threatening chronic disease, psoriasis imposes a sizeable social and financial burden to patients, health care systems, and society overall.10 Patients suffering from psoriasis frequently miss working hours and may experience diminished productivity associated with increased symptom severity or comorbidities.11–13 For instance, in the US, Americans with psoriasis lose approximately 56 million working hours, and $2–$3 billion per annum is spent on treatment of the disease.14 Moreover, it has been estimated that the 3-month drug cost per responder is approximately $10,000–$13,000 (cost refers to 2011 US$).15
Hence, it makes sense to manage psoriasis not only from a humanistic but also from an economic perspective as well. To therapeutically address the significant morbidity and burden associated with psoriasis, several scientific guidelines were developed in Europe and elsewhere.16–20 In accordance with the existing guidelines, systemic agents, such as the conventional therapies (cyclosporine, methotrexate, retinoids, and phototherapy) and the biologic agents, including the tumor necrosis factor (TNF) antagonists (adalimumab, etanercept, and infliximab), as well as the interleukin 12/23 antagonist ustekinumab, are recommended for the treatment of moderate to severe PP. Biologics represent established treatment options for moderate to severe psoriasis. The efficacy of these agents is well known and has been previously assessed in the relevant literature, while their use has improved the long-term management of the disease and patient outcomes.21–31
Cost-containment measures implemented across health care systems in the European Union as part of the financial austerity have affected market access to effective therapies for pharmaceuticals in general, including therapies for PP. Greece, in particular, is going through one of the most significant economic crises in its modern history and resources are under severe scrutiny, as the country has been affected by the financial turmoil more than any other European country.32 In this environment, it has been facing several challenges relating to the organization, financing, and delivery of health care services, with a fixed annual public pharmaceutical expenditure in the context of a memorandum signed with its international lenders. Consequently, the National Organization for Health Care Services (EOPYY— in Greek), the main health care payer in Greece, has to meet an inelastic and predetermined budget, imposing strict constraints. In this context, a clear picture of the financial impact regarding the reimbursed products is considered important to support relevant decision making by EOPYY. To this effect, an economic analysis of the available biologic treatments for PP in Greece was conducted. The aim of the study was to estimate the annual per-patient cost of treatment with the biologic agents adalimumab, etanercept, infliximab, and ustekinumab in the management of PP in Greece. The current manuscript presents the results of this economic analysis.
in Greek), the main health care payer in Greece, has to meet an inelastic and predetermined budget, imposing strict constraints. In this context, a clear picture of the financial impact regarding the reimbursed products is considered important to support relevant decision making by EOPYY. To this effect, an economic analysis of the available biologic treatments for PP in Greece was conducted. The aim of the study was to estimate the annual per-patient cost of treatment with the biologic agents adalimumab, etanercept, infliximab, and ustekinumab in the management of PP in Greece. The current manuscript presents the results of this economic analysis.
Methods
The present economic model is a cost-minimization analysis assuming similar efficacy at 1 year (ie, the time horizon of the model) for biologic treatments in scope.22 The model attempted to estimate the direct treatment costs of patients with PP. The main items considered include drug costs and their administration.
Costs associated with monitoring were not considered. The efficacy of biologics for the 1-year perspective was considered roughly equal between treatments, as was the cost for managing adverse events, and thus was not considered in the model.22 The time horizon was limited to 1 year for both new and existing patients, and the perspective of the analysis was that of the payer (EOPYY); other costs that quantify the indirect burden (eg, productivity loss) associated with each therapy were not taken into account in the present analysis.
A model was developed in Microsoft Excel® where patients were classified as either responders or nonresponders depending on whether or not they achieved the minimum improvement criterion for PP, namely the Psoriasis Area and Severity Index (PASI) criterion. PASI combines the assessment of the severity of disease into a single score in the range 0 (no disease) to 72 (maximal disease). Clinical improvements are generally reported in terms of the number of people reaching a specified percentage reduction in PASI from their baseline score (for example, PASI 75 is a 75% reduction from baseline score). It is generally recognizable that an achievement of a PASI 75 indicates that severe psoriasis has responded to treatment. Time to assessment of responder status followed the guidance from the respective Summary of Product Characteristics (SPC); ie, at 16 weeks for adalimumab, 12 weeks for etanercept, 14 weeks for infliximab, and 28 weeks for ustekinumab.33–36 The model assumed that nonresponders discontinued treatment at the above time points for each respective agent, while responders retained clinical efficacy and continued treatment with the same biologic agent throughout the study time horizon.
Treatment switching was not allowed in the present model. The prices of drugs were set based on the relevant price bulletin issued by the Ministry of Health.37 The cost of drugs was calculated, taking into account the available pack size of the agents under study, the unit cost per pack, and the required dose per SPC. In the case of ustekinumab, for those patients weighing more than 100 kg the base-case scenario assumed that a double dose was used (90 mg) as described in the respective SPC.33–36 As etanercept is licensed for intermittent as well as continuous treatment, the model incorporated the percentage of patients receiving either of those, as well as the time for which responders remained off treatment (treatment holiday), where applicable. Estimates of the time period for which the responders remain on treatment and the dosage schedule for etanercept were collected through a field based survey presented in the Supplementary materials (Table S1). The dosage scheme for the rest of the comparators is presented in Tables S2–S4. The unit costs of parameters used in the model are presented in Table 1. The total therapy cost was based on a per-vial analysis, assuming that there is drug wastage. Considering the annual time horizon of the model, no discount rate was applied. Cost calculations are presented in the appendix.
  | Table 1 Unit costs per item used in the model |
Due to the lack of detailed data from local registries, the model inputs were based on a nationwide survey of physicians. In particular, field work was performed by a specialized independent agency from June to September 2013 with dermatologists treating patients with PP; the authors had no involvement in the process of data collection. Data were collected by 10-minute telephone interviews conducted by trained interviewers using a structured questionnaire comprised of questions designed to collect information regarding patient demographics and physician treatment patterns. Participants had no prior knowledge of the actual content of the questionnaire, and researchers did neither interfere nor guide the answers throughout the process. The sampling methodology followed was simple random sampling with screener. A screening question was used to screen physicians who prescribe biologic agents for the treatment of PP. Only physicians prescribing biologics for psoriasis were included in the sample. In accordance with the inclusion criteria, the sample examined in this study comprised only patients with moderate to severe psoriasis. Hence, patients who were diagnosed with other diseases (ulcerative colitis, rheumatoid diseases, etc) were not taken into account. Data analysis was performed independently by the authors using the Excel 2007 software.
It is generally known that economic data are truncated at zero and do not follow normal distributions, and consequently hypothesis testing would be invalid if conducted with conventional approaches (ie, 95% confidence intervals). As such, in the present model, bias-corrected uncertainty intervals were calculated using the percentile method of nonparametric simulation.38 Probability distributions were therefore specified around the main model parameters. In particular, all cost components were associated with a gamma distribution and a 10% of coefficient of variation (the ratio of mean/standard deviation) around the mean was used for probabilistic sensitivity analysis (1000 bootstrap replications). Since the variance-covariance matrix was unknown, there was not any correlation pattern among variables in each bootstrap experiment. Of course, due to the probabilistic nature of all parameters, the cost of comparators can be slightly different in each experiment, and the mean of the 1,000 estimates is expected to be a good approximation of the true population mean cost per arm based on the cost assumptions.
In addition, in accordance with the recommended guidelines,39 several one-way sensitivity, and different scenario analyses were undertaken. In particular, the model parameters which varied were the mean weight of patients (±10%, mean 80.7 kg, maximum 89.0 kg, minimum 73.0kg), the dosage schedule for ustekinumab (45 mg for all patients), the holiday period for the intermittent use of etanercept (10 months on treatment/2 months off treatment cycles), and the percentage of etanercept patients starting at 2×50 mg weekly (50%, 75%) for the first 3 months of therapy.
Sensitivity analysis concerning the weight of patients was based on a reasonable assumption of ±10%, while the rest of sensitivity analyses were based on expert opinions. Furthermore, as per the relevant Summary of Product characteristics, dosage schedules for infliximab and adalimumab are “fixed”; ie, no variations are allowed. Hence, as this analysis is based on an as per SPC use, no variations in dosage schedules for these two agents were taken into account. On the other hand, for ustekinumab (weight-based dosing) and etanercept (different starting regimens, continuous or intermittent use), dose variations may be applicable as per the relevant SPC.
Results
The sample of physicians included in the study consisted of 29 dermatologists. 62.1% of participants originated from Athens, 17.2% from Salonika, and the remaining 20.7% from urban areas, Patras, Larisa, and Crete island. Approximately half of the participants (51.7%) were female; 48.3% were male. The majority of participants were employed in the private sector (55.2%), followed by the doctors who work in public hospitals (31.0%). It should be mentioned that due to a recent reform concerning the primary and secondary healthcare system in Greece, a unified health insurance Fund (ie, EOPYY), operating also as a provider of healthcare services was established. Hence, the remaining 14.8% of participants represent contracted EOPYY doctors across the country. In accordance with the survey results, these 29 participants saw 745 patients per month in total during the past 12 months. Every participant saw 26 patients per month on average, while only 10.3% of them examined more than 80 patients per month on biologic treatment in the last year. There was a relatively high concentration of the sample in a limited number of doctors, as 56% of patients in the sample were treated by 20% of physicians. Regarding patients, 78.6% were from Athens, 16.6% were from Salonika, and the remaining 4.7% were from urban areas.
Given that the disease prevalence is about 2.0% and assuming that patients requiring systemic therapy account for approximately 20%–30% of the total moderate to severe disease cases,40,41 with a satisfactory long-term response/tolerability to traditional systemic therapies achieved in 40% of patients with moderate to severe psoriasis,42 it was estimated that the sample used was representative of the entire psoriasis population on biologics. The mean weight of patients was reported at 80.7 kg on average for the entire sample; 89.1 kg for males (standard deviation 10.8 kg, range 75–125 kg) and 72.3 kg for females (standard deviation 9.7 kg, range 60–110 kg). This is in agreement with data from the literature showing that psoriasis patients are more likely to be overweight compared to the general population.43 The distribution of weight across all patients groups was positively skewed for both sexes (skewness: 1.46/1.94).
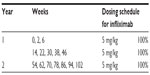
Regarding treatment regimens, the induction schedules were taken into consideration for new patients, as per respective SPC. Existing patients were considered those who were on treatment for 1 year when entering in the model, and the analysis for them reflects the second year on treatment with a yearly time horizon. The dose of adalimumab was an initial dose of 80 mg, followed by 40 mg given every other week starting 1 week after the initial dose. For etanercept, it was reported that 36.9% of patients started with 50 mg/twice weekly for the first 12 weeks, while the remaining 63.1% started with 50 mg/week. After the first 12 weeks, all etanercept patients were treated with 50 mg/week. In addition, etanercept was given intermittently in 11.2% of patients; the base-case scenario considered that new patients remained on continuous treatment for the first 24 weeks before going on treatment holiday, thus allowing etanercept to approach its maximum efficacy potential.2,44–46 Of the intermittent etanercept patients, 88.5% remained on treatment holiday for 12 weeks, while the remaining 11.5% remained off the drug for 8 weeks before reinitiating etanercept at 50 mg/week. For patients on infliximab, the dosing schedule was intravenous infusion of 5 mg/kg on weeks 0, 2, 6, and every 8 weeks thereafter; the total dose was calculated for the mean weight of patients. Treatment with ustekinumab started with dosing on weeks 0, 4 and every 12 weeks thereafter. It was estimated from the field research that 25% of patients undergoing ustekinumab therapy received the dosage of 90 mg every 12 weeks, while the remaining 75% received 45 mg every 12 weeks.
The results of the economic analysis for new and existing patients for the base-case scenario are presented in Table 2. According to these, the annual per-patient cost for new and existing patients is lower with etanercept than with the other agents in the comparison for both responders and nonresponders. For existing patients (ie, patients continuing treatment after the first year) the cost of treatment with etanercept becomes even lower (€9,462) compared to that of new patients (€10,415), as the biweekly regimen is not applicable after the first 12 weeks of treatment and after the first year a number of patients may go on treatment holiday twice per year (assuming, as per results above, that these patients remain off treatment for 2 or 3 consecutive months and receive treatment for 6 months consecutively). Results did not change significantly under several one-way sensitivity and scenario analyses undertaken, presented in Table 3.
Discussion
Understanding the relative benefits and costs of available treatments for people with moderate to severe PP is important in order to ensure that patients receive an acceptable level of care within the framework of constrained resources. Since the benefit of adalimumab, etanercept, infliximab, and ustekinumab has been acknowledged by authorities, physicians, medical societies, and payers, their use has become a part of standard medical practice.44,45 This economic analysis was undertaken to compare the mean annual cost of treatment of biologic agents approved for the treatment of PP (namely adalimumab, etanercept, infliximab, and ustekinumab) in the Greek setting. The analysis indicated that etanercept was associated with lower annual cost per patient compared to other agents. Cost minimization analysis is used to compare two health technologies that have proven to be equivalent in terms of survival and overall therapeutic effect. In such cases, the focus of the analysis shifts to their treatment cost to choose the least costly as the preferable therapeutic option. It must be noted, that in the context discussed here, the equivalence in the efficacy of the treatment regimens considered is not yet fully established by head-to-head trials. Hence, the economic conclusions drawn from the present analysis are based on the premise of comparable efficacy, which is a prerequisite.
The determination of cost was affected mainly by the drug prices and the dosage schedules. In this context, the present analysis focused only on economic implications, without taking into account any off-label dose escalation or differences in efficacy. Thus, our conclusions are driven mainly by differences in the cost and doses of the agents under evaluation. In economic studies such as the present, the choice of time horizon depends on the research question and can range from a few weeks to several years (eg, remaining life expectancy). The time horizon chosen should be long enough to reflect all outcomes of interest associated with the alternative therapies. In the present analysis, as the effectiveness of comparators has been considered equivalent, the focus shifted only in their financial impact in the health care setting for payers; hence, 1 year was considered a reasonable period to study the comparators.
According to the base-case analysis, biologic treatments were prescribed as per label and, in the case of etanercept (the only biologic approved for the treatment of PP with the option for intermittent or continuous use) real-world treatment pattern data were collected by means of a nationwide field research with dermatologists. The cost of these agents for existing patients was lower compared to new patients as the induction schedule was not applicable for the former.
Based on the findings from a recent systematic literature review, dose escalation with the aforementioned comparators may lead to greater efficacy in some cases, while dose reduction may result in reduced efficacy.47 We chose to base our analysis on the dosing schedules adopted in Greece based on the approved label for each biologic and on data from a local survey of clinical practice.
In the international literature, many different economic studies or reviews have examined the aforementioned comparators in terms of cost-effectiveness, and such comparisons are becoming increasingly relevant; however, the results remain inconclusive due to several factors, such as the different management of patients across countries, differences in dosage schedule, relative prices, time horizon of models, perspective of analysis, assumptions concerning the education of patients, sequences of biologic treatment, and conflicts due to funding of research.15,48–52 This fact imposes the need to interpret the results of these comparisons with caution. Also, methodological issues set aside, the cost-effectiveness of health technologies might vary from country to country simply due to differences in clinical practice patterns and/or relative prices; hence, extrapolations are not recommended.53 Moreover, long-term data from appropriately designed studies on the comparative efficacy and safety of such treatments are lacking; for a disease requiring life-long treatment, such as psoriasis, the use of short-term data may be misleading. For the above reasons, a straightforward approach is to compare only the treatment cost of the agents under consideration and to declare the least expensive one. This type of analysis, as the one we conducted here, has already been proposed by several national agencies to compare this specific group of agents.54
This is the second study conducted in Greece for patients with moderate to severe PP, and its results are somewhat inconsistent with those of the previous one.55 The differences in the results can be interpreted on the basis of the different assumptions used for the two models. For example, the previous analysis did not include the additional drug cost for patients receiving 90 mg of ustekinumab (ie, those weighing over 100 kg); furthermore, it assumed that all patients starting on etanercept receive the 50 mg biweekly dose and that all etanercept patients receive continuous treatment (ie, no intermittent dosing schedule). Importantly, the previous model also implemented a hypothetical projection regarding the use of ustekinumab, as this biologic was just becoming available in the Greek market and data on its real-world use were not available for Greece. On the other hand, in our analysis all these parameters were derived from the survey of dermatologists, capturing actual clinical practice, with ustekinumab having been available in the Greek market for over 3 years. This approach may not be as robust as, for instance, a real-world patient registry, but is quite sufficient in giving reasonable indications as the sample of physicians and patients was representative.
To test the robustness of our results, several one-way sensitivity and different scenario analyses were undertaken to show how different treatment approaches may influence the annual treatment cost. These analyses aimed at including the most plausible scenarios applied in daily practice for these biologics when prescribed as per the approved label. In this context, the results presented here may be of value for both decision-makers and Social Funds, as well as physicians faced with prescribing-related cost-containment measures. Furthermore, results are presented in a format that may allow for the direct calculation of costs upon changes in the prices of the concerned biologics (Table S5).
Regarding the limitations of the present study, it must be noted that modeling-based analyses, such as the one employed here, involve by definition a simplification of the process they try to emulate, and the present one is no exception. Much of the data entered in the model resulted from a nationwide survey that was based on questionnaire-driven interviews conducted with dermatologists across the country. Even though the sample size of dermatologists appears relatively small, it may be considered a representative one as it includes input from both public hospital and EOPYY physicians across Greece managing a satisfactory sample of patients with moderate to severe psoriasis. This approach has known limitations, such as participant recall bias and misunderstanding of questions. Nonetheless, it must be noted that this type of data collection represents a reasonable, second-best approach in the absence of local real-world data, such as those derived by registries, and was conducted by an independent company specializing in this type of research.
Moreover, our model did not include the cost related to treatment failure (eg, increased hospital visits, reduced productivity), as this was considered out of scope for the purpose of the study. In terms of the safety profile of the biologics considered, even though available data may favor etanercept over the other anti-TNFs regarding certain adverse events such as tuberculosis, nontuberculosis opportunistic infections, and lymphoma56–60 which, although rare, may be associated with increased management costs, our analysis assumed a comparable safety profile and thus did not include adverse event-related costs; this approach is consistent with previous economic analyses conducted.51,61–63
Another limitation of the study is that treatment switching between biologic agents is not considered in the present model. Nonetheless, it must be noted that in clinical practice both agents may be used in a sequence, an approach which has not been evaluated in the Greek model due to limitations concerning the data availability. This is a common issue in small countries like Greece, since there is no available registry/database which could be used for analytical purposes. In addition, in the present analysis the cost of nonresponders has been estimated until treatment discontinuation; thus, no further costs have been incorporated in the model for this type of patient.
Despite the above limitations, the model used incorporates real-life data and, under its specific scope, presents useful results of practical value for both the payer and the prescriber on the cost of treatment of PP with biologics in Greece.
Conclusion
The cost of treatment with etanercept may be lower than that with other biologic agents licensed for the treatment of moderate to severe PP in Greece. Further analysis with real-world data on effectiveness, safety, and utility is needed to confirm the accuracy, completeness, and appropriateness of the results in the local setting. Results presented must be viewed strictly in the light of the setting and time undertaken, the data, and the assumptions utilized and may change if some of these also change over time.
Acknowledgments
The authors would like to thank Tsekouras Vasilis from Pfizer Hellas for his in-depth review of the manuscript, Bilitou Katerina from Pfizer Hellas for reviewing and validating the economic model, and Kalpaxoglou Michalis from Ipsos for carrying out the market research and analyzing the sample.
VF conducted the analyses, interpreted the results, and wrote the manuscript. ER and EV supervised the study and interpreted the results. NM supervised the study and is the guarantor for the overall content.
Nothing contained in this paper is intended to guarantee the appropriateness of any medical treatment or to be used for therapeutic purposes or as a substitute for a health professional’s advice.
The authors or Pfizer Hellas have no access to any personal information of the participating physicians. Each one of the recruiters of IPSOS Company declared that the interviews were conducted in compliance with the law 2472/97.
Disclosure
For the present study, VF has received an unrestricted grant from Pfizer. ER and EV are full-time employees at Pfizer Hellas. NM and VF have received honoraria for lectures and seminars from Pfizer. The authors report no further conflicts of interest in this work.
References
Nestle FO, Kaplan DH, Barker J. Psoriasis. NEJM. 2009;361(5):496–509. | |
Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303(1):1–10. | |
World Health Organization. The known health effects of UV [webpage on the Internet]. Available at: http://www.who.int/uv/faq/uvhealtfac/en/index1.html. Accessed November 13, 2014. | |
Enamandram M, Kimball AB. Psoriasis epidemiology: the interplay of genes and the environment. J Invest Dermatol. 2013;133(2):287–289. | |
Parisi R, Symmons DP, Griffiths CE, Ashcroft DM; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133(2):377–385. | |
Bassukas ID, Mavridou KA, Evangelou T, Gaitanis G. The prevalence of psoriasis among elderly individuals: more questions than answers [webpage on the Internet]. Ageing Res. 2011;2(1). Available at: http://www.pagepress.org/journals/index.php/ar/article/view/ar.2011.e1/html_93. Accessed November 13, 2014. | |
Rigopoulos D, Gregoriou S, Katrinaki A, et al. Characteristics of psoriasis in Greece: an epidemiological study of a population in a sunny Mediterranean climate. Eur J Dermatol. 2010;20(2):189–195. | |
Samoutis A, Kaitelidou D, Siskou O. Moderate and severe plaque psoriasis: a quality of life and cost-of-illness study from Greece. Intl J Caring Sci. 2012;5(2). | |
Kyriakis KP, Palamaras I, Pagana G, Terzoudi S, Evangelou G. Lifetime prevalence fluctuations of chronic plaque psoriasis and other non-pustular clinical variants. J Eur Acad Dermatol Venereol. 2008;22(12):1513–1514. | |
Raho G, Koleva DM, Garattini L, Naldi L. The burden of moderate to severe psoriasis: an overview. Pharmacoeconomics. 2012;30(11):1005–1013. | |
Jacobs P, Bissonnette R, Guenther LC. Socioeconomic burden of immune-mediated inflammatory diseases – focusing on work productivity and disability. J Rheumatol Suppl. 2011;88:55–61. | |
Lewis-Beck C, Abouzaid S, Xie L, Baser O, Kim E. Analysis of the relationship between psoriasis symptom severity and quality of life, work productivity, and activity impairment among patients with moderate-to-severe psoriasis using structural equation modeling. Patient Prefer Adherence. 2013;7:199–205. | |
Kimball AB, GuÉrin A, Tsaneva M, et al. Economic burden of comorbidities in patients with psoriasis is substantial. J Eur Acad Dermatol Venereol. 2011;25(2):157–163. | |
International Federation of Psoriasis Association. Psoriasis is a serious disease deserving global attention [webpage on the Internet]. Available at: http://www.ifpa-pso.org/getfile.ashx?cid=279366&cc=3&refid=18. Accessed November 13, 2014. | |
Liu Y, Wu EQ, Bensimon AG, et al. Cost per responder associated with biologic therapies for Crohn’s disease, psoriasis, and rheumatoid arthritis. Adv Ther. 2012;29(7):620–634. | |
Pathirana D, Ormerod AD, Saiag P, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23 Suppl 2:1–70. | |
Giannetti A. Commentary on the European S3-guidelines on the systemic treatment of psoriasis. J Eur Acad Dermatol Venereol. 2010;24(3):368; author reply 368–369. | |
Pathirana D, Nast A, Ormerod AD, et al. On the development of the European S3 guidelines on the systemic treatment of psoriasis vulgaris: structure and challenges. J Eur Acad Dermatol Venereol. 2010;24(12):1458–1467. | |
American Academy of Dermatology, 2008. Guidelines of care for the management of psoriasis and psoriatic arthritis. Available at: https://www.aad.org/File%20Library/Global%20navigation/Education%20and%20quality%20care/Guidelines-psoriasis-sec-2.pdf. Accessed October 15, 2014. | |
Hsu S, Papp KA, Lebwohl MG, et al; National Psoriasis Foundation Medical Board. Consensus guidelines for the management of plaque psoriasis. Arch Dermatol. 2012;148(1):95–102. | |
Reich K, Burden AD, Eaton JN, Hawkins NS. Efficacy of biologics in the treatment of moderate to severe psoriasis: a network meta-analysis of randomized controlled trials. Br J Dermatol. 2012;166(1):179–188. | |
Galván-Banqueri M, Marín Gil R, Santos Ramos B, Bautista Paloma FJ. Biological treatments for moderate-to-severe psoriasis: indirect comparison. J Clin Pharm Ther. 2013;38(2):121–130. | |
Kim IH, West CE, Kwatra SG, Feldman SR, O’Neill JL. Comparative efficacy of biologics in psoriasis: a review.Am J Clin Dermatol. 2012;13(6):365–374. | |
Weinberg JM. An overview of infliximab, etanercept, efalizumab, and alefacept as biologic therapy for psoriasis.Clin Ther. 2003;25(10):2487–2505. | |
Tutrone WD, Saini R, Weinberg JM. Biological therapy for psoriasis: an overview of infliximab, etanercept, efalizumab and alefacept. IDrugs. 2004;7(1):45–49. | |
Saini R, Tutrone WD, Weinberg JM. Advances in therapy for psoriasis: an overview of infliximab, etanercept, efalizumab, alefacept, adalimumab, tazarotene, and pimecrolimus. Curr Pharm Des. 2005;11(2):273–280. | |
Weinberg JM, Bottino CJ, Lindholm J, Buchholz R. Biologic therapy for psoriasis: an update on the tumor necrosis factor inhibitors infliximab, etanercept, and adalimumab, and the T-cell-targeted therapies efalizumab and alefacept. J Drugs Dermatol. 2005;4(5):544–555. | |
Raval K, Lofland JH, Waters H, Piech CT. Disease and treatment burden of psoriasis: examining the impact of biologics. J Drugs Dermatol. 2011;10(2):189–196. | |
Langley RG. Effective and sustainable biologic treatment of psoriasis: what can we learn from new clinical data?J Eur Acad Dermatol Venereol. 2012;26 Suppl 2:21–29. | |
Sivamani RK, Goodarzi H, Garcia MS, et al. Biologic therapies in the treatment of psoriasis: a comprehensive evidence-based basic science and clinical review and a practical guide to tuberculosis monitoring. Clin Rev Allergy Immunol. 2013;44(2):121–140. | |
Baker EL, Coleman CI, Reinhart KM, et al. Effect of Biologic Agents on Non-PASI Outcomes in Moderate-to-Severe Plaque Psoriasis: Systematic Review and Meta-Analyses. Dermatol Ther (Heidelb). 2012;2(1):9. | |
Kentikelenis A, Karanikolos M, Papanicolas I, Basu S, McKee M, Stuckler D. Health effects of financial crisis: omens of a Greek tragedy. Lancet. 2011;378(9801):1457–1458. | |
European Medicines Agency. EPAR summary for the public: Humira (adalimumab) [webpage on the Internet]. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/000481/WC500050865.pdf. Accessed November 13, 2014. | |
European Medicines Agency. EPAR summary for the public: Enbrel (etanercept) [webpage on the Internet]. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/000262/WC500027364.pdf. Accessed November 13, 2014. | |
European Medicines Agency. EPAR summary for the public: Remicade (infliximab) [webpage on the Internet]. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/000240/WC500050883.pdf. Accessed November 13, 2014. | |
European Medicines Agency. EPAR summary for the public: Stelara (ustekinumab) [webpage on the Internet]. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/000958/WC500058509.pdf. Accessed November 13, 2014. | |
Ministry of Health, price bulletin 81505/30-08-2013, YA. Available at: http://www.moh.gov.gr/articles/times-farmakwn/deltia-timwn/1860-diorthwtiko-deltio-timwn-30-08-2013. Accessed August 10, 2013. Greek. | |
Mauskopf JA, Sullivan SD, Annemans L, et al. Principles of good practice for budget impact analysis: report of the ISPOR Task Force on good research practices – budget impact analysis. Value Health. 2007;10(5):336–347. | |
Lebwohl M. Psoriasis. Lancet. 2003;361(9364):1197–1204. | |
Schon MP, Boehncke WH. Psoriasis. NEJM. 2005;352(18):1899–1912. | |
Nijsten T, Margolis DJ, Feldman SR, Rolstad T, Stern RS. Traditional systemic treatments have not fully met the needs of psoriasis patients: results from a national survey. J Am Acad Dermatol. 2005;52(3 Pt 1):434–444. | |
Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes. 2012;2:e54. | |
Nast A, Boehncke WH, Mrowietz U, et al; Deutsche Dermatologische Gesellschaft (DDG); Berufsverband Deutscher Dermatologen (BVDD). S3 – Guidelines on the treatment of psoriasis vulgaris (English version). Update. J Dtsch Dermatol Ges. 2012;10 Suppl 2:S1–S95. | |
Smith CH, Anstey AV, Barker JN, et al; (Chair of Guideline Group). British Association of Dermatologists’ guidelines for biologic interventions for psoriasis 2009. Br J Dermatol. 2009;161(5):987–1019. | |
Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58(5):826–850. | |
Brezinski EA, Armstrong AW. Off-label biologic regimens in psoriasis: a systematic review of efficacy and safety of dose escalation, reduction, and interrupted biologic therapy. PLoS One. 2012;7(4):e33486. | |
Villacorta R, Hay JW, Messali A. Cost effectiveness of moderate to severe psoriasis therapy with etanercept and ustekinumab in the United States. Pharmacoeconomics. 2013;31(9):823–839. | |
Ahn CS, Gustafson CJ, Sandoval LF, Davis SA, Feldman SR. Cost effectiveness of biologic therapies for plaque psoriasis. Am J Clin Dermatol. 2013;14(4):315–326. | |
Nelson AA, Pearce DJ, Fleischer AB Jr, Balkrishnan R, Feldman SR. Cost-effectiveness of biologic treatments for psoriasis based on subjective and objective efficacy measures assessed over a 12-week treatment period. J Am Acad Dermatol. 2008;58(1):125–135. | |
Ferrándiz C, García A, Blasco AJ, Lázaro P. Cost-efficacy of adalimumab, etanercept, infliximab and ustekinumab for moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2012;26(6):768–777. | |
Samarasekera E, Sawyer L, Parnham J, Smith CH. Guideline Development G. Assessment and management of psoriasis: summary of NICE guidance. BMJ. 2012;345:e6712. | |
Drummond M, Barbieri M, Cook J, et al. Transferability of economic evaluations across jurisdictions: ISPOR Good Research Practices Task Force report. Value Health. 2009;12(4):409–418. | |
Iannazzo S, De Francesco M, Gomez-Ulloa D, Benucci M. A review of cost-effectiveness evaluations as part of national health technology assessments of biologic DMARDs in the treatment of rheumatoid arthritis. Expert Rev Pharmacoecon Outcomes Res. 2013;13(4):455–468. | |
Salmon-Ceron D, Tubach F, Lortholary O, et al; RATIO group. Drug-specific risk of non-tuberculosis opportunistic infections in patients receiving anti-TNF therapy reported to the 3-year prospective French RATIO registry. Ann Rheum Dis. 2011;70(4):616–623. | |
Tubach F, Salmon D, Ravaud P, et al; Research Axed on Tolerance of Biotherapies Group. Risk of tuberculosis is higher with anti-tumor necrosis factor monoclonal antibody therapy than with soluble tumor necrosis factor receptor therapy: The three-year prospective French Research Axed on Tolerance of Biotherapies registry. Arthritis Rheum. 2009;60(7):1884–1894. | |
Mariette X, Tubach F, Bagheri H, et al. Lymphoma in patients treated with anti-TNF: results of the 3-year prospective French RATIO registry. Ann Rheum Dis. 2010;69(2):400–408. | |
Piaserico S, Conti A, Lo Console F, et al. Efficacy and safety of systemic treatments for psoriasis in elderly patients. Acta Derm Venereol. 2014;94(3):293–297. | |
Toh S, Li L, Harrold LR, et al. Comparative safety of infliximab and etanercept on the risk of serious infections: does the association vary by patient characteristics? Pharmacoepidemiol Drug Saf. 2012;21(5):524–534. | |
Pearce DJ, Higgins KB, Stealey KH, et al. Adverse events from systemic therapies for psoriasis are common in clinical practice. J Dermatolog Treat. 2006;17(5):288–293. | |
Sizto S, Bansback N, Feldman SR, Willian MK, Anis AH. Economic evaluation of systemic therapies for moderate to severe psoriasis. Br J Dermatol. 2009;160(6):1264–1272. | |
National Institute for Health and Clinical Excellence. Psoriasis: The assessment and management of psoriasis. Available at: https://www.nice.org.uk/guidance/cg153/resources/guidance-psoriasis-pdf. Accessed October 15, 2014. | |
Fragoulakis V, Kourlaba G, Goumenos D, Konstantoulakis M, Maniadakis N. Economic evaluation of intravenous iron treatments in the management of anemia patients in Greece. Clinicoecon Outcomes Res. 2012;4:127–134. | |
Avgerinou G, Bassukas I, Chaidemenos G, et al. Budget impact analysis of ustekinumab in the management of moderate to severe psoriasis in Greece. BMC Dermatol. 2012;12:10. |
Supplementary materials
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.