Back to Journals » Degenerative Neurological and Neuromuscular Disease » Volume 14
Transient Neonatal Myasthenia Gravis Born to a Mother with Asymptomatic MG: A Case Report
Authors Yang J, Pan L, Liu Y, Wang Y
Received 5 December 2023
Accepted for publication 26 February 2024
Published 1 March 2024 Volume 2024:14 Pages 15—19
DOI https://doi.org/10.2147/DNND.S451611
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Thomas Müller
Jinrong Yang,* Liping Pan,* Yaping Liu, Yanrong Wang
Department of Neonatology, Shenzhen Third People’s Hospital, The Second Affiliated Hospital of Southern University of Science and Technology, Shenzhen, Guangdong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yanrong Wang, Department of Neonatology, Shenzhen Third People’s Hospital, The Second Affiliated Hospital of Southern University of Science and Technology, 29 Buji Bulan Road, Shenzhen, Guangdong Province, 518112, People’s Republic of China, Tel +86-13925271719, Email [email protected]
Abstract: Myasthenia gravis (MG) is an autoimmune disease which can impact pregnancy. We describe a transient neonatal myasthenia gravis (TNMG) born to an asymptomatic mother aged 26. The newborn presented cyanosis and generalized muscular weakness quickly after birth. Nasal continuous positive airway pressure (nCPAP) ventilation was performed immediately. On day 3, detailed family history showed that the neonate’s 50-year-old maternal grandmother was diagnosed as ocular MG at the age of 40. Ryanodine receptor calcium release channel antibody (RyR-Ab) and acetylcholine receptor antibody (AChR-Ab) tested on day 5 were positive. However, neostigmine tests were negative for the neonate. Intravenous immunoglobulin (IVIG) and oral pyridostigmine were administered. The infant was weaned from the ventilator on day 7. On day 10, the neonate’s asymptomatic mother was confirmed to have positive AChR-Ab either. The neonate regained the capability of bottle feeding on day 17 and discharged on day 26. Asymptomatic pregnant women with MG family history can also deliver infants who develop TNMG. Testing AChR antibodies in pregnant women with a family history of MG should be necessary as TNMG was a life-threatening disease. With timely diagnosis and accurate treatment, TNMG can be effectively relieved.
Keywords: transient neonatal myasthenia gravis, asymptomatic MG, IVIG, pyridostigmine
Background
Transient neonatal myasthenia gravis (TNMG) is considered as a rare disorder and may be present in 10–15% of newborns born to women with MG, either active or, less commonly, in remission.1 It is an antibody-mediated disease caused by the transplacental transmission of maternal antibodies directed against the acetylcholine receptor (AChR) and, less frequently, muscle-specific kinase (MuSK), resulting in impaired neuromuscular transmission.2 TNMG’s mainly clinical manifestation is cyanosis and poor feeding, usually resolves in the first two months of life. Respiratory muscles may be involved leading to respiratory distress which ventilatory support is required. Here, we present a case of a neonate with AChR-antibody positive showing severe generalized muscular weakness who needed tube feeding and ventilatory support and pharmaceutic treatment.
Case Report
A 3350g neonate, the first child of non-consanguineous parents, was transferred to the neonatal intensive care unit (NICU) 32 minutes after birth due to dyspnea. He was born to a 26-year-old G1P1 mother after a well-followed pregnancy, in which the mother felt normal fetal movements. The mother was diagnosed with hepatitis B carrier one year before and was asymptomatic without treatment during pregnancy.
A cesarean section, due to cephalopelvic disproportion and abnormal results of fetal heart rate monitoring, was performed at 39+3 weeks of gestation. The delivery was uneventful, with Apgar scores of 8 and 8 at one and five minutes, respectively. Ten minutes after birth, he was cyanotic, apathetic and had mild grunting.
On admission to the NICU, the vital signs were as follows: temperature 36.5°C, pulse 150 beats/min, blood pressure 68/48 mmHg, respiratory rate 65 breaths/min and arterial oxygenation 80%. The sucking, swallowing, moro and grasp reflexes were decreased or absent (Figure 1A). He remained apathetic and required gastric tube mix feeding and intermittent oral suction due to inability to clear airway secretions. Nasal continuous positive airway pressure (nCPAP) ventilation was performed immediately, and low ventilatory parameters were rapidly achieved (oxygen concentrate 21%, PEEP 6cm H2O) (Figure 1B).
As the clinical symptoms of the neonate might attribute to several causes, an extended diagnostic tests were performed, including blood glucose; liver, kidney, and thyroid function; muscle enzymes; arterial blood gas; infection and coagulation parameters; ammonia in blood and urine; TORCH in blood; chest x-ray, video electroencephalogram, cerebral ultrasound and cerebral computer tomography (CT). No abnormalities were found except that the blood gases revealed respiratory acidosis and elevated creatine kinase (682U/L, normal<170U/L) properly revealed myopathy.
On day 3, we were informed that the neonate’s 50-year-old maternal grandmother had a 10-year history of ocular myasthenia gravis (OMG), serologic testing for MG antibodies were performed including muscle-specific receptor tyrosine kinase receptor antibody (MuSK-Ab), Titin antibody (Titin-Ab), ryanodine receptor calcium release channel antibody (RyR-Ab), acetylcholine receptor antibody (AChR-Ab) and lipoprotein receptor-related membrane protein 4(LRP-4). On day 5, AChR-Ab (13.72 nmol/L, normal<0.40) and RyR-Ab showed positive by Enzyme-linked immunosorbent assay (ELISA). Prostigmine test result was negative but he was diagnosed with TNMG according to the clinical characteristics and the serological examination. A lower AChR-Ab titer (3.03nmol/L) was found in the asymptomatic mother on day 10 (Table 1).
 |
Table 1 Muscle-Specific Antibody Receptor for MG Between the Neonate and Mother |
On day 7 to 9, high dose intravenous immunoglobulin (IVIG,2.2g/kg.total) and oral pyridostigmine were administered (5mg/each time, 4 times a day for 4 days,3mg/each time, 8 times a day for 7 days later, and then reduced to 5mg/each time, 4 times a day for 3 days, maintenance 5mg/each time, 3 times a day at last) (Table 2). The infant was successfully weaned from the ventilator with spontaneous movements enhancement. He started bottle feeding at 17 days of age. A follow-up test showed that the CK is normal within one week. AChR-Ab decreased from 13.72 nmol/L to 5.80 nmol/L after the treatment and thorax CT revealed swollen thymus by 25 days of age. He was discharged home on day 26 with disappearance of clinical symptoms (Figure 1C) and no abnormality was found in whole-exome sequencing. On follow-up one month later, he had no neurologic abnormalities with muscular tension similar to a normal baby from a video. The neonate oraled pyridostigmine 1.5 months more after discharge. The boy had normal gross motor function development except a little poor head erection when 3 months old. Figure 2 shows the timeline of clinical symptoms and interventions.
 |
Table 2 Details of Drug Use in This Case |
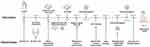 |
Figure 2 Timeline of clinical symptoms and interventions. |
Discussion
MG is an autoimmune disease, characterized by fatigue ability of skeletal muscles and muscle weakness.3 The disease is antibody-mediated, caused by auto-antibodies targeting components of the neuromuscular junction. The majority of patients (about 85%) have antibodies against the muscle AChR.4 TNMG presents shortly after birth in infants of mothers who have autoimmune MG. The circulating maternal auto antibodies cross the placenta to the fetus, leading to an affected child after birth. TNMG can be present in 10–15% of newborns born to women with MG5,6 either active or, less commonly, in remission.2 So far, there seems to be no report about a mother who has not been diagnosed and has no symptoms giving birth to a neonatal MG.
TNMG is one of the few treatable neuromuscular disorders in newborns. It is a self-limited but potentially life-threatening disease without prompt diagnosis, adequate respiratory support, and proper treatment. To evaluate a hypotonia infant is a challenge, but a good detailed family history always contributes to the diagnosis, as well as the case.
It is reported that TNMG can occur in those babies of MG mothers with AChR and MuSK antibodies, but also in patients without detectable muscle antibodies. There is no direct correlation between severity of mother’s MG and risk for TNMG, nor is there a correlation to antibody concentration in the mother.7 It showed that antibody-depleting therapy (IVIG and pyridostigmine) resulted in a lower concentrate AChR-Ab which contributed to a clinical improvement in our case.
The infant pulled through and developed well after the neonatal period. In order to verify the diagnosis and therapeutic effect, we performed MG antibody detection again after treatment on day 26 which showed decreased AChR-Ab titer. In combination with the clinical presentation, we diagnosed TNMG (TNMG). It is perplexing that our neonate had a higher concentrate AChR-Ab titer than the mother and RyR-Ab was negative in the mother whereas positive in the neonate. The mother was asymptomatic before, during, and after pregnancy. The genetic mechanism for the TNMG case may be intriguing. Besides, RyR-Ab is considered to be present in the MG patients, particularly those with thymoma.8 Ocular myasthenia gravis (OMG) and family history showed on other autoimmune disorders such as Von Hippel-Lindau Disease (VHL).9 Whether the neonate’s swollen thymus will develop to thymoma or normal is still unknown. A long-term follow-up is necessary.
Conclusions
The case has important clinical management value to pediatricians and obstetricians. It indicates that asymptomatic pregnant women with MG family history can deliver infants who may develop TNMG. Testing AChR antibodies in pregnant women with a family history of MG may be necessary. Too much emphasis is given to family history. If antibodies in mother are present, clinical monitoring and treatments of the neonate is probably necessary during and after birth.
Data Available Statement
All the necessary data that help the results of the case report are incorporated in the manuscript.
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. Informed consent was obtained from the neonate’s parents for the publication of this case report. The study has received ethical approval from Ethics Committee of Shenzhen Third People’s Hospital (no. 2022-083-02).
Acknowledgment
We would like to thank postdoctoral research fellow Mengyun Cai from the Institute of Translational Medicine of the First Poeple’s Hospital of Foshan for guiding us in drawing figures and tables. We are grateful to the patient and her family for consenting to this study.
Author Contributions
All authors made a significant contribution to the case reported, whether that is in the conception, study design, acquisition of data, execution, analysis and interpretation; took part in drafting, revising and critically reviewing the article; all authors have agreed on the journal to which the article has been submitted.
Funding
This work is supported by the Foundation (no. 22240G1034) of Shenzhen Third People’s Hospital.
Disclosure
None of the authors has any conflict of interest to disclose for this work.
References
1. Peragallo JH. Pediatric myasthenia gravis. Semin Pediatr Neurol. 2017;24(2):116–121. doi:10.1016/j.spen.2017.04.003
2. Hamel J, Ciafaloni E. An update: myasthenia gravis and pregnancy. Neurol Clin. 2018;36(2):355–365. doi:10.1016/j.ncl.2018.01.005
3. Gilhus NE, Skeie GO, Romi F, Lazaridis K, Zisimopoulou P, Tzartos S. Myasthenia gravis -autoantibody characteristics and their implications for therapy. Nat Rev Neurol. 2016;12(5):259–268. doi:10.1038/nrneurol.2016.44
4. Lazaridis K, Tzartos SJ. Autoantibody Specificities in Myasthenia Gravis; Implications for Improved Diagnostics and Therapeutics. Front Immunol. 2020;11:212–224. doi:10.3389/fimmu.2020.00212
5. Belasco C, Carbillon L, Louaib D, Gaudelus J, Uzan M. Neonatal myasthenia gravis. Arch Pediatrie. 2000;7(3):263–266. doi:10.1016/S0929-693X(00)88742-4
6. Hoff JM, Daltveit AK, Gilhus NE. Myasthenia gravis in pregnancy and birth: identifying risk factors, optimising care. Eur J Neurol. 2007;14:38–43. doi:10.1111/j.1468-1331.2006.01538.x
7. Townsel C, Keller R, Johnson K, et al. Seronegative maternal ocular myasthenia gravis and delayed transient neonatal myasthenia gravis. AJP Rep. 2016;6(01):e133–e136. doi:10.1055/s-0036-1579624
8. Takamori M, Motomura M, Kawaguchi N, et al. Anti-ryanodine receptor antibodies and FK506 in myasthenia gravis. Neurology. 2004;62(10):1894–1896. doi:10.1212/01.wnl.0000125254.99397.68
9. Norata D, Peri M, Giammalva GR. Immunological aspects of von Hippel-Lindau disease: a focus on neuro-oncology and myasthenia gravis. Diagnostics. 2023;13(1):144. doi:10.3390/diagnostics13010144
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

