Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 19
Therapeutic Effects of Lifei Decoction in a Murine Model of COPD Induced by LPS and Cigarette Smoke
Authors Lu L, Zhu C, Xu J, Hu Y, Dai J, Wang S, Wei T
Received 26 November 2023
Accepted for publication 12 March 2024
Published 18 April 2024 Volume 2024:19 Pages 957—967
DOI https://doi.org/10.2147/COPD.S449521
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Liguo Lu,1,2 Chengdong Zhu,3 Jian Xu,4 Yulan Hu,2 Juxiang Dai,2 Sheng Wang,2 Tao Wei1,5
1Jiangsu Key Laboratory of Immunity and Metabolism, Department of Pathogenic Biology and Immunology, Xuzhou Medical University, Xuzhou, People’s Republic of China; 2Department of Respiratory and Critical Care Medicine, Shuyang Hospital, The Affiliated Shuyang Hospital of Xuzhou Medical University, Suqian, People’s Republic of China; 3Department of Traditional Chinese Medicine, Shuyang Hospital, The Affiliated Shuyang Hospital of Xuzhou Medical University, Suqian, People’s Republic of China; 4Taian Maternal and Child Health Hospital, Tai An, Shandong, People’s Republic of China; 5Public Experimental Research Center, Xuzhou Medical University, Xuzhou, Jiangsu, People’s Republic of China
Correspondence: Tao Wei, 209 Tongshan Road, Xuzhou City, Jiangsu Province, People’s Republic of China, Tel +8613815303574, Email [email protected]
Introduction: The Lifei Decoction (LD) is a commonly utilized Chinese medicine for the treatment of sepsis and bronchial inflammation. However, its therapeutic potential in chronic obstructive pulmonary disease (COPD) remains unknown. Therefore, the objective of this study was to investigate the therapeutic efficacy and underlying mechanism of LD in a mouse model of COPD induced by cigarette smoke (CS) combined with lipopolysaccharide (LPS).
Methods: Hematoxylin-eosin (H&E) staining was employed to observe the pathological alterations in lung tissue, while ELISA was utilized for the detection of levels of inflammatory factors in both lung tissue and bronchoalveolar lavage fluid (BALF). Additionally, Western blot analysis was conducted to assess the expression of p-NF-κB, GDF11, ZO-1, and Occludin-1 proteins. The changes in intestinal flora were evaluated using the viable bacteria count method.
Results: The administration of LD demonstrates significant efficacy in mitigating pulmonary tissue damage in a murine model, while concurrently inhibiting the activation of the inflammatory pathway NF-κB to attenuate the levels of pro-inflammatory factors. Moreover, LD exhibits the capacity to enhance the expression of intestinal functional proteins ZO-1 and Occludin-1, thereby rectifying dysbiosis within the gut microbiota.
Conclusion: The LD shows great promise as a potential treatment for COPD.
Keywords: COPD, lifei decoction, inflammation, intestinal flora
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the chronic respiratory disorders worldwide, with a global incidence rate of 11.7%, resulting in approximately 3.2 million deaths annually.1 In China, it has emerged as the fourth leading cause of mortality, imposing a substantial economic burden on society.2 This condition typically arises from prolonged exposure to toxic particles or gases, triggering abnormal inflammation in the airways or lungs and subsequently impairing alveolar function.3 Within modern society, cigarette smoking (CS) stands out as a critical risk factor for COPD due to its inclusion of reactive oxygen and other chemical components that activate non-specific inflammatory responses, thereby exacerbating disease progression.3 The prevalence of COPD in long-term heavy smokers has been discovered to reach as high 50%, and the mitigation of inflammation can control symptom management and impede disease progression.1 Currently employed treatment options for COPD encompass cough expectorants, glucocorticoids, bronchodilators, among others.4 However, their effectiveness remains limited or they may induce severe side effects. Consequently, there is an urgent need to discover safer and more efficacious anti-COPD medications.
In addition to inflammation, recent studies have revealed a close association between an imbalance in intestinal flora and the occurrence of respiratory diseases, including asthma, COPD, and lung cancer.5,6 It has been observed that the composition differs of gut microbiota between COPD patients and healthy individuals.7 The fecal microbiota transplantation experiment further demonstrated that the dysbiosis of gut microbiota aggravated the symptoms of COPD in mice.8 These findings strongly substantiate the pivotal role of gut microbiota in the advancement of COPD. The dysbiosis of intestinal microbiota can impair the integrity of the intestinal barrier, thereby increasing the permeability of the mucosal lining and allowing bacteria or their metabolites, such as lipopolysaccharide (LPS), to enter the systemic circulation.9 The LPS can then modulate the inflammatory immune response in the lungs, inducing or exacerbating COPD progression.8 The modulation of the gut microbiota and its metabolites, therefore, presents a highly promising therapeutic approach for the progression.
Traditional Chinese medicine offers advantages in alleviating symptoms of COPD, reducing the frequency of acute exacerbations, and enhancing quality of life.10 Lifei Decoction (LD) is an empirical prescription in traditional Chinese medicine that comprises Rheum officinale, mirabilite, Magnolia officinalis, Forsythia, Scutellaria, almond, Bletilla striata and notoginseng.11 The studies have demonstrated that LD effectively inhibits the activation of NF-κB, a pivotal inflammatory pathway, and significantly reduces the levels of pro-inflammatory cytokines IL-Iβ, IL-6, and TNF-A.11 It exhibits significant therapeutic effects in conditions such as sepsis, bronchial inflammation, and bronchial asthma primarily due to its exceptional anti-inflammatory properties.11 Additionally, research has demonstrated that LD possesses the ability to restore the impaired intestinal barrier in sepsis-induced rats through modulation of ZO-1 and Occludin-1 proteins expression.12 Considering its beneficial role in anti-inflammation and intestinal repair mechanisms, LD holds promise as a potential strategy for COPD treatment.
In this study, a mouse model of COPD was established by exposing the mice to CS and stimulating them with LPS. The therapeutic efficacy of LD on COPD mice and its potential mechanism were assessed by evaluating lung tissue pathology, levels of inflammatory factors, expression of key proteins, and intestinal flora in the mice. This study aims to identify a safer and more effective treatment for COPD.
Materials and Methods
Drug and Reagents
The Enzyme-linked immunosorbent assay (ELISA) kits for IL-1β, IL-6 and TNF-α were procured from Quanzhou Ruixin Biotechnology Co., LTD (Quanzhou, China). The INF-γ ELISA kit was acquired from Shanghai Coibo Biotechnology Co., LTD (Shanghai, China). The primary antibodies targeting proteins GDF11, p-NF-κB, NF-κB, Occludin, ZO-1, and β-actin were acquired from Proteintech Group, Inc (Wuhan, China). Dexamethasone (DEX) was purchased from Shanghai Aladdin Biochemical Technology Co., LTD. (Shanghai, China). The lipopolysaccharide (LPS)was procured from MedChemExpress Inc. (Shanghai, China). The Lifei Decoction was provided by Shuyang People’s Hospital Affiliated to Xuzhou Medical University.
Preparation of Lifei Decoction
The main components of Lifei Decoction included Rheum officinale, mirabilite, Magnolia officinalis, Forsythia, Scutellaria, almond, Bletilla striata and notoginseng. The extraction process was conducted as follows: Rheum officinale, mirabilite, Magnolia officinalis, Forsythia, Scutellaria, almond, Bletilla striata and notoginseng (in a quality ratio of 2:1:1:1:1:1:1:1) were immersed in water. The crude drug should be mixed with 20mL of distilled water per gram and subjected to boiling for 1 hour in order to concentrate the filtrate to a concentration of 1 g/mL (representing the content of crude drug in the decoction). Finally, the decoction was stored at 4°C for future use.
Animals
The adult male ICR mice, weighing 20±2 g, were obtained from Shanghai Laboratory Animal Co., LTD. They were housed in a specific pathogen-free (SPF) environment and provided with ad libitum access to food and water on a 12-hour light/dark cycle. All experimental procedures were approved by the Animal Ethics Committee of Xuzhou Medical University and complied with the guidelines outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Following a one-week acclimation period, 40 mice were randomly assigned into four groups (each consisting of ten mice): Control group (exposed to normal air and administered normal saline orally); COPD group (exposed to CS and treated with LPS); DEX group (exposed to CS, treated with LPS, and administered DEX orally); LD group (exposed to CS, treated with LPS, and administered LD orally). The COPD mice model was established in the COPD, LD, and DEX groups by intratracheal instillation of LPS (7.5 μg dissolved in 50 μL saline) on days 1 and 14 and continuous exposure to CS for 12 weeks. According to Li et al, In the LD group, oral administration of LD (1.73 g/kg/day) was initiated at week 8 and continued for a duration of 4 weeks. The DEX group commenced oral administration of DEX (1 mg/kg/day) at week 10 for a period of 2 weeks.13 Saline of equal volume was given to Control and COPD groups. Commercially available Plum brand cigarettes (Guangdong Tobacco Industry Company, China), producing 11 mg of tar, 1.0 mg of nicotine, and 13 mg of CO per cigarette were used for establishing the mouse COPD model. Body weights of mice were recorded every biweekly throughout the experimental period.
The Pathological Examination of Lung Tissue
The lower lobe tissue of the right lung in mice was fixed in 4% paraformaldehyde for a duration exceeding 48 hours. Subsequently, the specimens were dehydrated and embedded in paraffin before being sectioned into slices with a thickness of 4 μm. These sections were then subjected to deparaffinization and stained using hematoxylin-eosin (H&E) following the instructions provided by the reagent manufacturer (Beyotime Biotechnology, China) Images were captured using an upright microscope (Olympus,Tokyo,Japan), and the extent of lung tissue damage was assessed based on Chunhua et al’s study.14
Bronchoalveolar Lavage Fluid Analysis
The left lobe lung tissue was washed twice with 1mL of phosphate-buffered saline (PBS). Subsequently, the collected bronchoalveolar lavage fluid (BALF) samples were placed on ice and centrifuged at 3000 rpm, 4°C for 10 minutes. The resulting cell precipitate was then resuspended in 0.3mL of PBS containing 0.1% fetal bovine serum (FBS). Neutrophils, macrophages, and lymphocytes were subsequently quantified using an automated hematology analyzer (Tecom, China).
Cytokine Assays
The mouse lung tissues were homogenized in ice-cold RIPA lysate, followed by incubation on ice for 30 minutes. Subsequently, the lysate was centrifuged at 12,000 rpm for 15 minutes at 4 °C to collect the supernatant containing total protein. Protein concentrations were determined using the BCA kit (Beyotime Biotechnology, Cat.No.: P0013B). The samples of BALF were obtained following the Method 2.5. Subsequently, the levels of IL-1β, IL-6, TNF-α, and INF-γ in these samples were determined according to the instructions provided by the ELISA kit.
Western Blot Analysis
The proteins from mouse lung and small intestine tissues were obtained following the protocol outlined in Method 2.6. Afterwards, the target proteins were separated by 10% SDS-PAGE and transferred onto nitrocellulose membranes. Following blocking, the membranes were incubated overnight at 4 °C with primary antibodies GDF11, p-NF-κB, NF-κB, Occludin, ZO-1 (all diluted 1:2000), and β-actin (diluted 1:10,000), followed by incubation with horseradish peroxidase-conjugated secondary antibodies for 2 hours at room temperature. After washing the membrane with TBST solution, images were captured using the Odyssey infrared fluorescence imaging system. Quantitative analysis was performed using Image J software.
Quantitative Real-Time PCR
The isolation of total RNA from mouse lung tissues was conducted utilizing Trizol (Thermo Fisher Scientific, USA), followed by cDNA synthesis employing a transcription kit ((Thermo Fisher Scientific, USA)). The real-time PCR analysis was conducted using the CFX96-C1000 system (Bio-Rad, Hercules, CA) and the QuantiTect SYBR Green RT-PCR kit (Qiagen, Germany). The expression levels of target genes were normalized against those of GAPDH and calculated using the 2−ΔΔCt method. The primers are listed in Table 1.
 |
Table 1 Primer Sequences for Quantitative Real-Time PCR Analysis |
Analysis of Gut Microbiota in Mice
After sacrificing the mice, fresh feces were collected and vigorously shaken in a centrifuge tube containing diluent for 5 minutes at a speed of 2,000 revolutions per minute (rpm). Subsequently, 10 μL of each shaken sample was inoculated onto the prepared specialized medium and evenly spread using an L-shaped rod. The culture media for Enterobacteriaceae and Enterococcus were incubated at 37°C for 24 hours, while the Bifidobacterium and Lactobacillus culture media were placed in anaerobic bags and incubated at 37°C for 48 hours. Suitable colonies were selected for colony counting, with results expressed as CFU/g.
Statistical Analysis
SPSS 21.0 software was utilized for data analysis, and the results were presented as Mean ± SEM. One-way analysis of variance (ANOVA) was employed to compare multiple groups. The LSD test was applied for assessing normal distribution and homogeneity of variance, while Dunnett’s T3 test was used in cases of heterogeneity of variance. Statistical significance was considered at P < 0.05 level. Graph Pad Prism 8.0 software was utilized to generate graphical representations.
Results
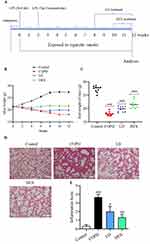
The Administration of LD Effectively Ameliorated the Structural Damage to Lung Tissue in COPD Mice
Patients with COPD commonly experience weight loss.15 The present study established a mouse model of chronic obstructive pulmonary disease (COPD), as depicted in Figure 1A, and monitored the weight fluctuations of the mice throughout the experimental period. It was observed that mice in the Control group exhibited gradual weight gain, while mice in the COPD group continued to experience weight loss. Conversely, administration of LD prevented this weight loss (Figure 1B and C). Furthermore, COPD mice displayed symptoms such as dull hair, reduced mobility, and respiratory distress, while these symptoms significantly improved after LD treatment, which aligned with the outcomes seen with DEX treatment. Next, H&E staining of lung tissue was conducted to evaluate the pathological damage. The findings revealed that in comparison with the Control group, the COPD group exhibited significantly thickened mucosal epithelium, along with a substantial infiltration of monocytes and neutrophils into the alveoli (Figure 1D), and also exhibited significantly higher lung injury scores (Figure 1E), which were effectively attenuated by LD treatment. The findings revealed the advantageous impact of LD on the treatment of COPD.
The LD Treatment Led to a Significant Improvement in Inflammation in COPD Mice
To evaluate the impact of LD on lung inflammation in COPD mice, we initially examined the quantity of inflammatory cells present in BALF samples. The findings indicate that inflammatory cells, including neutrophils, macrophages and lymphocytes, was significantly higher in the BALF of COPD group compared to Control group (Figure 2A). Correspondingly, levels of IL-1β, IL-6, TNF-α and INF-γ were also notably elevated in both BALF (Figure 2B) and lung tissue (Figure 2C) from COPD group as opposed to Control group, following administration of LD and D treatments, not only were inflammatory cell counts reduced but levels of associated cytokines within were also significantly decreased. These results suggest that LD may measure against progression of COPD. The results imply that LD exerts a protective the progression of COPD through its anti-inflammatory properties.
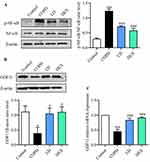
The Impact of LD on NF-κB and GDF11 Protein Levels in the Lung Tissue of COPD Mice
Due to the significant inhibitory effect of LD on cytokines observed, we further investigated its potential mechanism of suppressing inflammation. NF-κB is a classical inflammatory regulatory pathway that plays a crucial role in immune response of various cells and organs. In this study, the p-NF-κB protein in lung tissue was examined via Western blot analysis, revealing a significant increase in the COPD group (Figure 3A). However, administration of LD and DEX resulted in inhibition of this protein’s expression, suggesting that LD may effectively reduce inflammation in COPD by suppressing NF-κB activation. GDF11 belongs to the transforming growth factor beta protein family studies have shown its close association with COPD development. Our findings revealed not only a substantial down-regulation of GDF11 protein (Figure 3B) but it’s also mRNA levels (Figure 3C) in lung tissues of COPD mice, both of which were attenuated by LD and DEX administration. These results suggest that LD may improve COPD by restoring GDF11 levels.
The Modulation of LD on Gut Microbiota with COPD Mice
Accumulating evidence suggests that the gut microbiota and/or its metabolites play a crucial role in the development of pulmonary inflammatory responses through the gut-lung axis.16 To investigate whether the beneficial effect of LD on COPD is associated with the regulation of gut microbiota, we employed selective medium to assess changes in specific bacterial populations including Enterococcus, Enterobacter, Lactobacillus, and Bifidobacterium. The results revealed that compared to the Control group, there was a significant increase in Enterobacteriaceae and Enterococcus levels in the intestinal tract of the COPD group, while Bifidobacterium and Lactobacillus levels were significantly decreased. However, imbalances were reversed by LD and DEX treatment, indicating an increase in beneficial bacteria and a decrease in opportunistic bacteria following LD treatment (Table 2).
 |
Table 2 The Enumeration of Gut Microbiome |
The imbalance of intestinal flora has been found to be prone to damaging the integrity of the intestinal barrier, thereby facilitating the translocation of LPS from the gut into the bloodstream and ultimately exacerbating COPD development. Here, we conducted a Western blot analysis to assess the levels of ZO-1 and occludin-1, which are key regulatory proteins involved in maintaining the functionality of the intestinal barrier. As depicted in Figure 4, the protein levels of ZO-1 and occludin-1 in the COPD group were significantly lower compared to those in the Control group. However, following LD treatment, these protein levels were markedly elevated. The findings suggest that LD can enhance the integrity of the intestinal barrier by upregulating tight junction-related proteins, thereby preventing the progression of COPD.
Discussion
Chronic obstructive pulmonary disease (COPD) is a chronic inflammatory disease of the airways characterized by obstruction and abnormal inflammation in the lungs.17 Prolonged exposure to cigarette smoke (CS) can result in persistent inflammation in the lung tissue and irreversible damage, which is considered a major factor in the development of COPD.18 Lipopolysaccharide (LPS) is a main proinflammatory component found in the cell wall of Gram-negative bacteria. Exposure to LPS can trigger acute inflammatory responses, contributing to the development and progression of various lung diseases, including asthma and COPD.19,20 Many studies have utilized a combination of CS exposure and LPS treatment to establish a COPD model in mice.20,21 In this study, the same method was employed to establish a mouse model of COPD, and then model mice exhibited weight loss following prolonged CS exposure, which is consistent with previous research and clinical manifestations of COPD. Subsequent histopathological analysis also confirmed severe lung tissue damage in the model mice. Treatment with LD and DEX not only prevented weight loss in the mice but also significantly improved lung tissue damage, providing preliminary evidence for the potential therapeutic use of LD in COPD treatment.
Clinically, the inhibition of inflammatory response and the discovery of novel targeted inhibitors have emerged as the primary focus of COPD therapy in recent decades.22 Extensive research has demonstrated that the infiltration of inflammatory cells, such as neutrophils, macrophages, and lymphocytes, plays a pivotal role in the accumulation of airway mucus and inflammation.23 These cells secrete various cytokines, serine proteases, and reactive oxygen species (ROS), which contribute to alveolar destruction and airway remodeling.24 In this study, a substantial increase in the number of neutrophils, macrophages, and lymphocytes in bronchoalveolar lavage fluid (BALF) of the model mice was observed. Furthermore, compared to the Control group, the levels of inflammatory markers, including IL-1β, IL-6, TNF-α, and INF-γ, in both BALF and lung tissue of the model group were significantly elevated. However, treatment with LD not only reduced the recruitment of inflammatory cells but also inhibited the secretion of inflammatory cytokines. These findings provide compelling evidence supporting the potential of LD to ameliorate COPD by suppressing inflammation. The nuclear factor kappa B (NF-κB) signaling pathway is a well-established molecular pathway that promotes the production of pro-inflammatory cytokines.25 Previous studies by Fan et al have demonstrated that the improvement of COPD by the resveratrol derivative Amurensin H is closely associated with the inhibition of NF-κB.26 Consistent with these findings, our data support the aforementioned conclusion, as the results of Western blot analysis reveal that LD significantly reduces the expression of phosphorylated NF-κB, indicating that its anti-inflammatory effects are mediated through the inhibition of NF-κB activation.
Growth differentiation factor 11 (GDF11) represents a pivotal member of the transforming growth factor β protein family, initially discovered by Nakashima et al in 1999.27 Emerging research has substantiated the crucial regulatory role of GDF11 in metabolic disorders, cancer, and COPD.28,29 Notably, Onodera et al have reported significantly diminished plasma GDF11 levels in COPD patients compared to healthy individuals.29 Furthermore, Tang et al have demonstrated that cigarette smoke extract expedite COPD progression by suppressing GDF11 expression, and similar outcomes were observed upon GDF11 knockdown.30 These compelling findings suggest that GDF11 may serve as a novel therapeutic target for COPD treatment. In our present investigation, we have observed a significant reduction in both GDF11 protein and mRNA levels in the lung tissue of the COPD mice, which were effectively restored upon LD administration. This intriguing observation suggests that LD exerts its therapeutic effects on COPD by modulating the intricate regulatory network of GDF11 expression. However, further comprehensive data are required to substantiate whether GDF11 is the target of LD.
The gastrointestinal tract harbors a dynamic and intricate ecosystem of microbial communities, playing a pivotal role in maintaining the delicate equilibrium of the host immune system.31 Given that distinct bacterial species can elicit diverse immune cell responses, ranging from pro-inflammatory to anti-inflammatory effects, any perturbations in the intestinal microbiota can precipitate disease or incite inflammatory infections.32,33 Compelling evidence substantiates the profound impact of intestinal dysbiosis on the progression of COPD via the intricate “gut-lung axis”34 Notably, smoking, a primary etiological factor of COPD, exerts deleterious effects on the gastrointestinal system, thereby promoting intestinal pathologies.35 Intriguingly, approximately 50% of patients afflicted with inflammatory bowel disease exhibit pulmonary inflammation or compromised lung function, despite lacking a history of acute or chronic respiratory ailments.36 Conversely, individuals with COPD manifest functional and structural alterations in the intestinal mucosa, augmented intestinal permeability, and a 2-3-fold increased susceptibility to inflammatory bowel disease.37 Consequently, researchers have increasingly focused on modulating the intestinal microflora as a promising avenue for ameliorating COPD. In our comprehensive investigation, we conducted an intricate analysis of the predominant bacterial composition in the intestinal tract of COPD mice, employing a meticulous enumeration of viable bacteria. The discerning results unveiled a substantial elevation in the populations of Enterococcus and Enterobacter, while the commendable presence of beneficial bacteria, namely Bifidobacterium and Lactobacillus, exhibited a marked reduction in COPD mice. This dysbiosis of the intestinal flora played a pivotal role in the disruption of the delicate intestinal barrier. Remarkably, we observed a significant downregulation of ZO-1 and Occludin-1, the key guardians of intestinal barrier function, in these mice. The consequential impairment of the intestinal barrier function facilitated the translocation of LPS into the lung tissue via the circulatory system, thereby exacerbating the progression of COPD. However, our groundbreaking findings demonstrated a remarkable reversal of all these deleterious conditions following LD administration, unequivocally indicating the profound potential of LD in rectifying the imbalanced intestinal flora and preventing the onset of COPD.
Conclusion
Our comprehensive investigation has unequivocally established LD as an efficacious and well-tolerated therapeutic agent with immense potential in the treatment of COPD. The therapeutic effect of LD may be attributed to its multifaceted mechanisms, including the modulation of intestinal flora imbalance, dampening of inflammatory responses, and augmentation of growth differentiation factors. Nonetheless, it is imperative to underscore the necessity for further rigorous experimental validation and meticulous clinical studies to substantiate the clinical significance and therapeutic value of LD in COPD.
Abbreviations
LD, Lifei Decoction; COPD, chronic obstructive pulmonary disease; BALF, bronchoalveolar lavage fluid; CS, cigarette smoking; DEX, Dexamethasone; GDF11, Growth differentiation factor 11; p- NF-κB, phosphorylated nuclear factor kappa B; IL-1β: Interleukin-1 beta; IL-6, Interleukin-6; TNF-α, Tumor Necrosis Factor-alpha; INF-γ, Interferon-gamma; ZO-1, Zonula occludens-1; LPS, lipopolysaccharide; H&E, Hematoxylin-eosin; ELISA, enzyme-linked immunosorbent assay; ANOVA, One-way analysis of variance.
Funding
This study was supported by Jiangsu Key Laboratory of Immunity and Metabolism (NO.XZSYSKF2021047) and Jiangsu Province large instruments open to share independent research projects(NO.KY14092202).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Blanco I, Diego I, Bueno P, Casas-Maldonado F, Miravitlles M. Geographic distribution of COPD prevalence in the world displayed by geographic information system maps. Eur Respir J. 2019;54:
2. Momtazmanesh S, Moghaddam SS, Ghamari S-H. Global burden of chronic respiratory diseases and risk factors, 1990-2019: an update from the global burden of disease study 2019. EClinicalMedicine. 2023;59:101936. doi:10.1016/j.eclinm.2023.101936
3. Christenson SA, Smith BM, Bafadhel M, Putcha N. Chronic obstructive pulmonary disease. Lancet. 2022;399:2227–2242. doi:10.1016/S0140-6736(22)00470-6
4. Halpin DMG, Criner GJ, Papi A, et al. Global initiative for the diagnosis, management, and prevention of chronic obstructive lung disease. The 2020 GOLD science committee report on COVID-19 and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2021;203:24–36. doi:10.1164/rccm.202009-3533SO
5. Wang L, Cai Y, Garssen J, Henricks PAJ, Folkerts G, Braber S. The bidirectional gut-lung axis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2023;207:1145–1160. doi:10.1164/rccm.202206-1066TR
6. Park EM, Chelvanambi M, Bhutiani N, Kroemer G, Zitvogel L, Wargo JA. Targeting the gut and tumor microbiota in cancer. Nat Med. 2022;28:690–703. doi:10.1038/s41591-022-01779-2
7. Bowerman KL, Rehman SF, Vaughan A, et al. Disease-associated gut microbiome and metabolome changes in patients with chronic obstructive pulmonary disease. Nat Commun. 2020;11:5886. doi:10.1038/s41467-020-19701-0
8. Lai H-C, Lin T-L, Chen T-W, et al. Gut microbiota modulates COPD pathogenesis: role of anti-inflammatory parabacteroides goldsteinii lipopolysaccharide. Gut. 2022;71:309–321. doi:10.1136/gutjnl-2020-322599
9. Wang J, Ren C, Jin L, Batu W. Seabuckthorn Wuwei Pulvis attenuates chronic obstructive pulmonary disease in rat through gut microbiota-short chain fatty acids axis. J Ethnopharmacol. 2023;314:116591. doi:10.1016/j.jep.2023.116591
10. Li J, Xie Y, Zhao P, et al. A Chinese herbal formula ameliorates COPD by inhibiting the inflammatory response via downregulation of p65, JNK, and p38. Phytomedicine. 2021;83:153475. doi:10.1016/j.phymed.2021.153475
11. Chen L, Li L, Zou S, Liao Q, Lv B. Tong‑fu‑li‑fei decoction attenuates immunosuppression to protect the intestinal‑mucosal barrier in sepsis by inhibiting the PD‑1/PD‑L1 signaling pathway. Mol Med Rep. 2021;24. doi:10.3892/mmr.2021.12480
12. Chen L, Li L, Han Y, Lv B, Zou S, Yu Q. Tong-fu-li-fei decoction exerts a protective effect on intestinal barrier of sepsis in rats through upregulating ZO-1/occludin/claudin-1 expression. J Pharmacol Sci. 2020;143:89–96. doi:10.1016/j.jphs.2020.02.009
13. Li D, Sun D, Yuan L, et al. Sodium tanshinone IIA sulfonate protects against acute exacerbation of cigarette smoke-induced chronic obstructive pulmonary disease in mice. Int Immunopharmacol. 2020;81:106261. doi:10.1016/j.intimp.2020.106261
14. Chunhua M, Long H, Zhu W, et al. Betulin inhibited cigarette smoke-induced COPD in mice. Biomed Pharmacother. 2017;85:679–686. doi:10.1016/j.biopha.2016.11.079
15. Lakshman Kumar P, Wilson AC, Rocco A, et al. Genetic variation in genes regulating skeletal muscle regeneration and tissue remodelling associated with weight loss in chronic obstructive pulmonary disease. J Cachexia, Sarcopenia Muscle. 2021;12:1803–1817. doi:10.1002/jcsm.12782
16. Song W, Yue Y, Zhang Q. Imbalance of gut microbiota is involved in the development of chronic obstructive pulmonary disease: a review. Biomed Pharmacother. 2023;165:115150. doi:10.1016/j.biopha.2023.115150
17. Bhatt SP, O’Connor GT. Screening for chronic obstructive pulmonary disease: challenges and opportunities. JAMA. 2022;327:1768–1770. doi:10.1001/jama.2022.3823
18. Tuder RM, Petrache I. Pathogenesis of chronic obstructive pulmonary disease. J Clin Invest. 2012;122:2749–2755. doi:10.1172/JCI60324
19. Lee EH, Shin MH, Gi M, et al. Inhibition of pendrin by a small molecule reduces lipopolysaccharide-induced acute lung injury. Theranostics. 2020;10:9913–9922. doi:10.7150/thno.46417
20. Liu C-H, Chen Z, Chen K, et al. Lipopolysaccharide-mediated chronic inflammation promotes tobacco carcinogen-induced lung cancer and determines the efficacy of immunotherapy. Cancer Res. 2021;81:144–157. doi:10.1158/0008-5472.CAN-20-1994
21. Zhou R, Luo F, Lei H, et al. Liujunzi Tang, a famous traditional Chinese medicine, ameliorates cigarette smoke-induced mouse model of COPD. J Ethnopharmacol. 2016;193:643–651. doi:10.1016/j.jep.2016.09.036
22. Brightling C, Greening N. Airway inflammation in COPD: progress to precision medicine. Eur Respir J. 2019;54:
23. Wang Y, Xu J, Meng Y, Adcock IM, Yao X. Role of inflammatory cells in airway remodeling in COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:3341–3348. doi:10.2147/COPD.S176122
24. Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J. 2003;22:672–688. doi:10.1183/09031936.03.00040703
25. Baker RG, Hayden MS, Ghosh S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22. doi:10.1016/j.cmet.2010.12.008
26. Fan Y, Zhang Z, Yao C, et al. Amurensin H, a derivative from resveratrol, ameliorates lipopolysaccharide/cigarette smoke-induced airway inflammation by blocking the syk/nf-κb pathway. Front Pharmacol. 2019;10:1157. doi:10.3389/fphar.2019.01157
27. Brun CE, Rudnicki MA. GDF11 and the mythical fountain of youth. Cell Metab. 2015;22:54–56. doi:10.1016/j.cmet.2015.05.009
28. Król W, Machelak W, Zielińska M. GDF11 as a friend or an enemy in the cancer biology? Biochim Biophys Acta Rev Cancer. 2023;1878:188944. doi:10.1016/j.bbcan.2023.188944
29. Onodera K, Sugiura H, Yamada M, et al. Decrease in an anti-ageing factor, growth differentiation factor 11, in chronic obstructive pulmonary disease. Thorax. 2017;72:893–904. doi:10.1136/thoraxjnl-2016-209352
30. Tang F, Ling C, Liu J. Reduced expression of growth differentiation factor 11 promoted the progression of chronic obstructive pulmonary disease by activating the AKT signaling pathway. Biomed Pharmacother. 2018;103:691–698. doi:10.1016/j.biopha.2018.04.091
31. Thaiss CA, Zmora N, Levy M, Elinav E. The microbiome and innate immunity. Nature. 2016;535:65–74. doi:10.1038/nature18847
32. Lynch SV, Pedersen O, Phimister EG. The human intestinal microbiome in health and disease. N Engl J Med. 2016;375:2369–2379. doi:10.1056/NEJMra1600266
33. Caballero S, Pamer EG. Microbiota-mediated inflammation and antimicrobial defense in the intestine. Annu Rev Immunol. 2015;33:227–256. doi:10.1146/annurev-immunol-032713-120238
34. Budden KF, Gellatly SL, Wood DLA, et al. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol. 2017;15:55–63. doi:10.1038/nrmicro.2016.142
35. Qu L, Cheng Q, Wang Y, Mu H, Zhang Y. COPD and gut-lung axis: how microbiota and host inflammasome influence COPD and related therapeutics. Front Microbiol. 2022;13:868086. doi:10.3389/fmicb.2022.868086
36. Vutcovici M, Bitton A, Ernst P, Kezouh A, Suissa S, Brassard P. Inflammatory bowel disease and risk of mortality in COPD. Eur Respir J. 2016;47:1357–2015. doi:10.1183/13993003.01945-2015
37. Rodriguez-Roisin R, Bartolome SD, Huchon G, Krowka MJ. Inflammatory bowel diseases, chronic liver diseases and the lung. Eur Respir J. 2016;47:638–2015. doi:10.1183/13993003.00647-2015
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.