Back to Journals » International Journal of Nanomedicine » Volume 14
A perspective on magnetic core–shell carriers for responsive and targeted drug delivery systems
Authors Albinali KE , Zagho MM, Deng Y, Elzatahry AA
Received 9 November 2018
Accepted for publication 15 January 2019
Published 6 March 2019 Volume 2019:14 Pages 1707—1723
DOI https://doi.org/10.2147/IJN.S193981
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Kholoud E Albinali,1 Moustafa M Zagho,1 Yonghui Deng,2 Ahmed A Elzatahry1
1Materials Science and Technology Program, College of Arts and Sciences, Qatar University, Doha, Qatar; 2Department of Chemistry, State Key Laboratory of Molecular Engineering of Polymers, Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, iChEM, Fudan University, Shanghai, People’s Republic of China
Abstract: Magnetic core–shell nanocarriers have been attracting growing interest owing to their physicochemical and structural properties. The main principles of magnetic nanoparticles (MNPs) are localized treatment and stability under the effect of external magnetic fields. Furthermore, these MNPs can be coated or functionalized to gain a responsive property to a specific trigger, such as pH, heat, or even enzymes. Current investigations have been focused on the employment of this concept in cancer therapies. The evaluation of magnetic core–shell materials includes their magnetization properties, toxicity, and efficacy in drug uptake and release. This review discusses some categories of magnetic core–shell drug carriers based on Fe2O3 and Fe3O4 as the core, and different shells such as poly(lactic-co-glycolic acid), poly(vinylpyrrolidone), chitosan, silica, calcium silicate, metal, and lipids. In addition, the review addresses their recent potential applications for cancer treatment.
Keywords: magnetic, mesoporous, core–shell, drug carriers, active targeting, passive targeting, controlled release, targeted cancer treatment
Introduction
Drug delivery technology has witnessed significant developments over the last four decades. Scientists have focused their efforts on developing a new class of highly efficient carrier materials that are capable of meeting the vital demands of the health care industry. The concept of drug delivery entails the transfer of a specific dose of different therapeutic agents such as synthetic or natural drugs, genes, and proteins to the desired site in the body within a predetermined time using a specific formula or different devices.1–6 The concentration of the medicinal formula should lie between minimal toxic concentrations and the minimal effective concentration. Moreover, drug carriers enhance the pharmacokinetic effect, protect the medicinal agent from degradation via enzymes, and carry lipophilic and hydrophilic drugs to meet the intended usage of the system.7 Targeted drug delivery is an approach to deliver the therapeutic agents to an intended organ or tissue to increase the efficacy and reduce toxicity.7
Two essential requirements must be fulfilled to have a successful drug delivery system. First, the system must have a minimal loss of activity and dose in the blood circulation system. Second, the therapeutic formula should act only on the desired tissues without harming other healthy cells.8 There are four routes to control drug release: erosion, diffusion, swelling,9,10 and by using external stimulation to initiate drug release.11,12 Drug delivery systems based on diffusion mechanisms are driven and controlled via a concentration gradient.13,14 Water swelling leads to improved drug mobility by offering larger pores and enhanced polymer flexibility. The drug release in this system depends on diffusion and dissolution mechanisms.15,16 Drug delivery systems depending on erosion have gained much attention especially with the evolution of biodegradable polymers. Using this approach, a physical, chemical, or material loss is used to regulate drug delivery.17–19 Finally, drug release can be controlled by chemical composition, pH value, and temperature.20 In this review, we will focus on the fourth class of drug delivery, which is mainly linked to the effect of an external factor and connected to the specific type of carriers, namely magnetic core–shell drug carriers. Here, some questions arise including:
- Are magnetic core–shell carriers considered promising drug carriers in drug delivery systems?
- Is using a drug carrier better than conventional drugs?
Drug carriers and drug targeting
During the 20th century, Paul Ehrlich introduced the idea of drug targeting. In the 1960s, Peter Paul synthesized the first nanoparticles (NPs) for drug targeting.21,22 In 1963, the use of magnetic nanocarriers was introduced. Meyers et al used an externally applied magnet to compile small iron particles to be injected into the leg veins of dogs.23,24 Several years later, specifically in the late 1970s, Widder et al23,25 declared the synthesis of magnetic microspheres using magnetite particles and albumin. These microspheres were enclosed with Adriamycin as an anticancer drug. They were magnetically directed to the tumor cells.23,25 Then, the researchers continued their trials to fabricate, modify, and coat these magnetic nanoparticles (MNPs) until 1996 when Lübbe et al declared for the first time the use of MNPs coated with anhydroglucose polymers and loaded with epirubicin. These MNPs were tested in a clinical trial on a group of patients with advanced cancers.23,26 The progressive approaches in the improvement of magnetic nanocarriers since these advancements have been impressive. They represent promising vehicles in drug delivery, especially in the treatment of tumors.
Drug nanocarriers
Nanocarriers are defined as small entities with size <500 nm.27,28 There are many types of nanocarriers such as polymers, micelles,29–38 liposomes,39 dendrimers,40–50 gold,51 carbon nanotubes,52–56 silicon, and iron oxide.57 They have been developed and employed as carriers for drugs or vehicles for the controlled release of drugs, especially for anticancer medicines.58–67 NPs are known as prospective and profitable drug carriers over conventional drugs for cancer therapy due to their promising characteristics such as the ability to be functionalized with drugs, increased therapeutic efficacy, enhanced drug stability, and capability to entrap lipophilic, hydrophilic, lipophobic, and hydrophobic drugs.7,68–71
Loading and release behavior is an essential parameter. A study conducted by Lian et al described the potential of using mesoporous silica thin films as an efficient drug carrier.72 The effect of loading methods, mesostructure, and morphologies were studied on two types of mesoporous silica thin films (three-dimensional hexagonal structure and two-dimensional hexagonal structure). Three loading methods were used to load fluorescein isothiocyanate molecules into the thin films. The loading methods were cleavable binding, physical adsorption, and entrapment. The results revealed that these mesoporous silica thin films exhibited a dissolution-controlled release contrary to the release behavior of mesoporous powders, which was a diffusion-controlled release. Furthermore, these mesoporous silica films can load an extra amount of guest molecules due to their high external surfaces.72
Drug targeting
Drug targeting can be achieved via two strategies: active targeting and passive targeting.7,73 Owing to some limitations of passive targeting, active targeting outperforms passive targeting.7
Active targeting
Active targeting entails the binding of a ligand such as antipodes, peptides, and vitamins to the surface of nanocarriers. The function of these ligands is to attach an individual receptor on the surface of the cell, via receptor-mediated endocytosis mechanism (RME).27 This RME mechanism consists of three steps. First, the ligand attaches to a suitable receptor on the cell, subsequently resulting in the formation of endosomes. Endosomes are compartments of a plasma membrane, which include the receptor and ligand complex. Finally, the endosomes transfer to the desired site, and the drug is released under the influence of pH difference or enzymes.7 Figure 1 describes the specific ligand-mediated active targeting.74
  | Figure 1 Illustration of a ligand-mediated active targeting. |
Passive targeting
Passive targeting exploits natural conditions of the target tissue or organ to direct the drug to the desired position. This targeting exploits the physiological nature of tumor cells. The tumors have leaky vessels, and these vessels have a high number of pores with a size of 100–800 nm. The significant number of pores leads to an extended vasculature because the gap junction between endothelial cells expands.7 Furthermore, tumor cells have a deficient lymphatic drainage. Consequently, drug carriers and therapeutic agents accumulate in the tumor cells.75
Figure 2 compares passive and active targeting. Passive targeting depends mainly on the leaky tumor vessels, while active targeting depends on the presence of ligands on the nanocarrier surface.75
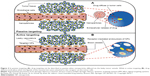  | Figure 2 In passive targeting (A), drug targeting can be described as passive when nanoparticles diffused via the leaky tumor vessels. While in active targeting (B), drug delivery took place when the ligands of the nanocarriers were attached to the receptor on the tumor cells. |
Fundamental features of targeted drug delivery systems
To achieve the anticipated impact of a drug, the drug should be in physical contact with its target and maintain contact with the targeted area for an adequate period of time. Not all drugs are suitable for drug delivery systems. For instance, some drugs exhibit a high specificity for their targets such as therapeutic antibodies. Other drugs have the same sites for toxicity. Hence, administering these drugs in a drug delivery system may induce either therapeutic or toxic effects. Furthermore, some drugs do not remain in the targeted sites long enough to cause the desired therapeutic action. The desired drug delivery system should exhibit fundamental features, which are briefly summarized in Table 1.76
  | Table 1 Fundamental characteristics of a targeted drug delivery system |
Magnetic drug delivery
The concept of magnetic drug delivery is to apply an external magnetic field to drive a drug carrier with a magnetic property to an intended site in the body. Among the different classes of MNPs, magnetite (Fe3O4) and maghemite (Ɣ-Fe20377) are the most used magnetic materials, especially in biomedical applications. They have inverse spinel crystal structures and physical properties.78 MNPs are characterized by magnetization (M), saturation magnetization (Ms), and coercivity (Hc). Ms is the magnetization value of an NP when it is subjected to an increased magnetic field. Also, Hc is the value of the outer magnetic field required to reduce the magnetic field of an NP down to zero. This parameter should be taken into consideration, mainly in biomedical applications where no residual magnetization is critical in hindering their coagulation and sustaining a long time of circulation.79 There are many reports that have discussed the recycling of MNPs.80 Other DDS studies illustrated that MNPs can be recycled at least 15 times without losing their potency.81
Potential of magnetic hyperthermia of magnetic drug delivery systems
Hyperthermia is a method of treating cancer which involves the application of excessive heat to kill tumor cells. The term “hyperthermia” is derived from Greek etymology and consists of two parts: “hyper” which literally translates to “rise” and “thermia” which literally translates to “heat.”82 Hyperthermia is a procedure carried out by locally generating and raising the temperature of tumor cells to a particular range, usually between 41°C and 46°C for a period of time (20–60 minutes) to kill tumor cells.83,84 Unlike other methods, hyperthermia is administered specifically with the goal of eliminating tumor cells while rendering minimal damage to other normal healthy cells. It is a very effective method and has outperformed other conventional procedures in cancer treatment. There are some drawbacks and challenges that must be addressed and overcome in order to achieve optimal therapy. There are two main challenges rendered by the hyperthermia approach to treating cancer: overheating of the tumor region and heterogeneity of the temperature distribution in the tumor mass.85
Using MNPs in hyperthermia (magnetic hyperthermia) will reduce the side effects of hyperthermia, as these MNPs can be injected into the tumor directly.85 The following studies represent the recent trends of synthesizing MNPs to be used as magnetic hyperthermia tools. Dias et al fabricated vortex iron oxide particles (VIPs) to be used in magnetic hyperthermia.86 Three types of VIPs were synthesized with deferent aspect ratios (VIP1, VIP3, and VIP6). Furthermore, the toxicity was also evaluated on human embryonic kidney cells (HEK293). Different concentrations of these MNPs were examined for 24 and 48 hours, respectively. HEK293 cells were treated with 100 μg/mL of these MNPs, followed by incubation for 24 hours. Following incubation, the cells were exposed to a magnetic field. Finally, the cells were stained using propidium iodide, and cell death was measured using flow cytometry. All MNPs induced cell death and the results were found to be exposure time-dependent and ranged from 10% to 65%.86
Magnetic nanocarriers: importance of coating and functionalization
MNPs are considered to be promising materials for biomedical applications. Coating them with biodegradable and biocompatible materials is essential to reducing their potential toxicity and protecting their magnetic core from corrosion.77,87 Moreover, biodegradable polymers can release the absorbed drugs at a rate determined by their degradation.87,88
Some studies illustrated the coating influence on the magnetization properties and it was claimed that surface disorder can control the magnetization of MNPs. It was observed through these studies that an inverse relationship exists between surface disorder and magnetization wherein the less the surface disorder, the more the magnetization.89 Other studies suggested a reduction in particle size to obtain higher magnetization.90
Magnetic core–shell nanocarriers
To consider a material as an efficient drug carrier, it should contain unoccupied sites to act as a reservoir to carry the drug as well as to enhance drug loading.91 Researchers have recently focused their efforts on developing efficient drug delivery materials that improve the cell internalization of medicines and reduce cytotoxicity.92,93 Moreover, a magnetic drug carrier should have individual properties, such as magnetization, low toxicity, and proper drug uptake and release.
Despite the available synthesis techniques of MNPs, the resulting MNPs may have insufficient magnetization especially when targeting some deep tissues in the body.94 Hence, a new strategy must be integrated to obtain better results. In addition to the magnetic core–shell spheres, other anisotropic shapes of MNPs can be synthesized such as nanorods, nanotubes, nanodiscs, nanoworms, and nanochains.94 These materials display better magnetic responsiveness and higher magnetic moment when compared to the magnetic core–shell spheres. Some researchers discussed the benefits of using alternating magnetic fields in drug delivery and magnetic hyperthermia, with a combination of MNPs.95 It was claimed that using AMF resulted in localized and deeper penetration in tumor cells. Furthermore, it can be beneficial in triggering drug release from encapsulated MNPs.95
In this context, this review addresses different types of magnetic core–shell drug carriers such as magnetic@polymer, magnetic@mesoporous silica, magnetic@calcium silicate (CS), magnetic@metal, and magnetic@liposomes core–shell materials. Figure 3 depicts an outline of different types of shells to encapsulate the MNPs for drug carriers, which will be discussed in the following sections.
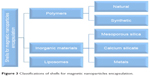  | Figure 3 Classifications of shells for magnetic nanoparticles encapsulation. |
The adsorption of drug molecules on the drug carrier represents an important parameter, and the physicochemical stability of the therapeutic agent is enhanced by this phenomenon.96 Many studies have been conducted to evaluate the adsorption behavior of drugs onto drug carriers and magnetic drug carriers. Previous studies investigated the adsorption–desorption behavior of many drugs such as methotrexate (MTX), doxorubicin (DOX), camptothecin, mitomycin C, and verapamil onto magnetically targeted carriers.96 The results illustrated that these drugs demonstrated a different behavior, which can be described as nonlinear behavior.96 For DOX administered to patients with hepatomas and metastases to the liver, for both intravenous and intra-arterial administrations (30 mg/m2), it was found that DOX plasma concentrations quickly decreased to the 100 ng/mL range.97
In vivo evaluation of magnetic core–shell nanocarriers
In vivo tests represent an important step in the evaluation process of drug carriers as it provides a closer perspective on how the drug carrier impacts the human body and organs. In vivo tests begin with the selection of a suitable animal model.98 The most widely used animal models are swine, daphnia magna, zebrafish, albino star rat strains, albino mouse strains, and chick embryos.99
In vivo tests were conducted on different types of MNPs. To illustrate, Fe3O4 MNPs efficacy was evaluated by treating nude mice suffering from blood cancer (leukemia cells K562) with daunorubicin-Fe3O4 MNPs.100 It was realized that these MNPs induced apoptosis and reduced the tumor growth. In addition, Albino rats suffering from sarcoma S-180 cells were injected with DOX-Fe3O4-citric acid-chitosan composites encapsulated with poly(lactic-co-glycolic acid) (PLGA).100 It was clearly observed that these MNPs were accumulated in the tumor cells, and the cancer cells size was reduced. Moreover, Kunming mice were injected with MPEG-PLGA loaded with evodiamine, and the tumor suppression was realized to be 50%.100 Recently, Li et al synthesized magnetic mesoporous silica core–shell nanocarriers via the sol-gel method.101 These MNPs were functionalized with matrix metalloproteinase (MMP)-2 enzyme responsive peptide. The in vivo results illustrated that accumulation and cell uptake of these MNPs in tumor cells were enhanced when an external magnetic field was applied. Furthermore, magnetic resonance imaging (MRI) test revealed the ability of these MNPs in the suppression of cancer cells growth.101
Polymer core–shell drug carriers
Polymer-based materials can be used in a wide range of applications.102–108 It is worth noting that the most remarkable known applications of polymer composites are the biomedical ones, including tissue engineering and wound healing.109–111 In addition, polymer-based nanoagents are the most reported organic-based photothermal carriers for cancer therapy.112 Due to their exceptional characteristics such as high energy density, low cost, structural diversity, and design flexibility, the conducting polymer matrices have been widely applied as supercapacitors.113
Natural and synthetic polymers
PLGA was utilized in biomedical applications for an extended period, and many records and studies have been conducted to assure its efficacy and safety.114–120 Furthermore, PLGA was approved by the European Medicine Agency121 and US Food and Drug Administration.87,122,123 It is widely used in drug delivery systems due to its desirable properties such as biodegradability and biocompatibility.87,121,124 There are many previous reports that have used PLGA to coat the magnetic particles for magnetic drug delivery systems. Because of the high magnetization values of MNPs, they tend to accumulate. To use MNPs in drug delivery systems, agglomeration can be prevented by inducing steric repulsion between the MNPs. As a result, the particles will be more stabilized and have less tendency to agglomerate.87,125 Many studies discussed the use of oleic acid in fabricating MNPs in order to enhance their stability.126 Furthermore, encapsulation of magnetite with PLGA enhanced the magnetite encapsulation efficacy to 60%.127 As an illustration of this approach, DOX-PLGA-MNPs were fabricated by Tansık et al,87 who reported the use of Fe3O4 as the core, and it was then coated with oleic acid and finally encapsulated with PLGA. They displayed a uniform spherical core–shell morphology with a diameter of 65 nm. Coating with oleic acid improves particle stability by preventing them from agglomeration as a result of their high magnetization. Due to their superparamagnetic properties, these MNPs could be targeted to a specific tissue using an external magnetic source. The drug release test showed a sustained release behavior of DOX, and 65% of the drug was released after 35 days at a pH =7.4. Furthermore, XTT cell proliferation assay was conducted on MCF-7 breast cancer cells using PLGA-MNPs before and after DOX loading. The cells were seeded at a concentration of 104, in a 96-well plate. In the XTT cell proliferation assay, an XTT-based colorimetric assay kit (2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5 carboxanilide) was used. The unloaded particles offered good biocompatibility even at a high dose of 250 μg/mL. They demonstrated significant toxicity at 500 μg/mL, and the DOX-loaded PLGA-MNPs displayed significant cell death. This study also reported that the superparamagnetic and biocompatibility characteristics of PLGA-MNPs allowed them to be applied in drug delivery systems. In contrast, the magnetic entrapment efficacy was decreased with increasing the content of oleic acid.87
Moreover, PLGA-MNPs have been reported to be utilized in aerosols to treat many lung diseases such as lung cancer, respiratory infections, chronic obstructive pulmonary disease, and asthma.128–131 Many aerosol chemotherapy drugs were evaluated in human and animal models as well as in vitro to investigate their efficiency in lung cancer treatment.130–134 A case in point, Verma et al declared the synthesis of MNPs loaded with flavonoid quercetin drug as inhalers and nebulizers for lung diseases.135 The MNPs were coated with PLGA for better biocompatibility, dispersion in the aquatic medium, and stability toward oxidation.136,137 This study lacks the desired specificity toward cancer cells, which is required to reduce the side effects. Thus, more specificity in drug release is required and can be obtained via surface modification or functionalization, which will be discussed in the upcoming studies.
To reduce the side effects of the drugs, advanced techniques and different materials are incorporated to restrict the drug release solely to the intended tissues. For example, a pH-responsive drug carrier to a trigger gains an advantage from the nature of the targeted tissues or cells. To get the optimum efficacy from the drug delivery system, it is necessary to control the drug release rate from the carriers. Chitosan is a low toxic, biocompatible, and biodegradable material, and it is widely used to encapsulate or functionalize the surface of the drug nanocarriers to enhance their pH-responsive drug release.124,138–147 It is soluble in the acidic medium; thus, it can induce pH sensitivity to control the drug release according to pH change.148 Moreover, it is used in the coating of MNPs to enhance their biodegradation.124 Montha et al122 declared the assembly of pH-responsive drug carriers, where chitosan has been chosen to stabilize Mn0.9Zn0.1Fe2O4/PLGA MNPs.122 In addition, DOX was selected to evaluate the efficacy of these MNPs. It was clarified that these chitosan-MNPs exhibited a core–shell morphology with a uniform size of about 25 nm and superparamagnetic properties. MTT assay was conducted on Hela cells using DOX and DOX-MNPs. Cell viability decreased progressively with increasing doses.
Nanoscale metal-organic frameworks (NMOFs) represent a new category of porous materials as they drag much attention in many fields, especially in drug delivery approaches owing to their promising properties such as large surface area, ultrahigh porosity, and tunable functionality.149–151 They can be prepared through highly efficient and more straightforward synthetic methods, especially when compared to other porous materials such as silica. Recent years have witnessed numerous studies aimed at improving the usefulness of these magnetic NMOFs in drug delivery systems.152–154 Some of these concepts aimed to deliver chemotherapeutics, but they showed weak encapsulation efficacy and fast drug release. Thus, an alternative approach is necessary to achieve enhanced efficiency. Many studies discuss the modification of NMOFs using polymers. For instance, Ren et al developed polyacrylic acid@zeolitic imidazolate framework-8 (PAA@ZIF-8) NPs as pH-responsive carriers.155 These PAA@ZIF-8 NPs exhibited an ultrahigh drug loading ability and pH-responsive drug release. The drug release tests were conducted on both neutral (pH =7.4) and acidic (pH =5.5) mediums and showed that these NPs possessed a pH-controlled drug release. The release at pH =5.5 was about 75.9% of DOX after 60 hours, while it was only 35.6% after 60 hours. Moreover, they noted a nontoxic effect to MCF-7 cells even at high concentrations.
In addition, Chowdhuri et al156 coated magnetic isoreticular metal-organic framework-3 with chitosan to achieve better degradability, enhanced drug loading, improved drug release, and pH-triggered release.156 Furthermore, the particles were functionalized with folic acid (FA) ligands to obtain more specificity owing to the presence of folate receptors in cancer cells compared to healthy cells. These MNPs were tested on Hela cancer cells, and the findings revealed that these MNPs exhibited a high encapsulation efficacy of DOX of about 96% and a pH-dependent DOX drug release.
The above studies revealed some promising findings and were considered reasonable, but more studies and more strategies must be incorporated to not only have responsive drug carriers but also provide carriers with the ability to localize drug administration to the intended cells only so as to minimize any adverse effects on the healthy cells, and to overcome the disadvantages of using conventional chemotherapy approaches. Furthermore, reducing the loss of the drug encapsulated in these MNPs represents a challenge. This can be addressed by introducing measures to increase their stability so as to minimize the amount of drug that escapes from the drug carriers inside the human body.157,158
Remarkably, the pH-responsive effect can also be achieved by using different combinations.122,123,159 Also, new strategies and functionalization methods are employed to achieve more specific targeting techniques and to have the ability to select only the intended cells. For example, Vivek et al developed a targeted breast cancer therapy using multifunctional Herceptin (Her)-Fe3O4@PLGA-poly(vinylpyrrolidone) (PVP) particles encapsulated with tamoxifen.123 Figure 4 describes the effective nanocombinations therapy. These particles offered superparamagnetic properties and high stability, which may be attributed to the presence of PVP. The drug entrapment efficacy reached about 87.5%. The packing capacity of tamoxifen into Her-Fe3O4@PLGA-PVP particles was 7% as a result of firm interaction between tamoxifen and PLGA and of the high surface area of the PLGA.158,160 Moreover, 96% of tamoxifen was released at pH =5, while only 4% was released at pH =7.4. On the other hand, MTT assay in vitro showed that the cell (MCF-7) viability was reduced from 88% to 31%. In addition, Her-Fe3O4@PLGA-PVP-loaded tamoxifen particles showed remarkable toxicity against cancer cells.
  | Figure 4 Schematic illustration describing the effective nanocombinations therapy. |
Inorganic core–shell drug carriers
The continuous discovery of new release trigger agents represent a step forward and open the door to utilize many drugs or to use one drug in multiple targeting aspects, and this leads to more selective and remarkable progress.
MNPs@mesoporous silica core–shell drug carriers
Mesoporous silica is a new carrier for different drugs with remarkable properties such as biocompatibility, high loading capacity due to its high specific surface area and high pore volume, and tunable pore size.161–165 Core–shell nanocomposites with mesoporous silica have attracted growing interest in recent years.166 Combining them with a magnetic core leads to benefits including magnetic cores to control these carriers and mesoporous silica to build such efficient vehicles.
Using mesoporous silica-based carriers is one of the exciting concepts, where specific materials are designed to be attached on the surface of the mesoporous silica to seal the entrances to the pores to assure minimum leakage of the therapeutic agents. A case in point, Yang et al167 reported the synthesis of mesoporous Fe3O4@mSiO2 core–shell nanocomposites (about 65 nm) with β-thiopropionate-poly(ethylene glycol) (PEG) as the gatekeeper and loaded with DOX. The gatekeeper polymer was synthesized with different concentrations and different molecular weights. The study showed that the drug release was mainly dependent upon the medium pH, concentration, and packaging of β-thiopropionate-PEG.
Different methods, materials, and drug release triggers have been proposed to apply the gatekeeper concept in mesoporous silica-based drug delivery systems. For instance, the following study developed a core–shell mesoporous-based drug that belongs to the glutathione-sensitive drug delivery category by grafting the glutathione cleavable diselenide linker on the surface of the mesoporous-based drug. It was emphasized that the drug release kinetics depends mainly on the amount of diselenide linker.168
The same concept was successfully applied to deliver DOX and safranin O using mesoporous MNPs coated with PEG.169 Safranin O is a biological stain dye, which has become a basic guide for identifying degenerative processes such as osteoarthritis. Moreover, introducing FA to this system promoted the targeting of cancer tissues. The Fe3O4@mSiO2-DOX@-Se-Se-FA nanocomposites showed an efficient encapsulation of DOX.168 The drug release rate was increased by increasing the amount of glutathione because of the presence of diselenide, which is sensitive to glutathione. Cell uptake and release were improved by adding FA. Moreover, the cytotoxicity of this carrier showed negligible toxicity (3.9%±0.38%), while after DOX encapsulation, the cell viability decreased. The selectivity of the prepared drug was examined using healthy cells (L02 and HUVEC) and cancer cells (Hela cells), and it demonstrated little toxicity on healthy cells, while recorded higher toxicity on Hela cells (about 65%±1.3%).
Layered double hydroxides (LDHs) are pH-sensitive materials that decompose in an acidic environment. Jiang et al170 prepared Fe3O4@mSiO2@LDH NPs via mixing method. In this subject, LDH was used to control the release rate. It was revealed that these MNPs showed sensible loading proportion as well as superparamagnetic properties. The release rate values of MTX-loaded MNPs were varied depending on pH value; 66% at pH =4.0 during 72 hours. On the contrary, the release rate of MTX was much slower (about 20.91%) in the neutral pH medium (pH =7.4). In vitro tests showed no significant toxicity of the drug carriers toward Hela cells, while it showed high toxicity after MTX loading. It also showed better toxicity than using MTX alone, which indicated the suitability of this carrier as a potential drug carrier as a result of the multidrug resistance of the cancer cells.
Synthesizing mesoporous drug carriers with multiple shell thicknesses could be beneficial. There are specific parameters that can be controlled by varying the shell thickness, like surface area, the amount of loaded drug, and the drug release kinetics. This approach was introduced by Madrid et al,171 wherein the fabrication of Fe3O4@mSiO2 composites with different shell thicknesses via hydrothermal and sol-gel techniques was reported. The intake and release efficiency were examined using ibuprofen drug. Transmission electron microscopic (TEM) images realized the formation of different shell thicknesses as clearly seen in Figure 5. These NPs were favored to be employed as drug carriers because they provided up to 80% of biocompatibility. The particles with higher shell thicknesses demonstrated more toxicity than those with lower ones (Figure 6). A dependency between surface area, drug loading, and shell thickness was observed through this study. The higher the shell thickness, the higher the surface area, and the higher the drug loading. On the other hand, the drug release results were the opposite, where the higher the shell thickness exhibited, the lower the drug release. The study provided an exciting concept reflecting the importance of designing the carrier to its function. This study ignored the effect of loaded drug content by using only one concentration of 20 μg/mL. This research would be more relevant if multiple concentrations were explored in determining the toxicity.
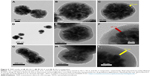  | Figure 5 TEM results of (A–C) SG-1, (D–F) SG-2, and (G–I) SG-3 composites. |
  | Figure 6 Ibuprofen release rate in different wall thicknesses. |
In the human body, enzymes play an essential role in regulating nearly all the body processes. So, any disorder in enzymes can be used to design a responsive drug carrier.172–174 For example, benefits of some enzymes like protease and phospholipase, which are responsible for the metastasis and invasiveness of the tumor cells, can be used.175–179 MMP protein is considered one of the significant changes in the cancerous cell’s microenvironment.180 Many attempts have been made to design a carrier with a responsive reaction to MMPs associated with tumor formation. However, these approaches were somewhat complicated.181–184 Thus, the upcoming report represents an easier synthesis process with promising findings.
A recent study conducted by Li et al101 summarized that enzyme responsive drug carrier could be fabricated easily to target tumor cells only. The study reported the synthesis of Fe3O4@mSiO2 nanoagents, which were then surface modified by Pro-Leu-Gly-Val-Arg peptide that have a responsive action to the presence of MMP-2 peptide (Gly-Gly-Pro-Leu-Gly-Val-Arg-Gly-Lys). After surface modification with peptide, these magnetic nanoagents were encapsulated with DOX. The prepared carriers were tested on two types of cells (NIH/3T3 normal cells and HT-1080 tumor cells) and showed high biocompatibility toward both normal and cancer cells. They provided better biocompatibility toward normal cells, even when they were encapsulated with the anti-tumor drug. Besides, the in vivo and in vitro examinations proved the enzyme dependent drug release. The accumulations of the nanoagents in the tumor sites were achieved using an external magnetic field.
MNPs@CS core–shell drug carriers
CSs attracted the attention of scientists because of their promising features such as biocompatibility, biodegradability, and bioactivity even more than other materials like gold and silica.185,186 Furthermore, they exhibit good drug loading efficiency and sustained drug release.187 Researchers reported many studies to enroll these materials in biomedical applications such as bone repairing and drug delivery systems.185,188,189
Lu et al synthesized a magnetic iron oxide-CS mesoporous core–shell nanocomposite as a promising vehicle for drug delivery applications.185 The synthesis process was conducted by ultrasound irradiation using two liquid phase systems in the presence of various contents of isooctane as an inert hydrophobic solvent (Figure 7). These nanocomposites showed novel properties such as high drug loading capacity, high pore volume, high specific surface area, excellent biocompatibility, and good superparamagnetic properties. Ibuprofen, docetaxel, and hemoglobin were used as drug models. Pore size distribution and specific surface area were found to be dependent on the concentration of isooctane. The highest surface area was obtained using 0.3 mL of isooctane, while the highest pore size distribution and pore size volume were obtained from 0.6 mL of isooctane. This suggests that the pore size distribution, pore volume, and surface area can be tuned using isooctane. The specific surface areas were 164, 474, and 427 m2/g for the nanocomposites fabricated without isooctane, with 0.3 mL, and 0.6 mL of isooctane, respectively, while the pore volumes were 0.77, 2.75, and 2.18 cm3/g, respectively, and pore size distributions were 9.5, 9.0, and 15.1 nm, respectively. This indicates the dependence of these parameters on the concentration of isooctane, where using 0.3 mL of isooctane shows better results than using larger concentrations. In addition, pore volume and specific surface area were higher for 0.3 mL of isooctane. TEM images also emphasize that using 0.3 mL of isooctane have better results than using 0.6 mL of isooctane, which illustrated that a part of these nanocomposites was collapsed in the case of 0.6 mL of isooctane because the cavity was so large. In addition, these materials are capable of loading both low molecular mass (docetaxel and ibuprofen) and high molecular mass drugs (hemoglobin) and are capable of loading both hydrophobic (docetaxel and ibuprofen) and hydrophilic (hemoglobin) drugs. Anticancer ability and toxicity assays were conducted on MCF-7 human breast cancer cells using these nanocomposites loaded with docetaxel. The carriers showed favorable biocompatibility even at high concentrations up to 500 μg/mL. The anti-cancer ability of docetaxel-loaded nanocomposites and free docetaxel nanocomposites are comparable.
  | Figure 7 (A) Synthesis of magnetic iron oxide–calcium silicate mesoporous core–shell nanocomposites with a hollow structure using two liquid phase system and ultrasound irradiation in the presence of isooctane. (B, C) TEM of the nanocomposites fabricated by using two liquid phase systems and ultrasound irradiation in the presence of isooctane. |
Other studies aimed to build multilayers instead of having only one shell layer. To illustrate, Lu et al190 fabricated a double shell magnetic nanocarrier consisting of iron oxide as the core and coated with two shells of silica and CS, and used ibuprofen as a drug model. These nanocomposites showed an excellent drug delivery and superparamagnetic behavior.
In summary, this section illustrated drug carriers based on silica and CS, either alone or by combining them. Controlling drug release is achieved by taking benefits from tumor nature like differences in pH or the presence of a substance like glutathione or protein. Magnetic cores can be coated with other materials or even metals to gain other properties or to target other therapeutics like bacteria. The next section briefly discusses the coating of magnetic cores with metals.
MNPs@metal core–shell drug carriers
More developed systems that target the microorganisms in the human body, which may cause infections or other types of diseases, have attracted the interest of researchers. Intracellular pathogens can cause many infections like meningitis, hepatitis, and tuberculosis. Treatment of these diseases with conventional drugs involves some health implications associated with prolonged exposure to the drug and its inherent toxicity.
Furthermore, MNPs can be utilized for the treatment of some infections related to the contamination of implants. It was reported previously that the superparamagnetic iron oxide nanoparticles (SPIONPs) alone might kill bacteria when they were used in concentrations above 100 μg/mL. Furthermore, SPIONPs grafted with carboxyl group killed about 44% of the pathogens.191,192
Candida is one of the fungal species, which is well known for causing not only systematic but also superficial infections. In this approach, Niemirowicz et al functionalized the MNPs with two types of antibiotics, which are amphotericin B and nystatin.193 These antibiotics were integrated to the surface of the MNPs and were examined by Candida species and human red blood cells to realize their hemolytic activities. The examination results revealed a low resistance of Candida species toward these modified antibiotics.
Gold nanostructures gained much attention in many biomedical applications such as thermal ablation of tumors, delivery of therapeutics, imaging, gene targeting, and cancer targeting. Therefore, combining gold and MNPs will provide a promising targeted drug delivery system for cancer therapy. For instance, Maleki et al192 discussed the antimicrobial activity and human cell cytotoxicity of superparamagnetic iron oxide–gold core–shell NPs functionalized with antimicrobial peptide (AMP) and cecropin mellitin (CM). The AMP-NPs showed promising applications in targeting pathogens as these NPs were based on the inhibition effect of CM. It was noticed that the AMP-NPs showed the minimal inhibitory concentration when examined on Escherichia coli. Furthermore, AMP-NPs have desirable properties such as targeting microorganisms without harming human body cells, where they did not show any pro-inflammation signs to concentrations up to 200 μg/mL.
MNPs@liposome core–shell drug carriers
Magnetic liposomes can be prepared by encapsulating MNPs with liposomes. Magnetic liposomes are considered promising for MRI, hyperthermia, and drug delivery due to their facile functionalization. Some limitations such as instability and tendency to aggregate in suspensions do exist. Interestingly, however, these limitations can be solved by the synthesis of liposomal cerasome carriers, which are spherical vesicles, consisting of lipid bilayer and covered with silica. Also, cerasomes are more stable than liposomes and can accommodate with both hydrophilic and hydrophobic drugs.194
The synthesis of magnetic liposomes to be employed as targeted drug carriers for cancer therapy was mentioned by Cao et al.194 In this approach, DOX hydrochloride was loaded into the magnetic cerasomes (MCs). The authors used the self-assembly and sol-gel approaches to load DOX and Fe3O4 NPs into the cerasomes to synthesize the DOX-loaded magnetic cerasomes (DLMCs). The schematic fabrication of DLMCs is shown in Figure 8. It was noted that the prepared DLMCs exhibited novel properties such as prolonged drug release and high stability. The drug release results showed that the DOX-encapsulated cerasomes exhibited a sustained release. Free DOX release was about 92.32% after 10 hours, while the rate of encapsulated DOX release was about 61% and it was extended to 120 hours. This sustained release is favored in cancer treatment. Furthermore, the MCs provided an excellent intake into the tumor cells, and this behavior can be ascribed to the strong response of the MCs to the magnetic field. The response of these DLMCs to the presence of magnetic field was proven by applying an external magnetic field to the dispersed MNPs in water which caused the DLMCs to exhibit superparamagnetic behavior. The Ms of DLMCs was 25.95 emu/g, which is considered low when compared to the Ms of Fe3O4 (69.65 emu/g). However, the value of 25.95 emu/g was sufficient to consider these DLMCs as efficient magnetic drug delivery carriers.
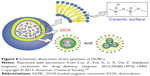  | Figure 8 Schematic illustration of the synthesis of DLMCs. |
Moreover, cell toxicity can be reduced by coating MNPs with silica, which results in an increase in hydrophilicity. Because of the presence of silanol group, silica surface is negatively charged, and this is not favorable especially for oncological therapeutic agents, whereas cationic particles are preferred because many cancer cells have a negatively charged surface. Thus, the synthesis of cationic magnetoliposomes is achieved by coating Fe2O3 MNPs with dual-chain lipids, especially zwitterionic phospholipids.195
The efficacy of DOX was enhanced by using MNP-supported lipid bilayers (SLBs) as a drug carrier. SLBs were studied by Mattingly et al195 to evaluate their potential use as drug carriers. Bilayers were prepared via self-assembly approach to allow their use as drug delivery carriers. These bilayers are composed of iron oxide–silica core–shell NPs and enclosed in a bilayer cationic lipid to host the amphiphilic DOX (Figure 9). The prepared NPs were tested in vitro against MCF-7 breast cancer cells. The MCF-7 cells were grown in a 12-well plate with each well containing 50,000 cells. The cells were incubated for 24 hours. Later, 50 μg of DOX-loaded SLBs were diluted and added to the wells. Surprisingly, the DOX-loaded SLBs showed a higher toxicity toward MCF-7 cells than using DOX alone. In addition, the DOX-loaded SLBs killed about 90% of MCF-7 breast cancer cells.
  | Figure 9 SLB-MNPs consisted of iron oxide–silica nanoparticles enclosed in a cationic lipid bilayer that contains the amphiphilic DOX. |
Besides the types mentioned in the previous sections, other inorganic materials are considered promising in the drug delivery systems. For instance, carbon nanotubes were used to deliver some drugs such as platinum drugs-cisplatin (IV) prodrug for cancer treatment as declared by Feazell et al.196 Other studies reported the successful usage of carbon nanotubes in the delivery of other drugs such as DOX, mitoxantrone, paclitaxel (PTX), quercetin, and FA.197 Moreover, there are other interesting approaches in drug delivery systems including quantum dots (QDs) synthesized from metals. QD is a nanocrystal material with size ranging from 2 to 10 nm and consists of semiconductors such as CdSe, ZnS, PbSe, PbS, InP, GaAs, and GaN. QDs are usually used in sensing and biological imaging. Due to their unique properties, research studies attempt to apply them in drug delivery systems. Due to their fluorescence properties, QDs are able to elucidate the pharmacodynamics and pharmacokinetics of drugs. Hence, combining drug with QD carriers will promote a more in-depth perspective of drug carrier biodistribution, drug release, and intracellular uptake using real-time monitoring. Integrating QDs in drug delivery systems can be achieved via two approaches: the first approach is loading the drug in a polymer or liposome that contains QDs and the second approach is linking the drug into the surface of QDs. Using QDs, scientists succeeded in delivering some chemotherapeutic drugs such as DOX and PTX.197
Conclusion
The magnetic drug carriers discussed in this review exhibit promising properties, particularly those modified with synthetic and natural polymers and bioactive materials. They show low toxicity and enhanced efficacy even though the results vary from one carrier to another. Another remarkable carrier is mesoporous silica. With their biocompatibility and ability to carry therapeutic agents like anti-tumor drugs, the presence of tunable pores leads to the attention surrounding these carriers. All critical findings in the previous studies can answer the question, “is using delivery systems better than using conventional drugs?” Although, it is not beneficial for some drugs as mentioned in this article, the benefits were obvious particularly in chemotherapy. The results show a high cell death against cancer cells in many drug carriers encapsulated with an anti-cancer drug. In some cases, they even show a higher cell death than that resulting from the use of the therapeutic agent alone.
With no doubts, the employment of these MNPs as vehicles in the magnetic-directed drug delivery systems will play a remarkable role in the enhancement of the drug efficacy of many diseases, especially in cancer treatment. Although some of these magnetic nanocarriers already exist in the market or have been used in clinical trials, some limitations are associated with the enhancement of the efficacy of drug loading and release. Moreover, further investigations need to be carried out with regard to the toxicity of nanocarriers, and this is a crucial step in the interpretation of these findings to a clinical endpoint. Herein lies a question of “what can be done to turn this potential into tangible outcomes, that is, formulations that can benefit patients?” Kinman Park.198,199
However, very few nano formulations are granted to be used in clinical practice when compared with the efforts made in this field. Therefore, the effective translation of a nanocarrier into clinical usage represents a challenge. Subsequently, some substantial issues must be addressed: first, enhanced NPs fabrication; second, standardization of NP efficacy with quantitative assessment; and third, establishment of a detailed profile of in-use formulations containing information about safety, immunogenicity, and toxicity. Another critical issue to be taken into account is investigating and understanding the interface between biological environments and Nano formulations. Finally, the adequate translation of these NPs into human medicine in commercial scale must be preceded with intensive research on the therapeutic development and preclinical estimations.199
Acknowledgments
The authors would like to acknowledge Qatar University for funding the project: GCC Co-Fund Program Grant #GCC-2017-001 and student grant QUST-1-CAS-2019-36. The publication of this article was funded by the Qatar National Library.
Author contributions
Kholoud E Albinali and Moustafa M Zagho wrote the manuscript draft and Yonghui Deng and Ahmed A Elzatahry initiated the idea, supervised the writing, and reviewed the manuscript. All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Jain KK. Drug delivery systems – an overview. Methods Mol Biol. 2008;437:1–50. | ||
Kim WJ, Kim SW. Efficient siRNA delivery with non-viral polymeric vehicles. Pharm Res. 2009;26(3):657–666. | ||
Pathak A, Patnaik S, Gupta KC. Recent trends in non-viral vector-mediated gene delivery. Biotechnol J. 2009;4(11):1559–1572. | ||
Degim IT, Celebi N. Controlled delivery of peptides and proteins. Curr Pharm Des. 2007;13(1):99–117. | ||
Tiwari G, Tiwari R, Sriwastawa B, et al. Drug delivery systems: an updated review. Int J Pharm Investig. 2012;2(1):2–11. | ||
Jain K. Drug Delivery Systems Kewal K. Jain K, editor. USA: Humana Press; 2008. | ||
Maiti S, Sen KK. Introductory chapter: drug delivery concepts. Adv Technol Deliv Ther. 2017:1–12. | ||
Cho K, Wang X, Nie S, Chen ZG, Shin DM. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008;14(5):1310–1316. | ||
Lin CC, Metters AT. Hydrogels in controlled release formulations: network design and mathematical modeling. Adv Drug Deliv Rev. 2006;58(12–13):1379–1408. | ||
Arifin DY, Lee LY, Wang CH. Mathematical modeling and simulation of drug release from microspheres: implications to drug delivery systems. Adv Drug Deliv Rev. 2006;58(12–13):1274–1325. | ||
Oupicky D, Bisht H, Manickam D, Zhou Q, Oupicky D, Bisht HS, MDSZQH. Stimulus-controlled delivery of drugs and genes. Expert Opin Drug Deliv. 2005;2(4):653–665. | ||
de Las Heras Alarcon C, Pennadam S, Alexander C. Stimuli responsive polymers for biomedical applications. Chem Soc Rev. 2005;34(3):276–285. | ||
Amsden B, Turner N. Diffusion characteristics of calcium alginate gels. Biotechnol Bioeng. 1999;65(5):605–610. | ||
Holowka EP, Bhatia SK. Drug Delivery:Materials Design and Clinical Perspective. Vol 4. New York, NY: Springer New York; 2014. | ||
Brazel CS, Peppas NA. Modeling of drug release from swellable polymers. Eur J Pharm Biopharm. 2000;49(1):47–58. | ||
Brazel CS, Peppas NA. Dimensionless analysis of swelling of hydrophilic glassy polymers with subsequent drug release from relaxing structures. Biomaterials. 1999;20(8):721–732. | ||
Siepmann J, Göpferich A. Mathematical modeling of bioerodible, polymeric drug delivery systems. Adv Drug Deliv Rev. 2001;48(2–3):229–247. | ||
Kimura H, Ogura Y. Biodegradable polymers for ocular drug delivery. Ophthalmologica. 2001;215(3):143–155. | ||
Gs D, Ghr R, Wilson RF, Chandy T. Colchicine encapsulation within poly(ethylene glycol)-coated poly(lactic acid)/poly(ε-caprolactone) microspheres-controlled release studies. Drug Deliv J Deliv Target Ther Agents. 2000;7(3):129–138. | ||
AS F, von Recum HA. Affinity-Based drug delivery. In: Engineering Polymer Systems for Improved Drug Delivery; 2013:429–452. | ||
Krukemeyer MG, Krenn V, Huebner F, Wagner W, Resch R. History and possible uses of nanomedicine based on nanoparticles and Nanotechnological progress. J Nanomed Nanotechnol. 2015;6(6):336. | ||
Kreuter J. Nanoparticles-a historical perspective. Int J Pharm. 2007;331(1):1–10. | ||
Barakat NS. Magnetically modulated nanosystems: a unique drug-delivery platform. Nanomedicine. 2009;4(7):799–812. | ||
Meyers PH, Cronic FR, Nice CM Jr. Experimental approach in the use and magnetic control of metallic iron particles in the lymphatic and vascular system of dogs as a contast and isotopic agent. Am J Roentgenol, Radium Ther Nucl Med. 1963;90:1068–1077. | ||
Widder KJ, Senyei AE, Ranney DF. Magnetically responsive microspheres and other carriers for the biophysical targeting of antitumor agents. Adv Pharmacol Chemother. 1979;16:213–271. | ||
Lübbe AS, Bergemann C, Huhnt W, et al. Preclinical experiences with magnetic drug targeting: tolerance and efficacy. Cancer Res. 1996;56(20):4694–4701. | ||
Din FU, Aman W, Ullah I, et al. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int J Nanomedicine. 2017;12:7291–7309. | ||
Neubert RH. Potentials of new nanocarriers for dermal and transdermal drug delivery. Eur J Pharm Biopharm. 2011;77(1):1–2. | ||
Dhar S, Gu FX, Langer R, Farokhzad OC, Lippard SJ. Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA-PEG nanoparticles. Proc Natl Acad Sci U S A. 2008;105(45):17356–17361. | ||
Eniola AO, da H, Hammer DA. Artificial polymeric cells for targeted drug delivery. J Control Release. 2003;87:15–22. | ||
Farokhzad OC, Cheng J, Teply BA, et al. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc Natl Acad Sci U S A. 2006;103(16):6315–6320. | ||
Gu F, Zhang L, Teply BA, et al. Precise engineering of targeted nanoparticles by using self-assembled biointegrated block copolymers. Proc Natl Acad Sci U S A. 2008;105(7):2586–2591. | ||
Chandran SS, Nan A, Rosen DM, Ghandehari H, Denmeade SR. A prostate-specific antigen activated N-(2-hydroxypropyl) methacrylamide copolymer prodrug as dual-targeted therapy for prostate cancer. Mol Cancer Ther. 2007;6(11):2928–2937. | ||
Sato H, Homma A, Okamachi A, et al. Novel hyaluronic acid-methotrexate conjugates for osteoarthritis treatment. Bioorg Med Chem. 2009;17(13):4647–4656. | ||
Luo Y, Prestwich GD. Synthesis and selective cytotoxicity of a hyaluronic acid-antitumor bioconjugate. Bioconjug Chem. 1999;10(5):755–763. | ||
Chau Y, Dang NM, Tan FE, Langer R. Investigation of targeting mechanism of new dextran-peptide-methotrexate conjugates using biodistribution study in matrix-metalloproteinase-overexpressing tumor xenograft model. J Pharm Sci. 2006;95(3):542–551. | ||
Farokhzad OC, Jon S, Khademhosseini A, Tran TN, Lavan DA, Langer R. Nanoparticle-aptamer bioconjugates: a new approach for targeting prostate cancer cells. Cancer Res. 2004;64(21):7668–7672. | ||
Yura H, Yoshimura N, Hamashima T, et al. Synthesis and pharmacokinetics of a novel macromolecular prodrug of tacrolimus (FK506), FK506-dextran conjugate. J Control Release. 1999;57(1):87–99. | ||
Bhatia S. Natural Polymer Drug Delivery Systems. Switzerland: Springer International Publishing; 2016. | ||
Tomalia DA, Naylor AM, Goddard WA. Starburst dendrimers: molecular-level control of size, shape, surface chemistry, topology, and flexibility from atoms to macroscopic matter. Angew Chem Int Ed Engl. 1990;29(2):138–175. | ||
Tomalia DA, Baker H, Dewald J, et al. A new class of polymers: starburst-dendritic macromolecules. Polym J. 1985;17(1):117–132. | ||
Zimmerman SC, Quinn JR, Burakowska E, Haag R. Cross-linked glycerol dendrimers and hyperbranched polymers as ionophoric, organic nanoparticles soluble in water and organic solvents. Angew Chem Int Ed. 2007;46(43):8164–8167. | ||
Zhou Z, Shen Y, Tang J, et al. Linear polyethyleneimine-based charge-reversal nanoparticles for nuclear-targeted drug delivery. J Mater Chem. 2011;21(47):19114. | ||
Fischer M, Vögtle F. Dendrimers: from design to application – a progress report. Angew Chem Int Ed. 1999;38(7):884–905. | ||
Zhang W, Nowlan DT, Thomson LM, Lackowski WM, Simanek EE. Orthogonal, convergent syntheses of dendrimers based on melamine with one or two unique surface sites for manipulation. J Am Chem Soc. 2001;123(37):8914–8922. | ||
Sideratou Z, Kontoyianni C, Drossopoulou GI, Paleos CM. Synthesis of a folate functionalized PEGylated poly(propylene imine) dendrimer as prospective targeted drug delivery system. Bioorg Med Chem Lett. 2010;20(22):6513–6517. | ||
Paleos CM, Tsiourvas D, Sideratou Z, Tziveleka L. Acid- and salt-triggered multifunctional poly(propylene imine) dendrimer as a prospective drug delivery system. Biomacromolecules. 2004;5(2):524–529. | ||
Kaminskas LM, Kelly BD, Mcleod VM, et al. Characterisation and tumour targeting of PEGylated polylysine dendrimers bearing doxorubicin via a pH labile linker. J Control Release. 2011;152(2):241–248. | ||
Chertok B, David AE, Yang VC. Polyethyleneimine-modified iron oxide nanoparticles for brain tumor drug delivery using magnetic targeting and intra-carotid administration. Biomaterials. 2010;31(24):6317–6324. | ||
Chen HT, Neerman MF, Parrish AR, Simanek EE. Cytotoxicity, hemolysis, and acute in vivo toxicity of dendrimers based on melamine, candidate vehicles for drug delivery. J Am Chem Soc. 2004;126(32):10044–10048. | ||
Patra CR, Bhattacharya R, Mukhopadhyay D, Mukherjee P. Fabrication of gold nanoparticles for targeted therapy in pancreatic cancer. Adv Drug Deliv Rev. 2010;62(3):346–361. | ||
Liu Z, Tabakman SM, Chen Z, Dai H. Preparation of carbon nanotube bioconjugates for biomedical applications. Nat Protoc. 2009;4(9):1372–1381. | ||
Dhar S, Liu Z, Thomale J, Dai H, Lippard SJ. Targeted single-wall carbon nanotube-mediated Pt(IV) prodrug delivery using folate as a homing device. J Am Chem Soc. 2008;130(34):11467–11476. | ||
Liu Z, Chen K, Davis C, et al. Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res. 2008;68(16):6652–6660. | ||
Wu W, Wieckowski S, Pastorin G, et al. Targeted delivery of amphotericin B to cells by using functionalized carbon nanotubes. Angew Chem Int Ed. 2005;44(39):6358–6362. | ||
Samorì C, Ali-Boucetta H, Sainz R, et al. Enhanced anticancer activity of multi-walled carbon nanotube–methotrexate conjugates using cleavable linkers. Chem Commun. 2010;46(9):1494–1496. | ||
Wong PT, Choi SK. Mechanisms of drug release in nanotherapeutic delivery systems. Chem Rev. 2015;115(9):3388–3432. | ||
Kaminskas LM, Mcleod VM, Kelly BD, et al. A comparison of changes to doxorubicin pharmacokinetics, antitumor activity, and toxicity mediated by PEGylated dendrimer and PEGylated liposome drug delivery systems. Nanomedicine. 2012;8(1):103–111. | ||
Studenovsky M, Ulbrich K, Ibrahimova M, Rihova B. Polymer conjugates of the highly potent cytostatic drug 2-pyrrolinodoxorubicin. Eur J Pharm Sci. 2011;42(1–2):156–163. | ||
Ho KS, Aman AM, Al-awar RS, Shoichet MS. Amphiphilic micelles of poly(2-methyl-2-carboxytrimethylene carbonate-co-d,l-lactide)-graft-poly(ethylene glycol) for anti-cancer drug delivery to solid tumours. Biomaterials. 2012;33(7):2223–2229. | ||
Huynh VT, Quek JY, de Souza PL, Stenzel MH. Block copolymer micelles with pendant bifunctional chelator for platinum drugs: effect of spacer length on the viability of tumor cells. Biomacromolecules. 2012;13(4):1010–1023. | ||
Yavuz MS, Cheng Y, Chen J, et al. Gold nanocages covered by smart polymers for controlled release with near-infrared light. Nat Mater. 2009;8(12):935–939. | ||
Nž K, Slowing II, Vsy L. Tuning the release of anticancer drugs from magnetic iron oxide/mesoporous silica core/shell nanoparticles. Chempluschem. 2012;77(1):48–55. | ||
Berlin JM, Leonard AD, Pham TT, et al. Effective drug delivery, in vitro and in vivo, by carbon-based nanovectors noncovalently loaded with unmodified paclitaxel. ACS Nano. 2010;4(8):4621–4636. | ||
Zhu RR, Qin LL, Wang M, et al. Preparation, characterization, and anti-tumor property of podophyllotoxin-loaded solid lipid nanoparticles. Nanotechnology. 2009;20(5):55702. | ||
Hosseinzadeh H, Atyabi F, Dinarvand R, Ostad SN. Chitosan-Pluronic nanoparticles as oral delivery of anticancer gemcitabine: preparation and in vitro study. Int J Nanomedicine. 2012;7:1851–1863. | ||
Wang W, Zhu R, Xie Q, et al. Enhanced bioavailability and efficiency of curcumin for the treatment of asthma by its formulation in solid lipid nanoparticles. Int J Nanomedicine. 2012;7:3667–3677. | ||
Ross JS, Schenkein DP, Pietrusko R, et al. Targeted therapies for cancer 2004. Am J Clin Pathol. 2004;122(4):598–609. | ||
Sun X, Li F, Wang Y, Liang W. Cellular uptake and elimination of lipophilic drug delivered by nanocarriers. Pharmazie. 2010;65(10):737–742. | ||
Abruzzo A, Zuccheri G, Belluti F, et al. Chitosan nanoparticles for lipophilic anticancer drug delivery: development, characterization and in vitro studies on HT29 cancer cells. Colloids Surf B Biointerfaces. 2016;145:362–372. | ||
Su CW, Chiang CS, Li WM, Hu SH, Chen SY. Multifunctional nanocarriers for simultaneous encapsulation of hydrophobic and hydrophilic drugs in cancer treatment. Nanomedicine. 2014;9(10):1499–1515. | ||
Lian H-Y, Liang Y-H, Yamauchi Y, Wu KC-W. A hierarchical study on Load/Release kinetics of guest molecules into/from mesoporous silica thin films. J Phys Chem C. 2011;115(14):6581–6590. | ||
Reichlin S. Handbook of Experimental Pharmacology. Vol 258;1969. | ||
Torchilin VP. Passive and active drug targeting drug delivery to tumors as an example. In: Schäfer-Korting M, editor. Drug Delivery. Handbook of Experimental Pharmacology. Berlin, Heidelberg: Springer;2009:3–53. | ||
Pawar PV, Domb AJ, Kumar N. Systemic targeting systems-EPR effect, ligand targeting systems. In: Domb AJ, Khan W, editors. Focal Controlled Drug Delivery. Boston, MA: Springer US; 2014:61–91. | ||
Petrak K. Essential properties of drug-targeting delivery systems. Drug Discov Today. 2005;10(23–24):1667–1673. | ||
Mcbain SC, Yiu HH, Dobson J. Magnetic nanoparticles for gene and drug delivery. Int J Nanomedicine. 2008;3(2):169–180. | ||
Estelrich J, Escribano E, Queralt J, Busquets MA. Iron oxide nanoparticles for magnetically-guided and magnetically-responsive drug delivery. Int J Mol Sci. 2015;16(4):8070–8101. | ||
Yoo D, Lee JH, Shin TH, Cheon J. Theranostic magnetic nanoparticles. Acc Chem Res. 2011;44(10):863–874. | ||
Wang L, Cole M, Li J, et al. Polymer grafted recyclable magnetic nanoparticles. Polym Chem. 2015;6(2):248–255. | ||
Pageni P, Yang P, Bam M, et al. Recyclable magnetic nanoparticles grafted with antimicrobial metallopolymer-antibiotic bioconjugates. Biomaterials. 2018;178:363–372. | ||
Gas P. Essential facts on the history of hyperthermia and their connections with Electromedicine. Prz Elektrotechniczny. 2017;87(12b):37–40. | ||
Chiriac H, Petreus T, Carasevici E, et al. In vitro cytotoxicity of Fe–Cr–Nb–B magnetic nanoparticles under high frequency electromagnetic field. J Magn Magn Mater. 2015;380:13–19. | ||
Hervault A, Thanh NT. Magnetic nanoparticle-based therapeutic agents for thermo-chemotherapy treatment of cancer. Nanoscale. 2014;6(20):11553–11573. | ||
Hedayatnasab Z, Abnisa F, Daud WMAW. Review on magnetic nanoparticles for magnetic nanofluid hyperthermia application. Mater Des. 2017;123:174–196. | ||
Dias CSB, Hanchuk TDM, Wender H, et al. Shape tailored magnetic nanorings for intracellular hyperthermia cancer therapy. Sci Rep. 2017;7(1):1–8. | ||
Tansık G, Yakar A, Gündüz U. Tailoring magnetic PLGA nanoparticles suitable for doxorubicin delivery. J Nanopart Res. 2014;16(1):2171. | ||
Arias JL, Gallardo V, Gómez-Lopera SA, Plaza RC, Delgado AV. Synthesis and characterization of poly(ethyl-2-cyanoacrylate) nanoparticles with a magnetic core. J Control Release. 2001;77(3):309–321. | ||
Costo R, Morales MP, Veintemillas-Verdaguer S. Improving magnetic properties of ultrasmall magnetic nanoparticles by biocompatible coatings. J Appl Phys. 2015;117(6):64311. | ||
Balakrishnan S, Bonder MJ, Hadjipanayis GC. Particle size effect on phase and magnetic properties of polymer-coated magnetic nanoparticles. J Magn Magn Mater. 2009;321(2):117–122. | ||
Chen T, Peng X, Dai H. Construction of site-specific core–shell structured nanocomposite for pH-controlled drug delivery. J Porous Mater. 2016;23(4):987–995. | ||
Pradhan P, Giri J, Rieken F, et al. Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. J Control Release. 2010;142(1):108–121. | ||
Ma Y, Liang X, Tong S, Bao G, Ren Q, Dai Z. Gold nanoshell nanomicelles for potential magnetic resonance imaging, light-triggered drug release, and photothermal therapy. Adv Funct Mater. 2013;23(7):815–822. | ||
Kralj S, Potrc T, Kocbek P, Marchesan S, Makovec D. Design and fabrication of magnetically responsive nanocarriers for drug delivery. Curr Med Chem. 2017;24(5):454–469. | ||
Mertz D, Sandre O, Bégin-Colin S. Drug releasing nanoplatforms activated by alternating magnetic fields. Biochim Biophys Acta Gen Subj. 2017;1861(6):1617–1641. | ||
Rudge S, Peterson C, Vessely C, Koda J, Stevens S, Catterall L. Adsorption and desorption of chemotherapeutic drugs from a magnetically targeted carrier (MTC). J Control Release. 2001;74(1–3):335–340. | ||
Y-Tn L, Chan KK, Harris PA, Cohen JL. Distribution of adriamycin in cancer patients. Tissue uptakes, plasma concentration after IV and hepatic Ia administration. Cancer. 2018;45(9):2231–2239. | ||
Chen H, Shao L, Ming T, et al. Understanding the photothermal conversion efficiency of gold nanocrystals. Small. 2010;6(20):2272–2280. | ||
Xie J, Lee S, Chen X. Nanoparticle-based theranostic agents. Adv Drug Deliv Rev. 2010;62(11):1064–1079. | ||
Malik A, Tahir Butt T, Zahid S, et al. Use of Magnetic Nanoparticles as Targeted Therapy: Theranostic Approach to Treat and Diagnose Cancer. J Nanotechnol. 2017;2017:1098765. | ||
Li E, Yang Y, Hao G, et al. Multifunctional Magnetic Mesoporous Silica Nanoagents for in vivo Enzyme-Responsive Drug Delivery and MR Imaging. Nanotheranostics. 2018;2(3):233–242. | ||
Zagho M, Almaadeed M, Majeed K. Thermal properties of TiO2NP/CNT/LDPE hybrid nanocomposite films. Polymers. 2018;10(11):1270. | ||
Kabalan L, Zagho MM, Al-Marri MJ, Khader MM. Experimental and theoretical studies on the mechanical and structural changes imposed by the variation of clay loading on poly(vinyl alcohol)/cloisite® 93A nanocomposites. J Vinyl Addit Technol. Epub Aug 10. | ||
Zagho MM, AlMaadeed MA, Majeed K. Role of TiO2 and carbon nanotubes on polyethylene, and effect of accelerated weathering on photo oxidation and mechanical properties. J Vinyl Addit Technol. 2019;25(1):19–25. | ||
Al-Marri MJ, Masoud MS, Nassar AMG, Zagho MM, Khader MM. Synthesis and characterization of poly(vinyl alcohol): Cloisite® 20A nanocomposites. J Vinyl Addit Technol. 2017;23(3):181–187. | ||
Majeed K, Al Ali AlMaadeed M, Zagho MM. Comparison of the effect of carbon, halloysite and titania nanotubes on the mechanical and thermal properties of LDPE based nanocomposite films. Chinese J Chem Eng. 2018;26(2):428–435. | ||
Zagho MM, Khader MM. The impact of clay loading on the relative intercalation of poly(vinyl alcohol)-clay composites. MSCE. 2016;04(10):20–31. | ||
Khan MI, Zagho MM, Shakoor RA. A brief overview of shape memory effect in thermoplastic polymers, smart polymer nanocomposites. In: Ponnamma D. editor. Smart Polymer Nanocomposites, Springer Series on Polymer and Composite Materials. Springer International Publishing AG; 2017:281–301. | ||
Zagho MM, Elzatahry A. Recent trends in electrospinning of polymer nanofibers and their applications as templates for metal oxide nanofibers preparation. In: Haider S, editor. Electrospinning – Material, Techniques, and Biomedical Application. InTech; 2016:3–24. | ||
Am A-E, Zagho MM, Elzatahry AA. Polymer-based electrospun nanofibers for biomedical applications. Nanomaterials. 2018;8(4):259. | ||
Zagho M, Hussein E, Elzatahry A. Recent overviews in functional polymer composites for biomedical applications. Polymers. 2018;10(7):739. | ||
Hussein EA, Zagho MM, Nasrallah GK, Elzatahry AA. Recent advances in functional nanostructures as cancer photothermal therapy. Int J Nanomedicine. 2018;13:2897–2906. | ||
Dawoud HD, Altahtamouni TM, Zagho MM, Bensalah N. A brief overview of flexible CNT/PANI super capacitors. J Mater Sci Nanotechnol. 2017;1(2):23–36. | ||
Wamocha HL. Synthesis and characterization of 5-FU loaded magnetic nanocomposite spheres for advanced drug delivery. 2010. | ||
Basu B, Katti DS, Kumar A, editors. Advanced Biomaterials. Hoboken, NJ: John Wiley & Sons, Inc.; 2009. | ||
Denardo GL, Denardo SJ, Goldstein DS, et al. Maximum-tolerated dose, toxicity, and efficacy of (131)I-Lym-1 antibody for fractionated radioimmunotherapy of non-Hodgkin’s lymphoma. J Clin Oncol. 1998;16(10):3246–3256. | ||
Marcos-Campos I, Asín L, Torres TE, et al. Cell death induced by the application of alternating magnetic fields to nanoparticle-loaded dendritic cells. Nanotechnology. 2011;22(20):205101. | ||
Yu CY, Zhang XC, Zhou FZ, Zhang XZ, Cheng SX, Zhuo RX. Sustained release of antineoplastic drugs from chitosan-reinforced alginate microparticle drug delivery systems. Int J Pharm. 2008;357(1–2):15–21. | ||
Panyam J, Zhou WZ, Prabha S, Sahoo SK, Labhasetwar V. Rapid endo-lysosomal escape of poly(DL-lactide-co-glycolide) nanoparticles: implications for drug and gene delivery. Faseb J. 2002;16(10):1217–1226. | ||
Acharya S, Sahoo SK. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv Drug Deliv Rev. 2011;63(3):170–183. | ||
Zhao J, Yang H, Li J, Wang Y, Wang X. Fabrication of pH-responsive PLGA(UCNPs/DOX) nanocapsules with upconversion luminescence for drug delivery. Sci Rep. 2017;7(1):18014. | ||
Montha W, Maneeprakorn W, Buatong N, Tang IM, Pon-On W. Synthesis of doxorubicin-PLGA loaded chitosan stabilized (Mn, Zn)Fe2O4 nanoparticles: biological activity and pH-responsive drug release. Mater Sci Eng C. 2016;59:235–240. | ||
Vivek R, Thangam R, Kumar SR, et al. HER2 targeted breast cancer therapy with switchable “Off/On” multifunctional “Smart” magnetic polymer core-shell nanocomposites. ACS Appl Mater Interfaces. 2016;8(3):2262–2279. | ||
Unsoy G, Khodadust R, Yalcin S, Mutlu P, Gunduz U. Synthesis of doxorubicin loaded magnetic chitosan nanoparticles for pH responsive targeted drug delivery. Eur J Pharm Sci. 2014;62:243–250. | ||
Andhariya N, Chudasama B, Mehta RV, Upadhyay RV. Biodegradable thermoresponsive polymeric magnetic nanoparticles: a new drug delivery platform for doxorubicin. J Nanopart Res. 2011;13(4):1677–1688. | ||
Khan US, Khattak NS, Rahman A, Khan F. Historical development of magnetite nanoparticles synthesis. J Chem Soc Pakistan. 2011;33(6):793–804. | ||
Douziech-Eyrolles L, Marchais H, Hervé K, et al. Nanovectors for anticancer agents based on superparamagnetic iron oxide nanoparticles. Int J Nanomedicine. 2007;2(4):541–550. | ||
O’Riordan TG. Aerosol delivery devices and obstructive airway disease. Expert Rev Med Devices. 2005;2(2):197–203. | ||
Hagerman JK, Hancock KE, Klepser ME. Aerosolised antibiotics: a critical appraisal of their use. Expert Opin Drug Deliv. 2006;3(1):71–86. | ||
Rao RD, Markovic SN, Anderson PM. Aerosol therapy for malignancy involving the lungs. Curr Cancer Drug Targets. 2003;3(4):239–250. | ||
Zarogoulidis P, Chatzaki E, Porpodis K, et al. Inhaled chemotherapy in lung cancer: future concept of nanomedicine. Int J Nanomedicine. 2012;7:1551–1572. | ||
Gagnadoux F, Hureaux J, Vecellio L, et al. Aerosolized chemotherapy. J Aerosol Med Pulm Drug Deliv. 2008;21(1):61–70. | ||
Roa WH, Azarmi S, Al-Hallak MH, Finlay WH, Magliocco AM, Löbenberg R. Inhalable nanoparticles, a non-invasive approach to treat lung cancer in a mouse model. J Control Release. 2011;150(1):49–55. | ||
Dames P, Gleich B, Flemmer A, et al. Targeted delivery of magnetic aerosol droplets to the lung. Nat Nanotechnol. 2007;2(8):495–499. | ||
Verma NK, Crosbie-Staunton K, Satti A, et al. Magnetic core-shell nanoparticles for drug delivery by nebulization. J Nanobiotechnology. 2013;11:1. | ||
Zheng SY, Li Y, Jiang D, Zhao J, Ge JF. Anticancer effect and apoptosis induction by quercetin in the human lung cancer cell line A-549. Mol Med Rep. 2012;5(3):822–826. | ||
di Carlo G, Mascolo N, Izzo AA, Capasso F. Flavonoids: old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999;65(4):337–353. | ||
Janes KA, Fresneau MP, Marazuela A, Fabra A, Alonso MJ. Chitosan nanoparticles as delivery systems for doxorubicin. J Control Release. 2001;73(2–3):255–267. | ||
Grenha A. Chitosan nanoparticles: a survey of preparation methods. J Drug Target. 2012;20(4):291–300. | ||
Tan ML, Choong PF, Dass CR. Review: doxorubicin delivery systems based on chitosan for cancer therapy. J Pharm Pharmacol. 2009;61(2):131–142. | ||
Prabaharan M. Chitosan-based nanoparticles for tumor-targeted drug delivery. Int J Biol Macromol. 2015;72:1313–1322. | ||
Javid A, Ahmadian S, Saboury AA, Kalantar SM, Rezaei-Zarchi S. Chitosan-coated superparamagnetic iron oxide nanoparticles for doxorubicin delivery: synthesis and anticancer effect against human ovarian cancer cells. Chem Biol Drug Des. 2013;82(3):296–306. | ||
Deng Z, Zhen Z, Hu X, Wu S, Xu Z, Chu PK. Hollow chitosan-silica nanospheres as pH-sensitive targeted delivery carriers in breast cancer therapy. Biomaterials. 2011;32(21):4976–4986. | ||
Hu X, Wang Y, Peng B. Chitosan-capped mesoporous silica nanoparticles as pH-responsive nanocarriers for controlled drug release. Chem Asian J. 2014;9(1):319–327. | ||
Chronopoulou L, Massimi M, Giardi MF, et al. Chitosan-coated PLGA nanoparticles: a sustained drug release strategy for cell cultures. Colloids Surf B Biointerfaces. 2013;103:310–317. | ||
Wang Y, Li P, Kong L. Chitosan-Modified PLGA nanoparticles with versatile surface for improved drug delivery. AAPS PharmSciTech. 2013;14(2):585–592. | ||
Guo M, Rong WT, Hou J, et al. Mechanisms of chitosan-coated poly(lactic-co-glycolic acid) nanoparticles for improving oral absorption of 7-ethyl-10-hydroxycamptothecin. Nanotechnology. 2013;24(24):245101. | ||
Assa F, Jafarizadeh-Malmiri H, Ajamein H, et al. Chitosan magnetic nanoparticles for drug delivery systems. Crit Rev Biotechnol. 2017;37(4):492–509. | ||
He C, Lu K, Liu D, Lin W. Nanoscale metal-organic frameworks for the co-delivery of cisplatin and pooled siRNAs to enhance therapeutic efficacy in drug-resistant ovarian cancer cells. J Am Chem Soc. 2014;136(14):5181–5184. | ||
Zhuang J, Kuo CH, Chou LY, Liu DY, Weerapana E, Tsung CK. Optimized metal-organic-framework nanospheres for drug delivery: evaluation of small-molecule encapsulation. ACS Nano. 2014;8(3):2812–2819. | ||
Wang XG, Dong ZY, Cheng H, et al. A multifunctional metal-organic framework based tumor targeting drug delivery system for cancer therapy. Nanoscale. 2015;7(38):16061–16070. | ||
Wu YN, Zhou M, Li S, et al. Magnetic metal-organic frameworks: γ-Fe2O3@MOFs via confined in situ pyrolysis method for drug delivery. Small. 2014;10(14):2927–2936. | ||
Bian R, Wang T, Zhang L, Li L, Wang C. A combination of tri-modal cancer imaging and in vivo drug delivery by metal–organic framework based composite nanoparticles. Biomater Sci. 2015;3(9):1270–1278. | ||
Ke F, Yuan Y-P, Qiu L-G, et al. Facile fabrication of magnetic metal–organic framework nanocomposites for potential targeted drug delivery. J Mater Chem. 2011;21(11):3843. | ||
Ren H, Zhang L, An J, et al. Polyacrylic acid@zeolitic imidazolate framework-8 nanoparticles with ultrahigh drug loading capability for pH-sensitive drug release. Chem Commun. 2014;50(8):1000–1002. | ||
Chowdhuri AR, Singh T, Ghosh SK, Sahu SK. Carbon dots embedded magnetic nanoparticles @chitosan @metal organic framework as a nanoprobe for pH sensitive targeted anticancer drug delivery. ACS Appl Mater Interfaces. 2016;8(26):16573–16583. | ||
Remant RB, Chandrashekaran V, Cheng B, et al. Redox potential ultrasensitive nanoparticle for the targeted delivery of camptothecin to HER2-positive cancer cells. Mol Pharm. 2014;11(6):1897–1905. | ||
Vivek R, Thangam R, Nipunbabu V. Multifunctional HER2-antibody conjugated polymeric nanocarrier-based drug delivery system for multi-drug-resistant breast cancer therapy. ACS Appl Mater Interfaces. 2014;6(9):6469–6480. | ||
Zhu YJ, Chen F. pH-responsive drug-delivery systems. Chem Asian J. 2015;10(2):284–305. | ||
Lü JM, Wang X, Marin-Muller C, et al. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev Mol Diagn. 2009;9(4):325–341. | ||
Zhao S, Zhang S, Ma J, et al. Double loaded self-decomposable SiO2 nanoparticles for sustained drug release. Nanoscale. 2015;7(39):16389–16398. | ||
Argyo C, Weiss V, Bräuchle C, Bein T. Multifunctional mesoporous silica nanoparticles as a universal platform for drug delivery. Chem Mater. 2014;26(1):435–451. | ||
Liu F, Wang J, Huang P. Outside-in stepwise functionalization of mesoporous silica nanocarriers for matrix type sustained release of fluoroquinolone drugs. J Mater Chem B. 2015;3:2206–2214. | ||
Wu S, Huang X, du X. pH- and redox-triggered synergistic controlled release of a ZnO-gated hollow mesoporous silica drug delivery system. J Mater Chem B. 2015;3:1426–1432. | ||
Abolhasani J, Hosseinzadeh Khanmiri R, Babazadeh M, Ghorbani-Kalhor E, Edjlali L, Hassanpour A. Determination of Hg(II) ions in sea food samples after extraction and preconcentration by novel Fe3O4@SiO2@polythiophene magnetic nanocomposite. Environ Monit Assess. 2015;187(9):554. | ||
Nasrallah GK, Zhang Y, Zagho MM, et al. A systematic investigation of the bio-toxicity of core-shell magnetic mesoporous silica microspheres using zebrafish model. Microporous Mesoporous Mater. 2018;265:195–201. | ||
Yang C, Guo W, Cui L, et al. pH-responsive magnetic core-shell nanocomposites for drug delivery. Langmuir. 2014;30(32):9819–9827. | ||
An N, Lin H, Yang C, et al. Gated magnetic mesoporous silica nanoparticles for intracellular enzyme-triggered drug delivery. Mater Sci Eng C. 2016;69:292–300. | ||
Giménez C, de la Torre C, Gorbe M, et al. Gated mesoporous silica nanoparticles for the controlled delivery of drugs in cancer cells. Langmuir. 2015;31(12):3753–3762. | ||
Jiang W, Wu J, Tian R, Jiang W. Synthesis and characterization of magnetic mesoporous core–shell nanocomposites for targeted drug delivery applications. J Porous Mater. 2017;24(1):257–265. | ||
Uribe Madrid SI, Pal U, Kang YS, Kim J, Kwon H, Kim J. Fabrication of Fe3O4@mSiO2 core-shell composite nanoparticles for drug delivery applications. Nanoscale Res Lett. 2015;10(1):217. | ||
Choi YR, Lee B, Park J, Namkung W, Jeong KS. Enzyme-Responsive Procarriers capable of transporting chloride ions across lipid and cellular membranes. J Am Chem Soc. 2016;138(47):15319–15322. | ||
Liu J, Jambhrunkar S, Kleitz F, Aa P, Bpbp R. Enzyme-responsive controlled release of covalently bound prodrug from functional mesoporous silica nanospheres. Angew Chem Int Ed. 2012;51:12486–12489. | ||
Zhang H, Fei J, Yan X, Wang A, Li J. Enzyme-responsive release of doxorubicin from monodisperse dipeptide-based nanocarriers for highly efficient cancer treatment in vitro. Adv Funct Mater. 2015;25(8):1193–1204. | ||
Weissleder R, Tung CH, Mahmood U, Bogdanov A. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat Biotechnol. 1999;17(4):375–378. | ||
Olson ES, Jiang T, Aguilera TA, et al. Activatable cell penetrating peptides linked to nanoparticles as dual probes for in vivo fluorescence and MR imaging of proteases. Proc Natl Acad Sci U S A. 2010;107(9):4311–4316. | ||
Lecaille F, Kaleta J, Brömme D. Human and parasitic papain-like cysteine proteases: their role in physiology and pathology and recent developments in inhibitor design. Chem Rev. 2002;102(12):4459–4488. | ||
Zheng F, Zhang P, Xi Y, Huang K, Min Q, Zhu J-J. Peptide-mediated core/satellite/shell multifunctional nanovehicles for precise imaging of cathepsin B activity and dual-enzyme controlled drug release. NPG Asia Mater. 2017;9(3):e366. | ||
Yang Y, Aw J, Chen K, et al. Enzyme-responsive multifunctional magnetic nanoparticles for tumor intracellular drug delivery and imaging. Chem Asian J. 2011;6(6):1381–1389. | ||
Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52–67. | ||
Ji T, Li S, Zhang Y, et al. An MMP-2 responsive liposome integrating antifibrosis and chemotherapeutic drugs for enhanced drug perfusion and efficacy in pancreatic cancer. ACS Appl Mater Interfaces. 2016;8(5):3438–3445. | ||
Huang S, Shao K, Kuang Y, et al. Tumor targeting and microenvironment-responsive nanoparticles for gene delivery. Biomaterials. 2013;34(21):5294–5302. | ||
Peng ZH, Kopeček J. Enhancing accumulation and penetration of HPMA copolymer-doxorubicin conjugates in 2D and 3D prostate cancer cells via iRGD conjugation with an MMP-2 cleavable spacer. J Am Chem Soc. 2015;137(21):6726–6729. | ||
Zou Z, He X, He D, et al. Programmed packaging of mesoporous silica nanocarriers for matrix metalloprotease 2-triggered tumor targeting and release. Biomaterials. 2015;58:35–45. | ||
Lu BQ, Zhu YJ, Ao HY, Qi C, Chen F. Synthesis and characterization of magnetic iron oxide/calcium silicate mesoporous nanocomposites as a promising vehicle for drug delivery. ACS Appl Mater Interfaces. 2012;4(12):6969–6974. | ||
Yang F, Lu J, Ke Q, Peng X, Guo Y, Xie X. Magnetic mesoporous calcium sillicate/chitosan porous scaffolds for enhanced bone regeneration and photothermal-chemotherapy of osteosarcoma. Sci Rep. 2018;8(1):7345. | ||
Zhang N, Molenda JA, Mankoci S, Zhou X, Murphy WL, Sahai N. Crystal structures of CaSiO3 polymorphs control growth and osteogenic differentiation of human mesenchymal stem cells on bioceramic surfaces. Biomater Sci. 2013;1(10):1101. | ||
Jain SK, Awasthi AM, Jain NK, Agrawal GP. Calcium silicate based microspheres of repaglinide for gastroretentive floating drug delivery: Preparation and in vitro characterization. J Control Release. 2005;107(2):300–309. | ||
Zhang M, Chang J. Surfactant-assisted sonochemical synthesis of hollow calcium silicate hydrate (CSH) microspheres for drug delivery. Ultrason Sonochem. 2010;17(5):789–792. | ||
Bq L, Zhu YJ, Cheng GF, Ruan YJ. Synthesis and application in drug delivery of hollow-core-double-shell magnetic iron oxide/silica/calcium silicate nanocomposites. Mater Lett. 2013;104:53–56. | ||
Webster TJ. The use of superparamagnetic nanoparticles for prosthetic biofilm prevention. Int J Nanomedicine. 2009;4:145–152. | ||
Maleki H, Rai A, Pinto S, et al. High antimicrobial activity and low human cell cytotoxicity of core-shell magnetic nanoparticles functionalized with an antimicrobial peptide. ACS Appl Mater Interfaces. 2016;8(18):11366–11378. | ||
Niemirowicz K, Tokajuk G, Durna B. Magnetic nanoparticles as a drug delivery system that enhance fungicidal activity of polyene antibiotics. Nanomedicine. 2016;12(8):2395–2404. | ||
Cao Z, Yue X, Li X, Dai Z. Stabilized magnetic cerasomes for drug delivery. Langmuir. 2013;29(48):14976–14983. | ||
Mattingly SJ, O’Toole MG, James KT, Clark GJ, Nantz MH. Magnetic nanoparticle-supported lipid bilayers for drug delivery. Langmuir. 2015;31(11):3326–3332. | ||
Feazell RP, Nakayama-Ratchford N, Dai H, Lippard SJ. Soluble single-walled carbon nanotubes as longboat delivery systems for platinum(IV) anticancer drug design. J Am Chem Soc. 2007;129(27):8438–8439. | ||
Chen S, Hao X, Liang X, et al. Inorganic nanomaterials as carriers for drug delivery. J Biomed Nanotechnol. 2016;12(1):1–27. | ||
Park K. Facing the truth about nanotechnology in drug delivery. ACS Nano. 2013;7(9):7442–7447. | ||
El-Boubbou K. Magnetic iron oxide nanoparticles as drug carriers: clinical relevance. Nanomedicine. 2018;13(8):953–971. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
