Back to Journals » Clinical Ophthalmology » Volume 13
Ab interno canaloplasty combined with trabecular bypass stenting in eyes with primary open-angle glaucoma
Authors Heersink M, Dovich JA
Received 14 May 2019
Accepted for publication 9 July 2019
Published 12 August 2019 Volume 2019:13 Pages 1533—1542
DOI https://doi.org/10.2147/OPTH.S215667
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Marius Heersink,1 Jesse A Dovich2
1University of Alabama School of Medicine, Birmingham, AL, USA; 2Pacific Eye Institute, Upland, CA, USA
Purpose: Evaluate outcomes of trabecular meshwork (TM) bypass (iStent® GTS100) with cataract extraction (CE) and TM-bypass + ab interno canaloplasty (CP) (VISCO360®) with CE in patients with primary open-angle glaucoma (POAG).
Setting: Private surgical center for a comprehensive ophthalmology practice
Design: Retrospective analysis of 186 eyes from 130 consecutive patients with 6 months follow-up.
Methods: Eligible eyes had POAG, indicated for CE, and had received CE + TM-bypass or CE + TM-bypass + CP. Exclusions: glaucomas not POAG, SLT within 6 months, or previous ALT. IOP, visual acuity, and medication use assessed at baseline, months 1, 3, and 6. Endpoints were mean reduction in IOP from baseline at 6 months, proportion with IOP reduction at 6 months of ≥20% and IOP <18 mmHg on same or fewer medications, mean medication reduction, and proportion medication independent.
Results: Eighty-six eyes comprised the CE + TM-bypass + CP group; 100 eyes in the CE + TM-bypass group. At 6 months: mean IOP reduction was 2.9±3.6 mmHg for CE + TM-bypass + CP and 1.7±3.1 mmHg for CE + TM-bypass group (P<0.05); the proportion with IOP reduction of ≥20% and an IOP <18 mmHg on the same or fewer medications was 46% for CE + TM-bypass + CP and 35% for CE + TM-bypass; for both CE + TM-bypass + CP and CE + TM-bypass, mean number of medications was decreased (0.9 and 0.7, P<0.0001) with 56% and 48% off all medication. The most common AE were inflammation (6%) for CE + TM-bypass + CP group and VA loss (8%) for CE + TM-bypass.
Conclusion: At six months, a greater proportion of CE + TM-bypass + CP patients achieved IOP reduction of ≥20% and an IOP <18 mmHg on the same or fewer medications than for TM-bypass + CE.
Keywords: 360-degree viscodilation, canaloplasty, MIGS, glaucoma, VISCO360,® viscodilation
Introduction
Microinvasive glaucoma surgery (MIGS) has several advantages over traditional glaucoma surgery: an ab interno approach through a clear corneal incision that spares the conjunctiva; minimal trauma to the target ocular tissue; IOP-lowering efficacy that justifies the approach; a favorable safety profile that generally avoids serious complications, and rapid post-operative recovery.1,2 MIGS interventions also help to eliminate or reduce issues with pharmacologic treatment such as medication compliance/adherence, adverse effects on the ocular surface, inadequate IOP control, and the financial burden of prescription medications.3–7 Traditional surgical interventions, while generally more effective at lowering IOP, are also more invasive, carry a higher risk of complications, and a higher rate of secondary surgical interventions compared with MIGS.8
MIGS can be performed as stand-alone procedures and/or in combination with cataract extraction (CE) depending on the approved indications.9 As approximately one-fifth of cataract patients suffer from comorbid glaucoma, the combined procedure is often an efficient and effective surgical option.10 Canaloplasty (ab interno) and trabecular bypass implants are two distinct and potentially complimentary MIGS.11 Canaloplasty involves the circumferential viscodilation of Schlemm’s canal (SC)12 with the goal to reestablish circumferential flow by dilating the canal and providing the aqueous humor access to greater numbers of collector channels.13 VISCO360® (Sight Sciences, Inc., Melo Park, CA) is a canaloplasty device designed for the delivery of small, controlled volumes of viscoelastic fluid through a flexible injection tube for up to 360° of SC.14 It utilizes an ab interno approach and can be used as a stand-alone procedure or combined with cataract extraction (CE). This technique preserves SC and the trabecular meshwork (TM) for future surgical interventions if needed. The device is a single-handed instrument providing the surgeon with a simplified and controlled visco canaloplasty procedure. The procedure allows access to treat 3 points of aqueous outflow resistance (ie, TM, SC, and collector channels). Promising results have been observed with ab interno viscodilation in terms of both reductions in IOP and the use of ocular hypotensive medications.14 Viscodilation can also be combined with other MIGS interventions.
Trabecular meshwork (TM-bypass) microbypass involves using a stent implanted into SC. Devices of this class have shown favorable reductions in both IOP and in the use of ocular hypotensive medications.15–17 One limitation of this approach is that the microbypass stents do not allow access to the entire 360° of SC; ie, only collector channels proximal to the implant are accessed and if the stent is placed in a region where the canal has collapsed or prolapsed into the ostia of collectors, the stent may be ineffective.
A few studies have been performed that compare the efficacy and safety of ab-interno canaloplasty as a stand-alone procedure versus in combination with CE.14,18 However, there are no published studies comparing TM-bypass with and without ab interno canaloplasty when both are used adjunctively to phacoemulsification. Moreover, no study has examined the effect of viscodilation in conjunction with TM-bypass on ocular hypotensive drop burden and IOP. The objective of this study was to evaluate the post-operative outcomes of TM-bypass in combination with CE and of TM-bypass + ab interno canaloplasty using the VISCO360® surgical system in combination with CE in a consecutive series of patients with primary open-angle glaucoma (POAG).
Materials and methods
This was a retrospective consecutive case series of patients with POAG indicated for CE who received either TM-bypass (CE + TM-bypass) or TM-bypass with canaloplasty after CE (CE + TM-bypass + Canaloplasty) between January 1, 2017 and May 1, 2017. The study was conducted at 1 investigative site in the United States. The study received Institutional Review Board (IRB) approval from the Aspire IRB and was conducted in accordance with federal and state laws and regulations including 45 CFR 46, the HIPPA Privacy Rule and in accord with the Declaration of Helsinki. The IRB did not require acquisition of patients’ informed consent due to the retrospective nature of the study.
Patient characteristics
Enrollment included 186 eyes from 130 patients (63.0±8.0 years old, 58% male). Included patients had been followed for at least six months (through November 1, 2017).
Inclusion/exclusion
The study included patients with POAG who were treated with CE + TM-bypass or CE + TM-bypass + Canaloplasty with at least 6 months of follow-up. Potential cases were identified by querying billing records for surgeries with the CPT Code for Canaloplasty and/or Trabecular Meshwork Bypass. Exclusion criteria included closed angle glaucoma or other forms of glaucoma not POAG, ischemic retinal conditions and neuropathies (ie, AION, NAION, BRVO, BRAO), previous incisional glaucoma surgery, SLT within 6 months prior to the study procedure, or previous ALT.
Surgical devices
The VISCO360® Viscosurgical System, a minimally invasive, ab interno approach, was used for the canaloplasty procedures. VISCO360® is a single-handed, non-implantable device that integrates an access cannula, customized microcatheter (200 µm in diameter), control wheel, and viscoelastic infusion pump into a single unit (Figure 1A). The iStent® GTS100 device (Glaukos Corporation, San Clemente, CA) was used (Figure 1B) for the TM bypass procedures. The stent consists of a heparin-coated titanium stent supplied with an inserter.15
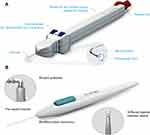 |
Figure 1 (A) VISCO360® viscosurgical system. Illustration of the device showing a handpiece with a microcatheter, control wheel for advancing and retracting the microcatheter, viscoelastic reservoir/infusion pump and a locking mechanism. Images are sourced from Sight Sciences, http://sightsciences.com and used with permission. (B) The iStent® GTS100 trabecular bypass system. Images are sourced from Glaukos Corp., https://www.glaukos.com and used with permission. |
Timepoints
Measurements and assessments were performed preoperatively and at months 1, 3, and 6. Patients continued their pre-operative IOP-lowering medication regimens through the 1-month post-op visit when adjustments (increase, reduce, stop) were made based on their IOP measurements.
Surgical procedures
Canaloplasty
Canaloplasty was performed after TM-bypass with both following CE. The VISCO360® cannula was introduced through the same 2.8 mm clear corneal incision used for phacoemulsification and the TM-bypass implantation. Under gonioscopic visualization, the system’s cannula was used to pierce the trabecular meshwork and enter Schlemm’s canal. The control wheel of the device was used to advance the microcatheter directly into and around the SC and facilitate the automatic delivery of a predetermined amount of viscoelastic fluid (Healon GV®, Johnson & Johnson Vision) to dilate 180° of SC in one direction. The device was then rotated 180° to access and viscodilate SC in the opposite direction.
TM-bypass
A single iStent device is implanted into the nasal aspect of SC. It creates a direct connection between SC and the anterior chamber through an opening in SC.15 The inserter provided with the device allows the stent to be implanted through a corneal incision into SC employing a sideways sliding technique. TM-bypass was performed after CE.
Post-operative treatment
Post-operative treatment consisted of topical tobramycin/dexamethasone qid for 1 week and tapered during the second week.
Endpoint assessment procedures
IOP was measured using Goldmann applanation tonometry. Visual acuity was measured using a Snellen chart at 20 feet. Adverse event assessment was performed at each visit by the principal investigator. Postop medical management and postop IOP were recorded. Demographic and baseline disease characteristics (age, gender, glaucoma type, procedure, IOP, number of IOP-lowering medications) were recorded for all patients. Fixed combination drops were counted as the number of component drugs.
The primary efficacy endpoint was the mean reduction in IOP from baseline at 6 months. The secondary endpoints were the proportion of eyes achieving treatment success defined as IOP reduction from baseline at 6 months of ≥20% and with an IOP <18 mmHg on the same or fewer IOP-lowering medications compared with the pre-operative values, the mean reduction in IOP-lowering medications from baseline, and the proportion of eyes achieving medication independence in each group. Safety endpoints included the rate of adverse events (intra-operative and post-operative) and re-interventions to treat glaucoma.
Statistics
Baseline and demographic characteristics were summarized by standard descriptive statistics (eg, means and standard deviations for continuous variables such as age, and percentages for categorical variables such as gender). Consistency in demographic make-up was evaluated using Student's t-tests (equal variance, two-sample, two-tailed) for continuous variables and Fisher’s exact test for categorical demographics.
Mean reduction in IOP (from pre-op to post-op) was determined for each timepoint. The change in mean number of medications pre-op to post-op was calculated for each timepoint as was the proportion of eyes achieving medication independence. Procedure success was defined as the percent of eyes that experienced a ≥20% IOP reduction and IOP <18 mmHg at 6 months with the same or fewer IOP-lower medications compared with the pre-operative values. Success rates were calculated at each follow-up timepoint. The rates of adverse events were calculated. Statistical tests (Student's t-tests and Z tests) were performed to evaluate if the study groups were statistically significantly different at each endpoint. Tukey’s adjustment to control the family-wise error rate was employed for all pairwise comparisons.
Sample size was based on the total number of eligible cases available with 6 months of postoperative follow-up and not on statistical power considerations.
Results
Eighty-six eyes were included in the CE + TM-bypass + Canaloplasty group and 100 eyes in the CE + TM-bypass group. There were 65 patients in each group and their baseline characteristics are shown in Table 1. There were no significant differences in demographic parameters. Where both eyes of a patient were included, in all instances the treatment group was the same for both eyes. Mean ages were 62.6 years for the CE + TM-bypass + Canaloplasty and 63.3 years for the CE + TM-bypass patients. Slightly more than half of the patients in each group were males. None of the eyes in the CE + TM-bypass + Canaloplasty group had undergone SLT while 7 (7%) of those in the CE + TM-bypass had undergone that procedure at baseline. This difference was not considered relevant as all laser procedures were at least six months prior to the study procedure (meeting the eligibility criteria) and the mean time since SLT was closer to 1 year (mean =11.1 months, Min 6 months, Max 19 months). However, baseline IOP for the SLT patients was lower on average than for the group as a whole (12.4 vs 15.3) and medication use at baseline was higher (2.0 vs 1.2). Analysis of the data with and without the seven SLT patients did not change the outcomes significantly.
 |
Table 1 Patient demographics and disease characteristics |
Mean IOP reduction at 6 months, the primary endpoint, was 2.9±3.6 mmHg in the CE + TM-bypass + Canaloplasty group and 1.7±3.1 mmHg for CE + TM-bypass group (Figure 2). The mean reductions in IOP at 1 and 3 months were 3.5 mmHg and 3.3 mmHg for CE + TM-bypass + Canaloplasty and 1.1 mmHg and 1.7 mmHg for CE + TM-bypass. Significantly greater reductions in mean pressure were achieved with Canaloplasty added to CE + TM-bypass at all three postop timepoints (all P-values <0.05).
Eyes receiving CE + TM-bypass + Canaloplasty had greater percent mean reductions in IOP at 1, 3, and 6 months compared with those receiving CE + TM-bypass alone (P<0.05; Figure 3). At 1 month, the percent mean reduction in IOP from the preop baseline was about 4-fold greater (19% versus 5%) for eyes receiving CE + TM-bypass + Canaloplasty compared with those receiving CE + TM-bypass alone. Both procedures produced significant IOP reductions from baseline that were maintained for 6 months (P≤0.007).
The proportion of eyes achieving treatment success (reductions of ≥20% and IOP <18 mmHg with the same or fewer numbers of medications than preop) for both cohorts is shown in Figure 4. Significant differences favoring CE + TM-bypass + Canaloplasty were observed at 1 month and 3 months (P<0.05). By 6 months, there were approximately 30% more treatment successes in the CE + TM-bypass + Canaloplasty group compared to CE + TM-bypass but the difference was no longer statistically significant.
The mean number of IOP-lowering medications at 3 and 6 months were significantly lower than at baseline for both treatment groups (Figure 5; P<0.0001). Eyes treated with CE + TM-bypass + Canaloplasty required on average about 1 medication less at 3 and 6 months than at preop baseline. The CE + TM-bypass eyes averaged about 0.5 and 0.7 medications less than baseline at 3 and 6 months. The difference between the two groups was significant at 3 months (P<0.001) but not at 6 months. Note that patients were kept on their preop IOP-lowering medication regimens until after their 1-month IOP assessments, so the number of medications was unchanged from baseline to 1 month.
Over half of the eyes in the CE + TM-bypass + Canaloplasty group were medication free 3 and 6 months postoperatively (48 of 86 or 56% at both timepoints); an increase from 14 (16%) eyes medication free preoperatively (Figure 6). While a higher proportion of eyes in the CE + TM bypass group were on zero medications at baseline (21%) a smaller proportion achieved this goal at 3 months (29 of 100 or 29%) and at 6 months (48 of 100 or 48% at 6 months). This difference was statistically significant at 3 months (P<0.001).
Few adverse events were observed with either group (Table 2) and all resolved within 3 months of the procedures (Table 2). The most common adverse events were inflammation (6%) for the CE + TM-bypass + Canaloplasty group and VA loss of >2 lines (8%) for CE + TM-bypass.
 |
Table 2 Adverse events |
Discussion
The results from this study demonstrate that the canaloplasty performed in combination with TM-bypass during phacoemulsification yields significantly better outcomes in lowering or in maintaining intraocular pressure and medication use compared with TM-bypass and phacoemulsification only. The mean baseline IOP-values were relatively low in both groups so the reductions in IOP were particularly noteworthy. Even though the mean preoperative IOP levels was slightly higher in the CE + TM-bypass + Canaloplasty group, eyes in this group achieved similar post-procedural mean IOP-values to those in the CE + TM-bypass.
A clinically meaningful IOP reduction is one that retards or prevents disease progression. AGIS demonstrated that there was a reduction in glaucoma progression for each 1 mmHg drop in IOP.19 In the current study, the added reduction from baseline in IOP in the CE + TM-bypass + Canaloplasty relative to CE + TM-bypass was observed to be clinically meaningful and statistically significant despite the relatively low mean baseline IOP (16.6 mmHg and 15.1 mmHg) for the groups. Mean reductions in IOP and percent reductions in IOP from baseline were greater in the CE + TM-bypass + Canaloplasty group compared with the CE + TM-bypass group (3.3 mmHg and 1.7 mmHg at 3 months, P<0.01; 2.9 mmHg and 1.7 mmHg at 6 months, P=0.019). These results indicate that adding an IOP-lowering procedure with a different mechanism of action, such as the dilation of Schlemm’s canal, can provide a clinically meaningful enhancement to cataract extraction and TM-bypass in reducing IOP.
Both procedures yielded significant reductions from baseline in the number of IOP-lowering medications needed after surgery. Although eyes in the CE + TM-bypass + Canaloplasty group had slightly higher needs for IOP-lowering medications at baseline (1.4 versus 1.2), they achieved a level of medication use as low or lower than that of their CE + TM-bypass counterparts. A substantial proportion of eyes were able to discontinue all IOP-lowering medications after both procedures. At month 3, a significantly lower proportion of eyes that received CE + TM-bypass + Canaloplasty were medication free compared with those receiving CE + TM-bypass alone with the trend continuing at 6 months.
Reduced reliance on IOP-lowering medications has potential benefits for glaucoma patients since pharmacologic treatment is associated with caveats such as poor medication compliance/adherence; adverse effects on the ocular surface; and heavy financial burden.3–7 Moreover, long-term ocular hypotensive use, and particularly multiple drops, has been implicated in higher failure rates for filtration surgery, should that be required in the future.20
Safety profiles with both procedures showed a low incidence of adverse events. The most common adverse events were inflammation for the CE + TM-bypass + Canaloplasty group (6%) and VA loss >2 lines for the CE + TM-bypass group (8%). These are common adverse events observed with phacoemulsification cataract surgery and are consistent with rates observed for the phaco only control groups of large randomized MIGS trials.17,21 All adverse events spontaneously resolved within 3 months after the procedures. The low incidence of adverse events with CE + TM-bypass + Canaloplasty suggests a favorable safety profile when canaloplasty is added to other IOP-lowering procedures. No eyes in either group required secondary surgical intervention.
While generally producing clinically significant reductions in IOP, TM-bypass alone may not adequately control IOP in some instances. One review of 3 RCTs and seven case series (including single and multiple iStent implants with and without phacoemulsification) found IOP reductions ranged between 16% and 33%.22 As with any procedure, some patients show a better IOP-lowering response than others. This response depends on factors such as stent placement, downstream collector channel patency, and episcleral venous pressure.23,24 In addition, the technique for finding the collector channels and determining their patency can be elusive. These factors affect the ability of the stent to promote aqueous humor outflow and produce an IOP reduction.
An intracanalicular scaffold (Hydrus, Ivantis, Inc.) has a somewhat similar mechanism of action combining some of the benefits of canaloplasty, ie dilation of the canal and aqueous access to more collector channels albeit for 90° rather than 360°, with trabecular bypass like the iStent.25 Canaloplasty, in contrast, dilates the full circumference of Schlemm’s canal accessing all patent collector channels, and moreover is thought to also dilate and allow flow through the distal outflow pathway as well.26 Although no direct comparisons are available, six-month results in a combined with phacoemulsification procedure are similar to those of the present study and in a head to head comparison as a standalone procedure, ab externo canaloplasty demonstrated better medication reduction.27,28
In the current study, IOP was reduced for up to 6 months in both treatment groups. Maintenance of IOP reduction is particularly important for glaucoma patients at risk for disease progression and vision loss.19 These patients typically require early and/or additional surgical intervention beyond topical meds to manage their elevated IOP. Ab interno canaloplasty is not only minimally invasive but one of the least destructive procedures to the anatomy of meshwork and Schlemm’s canal sparing the TM, internally, and the conjunctiva, externally, should additional procedures be needed.29 Outcomes for IOP and medication reduction with canaloplasty are comparable to those of MIGS implants but with the advantage of an implantless procedure. Like many MIGS, canaloplasty benefits from a favorable safety profile. The most frequent complications associated with the procedure noted in current literature are hyphema or microhyphema, cataract formation, IOP spikes, and hypotony, although with the exception of a single case of hyphema, none were observed in this study.
The current study was limited by its retrospective design, which carries the risks of selection bias, incomplete data, and variability in assessment methodology. However the risk of selection bias was mitigated by including all patients meeting the minimal inclusion and exclusion criteria within a pre-defined time period. While a single-center could be considered a limitation, this, along with the procedures being performed by a single surgeon, limited the potential variability in methodology. Follow-up was limited to 6-months however this ensured a complete data set for analysis and does not preclude additional follow-up of these cohorts as data becomes available. The strength of a retrospective, observational study is that the data is derived from real patients in a “real world” setting rather than under the artificial structure of a prospective clinical trial. The generalizability gained by this design should be weighed against the inherent weaknesses. The sample size was not determined based on statistical power calculations but was instead the total of all available and eligible cases. The study should thus be considered hypothesis generating rather than hypothesis testing. However, the sample was adequate to demonstrate statistically significant differences between the two groups and certainly informs the design of a larger prospective trial. Future research may compare the safety and efficacy of these two procedures, both alone and combined with phacoemulsification, in a prospective randomized manner and with longer (12–24 month) follow-up. The eye was considered the unit of analysis and in this study; 56 of the 130 patients contributed both eyes to the analysis. This is a potential confounder due to the lack of independence for these bilateral cases. However, the observed differences were generally sufficiently robust to overcome this weakness. Nevertheless, this limitation should be considered in the interpretation of results. Despite these limitations, this is the first report showing the additional benefit provided by viscocanaloplasty used adjunctively with a trabecular bypass stent in patients with POAG.
Conclusion
These six-month follow-up results in patients with POAG demonstrate that both canaloplasty performed in combination with TM-bypass during phacoemulsification and TM-bypass only with phacoemulsification allow significant and meaningful medication reductions while greater percent reductions in IOP are achieved with canaloplasty performed in combination with TM-bypass during phacoemulsification compared with TM-bypass alone with phacoemulsification. A greater proportion of CE + TM-bypass + CP patients achieved treatment success (IOP reduction of ≥20% and an IOP <18 mmHg on the same or fewer medications) than for TM-bypass + CE.
Acknowledgments
We are grateful to Julie Crider, PhD (Collaborative Medical Writing, LLC) for medical writing services that included discussing the context, background, and importance of the work with the authors and preparing a draft manuscript based on this discussion. The original draft has been revised extensively. We thank Kavita Dhamdhere, MD PhD and Jaime E. Dickerson, PhD for assistance in statistical analysis and preparing the final draft manuscript. The manufacturer of the VISCO360® device, Sight Sciences, provided financial assistance to support writing, editorial, and statistical analysis.
Disclosure
Mr Marius Heersink reports non-financial support from Sight Sciences, Inc., during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Pillunat LE, Erb C, Junemann AG, Kimmich F. Micro-invasive glaucoma surgery (MIGS): a review of surgical procedures using stents. Clin Ophthalmol. 2017;11:1583–1600. doi:10.2147/OPTH.S135316
2. Saheb H, Ahmed II. Micro-invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol. 2012;23:96–104. doi:10.1097/ICU.0b013e32834ff1e7
3. Feehan M, Munger MA, Cooper DK, et al. Adherence to glaucoma medications over 12 months in two US community pharmacy chains. J Clin Med. 2016;5. doi:10.3390/jcm5090079
4. Kashiwagi K, Matsubara M. Reduction in ocular hypotensive eyedrops by ab interno trabeculotomy improves not only ocular surface condition but also quality of vision. J Ophthalmol. 2018;2018:8165476. doi:10.1155/2018/8165476
5. Baudouin C, Labbe A, Liang H, et al. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29:312–334. doi:10.1016/j.preteyeres.2010.03.001
6. Rosin LM, Bell NP. Preservative toxicity in glaucoma medication: clinical evaluation of benzalkonium chloride-free 0.5% timolol eye drops. Clin Ophthalmol. 2013;7:2131–2135. doi:10.2147/OPTH.S41358
7. Prevent Blindness. Glaucoma costs reach $5.8 billion annually. December 16, 2013. Available from: https://www.preventblindness.org/glaucoma-costs-reach-5-point-8-billion-annually.
8. Richter GM, Coleman AL. Minimally invasive glaucoma surgery: current status and future prospects. Clin Ophthalmol. 2016;10:189–206. doi:10.2147/OPTH.S80490
9. Caprioli J, Kim JH, Friedman DS, et al. Special commentary: supporting innovation for safe and effective minimally invasive glaucoma surgery. Ophthalmology. 2015;122(9):1795–1801. doi:10.1016/j.ophtha.2015.02.029
10. Ianchulev T, Litoff D, Ellinger D, Stiverson K, Packer M. Office-based cataract surgery: population health outcomes study of more than 21,000 cases in the United States. Ophthalmology. 2016;123:723–728. doi:10.1016/j.ophtha.2015.12.020
11. Vinod K, Gedde SJ. Clinical investigation of new glaucoma procedures. Curr Opin Ophthalmol. 2017;28:187–193. doi:10.1097/ICU.0000000000000336
12. Koerber N. Canaloplasty-A new approach to nonpenetrating glaucoma surgery. Tech Ophthalmol. 2007;5:102–106. doi:10.1097/ITO.0b013e3181565083
13. Francis BA, Singh K, Lin SC, et al. Novel glaucoma procedures: a report by the American Academy of Ophthalmology. Ophthalmology. 2011;118:1466–1480. doi:10.1016/j.ophtha.2011.03.028
14. Ondrejka S, Körber N. 360°ab-interno Schlemm’s canal viscodilation in primary open-angle glaucoma. Clin Ophthalmol. 2019;13:1235–1246. doi:10.2147/OPTH.S203917
15. Craven ER, Katz LJ, Wells JM, Giamporcaro JE; iStent Study Group. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: two-year follow-up. J Cataract Refract Surg. 2012;38:1339–1345. doi:10.1016/j.jcrs.2012.03.025
16. Samuelson TW, Chang DF, Marquis R, et al. A Schlemm canal microstent for intraocular pressure reduction in primary open-angle glaucoma and cataract. The HORIZON study. Ophthalmology. 2019;126:29–37. doi:10.1016/j.ophtha.2018.05.012
17. Samuelson TW, Sarkisian SR
18. Gallardo MJ, Supnet RA, Ahmed IIK. Circumferential viscodilation of Schlemm’s canal for open-angle glaucoma: ab-interno vs ab-externo canaloplasty with tensioning suture. Clin Ophthalmol. 2018;12:2493–2498. doi:10.2147/OPTH.S178962
19. The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130(4):429–440. doi:10.1016/s0002-9394(00)00538-9
20. Broadway DC, Grierson I, O’Brien C, Hitchings RA. Adverse effects of topical antiglaucoma medication. II. The outcome of filtration surgery. Arch Ophthalmol. 1994;112:1446–1454. doi:10.1001/archopht.1994.01090230060021
21. Vold S, Ahmed IIK, Craven ER, et al. Two-year COMPASS trial results: supraciliary microstenting with phacoemulsification in patients with open-angle glaucoma and cataracts. Ophthalmology. 2016;123:2103–2112. doi:10.1016/j.ophtha.2016.06.032
22. Le K, Saheb H. iStent trabecular micro-bypass stent for open-angle glaucoma. Clin Ophthalmol. 2014;8:1937–1945. doi:10.2147/OPTH.S45920
23. Craven ER. Trabecular micro-bypass shunt (iStent): basic science, clinical, and future. Middle East Afr J Ophthalmol. 2015;22(1):30–37. doi:10.4103/0974-9233.148346
24. Fernandez-Barrientos Y, Garcia-Feijoo J, Martinez-de-la-Casa JM, Pablo LE, Fernandez-Perez C, Garcia-Sanchez J. Fluorophotometric study of the effect of the Glaukos trabecular microbypass stent on aqueous humor dynamics. Invest Ophthalmol Vis Sci. 2010;51(7):3327–3332. doi:10.1167/iovs.09-3972
25. Camras LJ, Yuan F, Fan S, et al. A novel Schlemm’s canal scaffold increases outflow facility in a human anterior segment perfusion model. Invest Ophthalmol Vis Sci. 2012;53:6115–6121. doi:10.1167/iovs.12-9570
26. Körber N. Ab interno canaloplasty for the treatment of glaucoma: a case series study. Spektrum Augenheilkd. 2018;32:223–227. doi:10.1007/s00717-018-0416-7
27. Fea AM, Rekas M, Au L. Evaluation of a Schlemm canal scaffold microstent combined with phacoemulsification in routine clinical practice: two-year multicenter study. J Cataract Refract Surg. 2017;43:886–891. doi:10.1016/j.jcrs.2017.04.039
28. Gandolfi SA, Ungaro N, Ghirardini S, Tardini MG, Mora P. Comparison of surgical outcomes between canaloplasty and Schlemm’s canal scaffold at 24 Months’ follow-up. J Ophthalmol. 2016;2016:2410469. doi:10.1155/2016/3410469
29. Khaimi MA. Canaloplasty: a minimally invasive and maximally effective glaucoma treatment. J Ophthalmol. 2015;2015:485065. doi:10.1155/2015/485065
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





