Back to Journals » Drug Design, Development and Therapy » Volume 9
Analysis of malignancies in patients after heart transplantation with subsequent immunosuppressive therapy
Authors Rivinius R , Helmschrott M, Ruhparwar A, Schmack B, Klein B, Erbel C, Gleissner CA, Akhavanpoor M, Frankenstein L, Darche FF , Thomas D , Ehlermann P, Bruckner T , Katus HA, Doesch A
Received 7 October 2014
Accepted for publication 31 October 2014
Published 17 December 2014 Volume 2015:9 Pages 93—102
DOI https://doi.org/10.2147/DDDT.S75464
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Editor who approved publication: Professor Shu-Feng Zhou
Rasmus Rivinius,1 Matthias Helmschrott,1 Arjang Ruhparwar,2 Bastian Schmack,2 Berthold Klein,2 Christian Erbel,1 Christian A Gleissner,1 Mohammadreza Akhavanpoor,1 Lutz Frankenstein,1 Fabrice F Darche,1 Dierk Thomas,1 Philipp Ehlermann,1 Tom Bruckner,3 Hugo A Katus,1 Andreas O Doesch1
1Department of Cardiology, Angiology and Pneumology, 2Department of Cardiac Surgery, 3Institute for Medical Biometry and Informatics, University of Heidelberg, Heidelberg, Germany
Objective: The aim of this study was to analyze the distribution of malignancies in patients after heart transplantation (HTX) and to evaluate the risk factors including immunosuppressive therapy with regard to the development of malignancies and survival. Special emphasis was placed on the effects of a mammalian target of rapamycin (mTOR) containing immunosuppressive regimen.
Methods: A total of 381 patients (age ≥18 years) receiving HTX were included in the present analysis. All patients were followed-up at the University of Heidelberg Heart Center, Heidelberg, Germany. Data were retrieved from the Heidelberg Registry for Heart Transplantation being collected between 1989 and 2014. According to center standard, all patients received induction therapy with anti-thymocyte globulin guided by T-cell monitoring since 1994. The initial immunosuppressive regimen consisting of cyclosporine A (CsA) and azathioprine (AZA) was replaced by CsA and mycophenolate mofetil (MMF) in 2001 and by tacrolimus (TAC) and MMF in 2006. Additionally, mTOR inhibitors (everolimus/sirolimus) were applied since 2003.
Results: Mean recipient age at HTX was 51.2±10.5 years and the mean follow-up period after HTX was 9.7±5.9 years. During follow-up, 130 patients developed a neoplasm (34.1% of total). Subgroup analysis revealed 58 patients with cutaneous malignancy only (15.2%), 56 patients with noncutaneous malignancy only (14.7%), and 16 patients with both cutaneous and noncutaneous malignancy (4.2%). Statistically significant risk factors associated with an increased risk of malignancy after HTX were older age (P<0.0001), male recipients (P=0.0008), dyslipidemia (P=0.0263), diabetes mellitus (P=0.0003), renal insufficiency (P=0.0247), and >1 treated rejection episode (TRE) in the first year after HTX (P=0.0091). Administration of CsA (P=0.0195), AZA (P=0.0008), or steroids (P=0.0018) for >1 year after HTX was associated with increased development of malignancy, whereas administration of MMF (P<0.0001) or mTOR inhibitors (P<0.0001) was associated with a lower risk for development of malignancy. Additionally, 5-year follow-up of cutaneous malignancy recurrence (P=0.0065) and noncutaneous malignancy mortality (P=0.0011) was significantly lower in patients receiving an mTOR inhibitor containing therapy after the development of a malignancy.
Conclusion: This study highlights the complexity of risk factors including immunosuppression with regard to the development of malignancies after HTX. mTOR-inhibitor-based immunosuppression is associated with a better outcome after HTX, particularly in cases with noncutaneous malignancy.
Keywords: immunosuppression, risk factors, cyclosporine A, tacrolimus, azathioprine, mycophenolate mofetil, mTOR inhibitor, steroids
Introduction
As survival of patients after heart transplantation (HTX) has been improving in the last decades, malignancy secondary to immunosuppressive therapy has become a major threat to the long-term quality of life and survival.1,2 Hence, the aim of this study was to investigate the distribution of malignancies in patients after HTX. Special emphasis was placed on the evaluation of risk factors, including immunosuppressive drug therapy, with regard to the occurrence of malignancies and survival after HTX.
Methods
Patients
All human studies were reviewed and approved by the ethics committee of the University of Heidelberg, Heidelberg, Germany, and were therefore performed in accordance with the ethical standards laid down in the 2008 Declaration of Helsinki. A total of 381 patients (age ≥18 years) receiving HTX were included in this retrospective study. All patients were followed-up at the University of Heidelberg Heart Center, Heidelberg, Germany. Data were retrieved from the Heidelberg Registry for Heart Transplantation being collected between 1989 and 2014. Taking the slow-growing nature of cancer into account, adequate length of follow-up after HTX is required. Hence, only patients who survived for a minimum of 2 years after HTX were included.
The mean recipient age at HTX was 51.2±10.5 years, and the mean follow-up period after HTX was 9.7±5.9 years. Three hundred patients were men (78.7% of total). The mean donor age was 38.9±13.5 years. One hundred and sixty-five donors were men (46.9%). The number of treated rejection episodes (TREs) in the first year after HTX was 1.0±1.6. Further patient characteristics are given in Table 1.
Principal diagnosis for HTX was cardiomyopathy in 217 patients (57.0% of total), coronary artery disease in 128 patients (33.6% of total), amyloidosis in 21 patients (5.5% of total), and valvular disease in 15 patients (3.9% of total).
Follow-up
All patients were routinely followed up at the University of Heidelberg Heart Center, Heidelberg, Germany. After initial hospital stay, patients were seen on a monthly basis during the first 6 months after HTX, then bimonthly until the end of the first year, and thereafter 3 times per year.
Noncutaneous malignancies were considered all forms of cancer not related to skin cancer. The following entities were included in the category of cutaneous malignancies: precancerous lesion, basal-cell carcinoma, squamous-cell carcinoma, and melanoma.
Tumor screening, including conventional chest radiograph in two planes, abdominal ultrasound, dermatological examination, urological/gynecological examination (including prostate-specific antigen or mammography in women [>45 years]), guaiac-based fecal occult blood testing, and cytomegalovirus (CMV) and Epstein–Barr virus (EBV) (viral load determination via polymerase chain reaction), was performed yearly. Endomyocardial biopsies were taken weekly during the first month after HTX, then biweekly for the next 3 months, then at 6-month and 1-year follow-up after HTX, and thereafter once a year (or if clinically indicated). Cardiac catheterization was performed 3 months after HTX, and subsequently, 1 year, 2 years, 5 years, and 10 years after transplantation or if clinically indicated.3 TRE were diagnosed according to the revised International Society for Heart and Lung Transplantation (ISHLT) classification.4
Immunosuppressive regimen
Patients after HTX had been receiving anti-thymocyte globulin intravenously (Genzyme GmbH, Neu-Isenburg, Germany), which was guided by the daily performed cluster of differentiation 4+ T-cell monitoring via fluorescence-activated cell sorting (FACS) to adjust the application rate, since 1994.5
The initial immunosuppressive regimen consisting of cyclosporine A (CsA) and azathioprine (AZA) was replaced by CsA and mycophenolate mofetil (MMF) in 2001, and by tacrolimus (TAC) and MMF in 2006. Additionally, mammalian target of rapamycin (mTOR) inhibitors (everolimus/sirolimus) were used from 2003.
The oral dose of CsA was titrated to reach the target trough levels of 50–225 ng/mL depending on time after HTX: <1.0 year after HTX, 90–225 ng/mL; >1.0 year after HTX, 70–110 ng/mL; and >2.0 years after HTX, 50–90 ng/mL. In combination with AZA, each CsA target trough level was increased by 25 ng/mL. AZA was titrated according to white blood cell count, usually by administering 25–150 mg/day. The MMF target trough level was 1.5–4.0 mg/L (alternatively mini-area under the curve 30–60 mg h L−1). TAC target trough levels ranged from 3 to 14 ng/mL depending on time after HTX: <1.0 year after HTX, 6–14 ng/mL; >1.0 year after HTX, 4–8 ng/mL; and >2.0 years after HTX, 3–6.5 ng/mL. In patients with significant renal insufficiency, calcineurin inhibitors (CNI) target trough levels were reduced by 30.0%–50.0%. Oral mTOR inhibitor therapy was titrated to achieve target trough levels of 3–8 ng/mL. Levels for sirolimus and everolimus were 4–8 ng/mL in patients <2.0 years after HTX and 3–6.5 ng/mL in patients >2.0 years after HTX. Steroids (prednisolone) were weaned off whenever possible 6 months after HTX.
Statistical analysis
Statistical analysis was performed with SAS (Version 9.1; SAS Institute, Cary, NC, USA). Student’s t-test was used for continuous variables and chi-squared test for categorical variables. A P-value of <0.05 was considered to be statistically significant. The Kaplan–Meier estimator using log-rank tests was applied for survival of patients and for recurrence of cutaneous neoplasia after HTX.
Univariate analyses were performed to evaluate the risk of the following variables: recipient age and sex, body mass index (BMI), coronary artery disease, arterial hypertension, dyslipidemia, diabetes mellitus, renal insufficiency, recipient CMV-positive serostatus, recipient EBV-positive serostatus, donor age, donor sex, and number of TREs in the first year after HTX. Further, the influence of the following immunosuppressive drugs was analyzed: CsA, TAC, AZA, MMF, steroids, and mTOR inhibitors.
Additionally, a multivariate analysis with a logistic regression model was performed to estimate the influence of the following four variables: recipient age, recipient sex, diabetes mellitus, and >1 TRE in the first year after HTX.
Results
Distribution of malignancies
All malignancies
Of the 381 included patients, there were 251 patients without posttransplant malignancy (65.9% of total) and 130 patients with posttransplant malignancy (34.1% of total). The patients developing a neoplasm were further subdivided into 58 patients with cutaneous malignancy only (15.2% of total), 56 patients with noncutaneous malignancy only (14.7% of total), and 16 patients with both malignancies (4.2% of total) (Figure 1A). The mean interval from transplantation until initial diagnosis of malignancy was 7.3±4.7 years, and from diagnosis of malignancy until last follow-up was 4.8±4.3 years.
Cutaneous malignancies
A total of 74 cutaneous malignancies were documented. Among these malignancies, there were 14 precancerous lesions (18.9% of subgroup), 30 basal-cell carcinomas (40.6% of subgroup), 28 squamous-cell carcinomas (37.8% of subgroup), and two melanomas (2.7% of subgroup) (Figure 1B). The mean interval from transplantation until initial diagnosis of a cutaneous malignancy was 7.0±4.1 years, and from diagnosis of cutaneous malignancy until last follow-up was 6.3±4.4 years.
Noncutaneous malignancies
Seventy-two noncutaneous malignancies were documented, including 58 carcinomas (80.5% of subgroup), eleven posttransplant lymphoproliferative diseases (PTLD, 15.3% of subgroup), and three other hematopoietic neoplasias (myeloma/leukemia, 4.2% of subgroup) (Figure 1C). The mean interval from transplantation until initial diagnosis of a noncutaneous malignancy was 7.7±5.3 years, and from diagnosis of malignancy until last follow-up was 3.2±3.5 years.
Risk factors
Mortality
A total of 114 out of 381 HTX recipients (29.9% of total) died during follow-up. Mortality rate in patients without development of a malignancy was significantly lower than in patients with a malignancy (no malignancy: 59 of 251 patients [23.5% of subgroup], malignancy: 55 of 130 patients [42.3% of subgroup]; P=0.0001).
Patients with only a cutaneous malignancy had a lower mortality rate than patients with only a noncutaneous malignancy (cutaneous malignancy: 18 of 58 patients [31.0% of subgroup], noncutaneous malignancy: 31 of 56 patients [55.4% of subgroup]; P=0.0087). Superior prognosis of patients with cutaneous malignancies only vs patients with noncutaneous malignancies only was confirmed by a Kaplan–Meier analysis (P=0.0007).
Sixteen patients had both cutaneous and noncutaneous malignancies (mortality rate was six of 16 patients, 37.5%).
Patients with malignancies vs patients without malignancies
On comparing patients with and without malignancies, statistically significant risk factors associated with an increased risk for malignancy after HTX were as follows: older recipient age (54.3±8.3 years vs 49.6±11.2 years; P<0.0001), male recipient sex (115 of 130 [88.5%] vs 185 of 251 [73.7%]; P=0.0008), and >1 TRE in the first year (41 of 119 [34.5%] vs 51 of 236 [21.6%]; P=0.0091). Further, metabolic factors such as dyslipidemia (97 of 130 [74.6%] vs 159 of 251 [63.3%]; P=0.0263), diabetes mellitus (59 of 130 [45.4%] vs 68 of 251 [27.1%]; P=0.0003), and renal insufficiency (86 of 130 [66.2%] vs 136 of 251 [54.2%]; P=0.0247), showed a higher risk for the development of a malignancy.
Regarding other comorbidities, including coronary artery disease prior to HTX or arterial hypertension, no statistically significant association with development of a malignancy was found. Additionally, there was no clear evidence in this study for a correlation between the development of a malignancy and positive recipient CMV or EBV serostatus (all P≥0.05; Table 1).
Cutaneous vs noncutaneous malignancies
Further subgroup analysis between cutaneous vs noncutaneous malignancies showed no statistically significant difference regarding recipient age or recipient sex (P=0.9857 and 0.3179, respectively). Neither did dyslipidemia (P=0.9235), diabetes mellitus (P=0.2654), or renal insufficiency (P=0.1259) differ statistically significantly.
Regarding positive recipient EBV serostatus, there were more patients with cutaneous than noncutaneous malignancies (44 of 58 [75.9% of subgroup] vs 32 of 56 [57.1% of subgroup]; P=0.0340).
Positive CMV recipient serostatus showed no significant difference between subgroups (P=0.2654).
Immunosuppressive drug therapy
Calcineurin inhibitors
Two different CNI agents (CsA and TAC) were compared separately, revealing a significant difference. Patients with CsA for >1 year (92 of 130 [70.8% of subgroup] vs 147 of 251 [58.6% of subgroup]) displayed a higher risk for cancer (P=0.0195) (Table 2). In patients with TAC application for >1 year, a lower risk for the development of a malignancy was found (P=0.0002). In these patients, 34 of 130 (26.2% of subgroup) vs 115 of 251 (45.8% of subgroup) developed a malignancy.
 
|
Table 2 Immunosuppressive drug therapy after HTX |
Azathioprine
Administration of AZA for >1 year was associated with a significantly increased risk for the development of a malignancy (P=0.0008). In patients with malignancy, 67 of 130 patients (51.5% of subgroup) were on AZA for >1 year, compared to only 85 of 251 patients in tumor-free patients (33.9%).
Mycophenolate mofetil
In contrast to AZA, the use of MMF for >1 year was associated with a reduced risk for the development of a malignancy (P<0.0001).
Steroids
Application of steroids for >1 year showed a higher risk for the development of a malignancy (P=0.0018).
mTOR inhibitors
Administration of mTOR inhibitor therapy for >1 year after HTX was associated with a statistically significant lower risk for the development of malignancies (19 of 130 patients [14.6% of subgroup] vs 85 of 251 patients [33.9% of subgroup]; P<0.0001).
Cutaneous malignancy recurrence after 2 years of follow-up (two of 22 [9.1% of subgroup] vs 13 of 41 [31.7% of subgroup]; P=0.0445) as well as after 5 years of follow-up (six of 19 [31.6% of subgroup] vs 22 of 31 [71.0% of subgroup]; P=0.0065) was significantly lower in patients with mTOR inhibitor therapy after the initial diagnosis of a tumor.
A lower mortality with noncutaneous malignancies was observed in patients receiving mTOR inhibitor therapy after the initial diagnosis of malignancy (six of 29 [20.7% of subgroup] vs 31 of 43 [72.1% of subgroup]; P<0.0001) (Table 3).
 
|
Table 3 Influence of mTOR inhibitor therapy |
The results regarding the lower cutaneous malignancy recurrence (P=0.0202) and lower noncutaneous malignancy mortality (P<0.0001) in patients with mTOR inhibitor therapy after the initial diagnosis of malignancy were confirmed by Kaplan–Meier analysis (Figures 2 and 3).
Multivariate risk factor analysis
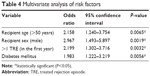
Multivariate analysis identified four independent risk factors for increased risk of malignancy: recipient age (odds ratio [OR] =2.158; P=0.0065); recipient sex (OR =2.967; P=0.0019); diabetes mellitus (OR =1.983; P=0.0056); and >1 TRE in the first year (OR =2.199; P=0.0032) (Table 4).
|
Table 4 Multivariate analysis of risk factors |
Discussion
Immunosuppressive regimens and treatment options have improved significantly, thereby resulting in better long-term survival rates. In patients with immunosuppressive therapy, occurrence of malignancies after HTX is a well-described phenomenon.6,7 Hence, this work aims at a better management of HTX patients to minimize tumor-associated mortality and recurrence of malignancies in order to further improve long-term survival after HTX.
The incidence of malignancy after HTX has been reported as being between 21.0% and 31.0% depending on duration and completeness of follow-up.3,8 In this long-term follow-up study, 130 patients (34.1%) developed posttransplant malignancy. These patients were further subdivided into 58 patients with cutaneous malignancy only, 56 patients with noncutaneous malignancy only, and 16 patients developing both types of malignancies. These numbers are in line with those of Crespo-Leiro et al who observed 50.7% cutaneous malignancies after transplantation.9
In comparison to cutaneous malignancies, mean interval from transplantation until initial diagnosis of tumor was longer in patients with noncutaneous malignancies. On the contrary, the mean interval from diagnosis of malignancy until last follow-up was longer in patients with cutaneous malignancies. Regarding mortality, patients without malignancy had the lowest mortality rate (23.5%), followed by skin cancer (31.0%), patients with both types of malignancies (37.5%), and noncutaneous malignancies (55.4%). These results are in accordance with the poor prognosis of solid tumors after transplantation in immunosuppressed patients.10,11 Hence, prognosis for post-HTX skin cancer is considerably more favorable than for other post-HTX neoplasias.9
These findings are comparable to those from nontransplanted patients, where skin cancer in general is associated with a lower mortality than noncutaneous cancer.
Demographic data of this study was comparable to those from the International Society for Heart and Lung Transplantation Registry and the Spanish Post-Heart-Transplant Tumor Registry regarding recipient age, percentage of male recipients, and occurrence of malignancies.9,12
In this study, older recipient age (P<0.0001), male recipient sex (P=0.0008), and >1 TRE in the first year (P=0.0091) were associated with an increased risk of malignancy after HTX.9 Also, metabolic factors such as dyslipidemia (P=0.0263), diabetes mellitus (P=0.0003), and renal insufficiency (P=0.0247) seemed to play an important role in the development of malignancies. This is in line with data from the Aerobic Center Longitudinal Study, which revealed a 56% greater age-adjusted risk for cancer mortality in patients with metabolic syndrome.13 However, we could not find a statistically significant correlation between the occurrence of a malignancy and coronary artery disease or arterial hypertension (all not significant, P≥0.05).
In accordance with above-mentioned findings, subsequent multivariate analysis identified four independent risk factors associated with an increased risk of malignancy: recipient age (OR =2.158; P=0.0065); male recipient sex (OR =2.967; P=0.0019); diabetes mellitus (OR =1.983; P=0.0056); and >1 TRE in the first year (OR =2.199; P=0.0032). Whereas age and sex are inevitable risk factors, diabetes mellitus and rejection episodes offer a therapeutic approach.
In fact, HTX itself was described as a risk factor, as the incidence of malignancies in patients after HTX is two- to threefold higher compared to patients after renal transplantation.14 This might be attributed to the fact that patients after HTX require higher doses of immunosuppressive therapy than patients after kidney transplant, resulting in a higher risk of malignancy.
EBV has been reported to be associated with lymphoproliferative disorders, and coinfections with CMV in recipients of solid-organ transplants have been described.15,16 Nevertheless, no difference between tumor and nontumor patients concerning positive recipient CMV or EBV serostatus was seen in this study (P≥0.05).
Also, the effects of different immunosuppressive agents were evaluated in this study. Administration of CsA (P=0.0195), AZA (P=0.0008), or steroids (P=0.0018) for >1 year after HTX was significantly associated with an increased risk for the development of a neoplastic lesion. This is in line with findings of Wilkinson et al reporting an increased frequency of posttransplant malignancies in patients treated with CsA, AZA, and steroids.17 CsA enhances tumor growth and increases the number of metastases by a direct cellular effect that is independent of its effect on the host’s immune cells, resulting in an increased production of growth factors.18 Increased occurrence of tumors in patients with AZA might be a consequence of the photosensitizing effects of AZA and its metabolites.19
In contrast to CsA, TAC, which is another CNI, showed a significantly lower risk for the development of a malignancy when applied for >1 year (P=0.0002). This might be explained by a shorter follow-up interval in patients on TAC (7.0±4.6 years) compared to CsA (11.8±5.8 years). Nevertheless, the use of TAC instead of CsA as primary immunosuppressant for heart transplant recipients led to a significant reduction in rejection episodes.20 Further, a combination of TAC and MMF was more efficacious in preventing rejection episodes than a combination of CsA and MMF.21,22 However, it may also be speculated that this reduction of rejection episodes may cause less malignancies, because in this study more than one TRE in the first year was associated with an increased risk for tumorigenesis (OR =2.199; P=0.0032).
Regarding the use of MMF for >1 year, there was a reduced risk for the development of a malignancy (P<0.0001). Crespo-Leiro et al showed a lower incidence of skin cancer with MMF than with AZA.9 These results were supported by O’Neill et al showing that the use of MMF in standard immunosuppressive regimens is associated with a significantly lower risk of developing malignancy.23
mTOR inhibitors have been shown to prevent tumors and even to reduce metastatic tumor growth by antiangiogenesis.24 In this study, the application of mTOR inhibitor therapy for >1 year after HTX was associated with a statistically significant lower risk for the development of malignancies (P<0.0001), which is in line with data from a multivariate analysis of posttransplant malignancies in patients receiving mTOR inhibitors for maintenance of immunosuppression, showing a significantly reduced risk of developing a posttransplant de novo malignancy in the 2-year follow-up.25
Further, our data show a significant lower cutaneous malignancy recurrence after 2 years of follow-up (P=0.0445) as well as after 5 years of follow-up (P=0.0065) in patients with mTOR inhibitor therapy after diagnosis of the initial malignancy. Stallone et al reported a complete remission of cancer in transplant patients with cutaneous malignancy after a switch from an immunosuppressive regimen based on CsA and MMF to a regimen based on mTOR inhibitors.26 Nevertheless, 1-year follow-up (P=0.1928) and overall follow-up (P=0.5520) were not significantly different statistically. This could be due to the fact that, on the one hand, the mean interval until recurrence of skin cancer is longer than 12 months, and, on the other, there is only a small number of remaining patients left within the final follow-up, making it more difficult to analyze short-term and very long-term effects.3
Most importantly, we observed lower mortality in patients with noncutaneous malignancies receiving a therapy containing an mTOR inhibitor after the initial diagnosis of malignancy (P<0.0001). These results were confirmed by Kaplan–Meier analysis (P<0.0001). This is supported by experimental work indicating the concept of mTOR inhibitors being able to simultaneously reduce the risk of cancer while avoiding allograft rejection.27,28 Nevertheless, the use of mTOR inhibitors is not benign, and such therapy is associated with significant side effects that need to be managed appropriately. Many of the side effects can be attenuated by decreasing the dose significantly and maintaining an overlap period with CNI during conversion for a longer period.29
These results regarding mTOR inhibitors strengthen the possibility of an equilibrium between efficient immunosuppressive drug therapy and control over the development of cancer, thereby offering new therapeutic strategies for the treatment of malignancies in clinical practice.30,31
Study limitations
These findings were derived from a single-center study. However, a study size with 381 patients was sufficient to compare results with multicenter studies and, in contrast to multicenter studies, all patients received an identical induction regimen.
Further, although the data were gathered prospectively, the retrospective nature of this analysis carries the limitations of all such study design.
Conclusion
As a result of the improvements in immunosuppressive regimen and follow-up treatment, long-term survival rates in patients after HTX have significantly increased. However, long-term prognosis is strongly affected by the occurrence of malignancy. This study highlights the complexity of risk factors and immunosuppressive drug therapy on the development of malignancies after HTX. Relevant risk factors for the development of malignancies were older recipient age, male recipient sex, and >1 TRE in the first year after HTX. Furthermore, diabetes mellitus was an independent metabolic risk factor associated with a higher risk for the development of neoplasms. This underlines the complexity of risk factors that are not only limited to immunosuppressive therapy.
However, most importantly, a therapy containing an mTOR inhibitor after the initial diagnosis of tumor was associated with lower cutaneous malignancy recurrence rates after 2 years and 5 years and lower noncutaneous malignancy mortality, and should therefore be considered as an essential part of a modern immunosuppressive therapy in patients after HTX.
Disclosure
AO Doesch received a research grant from Novartis Pharma GmbH, Nuremberg, Germany. The authors report no conflicts of interest.
References
Hunt SA. Taking heart-cardiac transplantation past, present, and future. N Engl J Med. 2006;355(3):231–235. | ||
Hauptman PJ, Mehra MR. It is time to stop ignoring malignancy in heart transplantation: a call to arms. J Heart Lung Transplant. 2005;24(8):1111–1113. | ||
Doesch AO, Müller S, Konstandin M, et al. Malignancies after heart transplantation: incidence, risk factors, and effects of calcineurin inhibitor withdrawal. Transplant Proc. 2010;42(9):3694–3699. | ||
Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24(11):1710–1720. | ||
Koch A, Daniel V, Dengler TJ, Schnabel PA, Hagl S, Sack FU. Effectivity of a T-cell-adapted induction therapy with anti-thymocyte globulin (Sangstat). J Heart Lung Transplant. 2005;24(6):708–713. | ||
Cole WH. The increase in immunosuppression and its role in the development of malignant lesions. J Surg Oncol. 1985;30(3):139–144. | ||
London NJ, Farmery SM, Will EJ, Davison AM, Lodge JP. Risk of neoplasia in renal transplant patients. Lancet. 1995;346(8972):403–406. | ||
El-Hamamsy I, Stevens LM, Carrier M, et al. Incidence and prognosis of cancer following heart transplantation using RATG induction therapy. Transpl Int. 2005;18(11):1280–1285. | ||
Crespo-Leiro MG, Alonso-Pulpón L, Vázquez de Prada JA, et al. Malignancy after heart transplantation: incidence, prognosis and risk factors. Am J Transplant. 2008;8(5):1031–1039. | ||
Goldstein DJ, Williams DL, Oz MC, Weinberg AD, Rose EA, Michler RE. De novo solid malignancies after cardiac transplantation. Ann Thorac Surg. 1995;60(6):1783–1789. | ||
Pham SM, Kormos RL, Landreneau RJ, et al. Solid tumors after heart transplantation: lethality of lung cancer. Ann Thorac Surg. 1995;60(6):1623–1626. | ||
Lund LH, Edwards LB, Kucheryavaya AY, et al; International Society for Heart and Lung Transplantation. International Society for Heart and Lung Transplantation. The Registry of the International Society for Heart and Lung Transplantation: thirtieth official adult heart transplant report-2013; focus theme: age. J Heart Lung Transplant. 2013;32(10):951–964. | ||
Jaggers JR, Sui X, Hooker SP, et al. Metabolic syndrome and risk of cancer mortality in men. Eur J Cancer. 2009;45(10):1831–1838. | ||
Opelz G, Döhler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant. 2004;4(2):222–230. | ||
Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant. 2004;4(6):905–913. | ||
Hodson EM, Jones CA, Webster AC, et al. Antiviral medications to prevent cytomegalovirus disease and early death in recipients of solid-organ transplants: a systematic review of randomised controlled trials. Lancet. 2005;365(9477):2105–2115. | ||
Wilkinson AH, Smith JL, Hunsicker LG, et al. Increased frequency of posttransplant lymphomas in patients treated with cyclosporine, azathioprine, and prednisone. Transplantation. 1989;47(2):293–296. | ||
Hojo M, Morimoto T, Maluccio M, et al. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature. 1999;397(6719):530–534. | ||
Hemmens VJ, Moore DE. Photochemical sensitization by azathioprine and its metabolites. Azathioprine and nitroimidazole metabolites. Photochem Photobiol. 1986;43(3):257–262. | ||
Ye F, Ying-Bin X, Yu-Guo W, Hetzer R. Tacrolimus versus cyclosporine microemulsion for heart transplant recipients: a meta-analysis. J Heart Lung Transplant. 2009;28(1):58–66. | ||
Meiser BM, Groetzner J, Kaczmarek I, et al. Tacrolimus or cyclosporine: which is the better partner for mycophenolate mofetil in heart transplant recipients? Transplantation. 2004;78(4):591–598. | ||
Helmschrott M, Beckendorf J, Akyol C, et al. Superior rejection profile during the first 24 months after heart transplantation under tacrolimus as baseline immunosuppressive regimen. Drug Des Devel Ther. 2014;8:1307–1314. | ||
O’Neill JO, Edwards LB, Taylor DO. Mycophenolate mofetil and risk of developing malignancy after orthotopic heart transplantation: analysis of the transplant registry of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006;25(10):1186–1191. | ||
Guba M, von Breitenbuch P, Steinbauer M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8(2):128–135. | ||
Kauffman HM, Cherikh WS, Cheng Y, Hanto DW, Kahan BD. Maintenance immunosuppression with target-of-rapamycin inhibitors is associated with a reduced incidence of de novo malignancies. Transplantation. 2005;80(7):883–889. | ||
Stallone G, Schena A, Infante B, et al. Sirolimus for Kaposi’s sarcoma in renal-transplant recipients. N Engl J Med. 2005;352(13):1317–1323. | ||
Geissler EK. The impact of mTOR inhibitors on the development of malignancy. Transplant Proc. 2008;40(10 suppl):S32–S35. | ||
Geissler EK. Fighting malignancy in organ transplant recipients. Transplant Proc. 2009;41(6 suppl):S9–S12. | ||
Kushwaha SS. mTOR inhibitors as primary immunosuppression after heart transplant: confounding factors in clinical trials. Am J Transplant. 2014;14(9):1958–1959. | ||
Valantine H. Is there a role for proliferation signal/mTOR inhibitors in the prevention and treatment of de novo malignancies after heart transplantation? Lessons learned from renal transplantation and oncology. J Heart Lung Transplant. 2007;26(6):557–564. | ||
Santoni M, Massari F, Cascinu S. Prophylactic use of mTOR inhibitors and other immunosuppressive agents in heart transplant patients. Cell Mol Immunol. 2014. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




