Back to Journals » Nature and Science of Sleep » Volume 15
Symptomatic, Drug-Resistant Narcolepsy Remission After Fenestration of an Arachnoid Cyst – A Case Report
Authors Künstler ECS , Schwab M, Schroeder HWS, Rupprecht S
Received 9 February 2023
Accepted for publication 21 June 2023
Published 9 November 2023 Volume 2023:15 Pages 925—930
DOI https://doi.org/10.2147/NSS.S407808
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Ahmed BaHammam
Erika CS Künstler,1,2 Matthias Schwab,1,2 Henry WS Schroeder,3 Sven Rupprecht1,2
1Department of Neurology, Jena University Hospital, Jena, Germany; 2Interdisciplinary Centre for Sleep and Ventilatory Medicine, Jena University Hospital, Jena, Germany; 3Department of Neurosurgery, University Medicine Greifswald, Greifswald, Germany
Correspondence: Erika CS Künstler, Department of Neurology, Jena University Hospital, Am Klinikum 1, Jena, D-07747, Germany, Tel +49 3641 9323525, Email [email protected]
Objective: The pathogenesis of different narcolepsy phenotypes remains unclear. In rare cases, narcolepsy can be attributable to secondary brain pathologies affecting the midbrain. These cases may elucidate the pathological background and the treatment of narcolepsy, but are often limited by poor objective symptom characterization and effects of therapeutic intervention, especially by modern diagnostic standards.
Methods: A young adult presented with excessive daytime sleepiness (EDS) that was refractory to classic narcolepsy medication. Diagnosis of narcolepsy was made based on the pathologically shortened sleep latencies in polysomnography and Multiple Sleep Latency Test (MSLT), together with confirmed sleep-onset REM-sleep (SOREM). Preserved hypocretin levels in cerebrospinal fluid, together with the absence of cataplectic events confirmed the diagnosis of narcolepsy type II. MRI revealed a large arachnoid cyst with compression of the midbrain.
Results: Six months after fenestration of the cyst, the patient’s EDS had vastly improved. No further SOREM was observed, and polysomnographic and MSLT sleep latencies normalized. No further drug treatment was required.
Conclusion: Symptomatic narcolepsy due to space-occupying lesions in the mesencephalon comprises a unique curative treatment option. Here, surgical intervention offers an effective curative therapeutic approach. However, differential diagnosis of symptomatic narcolepsy requires special consideration.
Keywords: symptomatic narcolepsy, arachnoid cyst, excessive daytime sleepiness, remission, case report
Introduction
Narcolepsy, a rare, chronic sleep disorder, is marked by excessive daytime sleepiness (EDS), accompanied by sleep paralysis, cataplexy, and/or hypnagogic/hypnopompic hallucinations and the presence of shortened sleep latencies and sleep onset REM (SOREM).1 “Classical” narcolepsy type I (ie, narcolepsy with cataplexy) has an autoimmune-mediated loss of hypocretinergic neurons in the hypothalamus. However, “symptomatic” forms of narcolepsy may arise from brain trauma, inflammatory, ischemic, tumorous or other space-occupying lesions affecting the midbrain1–3 For the latter, therapeutic intervention may come into consideration. Given the chronic nature of narcolepsy and the life-long need for medical treatment, alternative therapeutic approaches, where possible, are attractive in treating such symptomatic narcolepsy cases. However, literature on the subject is very scarce. The few reports which address this question4–6 are outdated by modern diagnostic standards, as symptom severity was not objectively quantified both pre- and post-intervention. The majority of these reports did not contain information about hypocretin levels and, consequently, the role of hypocretinergic dysfunction in the pathogenesis of symptomatic narcolepsy remains unclear. Furthermore, the sustained use of medication made it difficult to quantify the effects of the intervention on the remittance of narcoleptic symptoms.6
We present a case of drug refractory symptomatic narcolepsy with effective surgical treatment in which symptoms remitted following microsurgical fenestration of a mesencephalic cyst. To the best of our knowledge, ours is the first case report which clearly and objectively describes narcoleptic symptoms using standardized tests both pre- and post-intervention as well as hypocretin estimation in accordance with modern diagnostic standards. As both the pre- and postoperative assessments were conducted without medication, this case allows the effects of the surgical intervention on the symptomatic narcolepsy to be precisely quantified, without potential confounding factors. Such information can be vital in informing decisions regarding intervention in similar cases.
Initial Case Presentation
An 18-year-old male presented with EDS, an imperative urge to sleep in monotonous situations, and concentration difficulties. The anamnestically reported sleepiness was confirmed by an Epworth Sleepiness Scale (ESS) score of 13/24 points. Cataplexy, hypnagogic/hypnopompic hallucinations, and sleep paralysis were negated. The progressively worsening EDS and concentration difficulties had started approximately 10 years previously. The patient had been diagnosed with Attentional Deficit/Hyperactivity Disorder (ADHD) four years before clinical presentation. This was transiently medicated with methylphenidate in single doses of 10 mg up to 60 mg per day. However, the patient claimed persisting symptoms of daytime sleepiness. Prior to presenting in the Interdisciplinary Centre for Sleep and Ventilatory Medicine, the patient was further unsuccessfully treated with various stimulants (Modafinil, 100 mg per day; Pitolisant, 36 mg per day; and Solriamfetol, 150 mg per day). These medications were primarily discontinued due to the side effects reported by the patient (anxiety, nausea and anxiety, and nausea respectively), rather than a lack of stimulating effect. Importantly, the patient was unmedicated both during the initial presentation and at follow-up eight months later. A schematic overview of the assessment procedure is given in Figure 1, and comprises our standard diagnostic workup for narcolepsy. A nightly polysomnography preceded each day of testing in order to exclude other sleep disorders and to ensure that the patient had sufficient sleep prior to each test. At initial presentation, the patient also underwent a lumbar puncture and cerebral MRI scan.
Initial Findings
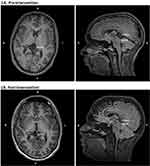
For ease of comparison, results of the initial and follow-up assessment are presented side-by-side in Table 1. During initial assessment, shortened REM latencies (of 16 and 17 minutes respectively) were detectable in two out of three polysomnographies. The Multiple Sleep Latency Test (MSLT) was discontinued after two trials as pathological sleep latencies (average: 6 minutes), and SOREMs were observed in both trials, as required for the diagnosis of a narcolepsy. In a Maintenance of Wakefulness Test (MWT), the patient could only maintain alertness in two of four trials, with subsequent pupillography indicating reduced alertness and vigilance. However, here an average sleep latency of 32 minutes was longer than expected, and it was surprising that the patient maintained wakefulness in two trials. Similarly, an average sleep latency of 26 minutes observed in the polysomnographies is also unusual in narcolepsy patients, and may have been caused by heightened physiological arousal due to the anxiety surrounding the first hospital stay. Periods of missing muscle atonia in REM-sleep were observed in all three nightly polysomnographic recordings. The patient had no sleep-related breathing disorder. Although the periodic leg movement (PLM) index of 6.3/h (reference: 0–5/h) was slightly elevated, there was no anamnestic evidence of Restless Legs Syndrome (RLS) symptoms. A lumbar puncture revealed a hypocretin-1 level of 278 pg/mL in the cerebrospinal fluid (reference: >200 pg/mL). The patient was furthermore negative for both human leukocyte antigen (HLA) haplotypes associated with narcolepsy (HLA-DQB1*06:02 and HLA-DRB1*15:01). Taken together with the anamnesis, this diagnostic workup revealing heightened sleep pressure and detection of several SOREMs implicated a narcolepsy type II (ie, narcolepsy without cataplexy) diagnosis. However, a cranial MRI scan revealed a large arachnoid cyst with impression of the thalamus and tectum (Figure 2A).
 |
Table 1 Summary of Findings at Initial Preoperative Presentation and Post-Operative Presentation 8 Months Later |
Therapeutic Intervention
Two months later, the patient underwent microsurgical intervention, with multiple fenestrations of the cyst walls being created to ensure ventricular communication.
Follow-Up Findings
Six months postoperatively, the patient reported a marked improvement in concentration abilities, as well as decreased EDS. An MRI scan showed a notable reduction in the size of the cyst (see Figure 2B).
The subjectively improved EDS was reflected by a decrease of the ESS score from 13 to 6 points, indicating normal levels of wakefulness. Both the MSLT and the MWT showed drastic improvements. SOREM was not further detectable in all five MSLT trails. Pupillography however once again revealed predominantly pathological values, indicative of residual impairment in tonic alertness. Comparable to the MSLT, nightly polysomnographies revealed normalization of REM latencies in all three nights, although the missing muscle atonia during REM sleep persisted. RLS symptoms were again negated, despite persistence of a slightly increased PLM index. Furthermore, the patient had an increased proportion of deep sleep, although more sleep fragmentation than during the initial assessment. We decided against further medication due to lack of subjective distress.
Discussion
The primary aim of this case report was to objectively quantify the degree to which indicators of symptomatic narcolepsy caused by a space-occupying lesion remitted following surgical intervention. The EDS and sleep quality showed considerable improvement, notably with normalisation of REM latency. Additionally, the proportion of deep sleep increased, likely leading to the subjective perception of sleep as being more restorative. This indicates that surgical intervention of space-occupying lesions of the midbrain offers an effective curative therapeutic approach in such cases of symptomatic narcolepsy. Whilst arachnoid cysts – a common incidental finding, seen in approximately 1.4% of MRI scans – are mostly without clinical relevance,7 the strategic location of the cyst in the third ventricle,8 and hypothalamus2,6 could result in narcolepsy without other clinical features and/or disabilities. Thus, our case illustrates the importance of detecting symptomatic narcolepsy – even if these cases are rare1 – as surgical intervention can offer an effective therapeutic approach.
Whilst most case reports on symptomatic narcolepsy do not contain information about hypocretin levels, those that do tend to find reduced levels, which – in rare cases – were reversed following intervention into the underlying neurological condition.2 However, in the case presented here, hypocretin-1 levels were within the normal range ie, phenotypically, our patient suffered from narcolepsy type II, which is marked by preserved hypocretin-1 levels. This could imply that whilst the patient’s hypocretin production was intact, the cyst’s compression of the midbrain interrupted projections to rostral parts of the central nervous system, leading to the narcoleptic symptoms. Missing muscle atonia during REM sleep, which is not directly attributable to the mesencephalic localisation of the cyst, could be caused by alterations of caudal hypocretinergic transmission to the REM-promoting centres in the upper brainstem. This could in turn suggest that if normal hypocretin production in narcolepsy type II is coupled with disturbed hypocretinergic transmission, this could play a pathomechanistic role in cases of “normal” narcolepsy type II. However, such an interpretation is speculative and warrants further investigation.
Taken together, this case report not only highlights the possible efficacy of surgical intervention in symptomatic narcolepsy, but, together with the preserved hypocretin levels, also hints at a potential pathomechanism of such cases, thereby opening up an interesting new avenue for future research.
Patient Consent for Publication
Prior to publication, full written informed consent was obtained from the patient to publish both case details and the accompanying images. Institutional approval was not necessary to publish case details.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. American Academy of Sleep Medicine. International Classification of Sleep Disorders.
2. Nishino S, Kanbayashi T. Symptomatic narcolepsy, cataplexy and hypersomnia, and their implications in the hypothalamic hypocretin/orexin system. Sleep Med Rev. 2005;9(4):269–310. doi:10.1016/j.smrv.2005.03.004
3. Vetrugno R, Stecchi S, Plazzi G, et al. Narcolepsy-like syndrome in multiple sclerosis. Sleep Med. 2009;10(3):389–391. doi:10.1016/j.sleep.2008.03.009
4. Nakano H, Ogashiwa M. Complete remission of narcolepsy after surgical treatment of an arachnoid cyst in the cerebellopontine angle. J Neurol Neurosurg Psychiatry. 1995;58(2):264. doi:10.1136/jnnp.58.2.264
5. Matsuda Y, Neshige R, Endo C, et al. A case of upper brainstem infarction developing symptomatic narcolepsy after the administration of anti-convulsant drugs. Rinsho Shinkeig. 1991;31(7):750–753.
6. Clavelou P, Tournilhac M, Vidal C, et al. Narcolepsy associated with arteriovenous malformation of the diencephalon. Sleep. 1995;18(3):202–205. doi:10.1093/sleep/18.3.202
7. Al-Holou WN, Terman S, Kilburg C, et al. Prevalence and natural history of arachnoid cysts in adults. J Neurosurg. 2006;118(2):222–231. doi:10.3171/2012.10.JNS12548
8. Autret A, Lucas B, Mondon K, et al. Sleep and brain lesions: a critical review of the literature and additional new cases. Neurophysiol Clin. 2001;31:356–375. doi:10.1016/S0987-7053(01)00282-9
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.