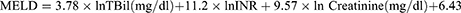Back to Journals » Hepatic Medicine: Evidence and Research » Volume 16
The Free Triiodothyronine, Gamma-Glutamyl Transpeptidase and Spontaneous Bacterial Peritonitis Index: A Novel Model for Predicting 1-Year Mortality in Patients with HBV-Related Hepatic Encephalopathy
Authors Lin L, Huang ZY, Liu K, Tong XC , Zhang ZX, Xue Y
Received 19 November 2023
Accepted for publication 12 January 2024
Published 22 January 2024 Volume 2024:16 Pages 1—9
DOI https://doi.org/10.2147/HMER.S450638
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Gerry Lake-Bakaar
Lin Lin,1,2,* Ze-yu Huang,1,* Kai Liu,1,3 Xue-cheng Tong,1,3 Zhi-xin Zhang,4 Yuan Xue1,3
1Institute of Hepatology, The Third People’s Hospital of Changzhou, Changzhou, People’s Republic of China; 2Department of Pharmacy, The Third People’s Hospital of Changzhou, Changzhou Medical Center, Nanjing Medical University, Changzhou, People’s Republic of China; 3Department of Infectious Diseases, The Third People’s Hospital of Changzhou, Changzhou Medical Center, Nanjing Medical University, Changzhou, People’s Republic of China; 4Department of Pulmonary Diseases, The Third People’s Hospital of Changzhou, Changzhou Medical Center, Nanjing Medical University, Changzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuan Xue; Zhi-xin Zhang, Email [email protected]; [email protected]
Background and Aims: Hepatic encephalopathy (HE) is characterized by neuropsychiatric manifestations in patients with decompensated cirrhosis (DC) and/or liver failure. This study aimed to investigate the predictive value of thyroid hormone in patients with HE.
Methods: Patients with DC and HE were enrolled, and multivariate logistic analysis was conducted to analyze the risk factors for 1-year mortality.
Results: Among the 81 patients with HBV-related DC and HE, 9 (11.1%) died within 3 months, and 15 (18.5%) died within the first year. More patients with FT3 < 3.5pmol/L had ascites (33.3% vs 8.9%, P< 0.01) and higher model for end-stage liver disease (MELD) (Z=3.669, P< 0.01). Additionally, free triiodothyronine (FT3) levels were lower in the non-survivor group (P< 0.01). FT3 exhibited a negative correlation with international normalized ratio and MELD (both P< 0.05). Multivariate analysis revealed that FT3, gamma-glutamyl transpeptidase (GGT), and spontaneous bacterial peritonitis (SBP) were independent risk factors for 1-year mortality of HE. A new model incorporating FT3, GTT, and SBP demonstrated superiority to MELD based on the AUROC (0.9 and 0.752, P=0.04).
Conclusion: Low FT3, but not thyroid-stimulating hormone and free tetraiodothyronine, was identified as an independent risk factor for 1-year mortality in patients with DC and HE. The newly proposed prognostic model, which includes FT3, GTT, and SBP, holds significant predictive value.
Keywords: hepatic encephalopathy, free triiodothyronine, model for end-stage liver disease, gamma-glutamyl transpeptidase, mortality
Introduction
Hepatic encephalopathy (HE) is characterized by a range of neuropsychiatric symptoms that occur in patients with decompensated cirrhosis (DC) and/or liver failure.1 Due to the severity of the underlying liver diseases and the high short-term mortality associated with HE, it is crucial to identify patients with a poor prognosis and determine the need for urgent liver transplantation. However, currently, there is no ideal prognostic biomarker or model available to predict the outcomes of HE.
Thyroid function tests are commonly used to assess the thyroid gland’s production capacity and the regulation of the hypothalamus-pituitary-thyroid axis.2 In clinical practice, the measurement of thyroid-stimulating hormone (TSH), tetraiodothyronine (T4), and triiodothyronine (T3) levels in the blood has been extensively studied in various diseases, including cancer, type 2 diabetes mellitus, non-alcoholic fatty liver disease (NAFLD), and acute-on-chronic liver failure.3–7 In patients with diabetes and NAFLD, the levels of free triiodothyronine (FT3) and total triiodothyronine (TT3) were found to be significantly higher, while free tetraiodothyronine (FT4) was lower compared to those without NAFLD.3 Furthermore, levels of FT3 and FT4 were significantly lower in patients with progressive liver fibrosis compared to those without progressive liver fibrosis.3 However, another study reported that FT3 levels were significantly higher in patients with F4 and F3 liver fibrosis compared to those with F2 fibrosis, and patients with severe and moderate NAFLD had higher FT3 levels compared to those with mild NAFLD.5
Liver plays a critical role in the metabolism of thyroid hormones.8 Various factors, such as inhibited activity of the type 1 deiodinase enzyme essential for converting T4 to T3, decreased thyroid-binding globulin, and impaired transport of thyroid hormone, can lead to thyroid hormone abnormalities. Growing evidence supports the association between serum FT3 levels and the severity of DC.4,8,9 Patients with DC at Child-Pugh stage C exhibited lower FT3 levels compared to those at stages A and B, and TSH showed a positive correlation with Child-Pugh score.8 Studies have reported that FT3 levels are lower in patients with DC and HE than in those without HE.4,9 Recently, data from a multicenter cohort showed that serum metabolites including thyroxine predicted advanced HE development.10 Findings from a small-scale study (17 cases) indicated that patients who died from HE had lower FT3 levels, suggesting that FT3 could serve as a prognostic indicator for the short-term outcome of HE,11 and its predictive value for long-term outcomes of HE remains unknown.
To date, limited knowledge is available regarding the value of thyroid function in predicting the 1-year outcome of DC and HE. In this study, we aimed to assess the predictive value of FT3, FT4, and TSH in determining 1-year mortality.
Materials and Methods
Patients
A recruitment was conducted, enrolling patients with HE (n=221) admitted to Changzhou Third Peoples’ Hospital, Changzhou Medical Center, Nanjing Medical University, between January 2010 and December 2021. The diagnosis and grading of HE were based on the West-Haven criteria.12 Underlying DC was defined by clinical manifestations, imaging diagnosis or endoscopic examination, along with complications such as ascites, HE, spontaneous bacterial peritonitis (SBP), upper gastrointestinal bleeding (UGIB), or hepatorenal syndrome.1 Patients with neurological and mental illnesses,1 malignancies,13 thyroid disease,2,8 acute or acute-on-chronic liver failure,14 or insufficient data for analysis were excluded.
Demographic, clinical and laboratory data were collected from the electronic medical records and the civil registration system. HE manifestations and DC complications were recorded. The study endpoint was 3-month and 1-year mortality following the diagnosis of HE.
The study was non-interventional, and posed no harm to the patients. Clinical data before March 2018 were collected retrospectively and data after March 2018 were collected prospectively. At admission, disease status and prognosis were informed to a family member or legal representative of the patients, and then the family member or legal representative signed to consent that clinical data could be anonymously collected for pure research. The patients who woke up were informed and consented that clinical data could be collected. The protocol was approved by the Ethics Committee of Changzhou Third Peoples’ Hospital, Changzhou Medical Center, Nanjing Medical University in accordance with the Declaration of Helsinki, 2013 (ethical approval code of NMUEC (2018) 506).
Model for End-Stage Liver Disease (MELD)
MELD15 which incorporates total bilirubin (TBil), international normalized ratio (INR) and creatinine, was analyzed using the following formula:
Statistical Analysis
The data were presented as median (interquartile range, IQR) and frequency for continuous and categorical data, respectively. Comparison between survivors and non-survivors was performed using Mann–Whitney U-tests and chi-square tests. Logistic regression analysis and Cox regression analysis were conducted to identify risk factors for 1-year mortality. Predictive accuracy was compared based on the area under the receiver operating characteristic curves (AUROCs), calculated using MedCalc Software version 20.1.0 (Mariakerke, Belgium). All analyses were performed using SPSS 25.0 software (Armonk, NY, USA), and a P-value <0.05 indicated statistical significance.
Results
Characteristics of Survivors and Non-Survivors with HBV-Related DC and HE
The analysis included data from 81 patients with HBV-related DC and HE. As shown in Table 1, 9 (11.1%) died within 3 months, and 15 (18.5%) patients died within the first year (Figure 1).
 |
Table 1 Baseline Characteristics of Patients with DC and HE |
 |
Figure 1 The flow-chart of this study. |
The non-survivor group had a higher proportion of patients with SBP (P < 0.01). Additionally, alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma glutamyl transferase (GGT), TBil, INR, and MELD scores were significantly higher (all P < 0.05), while serum FT3 levels were lower in the non-survivor group (P < 0.01). No significant difference was found in FT4, TSH, TT3 and TT4 (all P >0.05). Compared with the survivor group, more patients had a lower FT3 (normal range: 3.5–7.4pmol/L) in the non-survivor group (66.7% vs 39.4%, χ2=3.682, P=0.05).
Characteristics of Patients with and without FT3< 3.5pmol/L
Compared with those with FT3 ≥ 3.5pmol/L, more patients with FT3 < 3.5pmol/L were male (55.6% vs 77.8%, P = 0.04) and had ascites (P < 0.01). Patients with FT3 < 3.5pmol/L had higher INR and MELD score (both P<0.01). FT4, TT3 and TT4 were also significantly lower in patients with FT3 < 3.5pmol/L (Table 2).
 |
Table 2 Characteristics of Patients with FT3 < 3.5pmol/L and T3 ≥ 3.5pmol/L |
Correlation Between FT3 and Other Laboratory Parameters
FT3 negatively correlated with INR and MELD (both P < 0.05), while FT4 showed no significant correlations with INR and MELD (both P > 0.05). There was no significant relationship observed between FT3 and TSH (r = 0.215, P = 0.054) (Figure 2).
Risk Factors for 1-Year Mortality of HE
In logistic regression analysis, univariate analysis revealed that ALT, AST, GGT, TBil, INR, C-reactive protein, FT3, FT4 and SBP were associated with death within the first year (all P < 0.1). Multivariate analysis then identified FT3 (OR: 0.167, 95% CI: 0.052–0.532, P < 0.01), GGT (OR: 1.010, 95% CI: 1.001–1.018, P = 0.02), and SBP (OR: 0.030, 95% CI: 0.002–0.522, P = 0.02) as independent risk factors for 1-year mortality in HE (Table 3).
 |
Table 3 Analysis of Risk Factors for 12-Month Mortality in Patients with DC and HE |
We also performed a Cox regression analysis, and data showed that FT3 (95% CI [0.103–0.596], P<0.01), GGT (95% CI [1.003–1.012], P<0.01), SBP (95% CI [0.038–0.463], P<0.01) were independent risk factors for 1-year mortality, which was consistent with results from logistic regression analysis (Supplementary Table 1).
Models Incorporating FT3 for Predicting Accumulative Mortality of HE
Through multivariate logistic analysis, a prognostic model incorporating FT3, GGT and SBP was developed to predict 1-year mortality in patients with HE. 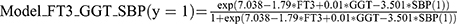 . This new model exhibited superior predictive performance compared to MELD, as indicated by the AUROC values (0.9 and 0.752, P = 0.04). Additionally, another model incorporating MELD and FT3 did not outperform MELD in predicting 12-month mortality (AUROC: 0.783 and 0.752, P = 0.54) (Figure 3).
. This new model exhibited superior predictive performance compared to MELD, as indicated by the AUROC values (0.9 and 0.752, P = 0.04). Additionally, another model incorporating MELD and FT3 did not outperform MELD in predicting 12-month mortality (AUROC: 0.783 and 0.752, P = 0.54) (Figure 3).
Using an optimal cut-off value of 0.14, the Model_FT3_GGT_SBP achieved a sensitivity of 86.67% and specificity of 78.79% (Youden index: 0.70). Patients were then categorized into two groups: the low-risk group (Model_FT3_GGT_SBP <0.14) and the high-risk group (Model_FT3_GGT_SBP ≥0.14). The high-risk group exhibited a poor prognosis (χ2 = 21.63, P <0.01) (Figure 4).
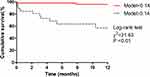 |
Figure 4 Kaplan-Meier analysis was performed to evaluate the prognostic value of Model_FT3_GGT_SBP in patients with decompensated cirrhosis and hepatic encephalopathy. |
Discussion
This study aimed to investigate the role of thyroid hormone in the outcome of HBV-related HE. We found that FT3, which negatively correlated with ALT, INR, MELD and WBC, was an independent risk factor for 1-year mortality in HE. Subsequently, a new model incorporating FT3, GGT and SBP was developed, demonstrating superior predictive ability compared to MELD for 1-year mortality in HE.
Previous investigations have reported a correlation between FT3 and liver function in various liver diseases. Studies have shown that low FT3, but not TSH and FT4, is associated with fibrosis progression in patients with non-alcoholic steatohepatitis.5,16 In patients with DC, FT3 was found to be negatively correlated with the Child-Pugh score.8 Clinically, the FT3 test, which is widely used due to its high sensitivity and low cost, is commonly employed to determine the presence of clinical or subclinical thyroid dysfunction in patients with HE. In our study, low FT3 levels were observed in patients with DC and HE, particularly in those who died, and there was a negative association between FT3 and MELD score. In addition, FT4, TT3 and TT4 are also lower in patients with FT3 < 3.5pmol/L, it is speculated that the synthesis of thyroid hormone and the regulation of the hypothalamus-pituitary-thyroid axis is impaired in DC. These findings suggest that FT3 is related to the severity of illness, underscoring the importance of recommending FT3 testing for patients with DC and HE.
Considering the relationship between FT3 and liver fibrosis, thyroid hormone replacement may serve as a potential therapeutic option. Low-dose T4 has been utilized as a therapy for the early stages of non-alcoholic steatohepatitis (NASH), while T3 may be more effective for advanced NASH.17 Thyroid hormone receptor-β agonists have shown efficacy in improving hepatic steatosis in patients with NASH,18 and halting liver fibrosis progression in mice with NASH.19 The impact of FT3 on hepatic stellate cells remains unclear.20 Additionally, it is speculated that reducing intrahepatic lipid levels and restoring mitochondrial function in hepatocytes may play a role.21 In our study, FT3 emerged as an independent risk factor for 1-year mortality in HE, suggesting that thyroid hormone replacement could be a potential treatment. However, the effectiveness and safety of this approach in patients with HE requires further investigation.
There are several limitations to this study. Firstly, it is a single-center study with a relatively small sample size. As we just focused on the outcomes of the patients with DC and HE, and data before March 2018 were collected retrospectively, selection bias including self-selection bias and sample-selection bias exist in the present study. A flow-chart of this study and inclusion and exclusion criteria are provided to reduce the bias, and a full analysis set of patients with DC and HE was analyzed at the analysis stage. To validate these findings, a multi-center study with a larger sample size is required. Secondly, the underlying mechanism that links FT3 and the development of HE remains unclear. Further research is needed to elucidate this relationship. Thirdly, the predictive performance of FT3 has not been compared in cases of overt and covert HE.
Conclusions
Low FT3 levels, but not TSH and FT4, were identified as independent risk factors for 1-year mortality in patients with DC and HE. The newly developed prognostic model, which incorporates FT3, GGT and SBP, demonstrates strong predictive value.
Abbreviations
HE, hepatic encephalopathy; DC, decompensated cirrhosis; INR, international normalized ratio; FT3, free triiodothyronine; FT4, free tetraiodothyronine; TSH, thyroid-stimulating hormone; T4, tetraiodothyronine; T3, triiodothyronine; TT3, total triiodothyronine; TT4, total tetraiodothyronine; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma glutamyl transferase; TBil, total bilirubin; CRP, C-reactive protein; INR, international normalized ratio; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; HBV, hepatitis B virus; SBP, spontaneous bacterial peritonitis; UGIB, upper gastrointestinal bleeding; OR, odds ratio; CI, confidence interval; IQR, interquartile range; MELD, model for end-stage liver disease; AUROC, area under the receiver operating characteristic curve; Model_1y_MELD_FT3_SBP_ALT, a model consisting of MELD, FT3, SBP and ALT.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
This study was supported by the Project of Changzhou Health Commission (Grant No. QN202128), the Science and Technology Project of Changzhou (Grant No. CE20225040), and Leading Talent of Changzhou “the 14th Five-Year Plan” High-Level Health Talents Training Project (2022CZLJ021).
Disclosure
The authors have no conflict of interest related to this publication.
References
1. Haussinger D, Dhiman RK, Felipo V, et al. Hepatic encephalopathy. Nat Rev Dis Primers. 2022;8(1):43. doi:10.1038/s41572-022-00366-6
2. Centre NG. Thyroid Function Tests: Thyroid Disease: Assessment and Management. Vol. NBK577224. London: National Institute for Health and Care Excellence (NICE); 2019.
3. Zhang Y, Li J, Liu H. Correlation between the thyroid hormone levels and nonalcoholic fatty liver disease in type 2 diabetic patients with normal thyroid function. BMC Endocr Disord. 2022;22(1):144. doi:10.1186/s12902-022-01050-2
4. Patira NK, Salgiya N, Agrawal D. Correlation of thyroid function test with severity of liver dysfunction in cirrhosis of liver. J Assoc Physicians India. 2019;67(3):51–54.
5. Guo W, Qin P, Li XN, et al. Free triiodothyronine is associated with hepatic steatosis and liver stiffness in euthyroid Chinese adults with non-alcoholic fatty liver disease. Front Endocrinol. 2021:12711956. doi:10.3389/fendo.2021.711956
6. Zhang J, Chen Y, Duan Z. The relationship between FT3 level and severity of HBV-ACLF. Crit Rev Eukaryot Gene Expr. 2022;32(6):47–56. doi:10.1615/CritRevEukaryotGeneExpr.2022041680
7. Alkhalaileh H, Wei R, Lee JKY, Jones J, Li J. Relationship between TSH and free thyroxine in outpatient cancer patient population. Endocrine. 2023. doi:10.1007/s12020-023-03399-3
8. Raj A, Pillai G, Divakar A, Shivam V, Nair A. Association of thyroid function and severity of illness in liver cirrhosis as measured by child-Pugh score. Cureus. 2023;15(3):e36618. doi:10.7759/cureus.36618
9. Punekar P, Sharma AK, Jain A. A study of thyroid dysfunction in cirrhosis of liver and correlation with severity of liver disease. Indian J Endocrinol Metab. 2018;22(5):645–650. doi:10.4103/ijem.IJEM_25_18
10. Bajaj JS, Tandon P, O’Leary JG, et al. Admission serum metabolites and thyroxine predict advanced hepatic encephalopathy in a multicenter inpatient cirrhosis cohort. Clin Gastroenterol Hepatol. 2023;21(4):1031–1040 e1033. doi:10.1016/j.cgh.2022.03.046
11. Guven K, Kelestimur F, Yucesoy M. Thyroid function tests in non-alcoholic cirrhotic patients with hepatic encephalopathy. Eur J Med. 1993;2(2):83–85.
12. Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American association for the study of liver diseases and the European association for the study of the liver. Hepatology. 2014;60(2):715–735. doi:10.1002/hep.27210
13. Fichtl A, Seufferlein T, Zizer E. Risks and benefits of TIPS in HCC and other liver malignancies: a literature review. BMC Gastroenterol. 2023;23(1):403. doi:10.1186/s12876-023-03047-0
14. Br VK, Sarin SK. Acute-on-chronic liver failure: terminology, mechanisms and management. Clin Mol Hepatol. 2023;29(3):670–689. doi:10.3350/cmh.2022.0103
15. Kamath PS, Kim WR; Advanced Liver Disease Study G. The model for end-stage liver disease (MELD). Hepatology. 2007;45(3):797–805. doi:10.1002/hep.21563
16. Manka P, Bechmann L, Best J, et al. Low free triiodothyronine is associated with advanced fibrosis in patients at high risk for nonalcoholic steatohepatitis. Dig Dis Sci. 2019;64(8):2351–2358. doi:10.1007/s10620-019-05687-3
17. Bruinstroop E, Dalan R, Cao Y, et al. Low-dose levothyroxine reduces intrahepatic lipid content in patients with type 2 diabetes mellitus and NAFLD. J Clin Endocrinol Metab. 2018;103(7):2698–2706. doi:10.1210/jc.2018-00475
18. Harrison SA, Bashir MR, Guy CD, et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, Phase 2 trial. Lancet. 2019;394(10213):2012–2024. doi:10.1016/S0140-6736(19)32517-6
19. Kannt A, Wohlfart P, Madsen AN, et al. Activation of thyroid hormone receptor-beta improved disease activity and metabolism independent of body weight in a mouse model of non-alcoholic steatohepatitis and fibrosis. Br J Pharmacol. 2021;178(12):2412–2423. doi:10.1111/bph.15427
20. He W, Huang C, Wang L, et al. The correlation between triiodothyronine and the severity of liver fibrosis. BMC Endocr Disord. 2022;22(1):313. doi:10.1186/s12902-022-01228-8
21. Alonso-Merino E, Martin Orozco R, Ruiz-Llorente L, et al. Thyroid hormones inhibit TGF-beta signaling and attenuate fibrotic responses. Proc Natl Acad Sci U S A. 2016;113(24):E3451–E3460. doi:10.1073/pnas.1506113113
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.