Back to Journals » Drug Design, Development and Therapy » Volume 18
The Safety and Efficacy of Remimazolam Compared to Dexmedetomidine for Awake Tracheal Intubation by Flexible Bronchoscopy: A Randomized, Double-Blind, Controlled Trial
Authors Chen Q, Qin B, Zhang M, Zhou Y, Shi X, Xie Y
Received 24 October 2023
Accepted for publication 14 March 2024
Published 28 March 2024 Volume 2024:18 Pages 967—978
DOI https://doi.org/10.2147/DDDT.S446222
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Georgios Panos
Qiumiao Chen,1 Bin Qin,2 Manqing Zhang,2 Yumei Zhou,2 Xiaotong Shi,2 Yubo Xie1,3
1Department of Anesthesiology, The First Affiliated Hospital of Guangxi Medical University, Nanning, People’s Republic of China; 2Department of Anesthesiology, College of Stomatology, Hospital of Stomatology, Guangxi Medical University, Nanning, People’s Republic of China; 3Guangxi Key Laboratory of Enhanced Recovery After Surgery for Gastrointestinal Cancer, The First Affiliated Hospital of Guangxi Medical University, Nanning, People’s Republic of China
Correspondence: Yubo Xie; Xiaotong Shi, Email [email protected]; [email protected]
Background: Remimazolam is a novel ultra-short-acting benzodiazepine sedative that has the potential to be an alternative for procedural sedation due to its rapid sedation and recovery, no accumulation effect, stable hemodynamics, minimal respiratory depression, anterograde amnesia effect, and specific antagonist. Here, we aimed to compare the safety and efficacy of remimazolam with dexmedetomidine for awake tracheal intubation by flexible bronchoscopy (ATI-FB).
Methods: Ninety patients scheduled for ATI-FB were randomly divided into three groups, each consisting of 30 cases: dexmedetomidine 0.6 μg/kg + sufentanil (group DS), remimazolam 0.073 mg/kg + sufentanil (group R1S), or remimazolam 0.093 mg/kg + sufentanil (group R2S). The primary outcome was the success rate of sedation. Secondary outcomes were MOAA/S scores, hemodynamic and respiratory parameters, intubation conditions, intubation time, tracheal intubation amnesia, and adverse events.
Results: The success rates of sedation in groups R2S and DS were higher than that in group R1S (93.3%, 86.7%, respectively, vs 58.6%; P = 0.002), and intubation conditions were better than those in group R1S (P < 0.05). Group R2S had shorter intubation times than groups R1S and DS (P = 0.003), and a higher incidence of tracheal intubation amnesia than group DS (P = 0.006). No patient in the three groups developed hypoxemia or hypotension, and there were no significant differences in oligopnea, PetCO2, or bradycardia (P > 0.05).
Conclusion: In conclusion, both DS and R2S had higher success rates of sedation, better intubation conditions, and minor respiratory depression, but R2S, with its shorter intubation time, higher incidence of anterograde amnesia, and ability to be antagonized by specific antagonists, may be a good alternative sedation regimen for patients undergoing ATI-FB.
Keywords: remimazolam, dexmedetomidine, difficult airways, awake tracheal intubation
Introduction
Awake tracheal intubation (ATI) is a recommended technique for airway management in patients with predictable difficult airways.1–3 However, when ATI induces a strong stress response in the body, patients may experience malignant cardiovascular events due to a sharp increase in catecholamine concentrations, as well as severe complications such as airway injury or failure of intubation due to coughing, irritability, and trunk twisting.4 As a result, in addition to perfect airway local anesthesia, appropriate sedatives and analgesics should be administered before intubation to reduce patients’ stress reaction and discomfort during tracheal intubation, thereby increasing patients’ tolerance of tracheal intubation, reducing complications, and improving the success rates of intubation.5,6 To ensure safety, patients with difficult airways need to maintain spontaneous breathing and minimize airway collapse during tracheal intubation. Hence, sedatives and analgesics should be quickly metabolized, minimally respiratory depressing, and can be antagonized.
Currently, frequently used regimens of sedatives and analgesics for awake tracheal intubation by flexible bronchoscopy (ATI-FB) include midazolam and dexmedetomidine alone or combined with opioids. Dexmedetomidine is the most widely used therapeutically for ATI because it results in arousable sedation and has little respiration and hemodynamic effects.7 Dexmedetomidine, on the other hand, has a slow metabolism and no specific antagonist. As a result, over-sedation with dexmedetomidine can be fatal in patients with difficult airways. Furthermore, dexmedetomidine has no pharmacologic anterograde amnesia, and patients may remember the unpleasant tracheal intubation procedure, which can have psychological consequences. Therefore, it is of great clinical significance to seek safe, effective, and comfortable sedatives for ATI-FB.
Remimazolam is a novel type of ultra-short-acting benzodiazepine that acts on the gamma-aminobutyric acid type A (GABAA) receptor,8,9 has a fast action onset, rapid metabolism, no accumulation, stable hemodynamics, mild respiratory depression, anterograde amnesia,10 and can be antagonized by flumazenil.8 Several clinical multicenter studies have shown that remimazolam is an ideal sedative for painless gastroenteroscopy and painless bronchoscopy, with good sedation, minimal respiratory depression and hemodynamic fluctuations, and few adverse events.11–14 However, no studies have been conducted to investigate the safety and efficacy of remimazolam for sedation undergoing ATI-FB. Therefore, this study aimed to compare the sedative effect, intubation conditions, intubation amnesia, and adverse events of remimazolam and dexmedetomidine for ATI-FB to provide a reference basis for further research into optimal sedatives for ATI-FB.
Materials and Methods
Study Design
This was a prospective, randomized, double-blind controlled study, which followed the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the Ethics Committee of the Hospital of Stomatology, Guangxi Medical University, and registered in the Chinese Clinical Trial Registry on Aug. 28, 2021 (ChiCTR2100050502). All patients signed a written informed consent form before the procedures.
Patients
Patients who underwent maxillofacial surgery at the College of Stomatology, Hospital of Stomatology, Guangxi Medical University from March 2022 to December 2022 were recruited for this study. Patients enrolled in this study had to meet the following inclusion criteria: American Society of Anesthesiologists (ASA) grade I–III; age 18–65 years; body mass index (BMI) ≤ 30 kg/m2; predictable difficult airway (Modified Mallampati grade III-IV).15,16 Patients with a long history of preoperative sedation or drug abuse, those with severe cardiac, pulmonary, hepatic, or renal disease prior to surgery, those with allergies to anesthetics, and those who were enrolled in another study within the 4 weeks before this trial were excluded. All patients included in this study were identified as having Modified Mallampati grade III-IV by experienced anesthesiologists during the preoperative interview. At the morning session on the day of the surgery, all anesthesiologists came to a consensus regarding whether or not the patient required awake tracheal intubation. Once it was determined that the patients required awake tracheal intubation, the researcher assessed the participant’s eligibility and signed written informed consent if the participant met the inclusion criteria and volunteered to participate in the trial.
Randomization and Blinding
Before this study, a research assistant who was not participating in this study generated random numbers in a 1:1:1 ratio for remimazolam groups (group R1S and group R2S) and dexmedetomidine group (group DS) using the Microsoft Excel-generated random number table. The results of randomization were sealed in sequentially numbered and opaque envelopes. After confirming the eligibility of the participants, an anesthesia assistant opened an envelope in sequence, prepared the intervention drug according to the instructions in the envelope, and performed anesthesia induction, who did not participate further in this study for blinding. To double-blind, the total volume of both dexmedetomidine and remimazolam liquids was configured as 25 mL, and there is no significant difference in their appearance. A researcher (an experienced anesthesiologist), unaware of the group assignment, was responsible for signing the patient’s informed consent, intubation procedures, and data collection. In addition to this researcher, patients, and the investigator who conducted the statistical analysis of the data were blinded. If there is a need to know about interventions for emergency rescue situations during the study, blindness will be broken.
Procedures
All patients in the three groups were awake nasotracheal intubated by flexible bronchoscopy (Zhejiang Youyi Medical Equipment Co. Ltd., model No. TIC-SD-III) under conscious sedation and local anaesthesia. Mask pre-oxygenation (100%, more than 6 L/min) was performed after the patients entered the operating room. The patency of the two nostrils was observed, and the nostrils with the better patency were selected for endotracheal intubation. Ephedrine and furacilin nasal drops were used to constrict nasal blood vessels on the intubated side, and 4% lidocaine aerosol (II) was sprayed along the surface and base of the patient’s tongue for 3–5 times (no more than 100 mg of lidocaine) for surface anesthesia. The absence of pain when suctioning secretions from the throat indicated that the supraglottic airway was perfectly anesthetized. At the beginning of anesthesia, patients in three groups received intravenous injections of penehyclidine hydrochloride 0.5 mg, and target-controlled infusions of sufentanil (Yichang Renfu Pharmaceutical Co. Ltd., approval No. H20054172, drug concentration configuration 2.5 μg/mL) 0.2 ng/mL. Meanwhile, patients in groups R1S and R2S received intravenous injections of remimazolam (Yichang Renfu Pharmaceutical Co. Ltd., approval No. H20200006, drug concentration configuration 1.0 mg/mL) 0.073 mg/kg and 0.093 mg/kg, respectively for 10 min. The doses of remimazolam in groups R1S and R2S are the ED50 and ED95 doses for sedation undergoing ATI-FB, respectively, which were determined by Dixon’s modified up-and-down method,17,18 and details were provided in Supplemental Data 1. Patients in group DS received intravenous injections of dexmedetomidine (Yangzijiang Pharmaceutical Group Co. Ltd., approval No. H20183219, drug concentration configuration 4 μg/mL) 0.6 μg/kg for 10 min. Endotracheal mucosal surface anesthesia was achieved by infusing 2% lidocaine 2 mL via cricothyroid puncture during the administration of sedatives and analgesics. At the end of the sedative infusion, the patient’s Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) score was assessed (Table 1), and nasotracheal intubation by flexible bronchoscopy was initiated. Considering that over-sedation may result in an emergency airway, if the level of sedation was insufficient, the anesthesiologist sprayed the rescue local anesthetic 2% lidocaine 3 mL in the vocal cords or/and trachea as needed during the operation, and the rescue sedative etomidate 6 mg intravenously could be administered after the flexible bronchoscopy entered the trachea, if necessary. After the tracheal bulge was visualized under direct vision, the tracheal tube was inserted while withdrawing the flexible bronchoscopy. The anesthesia machine was connected immediately after the exit of the flexible bronchoscopy to observe and record the tidal volume (the average of three consecutive tidal volumes), and the location of the tracheal tube was determined by monitoring the partial pressure of end-tidal carbon dioxide (PetCO2) and auscultation of both lungs. Then propofol 2 mg/kg and cisatracurium 0.2 mg/kg were administered intravenously for anesthesia induction, followed by machine-controlled breathing. Tracheal intubation was performed by the same anesthesiologist in the three groups, who also assessed sedation scores, intubation conditions and satisfaction, and other data collection. Oligopnea was defined as respiratory rate (RR) < 8 breaths/min.19 Hypoxemia was defined as peripheral pulse oxygen saturation (SpO2) < 90% for at least 10 s.20 In the presence of oligopnea and hypoxemia, the following maneuvers were carried out as required: verbal and tactile stimulation, oxygen delivery increased to 10 L/min, chin lifts, face mask, and manual ventilation. Hypotension was defined as systolic blood pressure (SBP) < 90 mmHg or 20% lower than baseline,21 and was treated with intravenous dopamine 2–4 mg. Hypertension was defined as SBP > 180 mmHg or 20% higher than baseline, and was treated with intravenous urapidil 5–10 mg. Bradycardia was defined as heart rate (HR) < 50 beats/min,22 and was treated with intravenous atropine 0.25–0.5 mg. Tachycardia was defined as HR > 120 beats/min and was treated with intravenous esmolol 10–20 mg. Patients were followed up 24 hours postoperatively for tracheal intubation amnesia and adverse events. Complications of sore throat and hoarseness were not included in this study because some patients underwent tracheotomies or indwelling endotracheal tubes after surgery, which is a significant influencing factor of postoperative sore throat and hoarseness.
 |
Table 1 MOAA/S Score, Intubation Comfort Score, Cough Score, Post-Intubation Score, and Satisfaction |
Outcome Measures
The primary outcome was the success rate of sedation, which needed the following requirements to be satisfied at the same time: a. collaborate to accomplish tracheal intubation; b. no rescue sedative; c. no rescue local anesthetic. Secondary outcomes included the following: ① Mean arterial pressure (MAP), HR, SpO2, and RR at T0 (5 min after arrival in the operation room, baseline level), T1 (immediately before intubation), T2 (immediately after intubation), T3 (1 min after intubation), and T4 (5 min after intubation). ② MOAA/S score at T0, T1, T2, and T3. ③ The concentrations of serum epinephrine and norepinephrine at T0 and T3 (venous blood was collected from patients and detected by ELISA). ④ Patients’ intubation conditions, including intubation comfort score, cough score, and post-intubation score (Table 1). ⑤ Intubation time (from insertion of the flexible bronchoscope into the nostril to exit of the flexible bronchoscope from the nostril after successful tracheal intubation). ⑥ Tracheal intubation amnesia assessed by the Modified Brice Scale23 at 24 hours postoperatively. ⑦ Anesthesiologist satisfaction score and patient satisfaction score (Table 1). ⑧ Respiratory depression-related parameters and adverse events, including PetCO2, tidal volume, oligopnea, hypoxemia, tongue base retropulsion, bradycardia, tachycardia, hypotension, hypertension, and postoperative nausea and vomiting (PONV).
Statistical Analysis
The sample size was calculated based on the success rates of sedation. According to our preliminary results (unpublished), the success rates of sedation in groups DS, R1S, and R2S were 67%, 50%, and 95%, respectively. Assuming an α level of 0.05 and a power of 0.90, PASS software (version 15) was used to estimate that 77 patients in total were required in our study. Considering the dropout rate was 10%, we assessed 91 patients in total. Finally, 90 patients were enrolled after 1 patient declined to participate.
Statistics were performed with SPSS software (version 25.0). Shapiro–Wilk test was used to test the normality of the data distribution. Levene’s test was used to test the homogeneity of variance. Data with normal distribution were presented as mean ± standard deviation (SD) and compared using analysis of variance, and subsequent pairwise comparisons were conducted using LSD-t test. Data with abnormal distribution were presented as median (25th and 75th percentile) and compared by Kruskal–Wallis test, and subsequent pairwise comparisons were performed by Nemenyi test. Categorical variables were described as n (%) and compared by Chi-square test, and subsequent pairwise comparisons were performed using Bonferroni correction. Repeated-measures data were statistically analyzed by repeated-measures analysis of variance. P value < 0.05 was considered statistically significant.
Results
The ED50 and ED95 Doses of Remimazolam for Sedation Undergoing ATI-FB
The ED50 and ED95 doses of remimazolam for sedation undergoing ATI-FB using Dixon’s modified up-and-down method were 0.073 (95% CI: 0.066–0.080) mg/kg and 0.093 (95% CI: 0.083–0.149) mg/kg, respectively (Figure S1).
Baseline Data and Airway Assessment in the Three Groups
Among the 90 patients enrolled, 1 case in group R1S that underwent tracheotomy after severe airway obstruction was excluded, and finally, 30 cases in group DS, 29 cases in group R1S, and 30 cases in group R2S were included (Figure 1). There were no significant differences in sex, age, BMI, ASA grade, mouth opening, head and neck movement, Modified Mallampati grade, thyromental distance, or disease types among the three groups (P > 0.05, Table 2).
 |
Table 2 Baseline Characteristics and Airway Assessment |
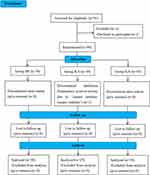 |
Figure 1 Enrollment flow diagram. |
Comparison of the Success Rates of Sedation and the Stress Response to Endotracheal Intubation Among the Three Groups
The success rates of sedation in groups R2S and DS were 93.3% and 86.7%, respectively, which were higher than that in group R1S (58.6%) (P = 0.002), and rescue local anesthetics were less than group R1S (P = 0.001). There were no statistically significant differences in the rescue sedative, and the concentrations of serum epinephrine and norepinephrine at T0 and T3 among the three groups (P > 0.05, Table 3). MOAA/S score, MAP, HR, SpO2, and RR changed over time in the three groups of patients, as shown in Figure 2. MOAA/S scores were lower in group R2S at T1, T2, and T3 compared to groups DS and R1S (P < 0.001), while the differences between groups DS and R1S at each time point were not statistically significant (P > 0.05). Compared to group DS, HR was higher in group R1S at T1, T2, T3, and T4, and higher in group R2S at T1 (P < 0.05). Compared to group R1S, HR was lower in group R2S at T2 (P < 0.05). There were no significant differences in MAP, SpO2, or RR among the three groups at each time point (P > 0.05, Table 4).
 |
Table 3 Comparison of the Success Rates of Sedation, the Rescue Local Anesthetic and Sedative, and the Concentrations of Serum Epinephrine and Norepinephrine Among the Three Groups |
 |
Table 4 The Fluctuation of MOAA/S Score, MAP, HR, SpO2, and RR in the Three Groups |
Comparison of Intubation Conditions, Tracheal Intubation Amnesia, and Satisfaction Among the Three Groups
Good intubation conditions were defined as intubation comfort score ≤ 2, cough score ≤ 1, and post-intubation score = 1. Compared with group R1S, groups R2S and DS had more patients with intubation comfort score ≤ 2 (18 vs 30, 27, respectively, P < 0.001), cough score ≤ 1 (17 vs 28, 26, respectively, P = 0.002), and post-intubation score = 1 (17 vs 27, 26, respectively, P = 0.006). When compared to groups R2S and DS, the differences in intubation comfort score ≤ 2, cough score ≤ 1, and post-intubation score = 1 were not statistically significant (P > 0.05). The success rates of nasotracheal intubation were 100% in the three groups. The intubation time in group R2S was significantly shorter than that in groups R1S and DS (P = 0.003), and the rate of tracheal intubation amnesia was higher than that in group DS (P = 0.006). The anesthesiologist satisfaction score in group DS was higher than that in group R1S (P = 0.02). The difference in patient satisfaction score was not statistically significant in any of the three groups (P > 0.05, Table 5).
 |
Table 5 Comparison of Intubation Conditions, Amnesia for Tracheal Intubation, and Satisfaction Among the Three Groups |
Comparison of Respiratory Depression-Related Parameters and Adverse Events Among the Three Groups
In group R1S, the incidence of hypertension was higher than that in group R2S (P = 0.002), and the incidence of tachycardia was higher than that in group DS (P = 0.032). The tidal volume in group R2S was lower than that in group R1S (P = 0.001). No patient in the three groups developed hypoxemia or hypotension, and there were no significant differences in oligopnea, tongue base retropulsion, PetCO2, bradycardia, or PONV among the three groups (P > 0.05, Table 6).
 |
Table 6 Comparison of Respiratory Depression-Related Parameters and Adverse Events Among the Three Groups |
Discussion
This was a double-blind, randomized controlled study to comprehensively evaluate the efficacy and safety of remimazolam versus dexmedetomidine for patients with difficult airways undergoing ATI-FB. We determined the ED50 and ED95 doses of remimazolam for sedation undergoing ATI-FB using Dixon’s modified up-and-down method, which were 0.073 mg/kg and 0.093 mg/kg, respectively (Figure S1). Therefore, the doses of remimazolam in groups R1S and R2S were 0.073 mg/kg and 0.093 mg/kg, respectively.
ATI-FB is an ideal intubation technique for dealing with predictably difficult airways.24,25 The patient’s quietness, compliance, and adequate local anesthesia of the airway are critical for the effective completion of this technique.1,26,27 The results of this study showed that the differences of epinephrine and norepinephrine at T3 were not statistically significant in the three groups, indicating that the three groups effectively suppressed the stress response of tracheal intubation under local anesthesia combined with appropriate sedatives and analgesics. The MOAA/S scores in group R2S at T1, T2, and T3 were lower than that in groups DS and R1S. In addition, group R2S had a higher success rate of sedation, required fewer rescue local anesthetics, and had better intubation conditions than group R1S, while being the same as group DS. The results showed that a higher dose of remimazolam (0.093 mg/kg) resulted in excellent sedation and was not inferior to dexmedetomidine, which is similar to the results of several earlier studies.28–30 When the MOAA/S score is > 2, the level of sedation is mild to moderate, and the patient can be aroused and maintain spontaneous respiration in this range of sedation, which can be considered to result in minimal risk of intubation for patients with difficult airways. The MOAA/S scores for the three groups were higher than 2, which is in line with the expected level of sedation. Group R2S had lower MOAA/S scores with deeper sedation, which may have rendered the patients more compliant during intubation, resulting in a shorter intubation time. Thus, remimazolam (0.093 mg/kg) combined with sufentanil provided better sedation and better intubation conditions for ATI-FB.
Respiratory depression-related parameters showed that the tidal volume in group R2S was lower than that in group R1S, but there was no difference compared with group DS. No patients in the three groups developed hypoxemia. SpO2 was maintained at over 99% at all time points. The incidence of oligopnea and tongue base retropulsion was similar among the three groups. The RR decreased over time but remained within the normal range, and there was no statistically significant difference in PetCO2 among the three groups. These results revealed that remimazolam caused dose-dependent respiratory depression; however, the respiratory depression was minor in all three groups and did not result in severe respiratory depression-related events. Chae et al31 also confirmed a significant dose dependence of remimazolam from loss of consciousness to respiratory depression when administering a single intravenous injection of remimazolam for anesthesia induction, and the ED50/ED95 for respiratory depression induced by remimazolam was 0.14/0.27 mg/kg, which is much higher than the remimazolam dose of 0.073/0.093 mg/kg in this study. Therefore, the doses of remimazolam used in both groups in this experiment caused minor respiratory depression. Although MAP and HR in all three groups tended to decrease over time, they were still within the normal range. After sedation, HR was higher in groups R1S and R2S than those in group DS immediately before intubation. As well, none of the three groups experienced hypotension, and the incidence rate of bradycardia was also similar among the three groups. But, in group R1S, patients had higher incidences of hypertension and tachycardia, as well as higher HR immediately after intubation, which was considered to be related to intubation stress. These results indicated that remimazolam had mild respiratory depression, minimal hemodynamic fluctuation, and no serious side effects, which were similar to the results of several previous clinical studies.32–34
Our results confirmed that remimazolam, like other benzodiazepines, had an anterograde amnesia effect,35–37 which was thought to be caused by the binding of remimazolam with the α1 of the GABAA receptor.38 Although intubation comfort scores, cough scores, and post-intubation scores were not lower in group DS than that in groups R1S and R2S, patient satisfaction scores seemed to be higher in groups R1S and R2S than that in group DS due to the rates of amnesia for tracheal intubation being higher in groups R1S and R2S. A similar outcome was also seen in the study,28 where the deeper, more consistent sedation and retrograde amnesia brought about by remimazolam resulted in median patient satisfaction scores that were higher in the remimazolam group than that in the dexmedetomidine group. The rate of intubation amnesia was more than 10% greater in the remimazolam high-dose group than that in the low-dose group, implying that remimazolam’s anterograde amnesia may be dose-dependent. A previous study has also demonstrated that benzodiazepines cause transient selective anterograde amnesia in a dose-dependent manner.39 Overall, remimazolam 0.093 mg/kg exhibited a better anterograde amnesic effect, which may contribute to higher patient satisfaction for ATI-FB.
Currently, there is debate about the benefit of additional sedation in patients with difficult airways. The main reason for this is that the depth of sedation used to perform “awake” tracheal intubation is a continuum from anxiolysis to almost complete loss of airway reflexes,40 and sensitivity to sedation varies among individuals, creating a gray zone between awake and sedated tracheal intubation and potentially resulting in the risks of tracheal intubation.27 One patient in group R1S developed airway obstruction after receiving intravenous remimazolam and sufentanil. Fortunately, the patient was fully conscious and resuscitated from danger after 2 minutes of intravenous flumazenil antagonizing remimazolam and intravenous nalmefene antagonizing sufentanil. Continued tracheal intubation of the patient may result in the risk of an emergency airway, so a tracheotomy was performed under local anesthesia before proceeding with the procedure, as the patient was scheduled to require a tracheotomy after surgery. At postoperative follow-up, the patient had no memory of the resuscitation as well as the tracheotomy procedure, again verifying that remimazolam had an excellent anterograde amnesia effect. “Awakening the patient” is one of the approaches used in the management of non-emergency airway/emergency airway,2 which requires specific antagonists to antagonize the efficacy of sedatives/analgesics, so that the patient wakes quickly to maintain effective ventilation. Based on this, the Difficult Airway Society guidelines for ATI in adults suggest minimal dosages of benzodiazepines or opioids if sedation is necessary.1 As a result, an ideal sedative for ATI should have a specific antagonist. This requirement is fulfilled by remimazolam, which can be antagonized by flumazenil and may be safer for ATI-FB.
This study has the following limitations: ① This was a single-center randomized controlled study involving only patients undergoing maxillofacial surgery, and more multicenter, large-sample studies are needed to validate the benefits of remimazolam sedation for ATI-FB. ② The study was not designed to evaluate the effects of remimazolam or dexmedetomidine alone, but in combination with sufentanil. Opioids can also inhibit tracheal intubation stress and respiration and have a synergistic effect with sedatives. ③ We assessed whether patients forgot the tracheal intubation procedure at 24 hours postoperatively, but unfortunately, there was no further follow-up to analyze the psychological impact on patients who remembered the tracheal intubation procedure. ④ In this study, tidal volume and PetCO2 were not continuously monitored, and a single tidal volume and PetCO2 after successful endotracheal intubation do not provide a complete picture of the effect of anesthetics on respiratory depression, nor do they allow for timely identification of inadequate ventilation in patients for further intervention.
Conclusion
In conclusion, both DS and R2S had higher success rates of sedation, better intubation conditions, and minor respiratory depression, but R2S, with its shorter intubation time, higher incidence of anterograde amnesia, and ability to be antagonized by specific antagonists, may be a good alternative sedation regimen for patients undergoing ATI-FB. However, additional studies should be conducted to expand the sample size to better evaluate the benefits and risks of remimazolam for patients undergoing ATI-FB.
Data Sharing Statement
All data generated and analyzed in the study are available from the corresponding author (Yubo Xie) upon reasonable request. The patients’ data anonymity will be preserved before distribution.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Our work was supported by Young Clinical Research Fund of the Chinese Stomatological Association (No. CSA-A2021-08), Special Fund of Neurotoxicity of General Anesthetics and Its Prevention and Treatment Innovation Team of the First Affiliated Hospital of Guangxi Medical University (No. YYZS2022001), Guangxi Clinical Research Center for Anesthesiology (No. GK AD22035214), and Guangxi Medical and Health Appropriate Technology Development and Popularization Project (No. S2021083).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ahmad I, El-Boghdadly K, Bhagrath R, et al. Difficult Airway Society guidelines for awake tracheal intubation (ATI) in adults. Anaesthesia. 2020;75:509–528. doi:10.1111/anae.14904
2. Apfelbaum JL, Hagberg CA, Connis RT, et al. 2022 American Society of anesthesiologists practice guidelines for management of the difficult airway. Anesthesiology. 2022;136:31–81. doi:10.1097/aln.0000000000004002
3. Mushambi MC, Athanassoglou V, Kinsella SM. Anticipated difficult airway during obstetric general anaesthesia: narrative literature review and management recommendations. Anaesthesia. 2020;75:945–961. doi:10.1111/anae.15007
4. Kaneko Y, Nakazawa K, Yokoyama K, et al. Subcutaneous emphysema and pneumomediastinum after translaryngeal intubation: tracheal perforation due to unsuccessful fiberoptic tracheal intubation. J clin anesth. 2006;18:135–137. doi:10.1016/j.jclinane.2005.10.006
5. Sequera-Ramos L, Laverriere EK, Garcia-Marcinkiewicz AG, Zhang B, Kovatsis PG, Fiadjoe JE. Sedation versus general anesthesia for tracheal intubation in children with difficult airways: a cohort study from the Pediatric Difficult Intubation Registry. Anesthesiology. 2022;137:418–433. doi:10.1097/aln.0000000000004353
6. Johnston KD, Rai MR. Conscious sedation for awake fibreoptic intubation: a review of the literature. Can J Anaesthesia. 2013;60:584–599. doi:10.1007/s12630-013-9915-9
7. Zhou LJ, Fang XZ, Gao J, Zhangm Y, Tao LJ. Safety and efficacy of dexmedetomidine as a sedative agent for performing awake intubation: a meta-analysis. Am J Therap. 2016;23:e1788–e800. doi:10.1097/mjt.0000000000000319
8. Kilpatrick GJ, McIntyre MS, Cox RF, et al. CNS 7056: a novel ultra-short-acting Benzodiazepine. Anesthesiology. 2007;107:60–66. doi:10.1097/01.anes.0000267503.85085.c0
9. Keam SJ. Remimazolam: first Approval. Drugs. 2020;80:625–633. doi:10.1007/s40265-020-01299-8
10. Wesolowski AM, Zaccagnino MP, Malapero RJ, Kaye AD, Urman RD. Remimazolam: pharmacologic considerations and clinical role in anesthesiology. Pharmacotherapy. 2016;36:1021–1027. doi:10.1002/phar.1806
11. Wang X, Hu X, Bai N, et al. Safety and efficacy of remimazolam besylate in patients undergoing colonoscopy: a multicentre, single-blind, randomized, controlled, Phase III trial. Front Pharmacol. 2022;13:900723. doi:10.3389/fphar.2022.900723
12. Zhou YY, Yang ST, Duan KM, et al. Efficacy and safety of remimazolam besylate in bronchoscopy for adults: a multicenter, randomized, double-blind, positive-controlled clinical study. Front Pharmacol. 2022;13:1005367. doi:10.3389/fphar.2022.1005367
13. Pastis NJ, Yarmus LB, Schippers F, et al. Safety and efficacy of remimazolam compared with placebo and midazolam for moderate sedation during bronchoscopy. Chest. 2019;155:137–146. doi:10.1016/j.chest.2018.09.015
14. Wang C, Gao Y, Li J, et al. Safety and effectiveness of the combination of remimazolam tosilate and propofol in gastroscopy: a multicenter, randomized controlled, single-blind clinical trial. Front Pharmacol. 2023;14:1124667. doi:10.3389/fphar.2023.1124667
15. Verma AK, Verma S, Barik AK, Kanaujia V, Arya S. Intubating conditions and hemodynamic changes during awake fiberoptic intubation using fentanyl with ketamine versus dexmedetomidine for anticipated difficult airway: a randomized clinical trial. Braz J Anesthesiol. 2021;71:259–264. doi:10.1016/j.bjane.2021.01.005
16. Hu R, Liu JX, Jiang H. Dexmedetomidine versus remifentanil sedation during awake fiberoptic nasotracheal intubation: a double-blinded randomized controlled trial. J Anesth. 2013;27:211–217. doi:10.1007/s00540-012-1499-y
17. Dixon WJ. Staircase bioassay: the up-and-down method. Neurosci Biobehav Rev. 1991;15:47–50. doi:10.1016/s0149-7634(05)80090-9
18. Pace NL, Stylianou MP. Advances in and limitations of up-and-down methodology: a précis of clinical use, study design, and dose estimation in anesthesia research. Anesthesiology. 2007;107:144–152. doi:10.1097/01.anes.0000267514.42592.2a
19. Pastis NJ, Hill NT, Yarmus LB, et al. Correlation of vital signs and depth of sedation by modified observer’s assessment of Alertness and Sedation (MOAA/S) Scale in bronchoscopy. J bronchol intervent pulmonol. 2022;29:54–61. doi:10.1097/lbr.0000000000000784
20. Wang L, Wu Q, Wang M, et al. The safety and efficacy of alfentanil combined with midazolam in fiberoptic bronchoscopy sedation: a randomized, double-blind, controlled trial. Front Pharmacol. 2022;13:1036840. doi:10.3389/fphar.2022.1036840
21. Li J, Wang X, Liu J, et al. Comparison of ciprofol (HSK3486) versus propofol for the induction of deep sedation during gastroscopy and colonoscopy procedures: a multi-centre, non-inferiority, randomized, controlled Phase 3 clinical trial. Basic Clin Physiol Pharmacol. 2022;131:138–148. doi:10.1111/bcpt.13761
22. Kim J, Lee S, Kim Y, Jeong JS. Remimazolam dose for successful insertion of a supraglottic airway device with opioids: a dose-determination study using Dixon’s up-and-down method. Can J Anaesthesia. 2023;70:343–350. doi:10.1007/s12630-022-02379-x
23. Mashour GA, Kent C, Picton P, et al. Assessment of intraoperative awareness with explicit recall: a comparison of 2 methods. Anesthesia Analg. 2013;116:889–891. doi:10.1213/ANE.0b013e318281e9ad
24. Aziz MF, Kristensen MS. From variance to guidance for awake tracheal intubation. Anaesthesia. 2020;75:442–446. doi:10.1111/anae.14947
25. Kamga H, Frugier A, Boutros M, Bourges J, Doublet T, Parienti JJ. Flexible nasal bronchoscopy vs. Airtraq(®) videolaryngoscopy for awake tracheal intubation: a randomised controlled non-inferiority study. Anaesthesia. 2023;78:963–969. doi:10.1111/anae.16042
26. Mirrakhimov AE, Torgeson E. Awake tracheal intubation: what can be done to maintain the skill? Can J Anaesthesia. 2023;70:1268–1269. doi:10.1007/s12630-023-02476-5
27. Vora J, Leslie D, Stacey M. Awake tracheal intubation. BJA Educ. 2022;22:298–305. doi:10.1016/j.bjae.2022.03.006
28. Kim H, Kim Y, Bae J, Yoo S, Lim YJ, Kim JT. Comparison of remimazolam and dexmedetomidine for intraoperative sedation in patients undergoing lower extremity surgery under spinal anesthesia: a randomized clinical trial. Reg Anesth Pain Med. 2024;49:110–116. doi:10.1136/rapm-2023-104415
29. Chen X, Xin D, Xu G, Zhao J, Lv Q. The efficacy and safety of remimazolam tosilate versus dexmedetomidine in outpatients undergoing flexible bronchoscopy: a prospective, randomized, blind, non-inferiority trial. Front Pharmacol. 2022;13:902065. doi:10.3389/fphar.2022.902065
30. Xiao Y, Wei R, Chen L, Chen Y, Kong L. Efficacy and safety of remimazolam for procedural sedation during ultrasound-guided transversus abdominis plane block and rectus sheath block in patients undergoing abdominal tumor surgery: a single-center randomized controlled trial. BMC Anesthesiol. 2022;22:381. doi:10.1186/s12871-022-01927-8
31. Chae D, Kim HC, Song Y, Choi YS, Han DW. Pharmacodynamic analysis of intravenous bolus remimazolam for loss of consciousness in patients undergoing general anaesthesia: a randomised, prospective, double-blind study. Br J Anaesth. 2022;129:49–57. doi:10.1016/j.bja.2022.02.040
32. Schüttler J, Eisenried A, Lerch M, Fechner J, Jeleazcov C, Ihmsen H. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: part I. Pharmacokinetics and clinical pharmacodynamics. Anesthesiology. 2020;132:636–651. doi:10.1097/aln.0000000000003103
33. Stöhr T, Colin PJ, Ossig J, et al. Pharmacokinetic properties of remimazolam in subjects with hepatic or renal impairment. Br J Anaesth. 2021;127:415–423. doi:10.1016/j.bja.2021.05.027
34. Hu B, Zhang M, Wu Z, et al. Comparison of remimazolam tosilate and etomidate on hemodynamics in cardiac surgery: a randomised controlled trial. Drug Des Devel Ther. 2023;17:381–388. doi:10.2147/dddt.S401969
35. Cornett EM, Novitch MB, Brunk AJ, et al. New benzodiazepines for sedation. Best Pract Res Clin Anaesth. 2018;32:149–164. doi:10.1016/j.bpa.2018.06.007
36. Wang L, Jing Q, Pei L, et al. Efficacy of continuous intravenous remimazolam versus midazolam in the extraction of impacted wisdom teeth: protocol of a randomised controlled trial. BMJ open. 2023;13:e067908. doi:10.1136/bmjopen-2022-067908
37. Shirozu K, Nobukuni K, Funakoshi K, et al. The effect of remimazolam on postoperative memory retention and delayed regeneration in breast surgery patients: rationale and design of an exploratory, randomized, open, propofol-controlled, single-center clinical trial: a study protocol. Medicine. 2021;100:e27808. doi:10.1097/md.0000000000027808
38. Kilpatrick GJ. Remimazolam: non-clinical and clinical profile of a new sedative/anesthetic agent. Front Pharmacol. 2021;12:690875. doi:10.3389/fphar.2021.690875
39. Hong YJ, Jang EH, Hwang J, et al. Effect of midazolam on memory during fiberoptic gastroscopy under conscious sedation. Clin Neuropharmacol. 2015;38:47–51. doi:10.1097/wnf.0000000000000067
40. Heidegger T, Schnider TW. “Awake” or “Sedated”: safe flexible bronchoscopic intubation of the difficult airway. Anesthesia Analg. 2017;124:996–997. doi:10.1213/ane.0000000000001748
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

