Back to Journals » International Journal of Nanomedicine » Volume 19
The Landscape of Exosomes Biogenesis to Clinical Applications
Authors Al-Madhagi H
Received 6 February 2024
Accepted for publication 16 April 2024
Published 22 April 2024 Volume 2024:19 Pages 3657—3675
DOI https://doi.org/10.2147/IJN.S463296
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Farooq A. Shiekh
Haitham Al-Madhagi
Biochemical Technology Program, Faculty of Applied Sciences, Dhamar University, Dhamar, Yemen
Correspondence: Haitham Al-Madhagi, Email [email protected]
Abstract: Exosomes are extracellular vesicles that originate from various cells and mediate intercellular communication, altering the behavior or fate of recipient cells. They carry diverse macromolecules, such as lipids, proteins, carbohydrates, and nucleic acids. Environmental stressors can change the exosomal contents of many cells, making them useful for diagnosing many chronic disorders, especially neurodegenerative, cardiovascular, cancerous, and diabetic diseases. Moreover, exosomes can be engineered as therapeutic agents to modulate disease processes. State-of-art techniques are employed to separate exosomes including ultracentrifugation, size-exclusion chromatography and immunoaffinity. However, modern technologies such as aqueous two-phase system as well as microfluidics are gaining attention in the recent years. The article highlighted the composition, biogenesis, and implications of exosomes, as well as the standard and novel methods for isolating them and applying them as biomarkers and therapeutic cargo carriers.
Keywords: Exosomes, extracellular vesicles, biomarkers, therapeutic cargo carriers, separation
Introduction
Exosomes, also known as extracellular vesicles (EVs), were initially observed half a century ago in plasma by Dr. Wolf, who denoted them as platelet dust‘.1 Since that time, investigations have revealed the presence of vesicles in all examined biological fluids, and in vitro cultivated cell lines have demonstrated varying degrees of vesicle release.2 Although these vesicles have undergone various nomenclatures over the years, they are now commonly referred to collectively as EVs. Three principal categories of EVs have been delineated based on their size and mechanism of release: exosomes (diameter less than 150 nm), microvesicles/shedding particles, and apoptotic bodies (both considered larger than 100 nm). The latter two types are discharged directly from the plasma membrane in viable and dying cells, respectively, and will not be further explored herein (Figure 1). This examination focuses on the smallest subset within this family—exosomes, which are vesicles released into the extracellular milieu following the fusion of late endosomes/multivesicular bodies (MVBs) with the plasma membrane. This fusion process was initially observed in rat reticulocytes in 19833 and subsequently in sheep reticulocytes in 1985.4 The term exosome was coined by Rose Johnstone, a trailblazer in the field, in 1987, as she found the process reminiscent of reverse endocytosis, wherein internal vesicular contents are released as opposed to external molecules being internalized within membrane-bound structures.5 However, comprehensive understanding of this process has largely been attained in recent years.6
 |
Figure 1 Comparison among exosomes, microvesicles and apoptotic bodies.7 The Figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0. |
Exosomes have emerged as pivotal mediators in cellular communication, orchestrating the transfer of functional proteins, metabolites, and nucleic acids to recipient cells.8 Their far-reaching influence spans diverse physiological processes, including immune responses,9 tissue repair,10 stem cell maintenance,11 central nervous system (CNS) communication,12 and their involvement in pathological processes such as cardiovascular diseases,13 neurodegeneration,14 cancer,15 and various types of inflammations.16
The clinical potential of exosomes is underscored by their suitability as diagnostic biomarkers and carriers of therapeutic cargo.17 Their diminished immunogenicity, attributed to biocompatibility and a protective bi-layered lipid structure safeguarding genetic cargo from degradation, renders them appealing as therapeutic vectors. Also, their petite size (Figure 2) and membrane composition enable them to traverse major biological barriers, including the blood-brain barrier. Active research in engineered exosome production focuses on assessing various therapeutic cargoes, enhancing target selectivity, and refining manufacturing processes.18 However, challenges persist in precisely targeting specific cell types or organs while minimizing off-target biodistribution and addressing concerns related to naturally incorporated cellular genetic impurities with potential immunogenicity.19,20 Overcoming these challenges necessitates an improved understanding of exosome biology to advance therapeutic exosome engineering.
 |
Figure 2 The scale of size of extracellular vesicles including exosomes in view of the most common biological macromolecules. Reprinted from J Chromatogr A, 1636, Liangsupree T, Multia E, Riekkola M-L. Modern isolation and separation techniques for extracellular vesicles. 461773, Copyright 2021, Creative Commons.21 |
Numerous review articles covered different aspects of exosome biology, separation and/or bioapplications.22–26 Nonetheless, papers covering exosomes biogenesis to clinical utilization are scarce. Therefore, it was sought in this review to provide a state-of-the art comprehensive updated investigation about the biology of exosomes involving their formation, uptake, dosing and biological actions on recipient cells. Moreover, the standard as well as novel approaches used for the isolation and their applications as diagnostic biomarkers and drug delivery agents for neurodegenerative diseases, cardiovascular disorders, cancers and diabetes are also covered.
What are Extracellular Vesicles?
The genesis of initial endosomes initiates with the invagination of the cell membrane during the early stage, facilitating the accumulation of bioactive substances within early sorting endosomes (ESEs). Subsequently, orchestrated by the endocytosis sorting complex and other requisite transport-related proteins, early endosomes undergo a transformation into late sorting endosomes (LSEs).27 Following a secondary invagination, LSEs progress to form multivesicular bodies (MVBs). Upon fusion of MVBs with the cell membrane, the internal cellular contents are extricated in the form of vesicles, which are identified as exosomes.28 The schematic representation of the biological origin of exosomes is delineated in Figure 3. The diversity in the formation of exosomes is currently a subject of extensive investigation, with predominant focus on both ESCRT-dependent and ESCRT-independent mechanisms. Recent findings, however, propose the involvement of certain components, such as four-transmembrane domain proteins and lipid rafts, in the formation of specific exosomes.29 Consequently, the precise mechanistic underpinnings remain a matter of ongoing debate.
Exosome biosynthesis culminates in the release of exosomes into the extracellular space through the fusion of MVB and the plasma membrane. This is mediated by Rab GTPases, v-SNAREs (eg, vesicle-associated membrane protein 7 and synaptobrevin homolog YKT6),30 tethering proteins (eg, early endosome antigen 1), and synaptotagmins.31 The exosomal envelope, which varies in composition,32 such as integrin proteins,33 enables the targeted delivery to specific cells. Exosomes can engage with the recipient cells through various modes, including receptor-mediated binding, membrane fusion, and endocytosis or phagocytosis. Upon internalization, the exosomes form endosomes, which they subsequently escape from, leading to the release and function of the exosomal cargo in the cytosol.34,35
 |
Figure 3 General mechanism of biogenesis and release of exosomes.22 The Figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0. |
Dosing of Exosomes
Exosome dosing is a challenging and controversial issue that has been explored in recent years. Various methods have been applied to measure exosome dosage, such as cell equivalents, protein concentration, and/or specific quantitative analytical tools, each having its own pros and cons. However, a standardized method for exosome dosing is still lacking and the current technologies are limited in their accuracy and precision in evaluating exosomes at the single-vesicle level. To enhance the accuracy in exosome dosing, it is suggested to use multiple methods. For instance, the protein method that measures total protein levels is quick and cheap, but it may also include proteins that are unrelated to exosomes and may not reflect bioactive components. Other methods for exosome dosing include Enzyme-linked immunosorbent assay (ELISA), cell equivalents, and flow cytometry. A review by Willis et al offers more information on methods related to exosome dosing.36 Regarding the use of exosomes in clinical trials, good manufacturing practices (GMPs) are essential. Indeed, exosomes used in clinical trials should adhere to GMPs. GMPs for exosomes involve three main factors, such as upstream cell culture, downstream purification process, and exosome quality control.37
Separation of Exosomes
Exosomes, ubiquitously released by nearly all cell types and notably present in biofluids, possess lipid bilayer membranes that facilitate the secure transport and conveyance of crucial biological signals originating from their respective cells. These signals not only exert influence on the body’s physiological state but also play a pivotal role in cell communication, immunoregulation,38 angiogenesis,39 tumorigenesis, and metastasis.40 Consequently, exosomes have emerged as promising tools for monitoring the occurrence and progression of cancer. Despite their potential, the comprehension of exosomal functions remains constrained. A primary impediment to research advancement lies in the absence of an efficient standardized isolation strategy for specific exosome subpopulations due to their inherent heterogeneity. Beyond the imperative to segregate intact and pure exosomes, there is a pressing need for further refinement of exosome isolation methodologies, focusing on achieving high purity, high throughput, reduced operational time, and enhanced repeatability.41
Presently, prevailing technologies for exosome isolation, such as ultrafiltration, immunoaffinity, and ultracentrifugation (widely considered the “gold standard” for exosome isolation), entail substantial costs, necessitate large sample volumes, pose the risk of protein contamination, involve intricate isolation procedures, and yield suboptimal results in terms of isolation efficiency, sample preservation, and exosome recovery and purity.42 The integration of microfluidics into exosome isolation has emerged as a consequential development, leveraging advancements in nanotechnologies. Optimized microfluidic chips hold considerable promise as tools for future research endeavors. Despite existing reviews that consolidate information on exosome isolation and purification, comprehensive examinations detailing the nuanced advantages and drawbacks of each technology are relatively scarce.43 The discussed current methods of exosomes isolation are summarized in Table 1.
 |
Table 1 The Most Commonly Employed Techniques for Isolating Exosomes and Their Corresponding Time, Purity and Yield |
Ultracentrifugation
Ultracentrifugation stands as a cornerstone technique for the isolation of exosomes, representing a widely recognized and extensively employed method in the field of extracellular vesicle research. This approach involves the high-speed centrifugation of biological samples to differentially separate particles based on their size, density, and mass. Typically, exosome isolation through ultracentrifugation comprises a series of centrifugation steps, starting with low-speed spins to eliminate cells and debris, followed by subsequent high-speed ultracentrifugation to pellet exosomes. This method is often regarded as the “gold standard” due to its ability to yield relatively pure exosome populations. Nevertheless, it is essential to acknowledge the associated limitations, such as lengthy processing times, the need for large sample volumes, and the potential for co-isolation of non-exosomal contaminants. Researchers have explored variations and optimizations to ultracentrifugation protocols, including the use of density gradients and sucrose cushions, to enhance specificity and efficiency in isolating exosomes from complex biological samples.44 Various pore size filters were applied to isolate exosomes with different molecular weights including 10 KDa,45 30 KDa,46 50 KDa,47,100 KDa.48–50
Despite its widespread use, the quest for alternative isolation techniques is fueled by the desire to address the inherent challenges and limitations of ultracentrifugation. Emerging technologies, such as size exclusion chromatography, immunoaffinity-based methods, and microfluidic approaches, are being increasingly explored for their potential advantages in terms of isolation efficiency, speed, and scalability. By comparing and integrating these diverse techniques, researchers aim to refine exosome isolation strategies and advance our understanding of their roles in health and disease.51,52
Size-Exclusion Chromatography (SEC)
Size exclusion chromatography (SEC) stands as an established method for the separation of macromolecules based on their molecular size or hydrodynamic volumes.53 The standard SEC configuration encompasses a porous stationary phase for chromatographic separation, with or without coupling to a pump for elution. This technique has been widely applied for the isolation of exosomes from diverse sample matrices originating from both prokaryotes and eukaryotes. These matrices include cell culture-derived specimens, blood-based samples (plasma and serum),15,16,21,54–56 urine,57,58 saliva,59 nasal lavage,60 synovial fluid,61 cerebrospinal fluids,62 and tear.59 In instances of diluted samples, particularly urine and cell culture-derived samples, concentration or removal of soluble contaminants through filtration-based techniques is often implemented before column injection.63
The predominant stationary phase materials employed for EV isolation and separation are cross-linked agarose beads, commercially designated as Sepharose® (CL-2B and CL-4B) and Sephacryl® S-400. Agarose beads are characterized by high scalability and flexibility, rendering them adjustable to diverse sample requirements. Some investigations have employed qEV SEC columns, available in various bed volumes and size exclusion limits (intra-pore sizes). Notably, Sepharose® CL-4B with a size exclusion limit of 42 nm has demonstrated suitability for isolating EVs from protein contaminants, such as albumin, in contrast to Sepharose® CL-2B,47,64 which has an exclusion limit of 75 nm. Enhanced resolution has been achieved with a larger column bed volume (1 mL vs 10 mL). Furthermore, studies by Arntz et al involved the isolation of plasma-derived EVs using two Sepharose® CL-2B SEC columns with identical stacking volumes but differing column lengths (56 mm vs 222 mm).65 Their findings indicated that utilizing the longer column resulted in a 90% reduction in protein and immunoglobulin contamination, while maintaining consistent EV particle size distribution and yields.65
Microfluidics
Microfluidic technology offers a promising approach for separating exosomes due to its precise manipulation of micro- and nanometer particles within minimal sample volumes. Specifically designed micro- and nanostructure microchips allow targeted exosome separation, purification, and collection. Microfluidics present several advantages over conventional methods. Firstly, the use of multiple microchannels enables efficient particle analysis, such as the orderly alignment of 2×106 cells/mL for single-cell analysis in a spiral microchannel. Secondly, microfluidic platforms facilitate cost-effective biological analyses, including non-invasive diagnostic tests (liquid biopsy), thanks to reduced reagent and sample consumption. Thirdly, microfluidic systems minimize contamination by consolidating multiple analysis steps onto a single chip. Lastly, functional and intact exosomes can be selectively captured using microfluidic devices, enhancing the efficiency of exosome separation.66,67 Currently there are three main forms of microfluidics systems for exosomes isolation: immunoaffinity-based, microsphere-mediated, and enhanced capture efficiency.68 Immunoaffinity being the most common form, it will be covered in detail as follows.
Affinity-based isolation, alongside charge-, density-, and size-based techniques, is a prominent method for isolating exosome. This approach capitalizes on the highly selective interactions between proteins or receptors on the EV membrane and corresponding ligands, such as antibodies. In developing affinity-based isolation, ligands like antibodies are immobilized or conjugated onto/into diverse solid media, including magnetic beads and polymeric materials like agarose beads and monolithic columns.45 Affinity-based isolation is categorized into affinity chromatography and immunocapture based on isolation mechanisms. Affinity ligands include bioaffinity ligands (eg, antibodies, peptides, and transmembrane proteins) and heparin.69
Immunoaffinity capture, a widely employed affinity-based technique, utilizes antibodies against EV surface proteins. Typically, these antibodies are covalently linked to magnetic beads through biotinylation. Commonly targeted surface proteins include CD9, CD63, and CD81, which are frequently enriched on exosome and EV surfaces.70 Immunoaffinity offers the advantage of selectively isolating EVs and the potential to isolate EVs from diverse cell types. For instance, anti-EpCAM conjugated magnetic beads have been used to target and isolate tumor-derived EVs expressing epithelial cell adhesion molecule (EpCAM).71,72 Other antibodies, such as anti-A33, anti-PSMA, anti-CD105, anti-CD171 (L1CAM), anti-CD34, anti-CD61, anti-CD41, and anti-CD235a, have also been employed for the isolation of specific EVs.73,74
Aqueous-Two Phase System
An aqueous-two phase system (ATPS) is a method of separating biomolecules or particles by using two incompatible solutes, such as polymers or salts, that form two immiscible phases in water.75 An ATPS can be used to isolate exosomes, which are nanoscale vesicles that carry biological information and have potential applications in drug delivery and diagnostics. Exosomes have different affinities for the two phases of an ATPS, depending on their surface properties and size. By adjusting the composition and concentration of the solutes, the partition coefficient of exosomes can be optimized to achieve high purity and recovery.76 Recently, through the utilization of ATPS, exosomes were isolated with high yield from CaCo2 cells in vitro,77 human plasma78 and human urine.79 This technique is cost-effective, easy to perform, rapid and scalable, making it a superior method over traditional chromatography and ultracentrifugation approaches.80
Clinical Applications
Scientists utilized the exosomes toward two main perspectives, ie as diagnostic markers since the composition of exosomes differ in normal and abnormal states. Even more, the scientists moved forward to engineer the exosomes as drug vehicles that is more specific, targetable, biocompatible and with no immune reactions.
Diagnostic Biomarkers
Being an intercellular communication way, and, at the same time, convey a range of biomolecules, such as proteins, lipids, nucleic acids, and metabolites, they have been utilized for diagnostic purposes. Exosomes can act as mediators of intercellular communication and influence various physiological and pathological processes. In recent years, exosomes have attracted considerable attention as potential diagnostic and therapeutic biomarkers for various diseases, especially cancers (Figure 4).81 One of the main advantages of exosomes as biomarkers is their accessibility and stability in various body fluids, such as blood, urine, saliva, cerebrospinal fluid, and breast milk. Exosomes can reflect the molecular profile and status of their parental cells, and thus provide valuable information about the origin, progression, and prognosis of diseases. For example, exosomes derived from tumor cells can carry tumor-specific antigens, oncogenic proteins, and mutated or aberrant nucleic acids, which can be detected by various techniques, such as ELISA, mass spectrometry, polymerase chain reaction (PCR), and next-generation sequencing (NGS).82,83 Table 2 provide a list of some exosomal proteins and miRNAs that were confirmed for their differentially existence in disease.
 |
Figure 4 Some of the diseases that exosome contents were found to be a diagnostic biomarkers.84 The Figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0. |
 |
Table 2 Examples of the Exosomal Contents That are Used in the Diagnosis of the Mentioned Diseases |
Therapeutic Cargo Carriers
A variety of diseases, such as respiratory, infectious, and cancerous diseases, are the target of exosome-based therapies in more than 150 clinical trials registered in ClinicalTrials.gov (searching for “exosome therapy” on www.clinicaltrials.gov). Exosomes from stem cells, mainly mesenchymal stem cells of different origins, are used in 31 of those trials as a substitute for mesenchymal stem cell therapy. These pre-clinical and clinical studies indicate that stem cell-derived exosomes may mimic some of the therapeutic benefits of their parent cells without the inherent limitations of stem cell therapy. This is attributable to many features involving being small in size, minimal risk of immune response and tumour formation, stable for long-term storage and transportation, no documented ethical issues, having various delivery routes, and engineerable to carry drug cargos.11
Neurodegenerative Diseases
Exosomes are small membrane-bound vesicles that can transfer various molecules between cells. They have been implicated in the pathogenesis and diagnosis of several central nervous system (CNS) diseases, such as Alzheimer’s disease (AD) and Parkinson’s disease (PD). However, they also have potential therapeutic applications for these disorders.
For AD, exosomes can be used to deliver anti-amyloid or anti-tau agents to the brain, or to modulate the neuroinflammation and neurogenesis processes. For example, exosomes derived from mesenchymal stem cells (MSCs) have been shown to reduce amyloid plaques and improve cognitive function in AD mouse models.109 Exosomes can also serve as biomarkers for early diagnosis of AD, as they carry disease-related proteins, such as amyloid-beta and tau, in the cerebrospinal fluid and blood.85
For PD, exosomes can be used to deliver neuroprotective or neurorestorative factors, such as dopamine, growth factors, or antioxidants, to the dopaminergic neurons that are degenerated in PD. For instance, exosomes from MSCs have been shown to enhance the survival and function of dopaminergic neurons and ameliorate the motor symptoms in PD animal models.86 Exosomes can also be used as biomarkers for PD, as they contain disease-related proteins, such as alpha-synuclein and DJ-1, in the cerebrospinal fluid and blood.87
Exosomes have promising roles in the treatment of AD and PD, as they can cross the blood-brain barrier, target specific cells, and modulate the pathological processes. However, more studies are needed to optimize the exosome isolation, characterization, and delivery methods, as well as to evaluate the safety and efficacy of exosome-based therapies in clinical trials.
Cardiovascular Diseases
Exosomes are nanosized vesicles that carry various molecules, such as proteins, lipids, and nucleic acids, and can mediate intercellular communication. They have been implicated in the pathophysiology and diagnosis of cardiovascular diseases (CVDs), such as myocardial infarction, heart failure, atherosclerosis, and hypertension. However, they also have potential therapeutic applications for these disorders.88 Exosomes can be used to deliver cardioprotective or regenerative factors, such as growth factors, cytokines, microRNAs, or antioxidants, to the injured or diseased heart. For example, exosomes derived from stem cells, such as mesenchymal stem cells, cardiac progenitor cells, or induced pluripotent stem cells, have been shown to improve cardiac function, reduce infarct size, inhibit fibrosis, and promote angiogenesis in animal models of CVDs.89 Exosomes can also be used to modify the immune response, inflammation, and oxidative stress in the cardiovascular system.90
Depending on their origin and content, exosomes can have either pro-atherogenic or anti-atherogenic effects on the vascular system. For example, some exosomes can promote inflammation, oxidative stress, lipid accumulation, and plaque formation, while others can inhibit these processes and enhance endothelial function, angiogenesis, and plaque stability.91 Therefore, exosomes can be used as a therapeutic option for atherosclerosis by either inhibiting the harmful exosomes or enhancing the beneficial exosomes. This can be achieved by manipulating the exosome biogenesis, uptake, or targeting, as well as by loading exosomes with drugs or modifying their surface with ligands. For instance, researchers have shown that exosomes from mesenchymal stem cells can reduce atherosclerosis in animal models by delivering anti-inflammatory and anti-oxidant molecules to the injured arteries.92 Exosomes can also be used as biomarkers for early diagnosis and prognosis of atherosclerosis, as they reflect the molecular and cellular status of their parent cells and tissues.93
Several in vivo studies and clinical trials have explored the use of exosomes as therapy for atherosclerosis. Some of these studies included the utilization of (i) MSC-derived exosomes, (ii) exosomes derived from endothelial progenitor cells, and (iii) exosomes derived from bone marrow-derived mesenchymal stem cells. These exosomes displayed positive actions toward atherosclerosis via reducing inflammation, promoting the migration of vascular endothelial cells, promoting the formation of new blood vessels and by inhibiting the proliferation of vascular smooth muscle cells.94
Cancer
Exosomes are nanosized vesicles that are released by various cell types and circulate in the body fluids. They are surrounded by a lipid bilayer and carry a diverse range of biomolecules, such as proteins (either embedded in the membrane or enclosed within the vesicle), RNA (including coding mRNA and different types of non-coding RNAs), DNA (both double-stranded and single-stranded), and glycans. Exosomes can interact with endothelial cells and modulate their permeability, leading to vascular leakiness that facilitates tumor cell extravasation.95 They can also affect the extracellular matrix (ECM) and activate the coagulation cascade, resulting in thrombosis that impairs blood flow and oxygen delivery. Moreover, exosomes can regulate the immune system by suppressing the anti-tumor immune response and enhancing the pro-tumor inflammatory response.96 Additionally, exosomes can induce the activation of cancer-associated fibroblasts (CAFs), which are key players in the tumor microenvironment. CAFs can also produce exosomes that stimulate the migration and invasion of cancer cells, thereby promoting tumor metastasis.15 Tumor-derived exosomes, taken up by organ-specific cells, play a crucial role in preparing the pre-metastatic niche. When treated with exosomes from lung-tropic models, bone-tropic tumor cells change their metastatic direction. Exosome proteomics reveals distinct integrin expression patterns: α6β4 and α6β1 are associated with lung metastasis, while αvβ5 is linked to liver metastasis. Targeting these integrins reduces exosome uptake and inhibits lung and liver metastasis.33
Exosomes have been investigated for their potential in cancer therapy, and several mechanisms have been identified for their role in cancer control. Some of the traced mechanisms are listed below and depicted in Figure 5:
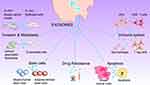 |
Figure 5 Different roles of cancer-derived exosomes against a variety of target cells. Reprinted from Lowry MC, Gallagher WM, O’Driscoll L. The Role of Exosomes in Breast Cancer. Clin Chem. 2015;61:1457–1465, by permission of Oxford University Press.97 |
- Direct administration to cancer cells: Exosomes can be administered directly to cancer cells to suppress tumor progression by inducing apoptosis, cell cycle arrest, or senescence. For example, exosomes derived from dendritic cells have been shown to induce apoptosis and inhibit proliferation of melanoma cells. Exosomes can also deliver anti-cancer drugs or siRNAs to cancer cells, enhancing their therapeutic efficacy and specificity.98
- Delivery to immune cells in the tumor microenvironment: Exosomes can be delivered to immune cells in the tumor microenvironment to modulate the immune response by either stimulating or inhibiting the anti-tumor immunity. For example, exosomes derived from tumor cells can suppress the activation and function of T cells, natural killer cells, and dendritic cells, thereby creating an immunosuppressive microenvironment. On the other hand, exosomes derived from immune cells can activate the cytotoxic activity of T cells and natural killer cells, thereby enhancing the anti-tumor immunity.99
- Antigen presentation: Exosomes can present antigens to immune cells, potentially eliciting an immune response against cancer cells². Exosomes can carry tumor-associated antigens (TAAs) or major histocompatibility complex (MHC) molecules on their surface, which can be recognized by antigen-presenting cells (APCs) or T cells, respectively. For example, exosomes derived from tumor cells or dendritic cells have been used as cancer vaccines to induce T cell-mediated immune responses against TAAs.100
- Cell signaling: Exosomes can initiate or suppress various signaling pathways in recipient cells, influencing processes such as tumor proliferation, invasion, angiogenesis, and drug resistance. Exosomes can transfer growth factors, cytokines, chemokines, or receptors to recipient cells, thereby activating or inhibiting the downstream signaling cascades. For example, exosomes derived from breast cancer cells can transfer HER2 to non-HER2-expressing cells, conferring them with increased proliferation and resistance to trastuzumab.101
- Therapeutic delivery: Exosomes can be utilized for the therapeutic delivery of small molecules, proteins, and RNAs to target cancer cells with high efficiency. Exosomes have several advantages over other nanocarriers, such as low immunogenicity, high biocompatibility, and ability to cross biological barriers. Exosomes can be engineered to load various therapeutic agents, such as doxorubicin, paclitaxel, or miRNAs, and to target specific cancer cells by modifying their surface with ligands, such as antibodies or peptides.102
These mechanisms demonstrate the diverse ways in which exosomes can be involved in cancer control, from directly influencing tumor cells to modulating the immune response in the tumor microenvironment. Further research and clinical trials are ongoing to explore the full potential of exosome-based cancer therapy.
Diabetes
Exosomes are small vesicles that can transfer molecules between cells and modulate various biological processes. They have been implicated in the pathophysiology and diagnosis of diabetes and its complications, as well as in the potential therapeutic applications for these disorders.103 Diabetes is a metabolic disease characterized by chronic hyperglycemia and impaired insulin secretion or action. Exosomes can reflect the metabolic status of the cells and tissues that produce them, and can carry biomarkers of diabetes, such as glucose, insulin, or glycated hemoglobin.104 Exosomes can also influence the insulin sensitivity and glucose uptake of the target cells, such as adipocytes, hepatocytes, or skeletal muscle cells, by transferring proteins, lipids, or microRNAs.105
Exosomes can regulate glucose metabolism, and this role was first identified in the context of physical exercise. Physical exercise is essential for DM care and has been shown to enhance insulin sensitivity in peripheral tissues and maintain β-cell function.106,107 Physical exercise or training can also trigger the rapid release of small EVs from skeletal muscle into the bloodstream, which suggests a link between exercise-induced exosome release and the improvement of insulin resistance and β-cell damage.108 Moreover, exosomes from muscles may help in DM management. Cardiomyocytes under glucose deprivation released exosomes with glucose transporter 1 (GLUT1) and GLUT4, and other enzymes involved in glucose metabolism, which can augment glucose uptake and subsequent glycolysis in adjacent endothelial cells.105,110 Exosomes from exercise contain miR-455, miR-29b, miR-323-5p, and miR-466, which can inhibit the expression of matrix metalloproteinase (MMP9) by binding to its 3′ region to prevent MMP9-mediated cardiac fibrosis, which may reverse diabetic cardiomyopathy. Previous reports isolated exosomes from MSCs to treat T2DM rat models, obtained good therapeutic effects in the early stage, and elucidated the underlying mechanisms. Furthermore, exosomes from INS-1 cells can transport neutral ceramidase to block palmitic acid (PA)-induced INS-1 cell apoptosis and boost insulin sensitivity in the PA-induced insulin-resistant cell model H4IIEC3.111,112 These data demonstrated the potential of physical exercise related exosomes in modulating glucose metabolism. Figure 6 summarizes the combined actions of stem cell, macrophage and cardiomyocytes-derived exosomes on β-cells.
 |
Figure 6 The impacts of stem cell, macrophage and cardiomyocytes-derived exosomes on pancreatic β- cells. Reprinted from Castaño C, Novials A, Párrizas M. Exosomes and diabetes. Diabetes Metab Res Rev. 2019;35:e3107. © 2018 John Wiley & Sons, Ltd.110 |
Exosomes, depending on their cargo, play a dual role in the regulation of insulin resistance in Type II Diabetes Mellitus (TIIDM). Exosomes containing Sonic Hedgehog (Shh) induce insulin resistance and elevate glucose levels in TIIDM by promoting M1 polarization of macrophages, enhancing their pro-inflammatory activity. Shh-loaded exosomes activate the Ptch-induced PI3K signaling pathway, leading to M1 polarization of macrophages. This polarization, in turn, reduces the expression levels of IRS-1 and HSL in adipocytes, contributing to insulin resistance and hyperglycemia in TIIDM.113 Moreover, it has been observed that cancer cells, particularly pancreatic tumor cells, secrete exosomes that can trigger the development of diabetes and negatively impact β cells. These tumor-derived exosomes stimulate insulin resistance by suppressing PI3K/Akt signaling, promoting the expression of FoxO1, and reducing GLUT4 expression in target cells. Inhibition of exosome secretion from pancreatic tumor cells or upregulation of GLUT4 expression is suggested as potential strategies to reverse insulin resistance.114 Adipose tissue also contributes to exosome secretion, with exosome-like vesicles from adipocytes playing a role in triggering insulin resistance. These vesicles serve as mediators between macrophages and adipose tissue, promoting the differentiation of monocytes to macrophages and inducing the secretion of pro-inflammatory cytokines like IL-6 and TNF-α. Silencing the TLR4/TRIF axis has been shown to prevent insulin resistance caused by these adipocyte-derived exosomes.115
Even concerning the diabetes complications, exosomes can greatly improve the prolonged wound healing occurring in diabetic patients via multiple mechanisms such as (i) enhancing cell proliferation and inhibiting apoptosis, (ii) resolve inflammation, (iii) promote angiogenesis, and (iv) encouraging collagen deposition.116–118
Several clinical trials are using exosomes as a therapeutic intervention against the four mentioned diseases and summarized in Table 3. The great majority of clinical trials are targeting cancers (81), followed by CVS (24), diabetes (9) and neurodegenerative diseases (8).
 |
Table 3 List of Some of the Registered Clinical Trials Using Exosomes Therapy for Managing Neurodegenerative Diseases, Cardiovascular Disorders, Cancers and Diabetes |
Future Prospective
Exosomes are nanoscale vesicles that play important roles in cell-to-cell communication and can modulate the function and fate of recipient cells. Exosomes have been shown to be altered in various diseases and can serve as potential biomarkers for diagnosis and prognosis. Moreover, exosomes can be engineered to deliver therapeutic molecules to target cells and tissues, offering a novel strategy for disease treatment. This review summarized the current knowledge on the composition, biogenesis, and implications of exosomes, as well as the methods for their isolation and application. Despite the rapid progress in exosome research, there are still many challenges and limitations that need to be addressed. Some of the key issues include the standardization and optimization of exosome isolation and characterization techniques, the elucidation of the mechanisms and specificity of exosome uptake and release, the evaluation of the safety and efficacy of exosome-based therapies in preclinical and clinical studies, and the development of scalable and cost-effective exosome production and purification methods. Future studies should also explore the diversity and heterogeneity of exosomes in different biological contexts and the potential interactions and synergies between exosomes and other extracellular vesicles.
Single exosome profiling involves multiple disciplines and aims to identify and characterize specific exosomes from patient samples. Recent advancements integrate machine learning and artificial intelligence (AI) techniques, significantly impacting rapid cancer screening. Additionally, this approach combines multi-omics for comprehensive profiling of important molecular expressions in cancer-related exosomes. By addressing early cancer biomarker detection challenges, it holds promise for precision medicine. In the future, this research may contribute to a platform for personalized cancer medicine.119,120 Moreover, with the aid of exosome barcoding technology, the profiling of surface proteins on individual exosomes is enabled. These tiny membrane-coated extracellular vesicles, ranging from 30 to 100 nanometers in size, play critical roles in various biological processes and can serve as important disease markers. The heterogeneity of exosomes makes it desirable to investigate them individually, but until now, this has been impractical. The proximity-dependent barcoding assay (PBA) overcomes this limitation by using antibody-DNA conjugates and next-generation sequencing. By analyzing the variable composition of surface proteins on exosomes derived from human body fluids or cell culture media, researchers can identify specific combinations of surface proteins associated with different sources. This approach allows exosomes to be quantified separately in mixed samples, serving as potential markers for tissue-specific engagement in diseases.121,122 Furthermore, exosome research should be integrated with other emerging fields such as nanotechnology, bioengineering, and artificial intelligence to create innovative and personalized solutions for various health problems.
Data Sharing Statement
Available upon request from the corresponding author.
Disclosure
The author declares no conflicts of interest in this work.
References
1. Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13(3):269–288. doi:10.1111/j.1365-2141.1967.tb08741.x
2. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi:10.1083/jcb.201211138
3. Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97:329–339. doi:10.1083/jcb.97.2.329
4. Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. 1985;101:942–948. doi:10.1083/jcb.101.3.942
5. Johnstone RM. Revisiting the road to the discovery of exosomes. Blood Cells Mol Dis. 2005;34:214–219. doi:10.1016/j.bcmd.2005.03.002
6. Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi:10.1146/annurev-cellbio-101512-122326
7. Alghamdi M, Alamry SA, Bahlas SM, Uversky VN, Redwan EM. Circulating extracellular vesicles and rheumatoid arthritis: a proteomic analysis. Cell Mol Life Sci. 2021;79:25. doi:10.1007/s00018-021-04020-4
8. Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487–514. doi:10.1146/annurev-biochem-013118-111902
9. Gangadaran P, Madhyastha H, Madhyastha R, et al. The emerging role of exosomes in innate immunity, diagnosis and therapy. Front Immunol. 2023;3:13.
10. Zhang M, Wan L, Li R, Li X, Zhu T, Lu H. Engineered exosomes for tissue regeneration: from biouptake, functionalization and biosafety to applications. Biomater Sci. 2023;11:7247–7267. doi:10.1039/D3BM01169K
11. Zhang K, Cheng K. Stem cell-derived exosome versus stem cell therapy. Nat Rev Bioeng. 2023;1:608–609. doi:10.1038/s44222-023-00064-2
12. Caruso Bavisotto C, Scalia F, Marino Gammazza A, et al. Extracellular Vesicle-Mediated Cell–Cell Communication in the Nervous System: focus on Neurological Diseases. Int J Mol Sci. 2019;20:434. doi:10.3390/ijms20020434
13. Jadli AS, Parasor A, Gomes KP, Shandilya R, Patel VB. Exosomes in Cardiovascular Diseases: pathological Potential of Nano-Messenger. Front Cardiovasc Med. 2021;2:8.
14. Men Y, Yelick J, Jin S, et al. Exosome reporter mice reveal the involvement of exosomes in mediating neuron to astroglia communication in the CNS. Nat Commun. 2019;10:4136. doi:10.1038/s41467-019-11534-w
15. Wortzel I, Dror S, Kenific CM. Exosome-Mediated Metastasis: communication from a Distance. Dev Cell. 2019;49:347–360. doi:10.1016/j.devcel.2019.04.011
16. Chan BD, Wong W-Y, Lee MM-L, et al. Exosomes in Inflammation and Inflammatory Disease. Proteomics. 2019;19:e1800149. doi:10.1002/pmic.201800149
17. Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9:19. doi:10.1186/s13578-019-0282-2
18. Bunggulawa EJ, Wang W, Yin T, et al. Recent advancements in the use of exosomes as drug delivery systems. J Nanobiotechnol. 2018;16:81. doi:10.1186/s12951-018-0403-9
19. Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9–17. doi:10.1038/s41556-018-0250-9
20. Ahn S-H, Ryu S-W, Choi H, You S, Park J, Choi C. Manufacturing Therapeutic Exosomes: from Bench to Industry. Mol Cells. 2022;45:284–290. doi:10.14348/molcells.2022.2033
21. Liangsupree T, Multia E, Riekkola M-L. Modern isolation and separation techniques for extracellular vesicles. J Chromatogr A. 2021;1636:461773. doi:10.1016/j.chroma.2020.461773
22. Krylova SV, Feng D. The Machinery of Exosomes: biogenesis, Release, and Uptake. Int J Mol Sci. 2023;24:1337. doi:10.3390/ijms24021337
23. Gong X, Chi H, Strohmer DF, Teichmann AT, Xia Z. Exosomes: a potential tool for immunotherapy of ovarian cancer. Front Immunol. 2023;13. doi:10.3389/fimmu.2022.1089410
24. Gao J, Li A, Hu J, Feng L, Liu L, Shen Z. Recent developments in isolating methods for exosomes. Front Bioeng Biotechnol. 2023;10. doi:10.3389/fbioe.2022.1100892
25. Rehman FU, Liu Y, Zheng M, Shi B. Exosomes based strategies for brain drug delivery. Biomaterials. 2023;293:121949. doi:10.1016/j.biomaterials.2022.121949
26. Chen Z, Xiong M, Tian J, Song D, Duan S, Zhang L. Encapsulation and assessment of therapeutic cargo in engineered exosomes: a systematic review. J Nanobiotechnol. 2024;22:18. doi:10.1186/s12951-023-02259-6
27. Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell. 2011;21:77–91. doi:10.1016/j.devcel.2011.05.015
28. Tschuschke M, Kocherova I, Bryja A, et al. Inclusion Biogenesis, Methods of Isolation and Clinical Application of Human Cellular Exosomes. J Clin Med. 2020;9:436. doi:10.3390/jcm9020436
29. Zhang Y, Bi J, Huang J, Tang Y, Du S. Exosome: a Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int J Nanomed. 2020;15:6917–6934. doi:10.2147/IJN.S264498
30. Np H, L A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75. doi:10.1007/s00018-017-2595-9
31. Langemeyer L, Fröhlich F, Ungermann C. Rab GTPase Function in Endosome and Lysosome Biogenesis. Trends Cell Biol. 2018;28:957–970. doi:10.1016/j.tcb.2018.06.007
32. Sancho-Albero M, Navascués N, Mendoza G, et al. Exosome origin determines cell targeting and the transfer of therapeutic nanoparticles towards target cells. J Nanobiotechnology. 2019;17:16. doi:10.1186/s12951-018-0437-z
33. Hoshino A, Costa-Silva B, Shen T-L, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi:10.1038/nature15756
34. Gurung S, Perocheau D, Touramanidou L, Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal. 2021;19:47. doi:10.1186/s12964-021-00730-1
35. Smith SA, Selby LI, Johnston APR, Such GK. The Endosomal Escape of Nanoparticles: toward More Efficient Cellular Delivery. Bioconjugate Chem. 2019;30:263–272. doi:10.1021/acs.bioconjchem.8b00732
36. Willis GR, Kourembanas S, Mitsialis SA. Toward Exosome-Based Therapeutics: isolation, Heterogeneity, and Fit-for-Purpose Potency. Front Cardiovasc Med. 2017;4:63. doi:10.3389/fcvm.2017.00063
37. Chen Y-S, Lin E-Y, Chiou T-W, Harn H-J. Exosomes in clinical trial and their production in compliance with good manufacturing practice. Tzu Chi Med J. 2019;32:113–120. doi:10.4103/tcmj.tcmj_182_19
38. Huang F, Wan J, Hao S, Deng X, Chen L, Ma L. TGF-β1-silenced leukemia cell-derived exosomes target dendritic cells to induce potent anti-leukemic immunity in a mouse model. Cancer Immunol Immunother. 2017;66:1321–1331. doi:10.1007/s00262-017-2028-5
39. Rossowska J, Anger N, Wegierek K, et al. Antitumor Potential of Extracellular Vesicles Released by Genetically Modified Murine Colon Carcinoma Cells With Overexpression of Interleukin-12 and shRNA for TGF-β1. Front Immunol. 2019;10:211. doi:10.3389/fimmu.2019.00211
40. Correa R, Caballero Z, De León LF, Spadafora C. Extracellular Vesicles Could Carry an Evolutionary Footprint in Interkingdom Communication. Front Cell Infect Microbiol. 2020;10:76. doi:10.3389/fcimb.2020.00076
41. An Y, Jin T, Zhu Y, Zhang F, He P. An ultrasensitive electrochemical aptasensor for the determination of tumor exosomes based on click chemistry. Biosens Bioelectron. 2019;142:111503. doi:10.1016/j.bios.2019.111503
42. LeBleu VS, Kalluri R. Exosomes as a Multicomponent Biomarker Platform in Cancer. Trend Cancer. 2020;6:767–774. doi:10.1016/j.trecan.2020.03.007
43. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977. doi:10.1126/science.aau6977
44. Konoshenko MY, Lekchnov EA, Vlassov AV, Laktionov PP. Isolation of Extracellular Vesicles: general Methodologies and Latest Trends. Biomed Res Int. 2018;2018:e8545347. doi:10.1155/2018/8545347
45. Cho S, Yang HC, Rhee WJ. Development and comparative analysis of human urine exosome isolation strategies. Process Biochem. 2020;88:197–203. doi:10.1016/j.procbio.2019.09.017
46. Guerreiro EM, Vestad B, Steffensen LA, et al. Efficient extracellular vesicle isolation by combining cell media modifications, ultrafiltration, and size-exclusion chromatography. PLoS One. 2018: 13:e0204276. doi:10.1371/journal.pone.0204276
47. Lane RE, Korbie D, Trau M, Hill MM. Optimizing Size Exclusion Chromatography for Extracellular Vesicle Enrichment and Proteomic Analysis from Clinically Relevant Samples. Proteomics. 2019;19:1800156. doi:10.1002/pmic.201800156
48. Park S, Lee K, Park IB, et al. The profiles of microRNAs from urinary extracellular vesicles (EVs) prepared by various isolation methods and their correlation with serum EV microRNAs. Diabetes Res Clin Pract. 2020;160:108010. doi:10.1016/j.diabres.2020.108010
49. Zhang M, Vojtech L, Ye Z, Hladik F, Nance E. Quantum Dot Labeling and Visualization of Extracellular Vesicles. ACS Appl Nano Mater. 2020;3:7211–7222. doi:10.1021/acsanm.0c01553
50. Tulkens J, De Wever O, Hendrix A. Analyzing bacterial extracellular vesicles in human body fluids by orthogonal biophysical separation and biochemical characterization. Nat Protoc. 2020;15:40–67. doi:10.1038/s41596-019-0236-5
51. Ramirez MI, Amorim MG, Gadelha C, et al. Technical challenges of working with extracellular vesicles. Nanoscale. 2018;10:881–906. doi:10.1039/C7NR08360B
52. Sunkara V, Kim C-J, Park J, et al. Fully Automated, Label-Free Isolation of Extracellular Vesicles from Whole Blood for Cancer Diagnosis and Monitoring. Theranostics. 2019;9:1851–1863. doi:10.7150/thno.32438
53. Yang Y, Wang Y, Wei S, et al. Extracellular vesicles isolated by size-exclusion chromatography present suitability for RNomics analysis in plasma. J Transl Med. 2021;19:104. doi:10.1186/s12967-021-02775-9
54. O’Neil EV, Burns GW, Ferreira CR, Spencer TE. Characterization and regulation of extracellular vesicles in the lumen of the ovine uterus†. Biol Reprod. 2020;102:1020–1032. doi:10.1093/biolre/ioaa019
55. Shukuya T, Ghai V, Amann JM, et al. Circulating MicroRNAs and Extracellular Vesicle–Containing MicroRNAs as Response Biomarkers of Anti–programmed Cell Death Protein 1 or Programmed Death-Ligand 1 Therapy in NSCLC. J Thorac Oncol. 2020;15:1773–1781. doi:10.1016/j.jtho.2020.05.022
56. Povero D, Yamashita H, Ren W, et al. Characterization and Proteome of Circulating Extracellular Vesicles as Potential Biomarkers for NASH. Hepatol Commun. 2020;4:1263–1278. doi:10.1002/hep4.1556
57. Zheng H, Guan S, Wang X, Zhao J, Gao M, Zhang X. Deconstruction of Heterogeneity of Size-Dependent Exosome Subpopulations from Human Urine by Profiling N-Glycoproteomics and Phosphoproteomics Simultaneously. Anal Chem. 2020;92:9239–9246. doi:10.1021/acs.analchem.0c01572
58. Guan S, Yu H, Yan G, Gao M, Sun W, Zhang X. Characterization of Urinary Exosomes Purified with Size Exclusion Chromatography and Ultracentrifugation. J Proteome Res. 2020;19:2217–2225. doi:10.1021/acs.jproteome.9b00693
59. Aqrawi LA, Galtung HK, Guerreiro EM, et al. Proteomic and histopathological characterisation of sicca subjects and primary Sjögren’s syndrome patients reveals promising tear, saliva and extracellular vesicle disease biomarkers. Arthritis Res Ther. 2019;21:181. doi:10.1186/s13075-019-1961-4
60. Bartel S, La Grutta S, Cilluffo G, et al. Human airway epithelial extracellular vesicle miRNA signature is altered upon asthma development. Allergy. 2020;75:346–356. doi:10.1111/all.14008
61. Foers AD, Chatfield S, Dagley LF, et al. Enrichment of extracellular vesicles from human synovial fluid using size exclusion chromatography. J Extracellu Vesicl. 2018;7:1490145. doi:10.1080/20013078.2018.1490145
62. Prieto-Fernández E, Aransay AM, Royo F, et al. A Comprehensive Study of Vesicular and Non-Vesicular miRNAs from a Volume of Cerebrospinal Fluid Compatible with Clinical Practice. Theranostics. 2019;9:4567–4579. doi:10.7150/thno.31502
63. Lozano‐Ramos I, Bancu I, Oliveira‐Tercero A, et al. Size‐exclusion chromatography‐based enrichment of extracellular vesicles from urine samples. J Extracellu Vesicl. 2015;4:27369. doi:10.3402/jev.v4.27369
64. Baranyai T, Herczeg K, Onódi Z, et al. Isolation of Exosomes from Blood Plasma: qualitative and Quantitative Comparison of Ultracentrifugation and Size Exclusion Chromatography Methods. PLoS One. 2015;10:e0145686. doi:10.1371/journal.pone.0145686
65. Arntz OJ, Pieters BCH, Van Lent PLEM, Koenders MI, Van Der Kraan PM, Van De Loo FAJ. An optimized method for plasma extracellular vesicles isolation to exclude the copresence of biological drugs and plasma proteins which impairs their biological characterization. PLoS One. 2020;15:e0236508. doi:10.1371/journal.pone.0236508
66. Bai J, Wei X, Zhang X, et al. Microfluidic strategies for the isolation and profiling of exosomes. TrAC Trend Analyti Chem. 2023;158:116834. doi:10.1016/j.trac.2022.116834
67. Kumar K, Kim E, Alhammadi M, et al. Recent advances in microfluidic approaches for the isolation and detection of exosomes. TrAC Trends Analyt Chem. 2023;159:116912. doi:10.1016/j.trac.2022.116912
68. Havers M, Broman A, Lenshof A, Laurell T. Advancement and obstacles in microfluidics-based isolation of extracellular vesicles. Anal Bioanal Chem. 2023;415:1265–1285. doi:10.1007/s00216-022-04362-3
69. Chen J, Li P, Zhang T, et al. Review on Strategies and Technologies for Exosome Isolation and Purification. Front Bioeng Biotechnol. 2022;9:1.
70. Filipović L, Spasojević M, Prodanović R, et al. Affinity-based isolation of extracellular vesicles by means of single-domain antibodies bound to macroporous methacrylate-based copolymer. New Biotechnol. 2022;69:36–48. doi:10.1016/j.nbt.2022.03.001
71. Hurwitz SN, Sun L, Cole KY, Ford CR, Olcese JM, Meckes DG. An optimized method for enrichment of whole brain-derived extracellular vesicles reveals insight into neurodegenerative processes in a mouse model of Alzheimer’s disease. J Neurosci Method. 2018;307:210–220. doi:10.1016/j.jneumeth.2018.05.022
72. Königsberg R, Obermayr E, Bises G, et al. Detection of EpCAM positive and negative circulating tumor cells in metastatic breast cancer patients. Acta Oncologica. 2011;50:700–710. doi:10.3109/0284186X.2010.549151
73. Sharma P, Ludwig S, Muller L, et al. Immunoaffinity‐based isolation of melanoma cell‐derived exosomes from plasma of patients with melanoma. J Extracellu Vesicl. 2018;7:1435138. doi:10.1080/20013078.2018.1435138
74. Katsu M, Hama Y, Utsumi J, et al. MicroRNA expression profiles of neuron-derived extracellular vesicles in plasma from patients with amyotrophic lateral sclerosis. Neurosci Lett. 2019;708:134176. doi:10.1016/j.neulet.2019.03.048
75. Chao Y. Emerging aqueous two-phase systems: from fundamentals of interfaces to biomedical applications. Chem Soc Rev. 2020;49:114–142. doi:10.1039/C9CS00466A
76. Kırbaş OK, Bozkurt BT, Asutay AB, et al. Optimized Isolation of Extracellular Vesicles From Various Organic Sources Using Aqueous Two-Phase System. Sci Rep. 2019;9:19159. doi:10.1038/s41598-019-55477-0
77. Torres-Bautista A, Torres-Acosta MA, González-Valdez J. Characterization and optimization of polymer-polymer aqueous two-phase systems for the isolation and purification of CaCo2 cell-derived exosomes. PLoS One. 2022;17:e0273243. doi:10.1371/journal.pone.0273243
78. Slyusarenko M, Nikiforova N, Sidina E, et al. Formation and Evaluation of a Two-Phase Polymer System in Human Plasma as a Method for Extracellular Nanovesicle Isolation. Polymers. 2021;13:458. doi:10.3390/polym13030458
79. Shin H, Park YH, Kim Y-G, Lee JY, Park J. Aqueous two-phase system to isolate extracellular vesicles from urine for prostate cancer diagnosis. PLoS One. 2018;13:e0194818. doi:10.1371/journal.pone.0194818
80. Yankov D. Aqueous two-phase systems as a tool for bioseparation – emphasis on organic acids. Phys Sci Rev. 2020;5. doi:10.1515/psr-2018-0067
81. Mosquera-Heredia MI, Morales LC, Vidal OM, et al. Exosomes: potential Disease Biomarkers and New Therapeutic Targets. Biomedicines. 2021;9:1061. doi:10.3390/biomedicines9081061
82. Lin J, Li J, Huang B, et al. Exosomes: novel Biomarkers for Clinical Diagnosis. Sci World J. 2015;2015:e657086. doi:10.1155/2015/657086
83. Miao M, Miao Y, Zhu Y, Wang J, Zhou H. Advances in Exosomes as Diagnostic and Therapeutic Biomarkers for Gynaecological Malignancies. Cancers. 2022;14:4743. doi:10.3390/cancers14194743
84. Cano A, Ettcheto M, Bernuz M, et al. Extracellular vesicles, the emerging mirrors of brain physiopathology. Int J Biol Sci. 2023;19:721–743. doi:10.7150/ijbs.79063
85. Fan Y, Chen Z, Zhang M. Role of exosomes in the pathogenesis, diagnosis, and treatment of central nervous system diseases. J Transl Med. 2022;20:291. doi:10.1186/s12967-022-03493-6
86. Abrishamdar M, Jalali MS, Yazdanfar N. The role of exosomes in pathogenesis and the therapeutic efficacy of mesenchymal stem cell-derived exosomes against Parkinson’s disease. Neurol Sci. 2023;44:2277–2289. doi:10.1007/s10072-023-06706-y
87. Liu S, Li L, Zhuang J, et al. Update on the application of mesenchymal stem cell-derived exosomes in the treatment of Parkinson’s disease: a systematic review. Front Neurol. 2022;2:13.
88. Guo D, Xu Y, Ding J, et al. Roles and Clinical Applications of Exosomes in Cardiovascular Disease. Biomed Res Int. 2020;2020:e5424281. doi:10.1155/2020/5424281
89. He N, Zhang Y, Zhang S, Wang D. Exosomes: cell-Free Therapy for Cardiovascular Diseases. J Cardiovasc Trans Res. 2020;13:713–721. doi:10.1007/s12265-020-09966-7
90. Wang J, Zhao C, Xiao J. Exosomes in Cardiovascular Diseases and Treatment: experimental and Clinical Aspects. J Cardiovasc Trans Res. 2019;12:1–2. doi:10.1007/s12265-018-9860-7
91. Wang H, Xie Y, Salvador AM, et al. Exosomes: multifaceted Messengers in Atherosclerosis. Curr Atheroscler Rep. 2020;22:57. doi:10.1007/s11883-020-00871-7
92. Patel N, Chin DD, Chung EJ. Exosomes in Atherosclerosis, a Double-Edged Sword: their Role in Disease Pathogenesis and Their Potential as Novel Therapeutics. AAPS J. 2021;23:95. doi:10.1208/s12248-021-00621-w
93. Wang C, Li Z, Liu Y, Yuan L. Exosomes in atherosclerosis: performers, bystanders, biomarkers, and therapeutic targets. Theranostics. 2021;11:3996–4010. doi:10.7150/thno.56035
94. Heo J, Kang H. Exosome-Based Treatment for Atherosclerosis. Int J Mol Sci. 2022;23:1002. doi:10.3390/ijms23021002
95. Tai Y, Chen K, Hsieh J, Shen T. Exosomes in cancer development and clinical applications. Cancer Sci. 2018;109:2364–2374. doi:10.1111/cas.13697
96. Paskeh MDA, Entezari M, Mirzaei S, et al. Emerging role of exosomes in cancer progression and tumor microenvironment remodeling. J Hematol Oncol. 2022;15:83. doi:10.1186/s13045-022-01305-4
97. Lowry MC, Gallagher WM, O’Driscoll L. The Role of Exosomes in Breast Cancer. Clin Chem. 2015;61:1457–1465. doi:10.1373/clinchem.2015.240028
98. Kim SB. Function and therapeutic development of exosomes for cancer therapy. Arch Pharm Res. 2022;45:295–308. doi:10.1007/s12272-022-01387-1
99. Zhu L, Sun H-T, Wang S, et al. Isolation and characterization of exosomes for cancer research. J Hematol Oncol. 2020;13:152. doi:10.1186/s13045-020-00987-y
100. Liu Y, Shi K, Chen Y, et al. Exosomes and Their Role in Cancer Progression. Front Oncol. 2021;3:11.
101. Mashouri L, Yousefi H, Aref AR, Mohammad AA, Molaei F, Alahari SK. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 2019;18:75. doi:10.1186/s12943-019-0991-5
102. Zhou Y, Zhang Y, Gong H, Luo S, Cui Y. The Role of Exosomes and Their Applications in Cancer. Int J Mol Sci. 2021;22:12204. doi:10.3390/ijms222212204
103. Nikfarjam S, Rezaie J, Zolbanin NM, Jafari R. Mesenchymal stem cell derived-exosomes: a modern approach in translational medicine. J Transl Med. 2020;18:449. doi:10.1186/s12967-020-02622-3
104. Sanganalmath SK, Dubey S, Veeranki S, Narisetty K, Krishnamurthy P. The interplay of inflammation, exosomes and Ca2+ dynamics in diabetic cardiomyopathy. Cardiovasc Diabetol. 2023;22:37. doi:10.1186/s12933-023-01755-1
105. Sun Y, Tao Q, Wu X, Zhang L, Liu Q, Wang L. The Utility of Exosomes in Diagnosis and Therapy of Diabetes Mellitus and Associated Complications. Front Endocrinol. 2021;2:12.
106. Ambery AG, Tackett L, Penque BA, Brozinick JT, Elmendorf JS. Exercise training prevents skeletal muscle plasma membrane cholesterol accumulation, cortical actin filament loss, and insulin resistance in C57BL/6J mice fed a western-style high-fat diet. Physiol Rep. 2017;5:e13363. doi:10.14814/phy2.13363
107. Narendran P, Jackson N, Daley A, et al. Exercise to preserve β-cell function in recent-onset Type 1 diabetes mellitus (EXTOD) - a randomized controlled pilot trial. Diabet Med. 2017;34:1521–1531. doi:10.1111/dme.13439
108. Safdar A, Tarnopolsky MA. Exosomes as Mediators of the Systemic Adaptations to Endurance Exercise. Cold Spring Harb Perspect Med. 2018; 8:a029827. doi:10.1101/cshperspect.a029827
109. Lakshmi S, Essa MM, Hartman RE, Guillemin GJ, Sivan S, Elumalai P. Exosomes in Alzheimer’s Disease: potential Role as Pathological Mediators, Biomarkers and Therapeutic Targets. Neurochem Res. 2020;45:2553–2559. doi:10.1007/s11064-020-03111-1
110. Castaño C, Novials A, Párrizas M. Exosomes and diabetes. Diabetes Metab Res Rev. 2019;35:e3107. doi:10.1002/dmrr.3107
111. Sun Y, Shi H, Yin S, et al. Human Mesenchymal Stem Cell Derived Exosomes Alleviate Type 2 Diabetes Mellitus by Reversing Peripheral Insulin Resistance and Relieving β-Cell Destruction. ACS Nano. 2018;12:7613–7628. doi:10.1021/acsnano.7b07643
112. Zhu M, Wu J, Gao J. Exosomes for diabetes syndrome: ongoing applications and perspective. Biomater Sci. 2022;10:2154–2171. doi:10.1039/d2bm00161f
113. Song M, Han L, Chen -F-F, et al. Adipocyte-Derived Exosomes Carrying Sonic Hedgehog Mediate M1 Macrophage Polarization-Induced Insulin Resistance via Ptch and PI3K Pathways. Cell Physiol Biochem. 2018;48:1416–1432. doi:10.1159/000492252
114. Ashrafizadeh M, Kumar AP, Aref AR, Zarrabi A, Mostafavi E. Exosomes as Promising Nanostructures in Diabetes Mellitus: from Insulin Sensitivity to Ameliorating Diabetic Complications. Int J Nanomed. 2022;17:1229–1253. doi:10.2147/IJN.S350250
115. Żbikowski A, Błachnio-Zabielska A, Galli M, Zabielski P. Adipose-Derived Exosomes as Possible Players in the Development of Insulin Resistance. Int J Mol Sci. 2021;22:7427. doi:10.3390/ijms22147427
116. Wu J, Chen L-H, Sun S-Y, Li Y, Ran X-W. Mesenchymal stem cell-derived exosomes: the Dawn of diabetic wound healing. World J Diabetes. 2022;13:1066–1095. doi:10.4239/wjd.v13.i12.1066
117. Dong J, Wu B, Tian W. How to maximize the therapeutic effect of exosomes on skin wounds in diabetes mellitus: review and discussion. Front Endocrinol. 2023;2:14.
118. Li D, Wu N. Mechanism and application of exosomes in the wound healing process in diabetes mellitus. Diabet Res Clin Pract. 2022;187:109882. doi:10.1016/j.diabres.2022.109882
119. Dhar R. Exosomes Barcoding: a smart approach for cancer liquid biopsy. J Liqu Biops. 2023;2:100129. doi:10.1016/j.jlb.2023.100129
120. Kalele K, Nyahatkar S, Mirgh D, Muthuswamy R, Adhikari MD. Exosomes: a Cutting-Edge Theranostics Tool for Oral Cancer. ACS Appl Bio Mater. 2024. doi:10.1021/acsabm.3c01243
121. Morales R-T-T, Ko J. Future of Digital Assays to Resolve Clinical Heterogeneity of Single Extracellular Vesicles. ACS Nano. 2022;16:11619–11645. doi:10.1021/acsnano.2c04337
122. Kulkarni M, Kar R, Ghosh S, et al. Clinical Impact of Multi-omics profiling of extracellular vesicles in cancer liquid biopsy. J Liqu Biops. 2024;3:100138. doi:10.1016/j.jlb.2024.100138
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
