Back to Journals » Neuropsychiatric Disease and Treatment » Volume 13
Supplementation of omega 3 fatty acids may improve hyperactivity, lethargy, and stereotypy in children with autism spectrum disorders: a meta-analysis of randomized controlled trials
Authors Cheng YS, Tseng PT , Chen YW, Stubbs B, Yang WC, Chen TY, Wu CK, Lin PY
Received 26 July 2017
Accepted for publication 1 September 2017
Published 4 October 2017 Volume 2017:13 Pages 2531—2543
DOI https://doi.org/10.2147/NDT.S147305
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Yu-Shian Cheng,1 Ping-Tao Tseng,1,2 Yen-Wen Chen,3 Brendon Stubbs,4–6 Wei-Chieh Yang,7 Tien-Yu Chen,8,9 Ching-Kuan Wu,1 Pao-Yen Lin10,11
1Department of Psychiatry, Tsyr-Huey Mental Hospital, Kaohsiung Jen-Ai’s Home, 2WinShine Clinics in Specialty of Psychiatry, 3Prospect Clinic for Otorhinolaryngology & Neurology, Kaohsiung, Taiwan, Republic of China; 4Physiotherapy Department, South London and Maudsley NHS Foundation Trust, 5Health Service and Population Research Department, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, 6Faculty of Health, Social Care and Education, Anglia Ruskin University, Chelmsford, UK; 7Department of Pediatrics, DA-AN Women and Children Hospital, Tainan, 8Department of Psychiatry, Tri-Service General Hospital, Taipei, 9School of Medicine, National Defense Medical Center, Taipei, 10Department of Psychiatry, Kaohsiung Chang Gung Memorial Hospital, Chang Gung University College of Medicine, 11Institute for Translational Research in Biomedical Sciences, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan, Republic of China
Aim: Deficiency of omega 3 fatty acids may be linked to autism spectrum disorder (ASD). Evidence about the potential therapeutic effects of supplementation of omega 3 fatty acids is lacking in ASD patients.
Methods: We searched major electronic databases from inception to June 21, 2017, for randomized clinical trials, which compared treatment outcomes between supplementation of omega 3 fatty acids and placebo in patients with ASD. An exploratory random-effects meta-analysis of the included studies was undertaken.
Results and conclusion: Six trials were included (n=194). Meta-analysis showed that supplementation of omega 3 fatty acids improved hyperactivity (difference in means =-2.692, 95% confidence interval [CI] =-5.364 to -0.020, P=0.048, studies =4, n=109), lethargy (difference in means =-1.969, 95% CI =-3.566 to -0.372, P=0.016, studies =4, n=109), and stereotypy (difference in means =-1.071, 95% CI =-2.114 to -0.029, P=0.044, studies =4, n=109). No significant differences emerged between supplementation of omega 3 fatty acids and placebo in global assessment of functioning (n=169) or social responsiveness (n=97). Our preliminary meta-analysis suggests that supplementation of omega 3 fatty acids may improve hyperactivity, lethargy, and stereotypy in ASD patients. However, the number of studies was limited and the overall effects were small, precluding definitive conclusions. Future large-scale randomized clinical trials are needed to confirm or refute our findings.
Keywords: poly-unsaturated fatty acid, omega 3, autism, pediatric
Introduction
Autism spectrum disorder (ASD) is a life-long neurodevelopment disorder, which is a great burden for the affected individuals, caregivers, and societies worldwide.1 The diagnosis of ASD is made predominantly by behavioral observation and is characterized by the presence of at least two core symptoms including restricted, repetitive patterns of behavior, interests, or activities and persistent difficulties in social communication and social interaction.2 The global prevalence of ASD is estimated to be between 0.76%1 and 1.46%.3 The exact etiology of ASD is complex and multifactorial.4 Numerous proposed mechanisms for ASD etiology exist, although none in isolation can explain all causes of the disorder.5
Over the past few decades, there has been an increase in the prevalence of ASD in several countries.6 Although the exact reason remains unknown, some proposed that heightened awareness and change in diagnostic criteria of ASD may partially account for the increase.7–9 In contrast, others suggested that changes in environmental (eg, pollution) or nutritional factors may be potential contributors.10,11 Recently, nutritional factors have become a focus of interest because of possible associations with ASD for which they are regarded as possible treatment targets. Since there remains a lack of effective medication for the ASD core symptoms and some effective medications have multiple side effects,12 the possibility of using nutritional supplements to improve ASD-associated symptoms has received much interest.13,14
Polyunsaturated fatty acids (PUFAs) have been a topic of interest for many psychiatric diseases. Accumulating evidence suggested that PUFA deficiency may be linked to some neurodevelopmental disorders, including schizophrenia, attention-deficit/hyperactivities disorder (ADHD), bipolar disorder, and ASD.10,15 PUFAs play an important role in brain functioning because of their anti-inflammatory properties and their ability to maintain appropriate function of brain cell membrane and myelin sheath.10 Since PUFAs cannot be produced by the human body, some studies have suggested that changes in dietary behaviors that caused an imbalance in PUFAs’ consumption may provide an explanation for recent increase in ASD prevalence.10 Omega 3 and omega 6 fatty acids are two of the most well-known PUFAs. While the former predominantly comes from seafood, the sources of the latter are animal or vegetable oils.16 Some studies reported that the optimal ratio of omega 6 to omega 3 consumption should be 1:1 to 4:1.17 However, recent changes in dietary habits may cause an increase in omega 6 fatty acid intake that may predispose some individuals with genetic vulnerability to certain psychiatric diseases.18 Therefore, many clinical trials have started to investigate the therapeutic potential of supplementation of omega 3 fatty acids in treating patients with psychiatric diseases.15
A recent meta-analysis found that supplementation of omega 3 fatty acids produced a small reduction in emotional liability and oppositional behaviors in ADHD patients.19 Although symptoms of ASD manifest mainly as social dysfunction, many ASD patients also suffer from other emotional or behavioral problems including hyperactivity and poor impulsivity.20 A high prevalence of anxiety disorders and ADHD was also found among ASD patients in previous studies.21,22
To the best of our knowledge, there have been two meta-analyses so far to assess the effects of supplementation of omega 3 fatty acids in ASD patient.23,24 The first meta-analysis, which included only two clinical trials with a very small total sample size (n=40), failed to show any benefits of supplementation of omega 3 fatty acids compared to placebo.23 A more recent meta-analysis by Horvath et al,24 which included more clinical trials, demonstrated no significant positive impact of supplementation of omega 3 fatty acids on most subscales of the Aberrant Behavior Checklist (ABC), despite an improved lethargy subscale. The authors concluded that supplementation of omega 3 fatty acids did not benefit patients with ASD. However, although five trials were included in the latter study, only patients from two out of the five trials (n=82) were included for the assessment of treatment outcomes (ie, change in ABC hyperactivities) in the omega 3 groups compared to those in the placebo groups. Moreover, the authors did not include non-English studies.25 In this updated meta-analysis, we aimed to conduct a comprehensive systematic review that investigated all studies on the effects of supplementation of omega 3 fatty acids with placebo controls in ASD patients.
Methods
The meta-analysis was conducted in accordance with the guideline of Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (Table S1 and Figure S1).26 The current meta-analysis followed our unpublished meta-analytic protocol, which fulfilled the requirement of the Institutional Review Board of the Tri-Service General Hospital (TSGHIRB: B-105-12).
Database searches and identification of eligible papers
Two independent authors (Y-SC and P-TT) searched the following electronic databases from inception to June 21, 2017, using the following chosen keywords: ([omega-3 fatty acids OR HUFAs OR eicosapentaenoic acid OR docosahexaenoic acid OR ethyl-eicosapentaenoic acid OR lipoic acid OR linoleic acid] AND [autism OR autism spectrum disorder OR ASD OR Asperger OR Pervasive development disorder OR PPD]) in PubMed, Cochrane Library, and ClinicalTrials.gov and ([omega-3 fatty acids] AND [autism OR autism spectrum disorder]) in ScienceDirect. In addition, to expand our search results, we hand searched the reference lists of specific review/original articles relevant to current topic.10,15,16,23
In the eligibility stage, the above two authors screened the articles via the titles and abstracts and came to consensus for the eligibility list. At the full-text review, the articles were scrutinized against the inclusion criteria and a final list was developed by the two authors. A third reviewer (P-YL) was available for mediation in the case of any inconsistencies.
Inclusion and exclusion criteria
The inclusion criteria were as follows: 1) randomized controlled trials (RCTs), 2) published articles investigating treatment effect of supplementation of omega 3 fatty acids in ASD children versus placebo, and 3) human studies. We considered articles published in any language. The exclusion criteria were as follows: 1) animal studies and 2) non-RCTs not comparing omega 3 supplement versus placebo in ASD children.
Methodological quality appraisal
To investigate the quality of recruited studies, we used the Jadad scale. The Jadad score ranges from 0 (poor quality) to 5 (high quality).27
Primary outcomes
The primary outcome was the changes of ASD severity rating scales or changes in secondary behavioral symptoms of ASD, including the ABC,28 Behavior Assessment System for Children (BASC),29 Clinical Global Impression – Improvement (CGI-I),30 and Social Responsiveness Scale (SRS).31
Secondary outcomes
The secondary outcomes of interest included the dropout rate and rate of discontinuation of treatment due to side effect.
Data extraction and management
The above two authors extracted the target data of primary and secondary outcomes and clinical variables of interest, such as mean age, gender distribution, and duration of omega 3 treatment. For CGI-I, we defined clinical improvement as CGI-I =1 or 2, which was used in most studies to represent significant clinical improvement in symptoms.
When data were not available in the articles, we tried to contact the authors up to two times over a month to request the data.
Meta-analysis
Due to the anticipated heterogeneity, a random-effects meta-analysis model was conducted rather than a fixed-effect one.32 The current meta-analysis was conducted on the platform of Comprehensive Meta-Analysis software, Version 3 (Biostat, Englewood, NJ, USA). For continuous data, we calculated the Hedges’ g and 95% confidence intervals (CIs) for studies using different outcome measures; we used the mean difference and 95% CI to represent the changes of outcome measures when studies used homogenous outcome measures. Finally, we calculated the odds ratio and 95% CIs for categorical data. Two-tailed P-values <0.05 are considered statistically significant.
Heterogeneity, publication bias, and sensitivity test
Heterogeneity was assessed with the Q statistic and corresponding P-value.33 The I2 statistic was interpreted as the proportion of heterogeneity estimated in a study.34 Furthermore, we examined the publication bias with visual inspection of funnel plots35 and Egger’s regression tests.36 For evidence of publication bias, the Duval and Tweedie’s trim-and-fill procedure, which is a validated model to estimate an effect size, was employed.37 We also conducted sensitivity analyses using the one study removal method.38 In brief, this method had been widely used in meta-analysis to detect any potential bias from any one outlier among the recruited studies in one subgroup meta-analysis via the removal of one of the recruited studies in each step.
Meta-regression and subgroup meta-analysis
In an effort to find out the potential sources of heterogeneity and confounding effects, we conducted meta-regression and subgroup meta-analyses. At first, if there are at least five sets of datasets, we performed meta-regression with the unrestricted maximum likelihood method for clinical variables of interest. Second, when there were at least three studies, we would perform subgroup meta-analysis.39
Results
Study selection
The search results are displayed in Figure 1. Among the 34 articles entering full-text evaluation, 28 articles meeting the exclusion criteria were excluded (reasons summarized in Table S2). Finally, six articles were included in the current meta-analysis (Table 1).25,40–44 Among them, although there were two studies conducted by the same authors, the study design and participants were different. Therefore, they were regarded as two different datasets.41,43
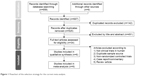  | Figure 1 Flowchart of the selection strategy for the current meta-analysis. |
Of the six articles, four articles provided data on changes of ABC scales25,41,43,44 (omega 3 participants =57, mean age =7.7, mean female proportion =9.3%; placebo participants =52, mean age =8.7, mean female proportion =14.6%), three articles provided changes of BASC40,42,43 (omega 3 participants =56, mean age =5.2, mean female proportion =16.1%; placebo participants =56, mean age =5.3, mean female proportion =21.5%), four articles provided changes of CGI40–43 (omega 3 participants =85, mean age =5.9, mean female proportion =14.1%; placebo participants =84, mean age =5.9, mean female proportion =19.1%), and three articles provided changes of SRS25,41,43 (omega 3 participants =50, mean age =7.3, mean female proportion =9.3%; placebo participants =47, mean age =8.4, mean female proportion =14.6%). Among the four studies that provided change of ABC scales, only one article provided both teachers’ rating and parents’ rating and most of other studies provided parents’ rating only. Therefore, we included only parents’ rating in our meta-analysis.
Methodological quality of included studies
For the six studies, the average Jadad score was 4.67 with a standard deviation of 0.52 (Table S3).
Meta-analysis investigating changes in ASD severity in patients treated with omega 3 or placebo
Primary outcome: changes of ABC
Among the four eligible articles (n=109) comparing changes of ABC scale severity between ASD children treated with omega 3 and those treated with placebo, there were only borderline significance of better treatment effects of the omega 3 treatment group in ABC scores of hyperactivities (k=4, Hedges’ g=−0.348, 95% CI =−0.716 to 0.021, P-value =0.064; difference in means =−2.692, 95% CI =−5.364 to −0.020, P=0.048) (Figure 2A) without significant heterogeneity (Q value =1.553, df=3, P-value =0.670, I2<0.001%, τ<0.001) or publication bias via inspection of funnel plot (Figure S2A) or Egger’s regression (t value =0.943, df=2, two-tailed P-value =0.445). Improvements in ABC lethargy (k=4, Hedges’ g=−0.447, 95% CI =−0.817 to −0.077, P-value =0.018; difference in means =−1.969, 95% CI =−3.566 to −0.372, P=0.016) (Figure 2A) were also noted; there was no any significant heterogeneity (Q value =1.597, df=3, P-value =0.660, I2<0.001%, τ<0.001) or publication bias via inspection of funnel plot (Figure S2B) or Egger’s regression (t value =0.639, df=2, two-tailed P-value =0.588). Furthermore, omega 3 resulted in improved ABC scores for stereotypy (k=4, Hedges’ g=−0.404, 95% CI =−0.773 to −0.035, P-value =0.032; difference in means =−1.071, 95% CI =−2.114 to −0.029, P=0.044) (Figure 2A) without significant heterogeneity (Q value =0.962, df=3, P-value =0.810, I2<0.001%, τ<0.001) or publication bias via inspection of funnel plot (Figure S2C) or Egger’s regression (t value =0.162, df=2, two-tailed P-value =0.886). Finally, this meta-analysis did not find any significant difference in ABC scores for inappropriate speech (k=4, Hedges’ g=−0.199, 95% CI =−0.629 to 0.232, P-value =0.366; difference in means =−0.210, 95% CI =−1.161 to 0.741, P=0.665) (Figure 2A), which did not have any significant heterogeneity (Q value =3.716, df =3, P-value =0.294, I2=19.268%, τ=0.198) or publication bias via inspection of funnel plot (Figure S2D) or Egger’s regression (t value =2.905, df=2, two-tailed P-value =0.101); similarly, there was no significant difference in treatment effect between omega 3 and placebo on ABC irritability (k=4, Hedges’ g=0.016, 95% CI =−0.348 to 0.380, P-value =0.931; difference in means =0.122, 95% CI =−2.020 to 2.265, P=0.911) (Figure 2A) without significant heterogeneity (Q value =0.029, df=3, P-value =0.999, I2<0.001%, τ<0.001) or publication bias via inspection of funnel plot (Figure S2E) or Egger’s regression (t value =0.821, df=2, two-tailed P-value =0.498).
Sensitivity test
Sensitivity analyses with one study removal test revealed that there was no change in the results of the meta-analysis of hyperactivities, inappropriate speech, and irritability scores. However, in the aspect of ABC stereotypy, the main result of meta-analysis became nonsignificant after the removal of study by Bent et al (2014)41 (Hedges’ g=−0.346, 95% CI =−0.870 to 0.179, P-value =0.197) or became only borderline significance after the removal of study by Amminger et al (2007)44 (Hedges’ g=−0.354, 95% CI =−0.745 to 0.038, P-value =0.077). Besides, the significant result of meta-analysis of ABC lethargy would become insignificant after removing data by Bent et al (2014)41 (Hedges’ g=−0.277, 95% CI =−0.799 to 0.246, P-value =0.300).
Meta-regression
There were insufficient data to perform meta-regression analyses.
Primary outcome: changes of BASC
Although three eligible articles compared changes in BASC scale severity between ASD children treated with omega 3 and those treated with placebo, only two studies provided information on BASC externalizing/internalizing problems40,43 and BASC functional communication/social,40,42 precluding meta-analysis. Nevertheless, these studies found no significant difference in total BASC scale or most BASC subscales between placebo and omega 3 groups. However, one study found significant favorable improvements in BASC functional communication according to teacher’s report in omega 3 groups.42 On the contrary, one study found a significantly less favorable change in BASC social skill by parents’ report in omega 3 group42 and another study found a significant worsening of BASC externalizing problems in the omega 3 group.40
Primary outcome: differences of clinical improvement as measurement of CGI
In the four eligible articles (n=169), the results of meta-analysis revealed that there was no significant difference in clinical improvement as reflected in CGI-I between omega 3 and placebo (odds ratio =1.255, 95% CI =0.574 to 2.748, P=0.569) (Figure 2B). There was no significant heterogeneity (Q value =2.350, df=3, P-value =0.503, I2<0.001%, τ<0.001) or publication bias via inspection of funnel plot (Figure S2F) or Egger’s regression (t value =0.449, df=2, two-tailed P-value =0.697).
Sensitivity test
No change in the overall results was noted after the removal of any one of the recruited study.
Meta-regression
Due to the paucity of data, meta-regression was not possible.
Primary outcome: changes of SRS
Among the three eligible articles (n=97), there was only borderline improved response by placebo in SRS total scores than those by omega 3 (k=3, Hedges’ g=0.367, 95% CI =−0.025 to 0.758, P-value =0.066; difference in means =3.143, 95% CI =−0.090 to 6.376, P=0.057) (Figure 2C) without significant heterogeneity (Q value =0.269, df=2, P-value =0.874, I2<0.001%, τ<0.001) or publication bias via inspection of funnel plot (Figure S2G) or Egger’s regression (t value =3.118, df=1, two-tailed P-value =0.198) was noted.
Sensitivity test
No change in the overall result was noted after the removal of any one of the recruited study.
Meta-regression
There were insufficient data for meta-regression analysis.
Secondary outcomes: dropout rate
Among the five eligible studies, no difference in dropout rate between children receiving omega 3 and those treated by placebo was noted (odds ratio =0.853, 95% CI =0.257 to 2.830, P=0.795) (Figure 3A). There was no significant heterogeneity (Q value =7.052, df=4, P-value =0.133, I2=43.275%, τ=0.880) or publication bias via inspection of funnel plot (Figure S2H) or Egger’s regression (t value =0.722, df=3, two-tailed P-value =0.522).
Sensitivity test
No change in the overall result was noted after the removal of any one of the recruited study.
Meta-regression
Meta-regression analysis revealed no significant association between dropout rate and clinical variables, including mean age (P=0.462), female proportion (P=0.848), and duration of omega 3 treatment (P=0.658). However, there was significantly positive association between dropout rate and daily dosage of omega 3 (slope =1.804, k=5, P=0.031), that is, the higher the daily dosage of omega 3, the higher dropout rate in the omega 3 treatment groups.
Secondary outcomes: difference of rate of discontinuation due to side effects
Meta-analysis demonstrated no significant difference in the rate of discontinuation due to side effects between children receiving omega 3 and those treated by placebo (odds ratio =0.734, 95% CI =0.147 to 3.666, P=0.707) (Figure 3B) without significant heterogeneity (Q value =1.964, df=2, P-value =0.375, I2<0.001%, τ<0.001) or publication bias via inspection of funnel plot (Figure S2I) or Egger’s regression (t value =1.794, df=1, two-tailed P-value =0.324).
Sensitivity test
No change in the overall result was noted after the removal of any one of the recruited study.
Meta-regression
There were insufficient data for meta-regression analysis.
Subgroup meta-analysis in treatment duration <1 or >1 year
There were insufficient data for subgroup meta-analysis comparing treatment duration of >1 or <1 year.
Discussion
Our preliminary meta-analysis recruited six studies in total (refer Table 2 for the characteristics of main outcome in each study) and suggests that supplementation of omega 3 fatty acids may improve scores on the ABC hyperactivity, ABC lethargy, and ABC stereotypy subscales (n=109). However, no significant differences in clinical improvement as measurement of CGI-I (n=169) or SRS total scores (n=97) were found between the omega 3 and placebo groups. The results of our study largely resemble those of two previous meta-analyses, although our study showed additional benefits of supplementation of omega 3 fatty acids on improving ABC hyperactivity and stereotypy subscales. In fact, the previous meta-analyses suggested that omega 3 groups had a more favorable outcome on ABC hyperactivity subscale, but the result was not significant.24 The meta-analysis by Horvath et al included only two trials for each ABC subscale meta-analysis, because the results of the ABC assessments were separated into two groups: parents’ ratings (n=82) and teachers’ or clinicians’ ratings (n=69). The main difference between this study and the more recent meta-analysis by Horvath et al is that our study had a larger sample size for each ABC subscale meta-analysis (n=109 versus n=82). It is possible that the effects of supplementation of omega 3 fatty acids on ABC hyperactivity and ABC stereotypy were not detected in the previous meta-analyses, because the sample size was too small.
Most studies included in our meta-analysis used dosages of omega 3 between 1.3 and 1.5 g/day with duration of treatment ranging from 6 to 24 weeks. Only one study used a much lower dosage of omega 3 (200 mg/day), but that study was only included for assessment of CGI-I due to a lack of other outcome data.42 Only one author declared competing interest among all the included trials.40 Most trials in our meta-analysis had fair study quality (average Jadad scores: 4.67), and there was no evidence of publication bias or significant heterogeneity between trials. However, most participants were males and younger than 12 years and most trials excluded nonverbal participants or those with severe intellectual disabilities; therefore, the study results may only be applicable to small subgroups of ASD patients.
Our meta-analysis suggests that supplementation of omega 3 fatty acid had a significant positive effect on ABC hyperactivity (k=4, difference in means =−2.692, 95% CI =−5.364 to −0.020, P=0.048), ABC lethargy (k=4, difference in means =−1.969, 95% CI =−3.566 to −0.372, P=0.016), and ABC stereotypy (k=4, difference in means =−1.071, 95% CI =−2.114 to −0.029, P=0.044) subscales when compared with the placebo (n=109). However, the effect sizes were small for all three outcome measurements (Hedges’ g=−0.348, 95% CI =−0.716 to 0.021, P-value =0.064 for ABC hyperactivities; Hedges’ g=−0.447, 95% CI =−0.817 to −0.077, P-value =0.018 for ABC lethargy; Hedges’ g=−0.404, 95% CI =−0.773 to −0.035, P-value =0.032 for ABC stereotypy). The ABC scale was initially designed to assess treatment effects on severely mentally retarded individuals28 but gradually adopted as an outcome measure used by pharmacological studies for autism treatment.45,46 When compared with SRS scores, the ABC was also frequently used for behavioral problems in other diagnostic groups such as disruptive behavioral disorders or intellectual disabilities47,48 and can provide more information on externalizing problems, such as hyperactivities and irritability.49 Our meta-analysis showed significant positive treatment effects of supplementation of omega 3 fatty acids relative to placebo not only on core symptoms of autism such as lethargy (social withdrawal) and stereotypy but also on other related secondary behavioral problems such as hyperactivity. Previous meta-analyses also found positive treatment effect on the ABC hyperactivity subscales (MD: 3.46, 95% CI: −0.97 to 7.70 and MD: 2.09, 95% CI: −0.91 to 5.09), but the results were not statistically significant.23,24 Similarly, although most studies showed trend favoring omega 3 groups on the ABC hyperactivity subscale, none of the results reached statistical significance. However, after combining the results of these studies, we found that supplementation of omega 3 fatty acids had significant treatment effect on the ABC hyperactivity subscale. In fact, the potential benefit of supplementation of omega 3 fatty acids on reducing hyperactivity was not only observed in ASD patients, several clinical trials focusing on ADHD also found greater reduction in hyperactivity with supplementation of omega 3 fatty acids.50,51 It was also proposed that children with hyperactivity may have difficulty in absorbing omega 3 fatty acids, which is essential for normal brain function.52 However, our study only showed an average of 2.69 points better improvement in the ABC hyperactivity subscale, with baseline score of 14.6–33.3 out of a total of 45 points in the included studies, and this treatment effect on hyperactivity was small when compared with treatment with risperidone (11.42 points greater improvement relative to placebo), aripiprazole (7.93 points greater improvement relative to placebo), or methylphenidate (9.6 points greater improvement relative to placebo) in other trials.47,53–56 Moreover, the effects’ size for improvement in ABC hyperactivity subscale was small and only reached borderline significance (Hedges’ g=−0.348, 95% CI =−0.716 to 0.021). Nevertheless, even though the improvement in hyperactivity with supplementation of omega 3 fatty acids in ASD patients was small compared with other medications, supplementation of omega 3 fatty acids was safer and had fewer side effects. Therefore, supplementation of omega 3 fatty acids may still be worth trying if the result could be confirmed by further studies.
As for individual study results on ABC lethargy and stereotypy, one study showed significant improvements in the omega 3 group compared to the placebo group on both the ABC lethargy and ABC stereotypy subscales,41 one study showed significant improvements in the omega 3 group compared to the placebo group only on ABC lethargy subscale25 and two studies did not find any significant difference between placebo and omega 3 groups.43,44 Overall, our meta-analysis showed a significant but small improvement on both subscales. Specifically, the ABC lethargy subscale in omega 3 groups had an average of 1.969 points improvement, with baseline scores 5.4 to 30 out of a total of 48 points among studies. The ABC stereotypy subscale in omega 3 groups had an average of 1.071 points better improvement, with baseline scores 2.8 to 14.4 out of a total of 21 points among studies. However, the effect sizes were also small for both ABC lethargy subscale (Hedges’ g=−0.447, 95% CI =−0.817 to −0.077, P-value =0.018) and ABC stereotypy subscale (Hedges’ g=−0.404, 95% CI =−0.773 to −0.035, P-value =0.032). Due to the relatively small improvement and lack of previous report about such treatment effects, these results need further investigation.
Our meta-analysis found no significant difference in clinical improvement as measurement of CGI-I between omega 3 and placebo (CGI-I =1 or 2) between placebo and omega 3 groups (n=169). Although a greater number of participants appeared to produce a positive response in most trials except one,43 none of these results reached statistical significance.41–43 It is possible that effects of supplementation of omega 3 fatty acids were too small to produce significant result on the CGI-I score as improvement in ABC hyperactivity, lethargy, and stereotypy. Similarly, our meta-analysis and none of the included studies found significant improvement in omega 3 groups in total SRS scores (n=97). Therefore, there is still lack of clear evidence to support the use of omega 3 in improving ASD core symptoms as measured by CGI-I scores or SRS scores.
Our preliminary data suggest that omega 3 is well tolerated among the treatment groups. Gastrointestinal discomfort and irritability were most commonly reported side effects in the omega 3 groups. Nevertheless, our meta-analysis did not find a difference between dropout rate or rate of discontinuation due to side effects between omega 3 and placebo groups. Similarly, none of the studies found significant difference in side effects between placebo and omega 3 groups and none reported serious side effects. However, our meta-regression found that there was significantly positive association between dropout rate and daily dosage of omega 3 (slope =1.804, k=5, P=0.031). Although, little information was provided on the relationship between dosage and side effects on individual studies, this result suggested that higher dosage of omega 3 treatment is associated with higher dropout rate. Further studies may need to explore the relationship between dosage of omega 3 and dropout rate, as this result may indicate that higher dosage of omega 3 treatment is less tolerable due to variety of reasons including medication side effects.
Limitations
There were some limitations in our meta-analysis. First, there were only six trials with a total of 194 participants. The sample size in this meta-analysis is still not sufficient to provide any robust evidence. Besides, we cannot exclude the possibility that certain negative findings in our meta-analysis may be due to the lack of adequate study power. Second, since the longest treatment duration was 24 weeks and the largest dosage of omega 3 was 1.5 g/day, the treatment effect above this dosage or this duration cannot be determined. Third, most of the included studies had many exclusion criteria such as severe medical illness, no language ability, and severe intellectual disability; therefore, the study result may not be applicable to patients with more severe disease and those with nonverbal autism. Fourth, since most participants were allowed to receive behavioral treatments and some trials did not exclude the use of other psychiatric medications, we cannot rule out the possibility that the observed improvement may also come from other interventions. Finally, as some authors only published statistically significant results and did not respond to our email request for more data, we were unable to obtain all the data from included RCTs. It is unclear how this may affect the results of our meta-analysis.
Conclusion
This preliminary meta-analysis suggests that supplementation of omega 3 fatty acids is well tolerated and may produce a small but positive effect on reducing hyperactivity in ASD patients. However, in the absence of significant improvement in CGI-I score and relatively small sample size, we still cannot draw reliable conclusion about potential effects of supplementation of omega 3 fatty acids on ASD patients. The finding that supplementation of omega 3 fatty acids improves lethargy and stereotypy is also preliminary and needs further investigations. Nevertheless, given relatively few side effects compared to those of other medications, we encourage further trials to continue to explore potential benefit of supplementation of omega 3 fatty acids in ASD patients and also to include wider range of participants such as lower functioning autism and older age groups.
Acknowledgments
The authors declare no funding were received in relation to the subject of this study. Y-SC and P-TT contributed equally as first authors.
Disclosure
The authors report no conflicts of interest in this work.
References
Baxter AJ, Brugha TS, Erskine HE, Scheurer RW, Vos T, Scott JG. The epidemiology and global burden of autism spectrum disorders. Psychol Med. 2015;45(3):601–613. | ||
Association AP. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013. | ||
Christensen DL, Baio J, Van Naarden Braun K, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years – autism and developmental disabilities monitoring network, 11 sites, United States, 2012. MMWR Surveill Summ. 2016;65(3):1–23. | ||
Chaste P, Leboyer M. Autism risk factors: genes, environment, and gene-environment interactions. Dialogues Clin Neurosci. 2012;14(3):281–292. | ||
Fakhoury M. Autistic spectrum disorders: a review of clinical features, theories and diagnosis. Int J Dev Neurosci. 2015;43:70–77. | ||
Boat TF, Wu JT, editors. Mental Disorders and Disabilities among Low-Income Children. Washington, DC: National Academies Press (US); 2015. | ||
Van Naarden Braun K, Christensen D, Doernberg N, et al. Trends in the prevalence of autism spectrum disorder, cerebral palsy, hearing loss, intellectual disability, and vision impairment, metropolitan atlanta, 1991–2010. PLoS One. 2015;10(4):e0124120. | ||
King M, Bearman P. Diagnostic change and the increased prevalence of autism. Int J Epidemiol. 2009;38(5):1224–1234. | ||
Lundstrom S, Reichenberg A, Anckarsater H, Lichtenstein P, Gillberg C. Autism phenotype versus registered diagnosis in Swedish children: prevalence trends over 10 years in general population samples. BMJ. 2015;350:h1961. | ||
van Elst K, Bruining H, Birtoli B, Terreaux C, Buitelaar JK, Kas MJ. Food for thought: dietary changes in essential fatty acid ratios and the increase in autism spectrum disorders. Neurosci Biobehav Rev. 2014;45:369–378. | ||
Lam J, Sutton P, Kalkbrenner A, et al. A systematic review and meta-analysis of multiple airborne pollutants and autism spectrum disorder. PLoS One. 2016;11(9):e0161851. | ||
Accordino RE, Kidd C, Politte LC, Henry CA, McDougle CJ. Psychopharmacological interventions in autism spectrum disorder. Expert Opin Pharmacother. 2016;17(7):937–952. | ||
Vancassel S, Durand G, Barthelemy C, et al. Plasma fatty acid levels in autistic children. Prostaglandins Leukot Essent Fatty Acids. 2001;65(1):1–7. | ||
El-Ansary AK, Bacha AG, Al-Ayahdi LY. Impaired plasma phospholipids and relative amounts of essential polyunsaturated fatty acids in autistic patients from Saudi Arabia. Lipids Health Dis. 2011;10:63. | ||
Bozzatello P, Brignolo E, De Grandi E, Bellino S. Supplementation with omega-3 fatty acids in psychiatric disorders: a review of literature data. J Clin Med. 2016;5(8):E67. | ||
Politi P, Rocchetti M, Emanuele E, Rondanelli M, Barale F. Randomized placebo-controlled trials of omega-3 polyunsaturated fatty acids in psychiatric disorders: a review of the current literature. Curr Drug Discov Technol. 2013;10(3):245–253. | ||
Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002;56(8):365–379. | ||
Simopoulos AP. Importance of the ratio of omega-6/omega-3 essential fatty acids: evolutionary aspects. World Rev Nutr Diet. 2003;92:1–22. | ||
Cooper RE, Tye C, Kuntsi J, Vassos E, Asherson P. The effect of omega-3 polyunsaturated fatty acid supplementation on emotional dysregulation, oppositional behaviour and conduct problems in ADHD: a systematic review and meta-analysis. J Affect Disord. 2016;190:474–482. | ||
Matson JL, Nebel-Schwalm MS. Comorbid psychopathology with autism spectrum disorder in children: an overview. Res Dev Disabil. 2007;28(4):341–352. | ||
Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry. 2008;47(8):921–929. | ||
Mattila ML, Hurtig T, Haapsamo H, et al. Comorbid psychiatric disorders associated with Asperger syndrome/high-functioning autism: a community- and clinic-based study. J Autism Dev Disord. 2010;40(9):1080–1093. | ||
James S, Montgomery P, Williams K. Omega-3 fatty acids supplementation for autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2011;(11):CD007992. | ||
Horvath A, Lukasik J, Szajewska H. Omega-3 fatty acid supplementation does not affect autism spectrum disorder in children: a systematic review and meta-analysis. J Nutr. 2017;147(3):367–376. | ||
Yui K, Koshiba M, Nakamura S, Kobayashi Y. Effects of large doses of arachidonic acid added to docosahexaenoic acid on social impairment in individuals with autism spectrum disorders: a double-blind, placebo-controlled, randomized trial. J Clin Psychopharmacol. 2012;32(2):200–206. | ||
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. | ||
Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. | ||
Aman MG, Burrow WH, Wolford PL. The Aberrant Behavior Checklist-Community: factor validity and effect of subject variables for adults in group homes. Am J Ment Retard. 1995;100(3):283–292. | ||
Reynolds CR, Kamphaus RW. Behavior Assessment System for Children. Circle Pines, MN: AGS Publishing; 2004. | ||
Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont). 2007;4(7):28–37. | ||
Constantino JN. The Social Responsiveness Scale. Los Angeles, CA: Western Psychological Services; 2002. | ||
Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97–111. | ||
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. | ||
Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta-analysis: I2 is not an absolute measure of heterogeneity. Res Synth Methods. 2017;8(1):5–18. | ||
Higgins JP, Green S. 10.4.3.1 recommendations on testing for funnel plot asymmetry. In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. 5.1.0. Cochrane Library; 2011. Available from: http://training.cochrane.org/handbook. Accessed September 21, 2017. | ||
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. | ||
Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. | ||
Tobias A. Assessing the influence of a single study in meta-analysis. Stata Tech Bull. 1999;47:15–17. | ||
Davey J, Turner RM, Clarke MJ, Higgins JP. Characteristics of meta-analyses and their component studies in the Cochrane Database of Systematic Reviews: a cross-sectional, descriptive analysis. BMC Med Res Methodol. 2011;11:160. | ||
Mankad D, Dupuis A, Smile S, et al. A randomized, placebo controlled trial of omega-3 fatty acids in the treatment of young children with autism. Mol Autism. 2015;6:18. | ||
Bent S, Hendren RL, Zandi T, et al. Internet-based, randomized, controlled trial of omega-3 fatty acids for hyperactivity in autism. J Am Acad Child Adolesc Psychiatry. 2014;53(6):658–666. | ||
Voigt RG, Mellon MW, Katusic SK, et al. Dietary docosahexaenoic acid supplementation in children with autism. J Pediatr Gastroenterol Nutr. 2014;58(6):715–722. | ||
Bent S, Bertoglio K, Ashwood P, Bostrom A, Hendren RL. A pilot randomized controlled trial of omega-3 fatty acids for autism spectrum disorder. J Autism Dev Disord. 2011;41(5):545–554. | ||
Amminger GP, Berger GE, Schäfer MR, Klier C, Friedrich MH, Feucht M. Omega-3 fatty acids supplementation in children with autism: a double-blind randomized, placebo-controlled pilot study. Biol Psychiatry. 2007;61(4):551–553. | ||
Kent JM, Kushner S, Ning X, et al. Risperidone dosing in children and adolescents with autistic disorder: a double-blind, placebo-controlled study. J Autism Dev Disord. 2013;43(8):1773–1783. | ||
Marcus RN, Owen R, Manos G, et al. Aripiprazole in the treatment of irritability in pediatric patients (aged 6–17 years) with autistic disorder: results from a 52-week, open-label study. J Child Adolesc Psychopharmacol. 2011;21(3):229–236. | ||
Loy JH, Merry SN, Hetrick SE, Stasiak K. Atypical antipsychotics for disruptive behaviour disorders in children and youths. Cochrane Database Syst Rev. 2012;(9):CD008559. | ||
Schmidt JD, Huete JM, Fodstad JC, Chin MD, Kurtz PF. An evaluation of the Aberrant Behavior Checklist for children under age 5. Res Dev Disabil. 2013;34(4):1190–1197. | ||
Karabekiroglu K, Aman MG. Validity of the aberrant behavior checklist in a clinical sample of toddlers. Child Psychiatry Hum Dev. 2009;40(1):99–110. | ||
Sinn N, Bryan J, Wilson C. Cognitive effects of polyunsaturated fatty acids in children with attention deficit hyperactivity disorder symptoms: a randomised controlled trial. Prostaglandins Leukot Essent Fatty Acids. 2008;78(4–5):311–326. | ||
Sinn N, Bryan J. Effect of supplementation with polyunsaturated fatty acids and micronutrients on learning and behavior problems associated with child ADHD. J Dev Behav Pediatr. 2007;28(2):82–91. | ||
Colquhoun I, Bunday S. A lack of essential fatty acids as a possible cause of hyperactivity in children. Med Hypotheses. 1981;7(5):673–679. | ||
Ghanizadeh A, Sahraeizadeh A, Berk M. A head-to-head comparison of aripiprazole and risperidone for safety and treating autistic disorders, a randomized double blind clinical trial. Child Psychiatry Hum Dev. 2014;45(2):185–192. | ||
Research Units on Pediatric Psychopharmacology Autism N. Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Arch Gen Psychiatry. 2005;62(11):1266–1274. | ||
Aman M, Rettiganti M, Nagaraja HN, et al. Tolerability, safety, and benefits of risperidone in children and adolescents with autism: 21-month follow-up after 8-week placebo-controlled trial. J Child Adolesc Psychopharmacol. 2015;25(6):482–493. | ||
Pearson DA, Santos CW, Aman MG, et al. Effects of extended release methylphenidate treatment on ratings of attention-deficit/hyperactivity disorder (ADHD) and associated behavior in children with autism spectrum disorders and ADHD symptoms. J Child Adolesc Psychopharmacol. 2013;23(5):337–351. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




