Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 11
A comparative trial of ice application versus EMLA cream in alleviation of pain during botulinum toxin injections for palmar hyperhidrosis
Authors Alsantali A
Received 24 October 2017
Accepted for publication 21 December 2017
Published 3 April 2018 Volume 2018:11 Pages 137—140
DOI https://doi.org/10.2147/CCID.S155023
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Adel Alsantali
Department of Dermatology, King Fahd Armed Forces Hospital, Jeddah, Saudi Arabia
Background: Botulinum toxin is a safe and effective therapy for palmar hyperhidrosis, but the associated pain from injections limits the usefulness of this method of treatment.
Purpose: To evaluate the efficacy of Eutectic Mixture of Local Anesthetics (EMLA) cream versus ice application in alleviation of pain during botulinum toxin injections for palmar hyperhidrosis.
Methods: In this prospective study, 23 patients underwent palm Botox injections to treat their excessive sweating. In each patient, EMLA cream was applied to one palm and ice was applied directly before the injections in the other palm. Pain was evaluated using a Visual Analog Scale.
Results: Statistically, there was a significant difference in pain control between EMLA cream group and ice application group (p<0.05). The average pain score on the hands where EMLA cream was applied was 8.9 (SD=0.81), whereas it was 4.8 (±0.9) in the ice group.
Conclusion: In this study, the successful use of ice application in reducing pain by 40% in comparison to EMLA cream during Botox toxin injection for palmar hyperhidrosis is demonstrated.
Keywords: ice, local anesthetics, EMLA, palmar hyperhidrosis, Botox injection, pain reduction
Introduction
Hyperhidrosis is an eccrine sweat gland disorder that is characterized by excessive sweating, which goes beyond that required for normal thermoregulation. Any area on the body can be affected by the hyperhidrosis, but the palms, soles, and axillae are the most commonly affected sites. Hyperhidrosis can be classified into primary (idiopathic) and secondary, ie, following other diseases including endocrine disturbances, febrile illnesses, or medication use.1 Also, hyperhidrosis can be classified according to the distribution of affected regions. Palmar hyperhidrosis often causes great emotional distress and occupational disability for the patients. About 2.8% of US population is affected by hyperhidrosis (1.4% axillary and 0.5% palmar), with the condition being equally prevalent in both males and females.2 Many therapeutic options are available for the treatment of primary hyperhidrosis including topical aluminum salts, iontophoresis, systemic anticholinergic drugs, sympathectomy, and local injections of botulinum toxin A (BTX-A).3
BTX-A is effective and safe in treating primary hyperhidrosis.4 BTX-A blocks the release of acetylcholine from the presynaptic nerve terminal, resulting in temporary and reversible local chemodenervation. Because of the intense pain generated by the 30–40 needle punctures needed on each hand, many patients cannot tolerate this pain and refuse this modality of treatment to control the excessive sweating in the palms.
The current methods to counter the pain induced by BTX-A injections into the palms with needles are either inadequate or too invasive. They include topical anesthesia, vibration anesthesia, cryoanalgesia, and nerve block.
Cooling by cold water, ice cubes, and frozen gel packs are often used to reduce pain from a variety of procedures such as laser therapy, injections, and insertion of intravenous catheters.5–7
The aim of the present study was to evaluate the efficacy of ice application in comparison to EMLA cream in reducing pain during BTX-A injections for palmar hyperhidrosis.
Methods
Patients and ethics
Twenty-three patients (13 women and 10 men) with bilateral palmar hyperhidrosis were included in the study. Patients younger than 18 years old, pregnant women, and patients with major psychiatric diseases were excluded. All patients signed the informed consent. The study protocol was approved by the scientific ethics committee in the King Fahd Armed Forces Hospital.
Study design
The study design was a within-patient prospective comparative trial. Coin tossing and concealed envelopes were used to decide on which hand Eutectic Mixture of Local Anesthetics (EMLA) cream was applied (right or left).
Clinical procedure
For each patient, EMLA cream was applied on one hand for 1 hour before the procedure. An ice cube was applied for few seconds (3–5 seconds) immediately before each Botox injection.
We used BTX-A 100 Units (Botox, Allergan, Irvine, CA, USA) diluted in 3 mL NaCl. The injections were administered with a 1 mL syringe, with a 30G needle (microlance 30G × ¾ 0.4×19 mm). The injection volume was 0.1 mL/cm2.
Pain measurement
Pain was assessed by a numeric pain rating scale, where 0 indicated no pain and 10 the worst possible pain. Each patient was asked to self-report the pain intensity during the procedure. The numbers of injections in each palm was counted and the total time taken to administer them, including pauses, was recorded.
Statistical analysis
Data were analyzed using GraphPad Prism 6 Software (Graph Pad Software Inc., La Jolla, CA, USA). All data are presented as the means ± SD of the mean.
The Wilcoxon signed-rank test for paired samples was used to determine whether changes in pain were statistically significant. All analyses were performed using SPSS for windows (SPSS version 11.51, SPSS Inc., Chicago, IL, USA). Statistical tools used to analyze data are as follows:
- Paired samples statistics
- Independent sample t-test
- One-way analysis of variance
Results
Twenty-three patients completed the study. Pain score was assessed immediately after the procedure. Table 1 shows the differences between the two groups (ice side and EMLA cream side). The average pain score on the hands where EMLA cream was applied was 8.9 (9.75–8.12), whereas the average pain score on the hands where ice was applied was 4.8 (5.84–3.90).
  | Table 1 Paired samples statistics Abbreviation: EMLA, Eutectic Mixture of Local Anesthetics. |
The Student’s t-test for the differences between the two groups was 26.79 with p-value (0.000), less than (0.05), which indicates that the differences are statistically significant. Figure 1 shows these results.
  | Figure 1 Mean pain score of the EMLA cream and the ice sides. Abbreviation: EMLA, Eutectic Mixture of Local Anesthetics. |
Table 2 shows the differences between the age in each group and the average pain score according to p-value of t-test. This indicates that there is no statistical difference between age and the application of EMLA or ice group at level (0.05), and means the age does not affect the feeling of pain in the two groups.
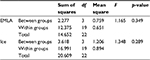  | Table 2 Analysis of variance Abbreviation: EMLA, Eutectic Mixture of Local Anesthetics. |
The pain score for female patients was higher than that for male patients, as shown in Table 3.
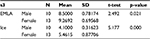  | Table 3 Group statistics Abbreviation: EMLA, Eutectic Mixture of Local Anesthetic. |
During the study, no side effects were reported other than discomfort and pain from ice application noticed in 2 patients out of 23 patients.
Discussion
Palmar hyperhidrosis, a socially embarrassing condition, can be treated with botulinum toxin (BTX). Unfortunately, these injections are very painful in the palms. Topical anesthesia is among the most commonly applied method to reduce the pain during BTX injection. Also, cryoanalgesia can allow injectors to inject BTX for palmar hyperhidrosis without use of local anesthesia. Cooling of the skin is simple and cheap form of anesthesia. The exact mechanism of action of anesthesia by cooling is unknown, but several mechanisms, such as decreased nerve conduction, reduction in muscle spasms, prevention of edema after injury, a decrease in the release of pain-production substances locally, release of endorphins, and pain inhibition through inhibitory interneurons (pain gate), have been suggested.8–10
The present study shows that the application of ice cube for few seconds prior to BTX injection result in a statistically significant pain reduction of 50%–40%. Pain reduction of four or more points on Numerical Rating Scale or 50% or more reduction on the Visual Analog Scale have been described as substantial improvement.11 Bechara et al12 achieved an average pain reduction of around 66% in 10 patients with axillary hyperhidrosis by applying ice cubes in a glove. A possible explanation for these differences might be that the palms are more sensitive to pain compared to the axillae. Skiveren et al13 reported 14%–19% pain reduction by using frozen gel packs during treatment of axillary hyperhidrosis. Bastami et al14 did not find a statistically significant pain reduction by using ice pack before arterial punctures.
Cold-induced analgesia has been shown to begin after the skin surface temperature lowers to ~13.6°C and stops when temperature rises to more than 15.6°C.15
Direct contact with ice cube for few seconds at each site of injection just before injection can decrease skin temperature to the target temperature zone. But using plastic bags or gloves can limit the effect of ice cube as a cryoanalgesic method as it decreases the cooling of skin by ice.
Some forms of cryoanalgesia are more effective than others. For example, France et al16 showed that pain from arterial punctures was not affected using ethyl chloride coolant vapor. But Irkoren et al17 showed that skin cooling with ethyl chloride spray significantly decreases the pain associated during forehead BTX injections. Moreover, crushed ice packs provide more effective cryoanalgesia than cold gel packs or cold water immersion.18,19 In addition, the quality of ice used and the practice of compressing the ice against the skin has a positive effect on skin cooling.20,21 After removal of any cooling method, the skin surface temperature will rise beyond the point of analgesia, so direct contact with ice cube for few seconds just before BTX injection ensures that the skin temperature is in the analgesia zone.
Although cooling can reduce the pain sensation, it can also induce pain and discomfort. Cold-induced pain was noticed in 2 patients. No other side effects have been reported during the study.
Cold-induced urticaria and cryoglobulinemia can be seen upon ice application, so the physician should be aware of these possible adverse effects while using any cooling method of analgesia.22–24
Conclusion
This study showed that the pain reduction achieved by applying ice cubes immediately before BTA injections was clinically and statistically significant and supports the use of cooling as pain relief method for multiple skin injections.
Disclosure
The author reports no conflicts of interest in this work.
References
Khurana R. Acral sympathetic dysfunction and hyperhidrosis. In: Low PA, editor. Clinical Autonomic Disorders. 2nd ed. Philadelphia, PA: LippincottRaven; 1997:809–818. | ||
Strutton DR, Kowalski JW, Glaser DA, Stang PE. US prevalence of hyperhidrosis and impact on individuals with axillary hyperhidrosis: results from a national survey. J Am Acad Dermatol. 2004;51(2):241–248. | ||
Grabell DA, Hebert AA. Current and emerging medical therapies for primary hyperhidrosis. Dermatol Ther (Heidelb). 2017;7(1):25–36. | ||
Rosell K, Hymnelius K, Swartling C. Botulinum toxin type A and B improve quality of life in patients with axillary and palmar hyperhidrosis. Acta Derm Venereol. 2013;93(3):335–339. | ||
Haynes JM. Randomized controlled trial of cryoanalgesia (ice bag) to reduce pain associated with arterial puncture. Respir Care. 2015;60(1):1–5. | ||
Watkins AA, Johnson TV, Shrewsberry AB, et al. Ice packs reduce postoperative midline incision pain and narcotic use: a randomized controlled trial. J Am Coll Surg. 2014;219(3):511–517. | ||
Fang L, Hung CH, Wu SL, Fang SH, Stocker J. The effects of cryotherapy in relieving postarthroscopy pain. J Clin Nurs. 2012;21(5–6):636–643. | ||
Ernst E, Fialka VJ. Ice freezes pain? A review of the clinical effectiveness of analgesic cold therapy. Pain Symptom Manage. 1994;9:56–59. | ||
Algafly AA, George KP. The effect of cryotherapy on nerve conduction velocity, pain threshold, and pain tolerance. Br J Sports Med. 2007;41(6):365–369. | ||
Herrera E, Sandoval MC, Camargo DM, Salvini TF. Motor and sensory nerve conduction are affected differently by ice pack, ice massage, and cold water immersion. Phys Ther. 2010;90(4):581–559. | ||
Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9:105–121. | ||
Bechara FG, Sand M, Altmeyer P, Sand D, Hoffmann K. Skin cooling for botulinum toxin A injection in patients with focal axillary hyperhidrosis: a prospective, randomized, controlled study. Ann Plast Surg. 2007;58(3):299–302. | ||
Skiveren J, Kjaerby E, Nordahl Larsen H. Cooling by frozen gel pack as pain relief during treatment of axillary hyperhidrosis with botulinum toxin a injections. Acta Derm Venereol. 2008;88(4):366–369. | ||
Bastami M, Azadi A, Mayel M. The use of ice pack for pain associated with arterial punctures. J Clin Diagn Res. 2015;9(8):JC07–JC09. | ||
Bugaj R. The cooling, analgesic, and rewarming effects of ice massage on localized skin. Phys Ther. 1975;55:11–19. | ||
France JE, Beech FJ, Jakeman N, Benger JR. Anaesthesia for arterial puncture in the emergency department: a randomized trial of subcutaneous lidocaine, ethyl chloride or nothing. Eur J Emerg Med. 2008;15(4):218–220. | ||
Irkoren S, Ozkan HS, Karaca H. A clinical comparison of EMLA cream and ethyl chloride spray application for pain relief of forehead botulinum toxin injection. Ann Plast Surg. 2015;75(3):272–274. | ||
Kanlayanaphotporn R, Janwantanakul P. Comparison of skin surface temperature during the application of various cryotherapy modalities. Arch Phys Med Rehabil. 2005;86(7):1411–1415. | ||
Chesterton LS, Foster NE, Ross L. Skin temperature response to cryotherapy. Arch Phys Med Rehabil. 2002;83(4):543–549. | ||
Kennet J, Hardaker N, Hobbs S, Selfe J. Cooling efficiency of 4 common cryotherapeutic agents. J Athl Train. 2007;42(3):343–348. | ||
Dykstra JH, Hill HM, Miller MG, Cheatham CC, Michael TJ, Baker RJ. Comparisons of cubed ice, crushed ice, and wetted ice on intramuscular and surface temperature changes. J Athl Train. 2009;44(2):136–141. | ||
Greaves MW. Pathology and classification of urticaria. Immunol Allergy Clin North Am. 2014;34(1):1–9. | ||
Retamozo S, Brito-Zerón P, Bosch X, Stone JH, Ramos-Casals M. Cryoglobulinemic disease. Oncology (Williston Park). 2013;27(11):1098–1105, 1110–1116. | ||
Ferri C. Mixed cryoglobulinemia. Orphanet J Rare Dis. 2008;3:25. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
