Back to Journals » Nature and Science of Sleep » Volume 15
Sleep and Its Disturbance in Parents of Children and Adolescents with Epilepsy: A Systematic Review and Meta-Analysis
Authors Tsai SY , Tsai HY, Lin YY, Chen SR, Kuo SY, Lou MF
Received 4 October 2023
Accepted for publication 18 December 2023
Published 28 December 2023 Volume 2023:15 Pages 1139—1152
DOI https://doi.org/10.2147/NSS.S437349
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Ahmed BaHammam
Shao-Yu Tsai,1,2 Han-Yi Tsai,3 Ying-Ying Lin,1 Su-Ru Chen,4 Shu-Yu Kuo,4 Meei-Fang Lou1
1School of Nursing, College of Medicine, National Taiwan University, Taipei, Taiwan; 2Department of Nursing, National Taiwan University Hospital, Taipei, Taiwan; 3Department of Nursing, Taipei Veterans General Hospital, Taipei, Taiwan; 4School of Nursing, College of Nursing, Taipei Medical University, Taipei, Taiwan
Correspondence: Shao-Yu Tsai, School of Nursing, College of Medicine, National Taiwan University, No. 1, Sec 1, Jen-Ai Road, Taipei, 10051, Taiwan, Tel +886-2-2351-7770, Fax +886-2- 2321-9913, Email [email protected]
Abstract: Sleep disturbances are commonly reported by parents of children and adolescents with epilepsy. However, evidence synthesis including quality and quantity of sleep in parents of children and adolescents with epilepsy is lacking. This systematic review and meta-analysis was conducted to quantify pooled mean estimates of parental sleep variables and to determine the prevalence of sleep disturbances in parents of children and adolescents with epilepsy. Five electronic databases, PubMed, Medline, Embase, PsychINFO, and CINAHL, were systematically searched from inception to September 2021. Eleven observational studies examining parents of pediatric patients aged < 18 years with epilepsy using a quantitative measure of sleep duration, sleep quality, or sleep disturbance were reviewed. Our results showed that the pooled nocturnal sleep duration was 5.93 hours (95% CI: 4.64 to 7.21 hours). Overall sleep quality as estimated by the bias-adjusted pooled Pittsburgh Sleep Quality Index total score was 6.65 (95% CI: 5.98 to 7.33). Parents of children with epilepsy had significantly higher Pittsburgh Sleep Quality Index total scores compared to parents of healthy children (differences in means 1.84, 95% CI: 1.29 to 2.39). The pooled estimated prevalence of parental sleep disturbances was 58.1% (95% CI: 45.7% to 69.6%). Our findings demonstrate a high prevalence of sleep disturbances with poor sleep quality and substantial reductions in sleep time in parents of children and adolescents with epilepsy. Healthcare professionals in pediatric neurology clinics should proactively initiate screening for sleep disturbances in parents of children and adolescents with epilepsy and refer parents to a sleep specialist when necessary.
Keywords: adolescents, children, epilepsy, parents, sleep
Introduction
Pediatric epilepsy is a complex and prevalent childhood neurological disorder characterized by unprovoked and repeated seizures, with approximately 1 in every 150 children being diagnosed with epilepsy in the first 10 years of life.1 It is a challenging condition that not only affects 0.3–1% of children worldwide,2–4 but also impacts their parents and families.5–7 Although seizures and their control are the main focus of clinical care, research shows that up to 80% of children and adolescents with epilepsy suffer from sleep disturbances, irrespective of whether seizures occur during nighttime sleep.8–10
Epilepsy itself or seizures are associated with nocturnal arousals, sleep fragmentation, excessive daytime sleepiness, and alterations in sleep architectures.11–13 Antiepileptic drugs are the main treatment for epilepsy, but most of these medications degrade sleep architecture, impair sleep quality, and aggravate daytime sleepiness.8 A recent systematic review and meta-analysis further demonstrated that compared to healthy peers, children and adolescents with epilepsy had reduced sleep duration and sleep efficiency and experienced more sleep problems, particularly in the domains of night waking, parasomnia, and sleep-disordered breathing.14
Sleep disturbances in pediatric epilepsy have been shown to have an adverse impact on sleep quality and quantity of parents, particularly when the child needs seizure monitoring and supervision during the night.6,15,16 Existing observational studies demonstrate that sleep disturbances such as poor sleep, shortened sleep, and nocturnal awakenings are common in parents of children with epilepsy, and that these sleep disturbances are experienced by parents across the child’s age span and disease severity.5,16,17 Shaki et al even found that mothers of children with epilepsy had a 7-fold increase in the occurrence of sleep disturbances when compared to mothers of children without epilepsy after controlling for potential confounding factors.17 Psychological distress and caregiving burden further contribute to the disturbance of sleep and impair daytime functioning in parents of children and adolescents with epilepsy.5,9 As demonstrated by Tsai et al’s study, up to 48.9% of mothers of children with epilepsy had clinically significant depressive symptoms, which together with child sleep disturbances, predicted poorer maternal sleep quality.18
Sleep is a vital need, with adequate and restorative sleep necessary for maintaining optimal health and well-being.19–21 Sleep disturbances in the general adult population have been associated with metabolic dysregulation, immune function alterations, impaired inflammatory responses, and reduced cognitive performances.20–22 These negative and potentially cumulative health consequences may have significant implications for parents of children with epilepsy who are particularly at long-term risk of sleep disruption and sleep loss due to the high demands of nocturnal caregiving and the increased prevalence of sleep problems in these children.4,14,23 Change of sleeping arrangements has also been reported in parents of children with epilepsy, with 63.6% parents not co-sleeping before the onset of their child’s seizures and 61.7% reporting decreased sleep quality and/or quantity with parent–child co-sleeping.23 However, there is currently no systematic review and meta-analysis examining sleep and its disturbances in parents of children and adolescents with epilepsy.
The purpose of this systematic review and meta-analysis was to quantify pooled mean estimates of parental sleep variables and to determine the prevalence of sleep disturbances in parents of children and adolescents with epilepsy. A greater understanding of the characteristics of sleep patterns and sleep disturbances in parents of children with epilepsy is needed because when the sleep of the parents is compromised, the parents’ ability to provide quality care for the child may also be hampered. We also assessed the study design and methodological quality of the included studies to inform future research directions and sleep intervention development.
Methods
Search Strategy
We performed a systematic review and meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.24 The study protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO) on October 04, 2021 (registration number: CRD42021276820). Five electronic databases, PubMed, Medline, Embase, PsychINFO, and CINAHL, were systematically searched from inception to September 2021. Examples of key terms included “sleep” OR “sleep disturbance” OR “Sleep problem” OR “sleep quality” OR “sleep duration” AND “epilepsy” OR “childhood epilepsy” OR “pediatric epilepsy” AND children OR adolescents AND parents OR mothers OR fathers OR caregivers. A full list of search terms is presented in Table S1. The search strategies with the keywords for each database are provided in Table S2.
Study Selection
Two investigators (HYT and YYL) independently searched and screened for all relevant articles in accordance with the eligibility criteria. Any disagreement was resolved through discussion with a third investigator (SYT). The initial search obtained 203 papers, with a total of 102 duplicates removed (Figure 1). The remaining 101 papers were screened through the titles and abstracts for further review based on the following inclusion criteria: 1) participants included parents or caregivers of pediatric patients aged <18 years with epilepsy, 2) articles included a quantitative measure of parents or caregivers’ sleep duration, sleep quality, or sleep disturbance, 3) not animal studies, and 4) not reviews, case reports, editorials, letters or comments. There were no language or study design restrictions. Studies involving parents or caregivers of adult epileptic patients aged 19 years and older or articles not published in peer-reviewed journals were excluded. After screening for eligibility using titles and abstracts, the full text of 19 articles with 9 from databases and registers and 10 from other methods were retrieved and assessed for inclusion. Hand searches were also performed on reference lists of included full-text articles. If more than one published article reported data from the same study, we adopted the one with the most complete data. Following this process, 11 articles were included in the final analysis (Figure 1). Details of the 8 excluded articles are provided in Table S3.
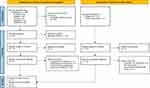 |
Figure 1 Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram for study selection and exclusion. Notes: PRISMA figure adapted from Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. Creative Commons.24 |
Data Extraction and Study Quality Assessment
Two investigators (HYT and YYL) separately extracted data and assessed the quality of the final included studies, with a third investigator (SYT) to adjudicate when discrepancies occurred. The data extraction included author names, publication year, study design, country where the study was conducted, sample size, % of maternal caregiver, the average age of the study sample, main sleep measure, and sleep variables. In case of data unavailability, we contacted the authors by e-mail for further information. To assess methodological quality, we used the critical appraisal checklist recommended by the Joanna Briggs Institute.25 The checklist has 8 items for cross-sectional study, 11 items for cohort study, 10 items for case–control study, and 9 items for quasi-experimental study with each item rated as “yes”, “no”, “unclear”, or “not applicable”. A quality score was assigned to each study and was computed as number of items with yes divided by total number of items. The inter-rater agreement between the two investigators (HYT and YYL) for study quality assessment was high (kappa = 0.89, p = 0.01).
Data Synthesis and Analysis
A series of meta-analyses was performed to obtain the overall effect size for each sleep variable using Comprehensive Meta-Analysis Software version 3.0 (https://www.meta-analysis.com/). Results were expressed as mean, mean difference, or prevalence rate with corresponding 95% confidence intervals (CIs) and with its statistical significance assessed by the Z statistic. Cochran’s Q and I2 statistics were used to evaluate the between-study heterogeneity, with a p value of <0.1 in the Cochrane’s Q test or an I2 value of >50% indicative of substantial heterogeneity.26 Random-effects models were used to meta-analyze data classified as of substantial heterogeneity. Otherwise, a fixed-effect model was used. Subgroup and meta-regression analyses were conducted to explore potential sources of heterogeneity on a range of study characteristics determined a priori, including sample size (both as a continuous and as a dichotomous variable; ≤50 versus >50), study region (western countries versus eastern countries), child age, and study quality score. These analyses were limited to cases where groups consisted of a minimum of two studies to ensure there was enough data for analysis. Sensitivity analyses were conducted by excluding the study with the highest mean value or prevalence rate to evaluate the robustness of our study findings. Because of the limited number of studies, we evaluated publication bias using funnel plots and Egger’s tests only for analyses consisting of 5 studies or more, with the Duval and Tweedie’s trim and fill procedure applied as appropriate.27,28
Ethical Consideration
This study was a systematic review and meta-analysis and did not prospectively collect data from human subjects. All the data included in the current study were from previously published research articles. According to the Declaration of Helsinki and local guidelines, no institutional review board approval is required for this study method.
Results
Characteristics of Included Studies
We finally identified four cross-sectional studies,6,15,16,18 five case–control studies,5,9,17,23,29 one cohort study,30 and one quasi-experimental study.31 Among the five case–control studies (Table 1), the comparison groups were composed of parents of healthy children (n = 4) or parents of children with nonepilepsy-related neurodisability (n = 1). Articles were all published in English and from seven different countries, with the United States contributing the majority of 36.4%. Sample sizes ranged from 12 to 234 parents of children with epilepsy. All the caregivers of children with epilepsy were parents and largely mothers (86.4%). Seven studies reported data on parental age, with a mean age of between 32 and 40 years. All studies measured sleep through subjective reports. A meta-analysis could only be performed on a few sleep variables due to the sparsity of data. Ten of the 11 included studies used the Pittsburgh Sleep Quality Index (PSQI) with a sleep measurement period over the previous month, of which one study only used a single sleep quality item from the PSQI.30 One study used validated daily phone diaries to recall activities over a 24-hour period for one weekday and one weekend day.29 One study also used Epworth Sleepiness Scale (ESS) to assess daytime sleepiness in parents.31
 |
Table 1 Study Participant Characteristics of the Included Studies |
The typology of seizures and/or their occurrence was reported in 6 studies. In the cross-sectional study by Tsai et al,18 45.9% of the children had experienced at least one seizure within the past 3 months. Wood et al reported the seizure syndrome of the children in their study as follows: idiopathic generalized (8%), symptomatic generalized (12%), cryptogenic partial (6%), and symptomatic partial (75%).15 In the case–control study by Larson et al,23 64.2% of the children had at least one seizure within the previous month, and 36.8% experienced daily seizures. Painter et al reported the seizure type of the children in their study as follows: partial epilepsy (56.7%), generalized epilepsy (33.3%), and unclassified (10.0%).29 In the case–control study by Reilly et al,9 67% of the children had at least one seizure per month, of which 52% had predominantly generalized seizures and 48% had predominately focal seizures. Yang et al reported the seizure type of the children in their study as follows: generalized seizures (75.21%) and focal seizures (24.79).5
Quality of Included Studies
The overall methodological quality of studies was mixed (Tables S4–S7), with three out of 11 studies fulfilling all the criteria of a high-quality, bias-free study.6,18,29 All four cross-sectional studies clearly described the study subjects and settings (item 2) and measured the exposure and outcome in a valid and reliable way (items 3 and 7). One cross-sectional study did not clearly define the study sample and the disease condition,16 while two other studies either did not identify or did not clearly specify the confounding factors.15,16 All five case–control studies clearly defined the study samples (items 1 and 3), measured the exposure and outcome using valid and reliable methods for cases and controls (item 4, 5, 8), identified confounding factors and stated the strategies to handle these factors (items 6 and 7), and used appropriate statistical analyses (item 10). The cohort study measured sleep in a valid and reliable manner but did not clearly identify confounding factors and did not describe the strategies for addressing incomplete follow-ups.30 The quasi-experimental study included multiple measurements of the outcome both before and after the intervention and had a complete follow-up but did not have a control group.31
Sleep Duration
Nocturnal sleep duration in parents of children with epilepsy was reported in four studies ranging from 4.5 to 6.5 hours per night,16–18,29 with one study specifically reporting that 25.6% of the parents in the study sample had an average nocturnal sleep of less than 6 hours.18 Because one study did not report the standard deviation16 and one showed significantly 41.66 minutes longer weekend sleep than weekday sleep without providing the raw data,29 estimates were therefore based on a meta-analysis of two studies with a total of 172 parents.17,18 Results indicated that the average nocturnal sleep duration for parents of children with epilepsy was 5.93 hours, with substantial heterogeneity across the two studies (95% CI: 4.64 to 7.21; Q = 22.16, p < 0.01; I2 = 95.48%).
Two case–control studies reported significantly higher scores on the PSQI sleep duration subscale in a total of 273 parents of children with epilepsy than 272 parents of healthy children,5,17 suggesting shorter sleep in parents of children with epilepsy than those of healthy children. Another case–control study did not find differences in the PSQI sleep duration subscale scores comparing 105 parents of children with epilepsy and 79 parents of healthy children.23 Painter et al also did not find differences in daily sleep duration between parents of children with and without epilepsy. Because only two case–control studies provided the PSQI sleep duration subscale numerical data,5,23 a meta-analysis of these two studies found that parents of children with epilepsy had significantly higher PSQI sleep duration subscale scores in comparison to parents of healthy children with no evidence of heterogeneity observed (differences in means = 0.20, 95% CI: 0.06 to 0.34; Q = 0.62, p = 0.42; I2 = 0%).
Sleep Quality
Seven studies reported sleep quality in a total of 740 parents of children and adolescents with epilepsy using the PSQI total score.5,6,9,15,18,23,31 Because one study did not find differences in PSQI total score after intervention and another study did not find PSQI total score differences between maternal and paternal caregivers,9,31 pre-intervention PSQI total score and maternal PSQI total score were included in the meta-analysis for sleep quality, respectively. The pooled mean estimate for PSQI total score across the seven studies was 7.03 (95% CI: 6.43 to 7.63; Q = 25.76, p < 0.01; I2 = 76.71%, Figure 2A), with substantial heterogeneity observed and evidence of publication bias detected in the funnel plot and Egger’s test (p = 0.02).
Subgroup and meta-regression analyses (Table 2) showed that study quality score was the only factor explaining the observed heterogeneity for PSQI total score (β = −0.09, p < 0.01). Using Duval and Tweedie’s trim and fill method to adjust for potentially unpublished reports revealed a slight decrease in the pooled mean for PSQI total score (random effect model point estimate: 6.65, 95% CI: 5.98 to 7.33, Figure 2B). Sensitivity analyses were conducted by excluding Ledet et al’s study (2016) in which the highest mean value (10.55) was reported. The pooled estimate changed to 6.76 (95% CI: 6.30 to 7.21) for the PSQI total score and remained statistically significant, showing similar results to our meta-analyses above.
 |
Table 2 Subgroup Analysis and Meta-Regression of Sleep Quality and Prevalence of Parental Sleep Disturbance |
Two case–control studies reported significantly higher PSQI total scores in parents of children with epilepsy than in parents of healthy children, suggesting poorer sleep quality in parents of children with epilepsy than those of healthy children.5,23 A meta-analysis of these two studies found similar results with no evidence of heterogeneity (differences in means = 1.84, 95% CI: 1.29 to 2.39; Q = 0.12, p = 0.72; I2 = 0%). One study specifically reported that the PSQI total score in parents of infants with epilepsy was significantly higher than other age groups, with no differences found between parents of healthy infants and preschool children, school-age children, or adolescents.5 Another study reported no significant differences in the PSQI total score between mothers and fathers of children with epilepsy.9
Sleep Latency
Only one cross-sectional study with 133 mothers of children with epilepsy reported a median sleep latency of 25 minutes.18 One case–control study reported significantly higher scores on the PSQI sleep latency subscale in 234 parents of children with epilepsy than 230 parents of healthy children,5 suggesting parents of children with epilepsy taking longer to fall asleep than those of healthy children. Another two case–control studies did not find differences in the PSQI sleep latency subscale scores comparing a total of 144 parents of children with epilepsy and 121 parents of healthy children.17,23 Because only two case–control studies provided the PSQI sleep latency subscale numerical data,5,23 a meta-analysis of these two studies found that parents of children with epilepsy had significantly higher PSQI sleep latency subscale scores in comparison to parents of healthy children with no evidence of heterogeneity (differences in means = 0.28, 95% CI: 0.15 to 0.42; Q = 0.01, p = 0.91; I2 = 0%).
Sleep Efficiency
Only one cross-sectional study with 133 mothers of children with epilepsy reported a mean sleep efficiency of 86.86%.18 Another study found mothers of children with epilepsy had significantly higher scores on the PSQI habitual sleep efficiency subscale than fathers.9 Because only two case–control studies provided the PSQI habitual sleep efficiency subscale numerical data,5,23 a meta-analysis of these two studies found that parents of children with epilepsy had significantly higher PSQI habitual sleep efficiency subscale scores in comparison to parents of healthy children with no evidence of heterogeneity (differences in means = 0.17, 95% CI: 0.03 to 0.32; Q = 1.16, p = 0.91; I2 = 0%), suggesting parents of children with epilepsy having poorer sleep efficiency than those of healthy children.
Nocturnal Wake Frequency
Only one study reported that parents of children with epilepsy awoke approximately 3 times during the night.16
Sleep Disturbances
Sleep disturbances in parents of children and adolescents with epilepsy were reported using various terms and definitions in 6 studies, including sleep problem, sleep disorder, disturbed sleep, sleep disturbance, and poor sleeper, with a prevalence between 37.6% and 100%. Five studies used the PSQI employing three different cut-off scores to define sleep disturbances (ie, ≥5, >5, ≥7).6,9,15,18,31 Nevertheless, the proportion of parents categorized as having sleep disturbances remained high (37.6%) under the highest PSQI cut-off of 7.6 One study reported that 59.9% of parents of children with epilepsy aged ≤5 years old perceived their sleep to be a problem but did not report how sleep problems were defined.16 One study using the PSQI cut-off of 5 found no significant differences in the proportion of mothers (62.0%) and fathers (44.0%) of children with epilepsy categorized as poor sleepers.9 The pooled estimated prevalence of parental sleep disturbance from the 6 studies was 58.1% (95% CI: 45.7% to 69.6%; Q = 26.33, p < 0.01; I2 = 81.01%, Figure 3A,), with substantial heterogeneity observed and no evidence of publication bias detected in the funnel plot (Figure 3B) and Egger’s test (p = 0.09).
Subgroup and meta-regression analyses (Table 2) showed that sample size as a continuous variable was the only factor explaining the observed heterogeneity for prevalence of parental sleep disturbance (β = −0.008, p < 0.01), while sample size analyzed as a dichotomous variable (≤50 versus >50) did not show such an association (p = 0.35). The pooled prevalence estimate became slightly lower to 52.5% (95 CI: 47.7% to 57.2%) after conducting a sensitivity analysis by omitting Ledet et al’s study with the highest prevalence rate.
Three case–control studies reported significantly higher scores on the PSQI sleep disturbance subscale in parents of epileptic children than parents of healthy children,17,23 suggesting more disturbed sleep in parents of children with epilepsy than those of healthy children. Because only two case–control studies provided the PSQI sleep disturbances subscale numerical data,5,23 a meta-analysis of these two studies found similar results that parents of children with epilepsy had significantly higher PSQI sleep disturbances subscale scores in comparison to parents of healthy children, with substantial heterogeneity observed (differences in means = 0.26, 95% CI: 0.03 to 0.49; Q = 6.23, p = 0.01; I2 = 83.9%).
Daytime Sleepiness
Only one interventional pilot study with 12 parents of children with epilepsy reported a mean score of 10.58 on the ESS and 58.0% of the parents had an ESS score ≥10 for both pre- and post-intervention,31 suggesting parents of children with epilepsy experienced significant daytime sleepiness.
Discussion
This study provides a systematic review of the literature and meta-analysis of available data for seven sleep quantity- and quality-related variables reported in parent caregivers of children and adolescents with epilepsy: sleep duration, sleep quality, sleep latency, sleep efficiency, nocturnal wake frequency, sleep disturbance, and daytime sleepiness. In total, 11 observational studies with 934 parents of epileptic children and adolescents that capture populations from different global regions and cultures were reviewed, with the majority of included studies having moderate to high quality. Our analyses indicate that parents of children and adolescents with epilepsy have inadequate sleep duration, poor sleep quality, reduced sleep efficiency, severe sleep disturbances, and excessive daytime sleepiness. Case–control studies provided additional evidence supporting that parents of children and adolescents with epilepsy have significantly poorer sleep quality, lower sleep efficiency, and more severe sleep disturbances compared to parents of children and adolescents without epilepsy. Findings from this study suggest that poor and altered sleep in parents of children and adolescents with epilepsy is an important target of intervention requiring further investigation.
Our analyses of sleep duration found parents of children and adolescents with epilepsy sleeping on average of 5.93 hours per night, which is below the national and international recommendations for healthy adults who should sleep 7 or more hours per night to promote optimal health.19 This pooled estimate for nocturnal sleep in our analyses was based on parental reporting rather than objective measures. Parents may actually obtain an even shorter sleep given that subjective report was shown to overestimate total sleep time when compared to objective actigraphy- or polysomnography-measured sleep.32 This finding is of concern because sleep duration of 7–8 hours per day was found most favorably associated with a variety of health outcomes, including reduced risk of all-cause mortality, incident cardiovascular disease, type 2 diabetes, depression, and obesity, according to a recent overview of systemic reviews.20 However, results from three case–control studies showed inconsistent differences in the PSQI sleep duration subscale scores between parents of children with and without epilepsy. It is possible that age of the children may explain some of the conflicting results. Obtaining an adequate amount of sleep may be equally challenging for parents of younger children regardless of whether the child has epilepsy.
Our pooled estimate for sleep duration in parents of children and adolescents with epilepsy was only for nocturnal sleep. Most studies included in our analyses used the PSQI in which daytime sleep is not part of the questionnaire, so daytime sleep data were not collected. Parents may nap during the day, particularly parents of younger children who often synchronize their sleep-wake schedule with the child. Among the 11 included studies, only one study recorded parental sleep and activity patterns over a 24-hour period,29 but this study did not report nocturnal sleep separate from daily sleep, making direct comparisons between studies difficult. This study found that both parents of children with and without epilepsy slept 41.66 minutes longer on weekends than on weekdays (p < 0.01). Maintaining a consistent sleep schedule is essential for adequate sleep, which would be particularly important to be addressed for parents of children with epilepsy because weekday-weekend sleep irregularities could perpetuate the already reduced and disrupted sleep in parents of children with epilepsy. Screening parental sleep hygiene knowledge and providing parents with information about healthy sleep practices can inform both future research and the development of behavioral-educational sleep interventions for parents of children and adolescents with epilepsy.
Our pooled PSQI global sleep quality score in this meta-analysis was 6.65, with 6 studies reporting an average PSQI score >55,6,9,18,23,31 which is the original PSQI cut-off score used to classify individuals as having poor sleep quality.33 Evidence from two case–control studies further showed that parents of children with epilepsy consistently having poorer sleep quality than parents of healthy children.5,23 Night wakings may impair sleep quality, but this measure yielded the least amount of data in the current review.16 Duration of sleep latency was also reported in only one study and was on average 25 minutes,18 which marginally fits within the cutoff for clinical significance because a sleep latency of >30 minutes often suggests some difficulties with sleep initiation.33 However, results from three case–control studies showed inconsistent differences in the PSQI sleep latency subscale scores between parents of children with epilepsy and those of healthy children.5,17,23 One possible explanation is that sleep latency is related to the homeostatic sleep pressure and is influenced by multiple factors such as daytime napping and physical activity34 which are not assessed in all of the included studies.
In our included studies, only one cross-sectional study reported that parents of children with epilepsy had a mean sleep efficiency of 86.86%.18 Two case–control studies reported that parents of children with epilepsy had poorer sleep efficiency than those of healthy children as evidenced by a higher PSQI habitual sleep efficiency subscale score.5,23 Sleep efficiency is the ratio of the total time spent asleep at night compared to the total amount of time spent in bed. A person with poor sleep efficiency is closely linked to taking longer to fall asleep, waking up in the middle of the night, or both. These findings of the sleep efficiency, along with our aforementioned analyses on sleep latency, suggest that reduced sleep efficiency in parents of children and adolescents with epilepsy more likely resulted from frequent nighttime wakings or increased wake after sleep onset rather than prolonged sleep latency. Sleep efficiency is an important dimension of the PSQI to examine global sleep quality and level of sleep disturbances. Parents of children and adolescents with epilepsy experience a decrease in sleep efficiency which likely contributes to the reduced overall sleep quality and high prevalence of sleep disturbances as well as daytime sleepiness.
The pooled prevalence of sleep disturbances in parents of children and adolescents with epilepsy is high (58.1%), with clear evidence of more disturbed sleep in parents of children with epilepsy than those of healthy children. Sleep disturbances in parents of children with epilepsy may influence not only their caregiver role but also their own health and ability to attend to daily tasks at work and home.16 Symptoms of depression and anxiety have been linked to parental sleep disturbances in both cross-sectional and case–control pediatric epilepsy studies.5,18 These symptoms are likely to co-occur or to have a bi-directional relationship with parental sleep disturbances. Altered sleeping arrangements are another factor that needs to be taken into account when interpreting findings on parental sleep disturbances. Approximately 22–63.6% of parents change to co-sleep with their child following epilepsy diagnosis or onset of seizure.23,35 Co-sleeping has been reported more frequently in families of children with epilepsy than those of healthy children and linked to poorer and/or shorter parental sleep.23 The high pooled prevalence of parental sleep disturbances of our meta-analysis, together with the 53–57.1% previously reported in children with epilepsy,13,36 suggest that sleep disturbance is a shared problem in parents and children with epilepsy. While our review and analysis focused on parents of children with epilepsy, a dyadic approach to assessing the sleep and sleeping arrangement in the parent and child may uncover how the dyads’ influence each other’s sleep, and may facilitate the development of more tailored sleep interventions that benefit both the parent and child.
Some limitations of this systematic review and meta-analyses should be acknowledged. First, although it is possible to conduct a meta-analysis with only two studies, having small numbers of studies could affect the assessment of between-study heterogeneity. Our subgroup and meta-regression analyses did not show the study region or child age as the source of heterogeneity. However, parental sleep quality and prevalence of parental sleep disturbance were negatively moderated by study quality score and sample size, respectively. Future studies should consider using rigorous study designs with an adequate sample size to generate high-quality evidence. Second, subgroup analyses such as gender differences were not analyzed because only two studies, not uniformly reporting the same sleep variables, considered gender, both with a sample size of <50 parents.9,17 Lastly, although we pre-registered the protocol in a publicly available repository (PROSPERO) and performed a comprehensive literature search, publication bias was still present for the sleep quality measure. All of the reviewed studies were also observational and many were cross-sectional, with the lack of daytime sleep data and data from objective sleep measures. Multi-day sleep diary or actigraphy assessments of sleep with a more transparent report of research findings are needed to avoid publication bias and to have a better understanding of sleep habits and sleep hygiene practices in parents of children and adolescents with epilepsy.
Conclusion
The results of our systematic review and meta-analysis demonstrate a high prevalence of sleep disturbances with poor sleep quality and substantial reductions in sleep time in parent caregivers of children and adolescents with epilepsy. Our findings suggest that healthcare professionals in pediatric neurology clinics should proactively initiate screening for sleep disturbances in parents of children and adolescents with epilepsy and refer parents to a sleep specialist when it is deemed necessary. Future prospective studies with objective sleep measurements are needed to validate our results on sleep quality, quantity, and disturbance in parents of children with epilepsy as well as sleep pattern differences in parents of children with and without epilepsy. Future studies should also include daytime sleep analysis and examine whether parents of children with epilepsy may use daytime napping to compensate for reduced and poor nocturnal sleep.
Data Sharing Statement
The template data collection forms, data extracted from included studies, or other materials used in this review are available from the corresponding author on reasonable request.
Acknowledgments
We gratefully thank contacted authors who provided clarifying information and assistance.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Ministry of Science and Technology, Taiwan (grant number MOST 110 - 2628 - B - 002 – 039). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors declare that they have no competing interests. The abstract of this paper was presented at the conference SLEEP 2023 as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in SLEEP as “Sleep and Its Disturbance in Parents of Children and Adolescents with Epilepsy: A Systematic Review and Meta-Analysis” (https://academic.oup.com/sleep/article/46/Supplement_1/A345/7182296).
References
1. Aaberg KM, Gunnes N, Bakken IJ, et al. Incidence and prevalence of childhood epilepsy: a nationwide cohort study. Pediatrics. 2017;139(5):e20163908. doi:10.1542/peds.2016-3908
2. Chiang KL, Cheng CY. Prevalence and neuro-psychiatric comorbidities of pediatric epilepsy in Taiwan: a national population-based study. Epilepsy Res. 2014;108(8):1451–1460. doi:10.1016/j.eplepsyres.2014.07.004
3. Russ SA, Larson K, Halfon N. A national profile of childhood epilepsy and seizure disorder. Pediatrics. 2012;129(2):256–264. doi:10.1542/peds.2010-1371
4. Gogou M, Haidopoulou K, Eboriadou M, Pavlou E. Sleep disturbances in children with rolandic epilepsy. Neuropediatrics. 2017;48(1):30–35. doi:10.1055/s-0036-1593611
5. Yang H, Feng Y, Zhu Z, Qiao Z, Xiao B, Feng L. Evaluation of anxiety, depression, and sleep quality among parents of children with epilepsy in Southern China. Epilepsy Behav. 2020;112:107340. doi:10.1016/j.yebeh.2020.107340
6. Liu PP, Yin P, Zhu YH, Zhang S, Sheng GM. The correlation of family resilience with sleep quality and depression of parents of children with epilepsy. J Pediatr Nurs. 2021;56:e49–e54. doi:10.1016/j.pedn.2020.07.016
7. Reilly C, Atkinson P, Memon A, et al. Parenting stress and perceived stigma in mothers of young children with epilepsy: a case-control study. Epilepsy Behav. 2018;89:112–117. doi:10.1016/j.yebeh.2018.10.016
8. Gibbon FM, Maccormac E, Gringras P. Sleep and epilepsy: unfortunate bedfellows. Arch Dis Child. 2019;104(2):189–192. doi:10.1136/archdischild-2017-313421
9. Reilly C, Atkinson P, Memon A, et al. Child and parental sleep in young children with epilepsy: a population-based case-control study. Epilepsia Open. 2018;3(3):383–391. doi:10.1002/epi4.12241
10. Ekinci O, Isik U, Gunes S, Ekinci N. Understanding sleep problems in children with epilepsy: associations with quality of life, attention-deficit hyperactivity disorder and maternal emotional symptoms. Seizure. 2016;40:108–113. doi:10.1016/j.seizure.2016.06.011
11. Manokaran RK, Tripathi M, Chakrabarty B, Pandey RM, Gulati S. Sleep abnormalities and polysomnographic profile in children with drug-resistant epilepsy. Seizure. 2020;82:59–64. doi:10.1016/j.seizure.2020.09.016
12. Chan SY. Sleep architecture and homeostasis in children with epilepsy: a neurodevelopmental perspective. Dev Med Child Neurol. 2020;62(4):426–433. doi:10.1111/dmcn.14437
13. Gupta G, Dang LT, O’Brien LM, Shellhaas RA. Parent-reported sleep profile of children with early-life epilepsies. Pediatr Neurol. 2021;128:9–15. doi:10.1016/j.pediatrneurol.2021.12.006
14. Winsor AA, Richards C, Bissell S, Seri S, Liew A, Bagshaw AP. Sleep disruption in children and adolescents with epilepsy: a systematic review and meta-analysis. Sleep Med Rev. 2021;57:101416. doi:10.1016/j.smrv.2021.101416
15. Wood LJ, Sherman EM, Hamiwka LD, Blackman MA, Wirrell EC. Maternal depression: the cost of caring for a child with intractable epilepsy. Pediatr Neurol. 2008;39(6):418–422. doi:10.1016/j.pediatrneurol.2008.08.007
16. Cottrell L, Khan A. Impact of childhood epilepsy on maternal sleep and socioemotional functioning. Clin Pediatr. 2005;44(7):613–616. doi:10.1177/000992280504400709
17. Shaki D, Goldbart A, Daniel S, Fraser D, Shorer Z. Pediatric epilepsy and parental sleep quality. J Clin Sleep Med. 2011;7(5):502–506. doi:10.5664/JCSM.1318
18. Tsai SY, Lee WT, Lee CC, Jeng SF, Weng WC. Sleep in mothers of children with epilepsy and its relation to their children’s sleep. Res Nurs Health. 2020;43(2):168–175. doi:10.1002/nur.22008
19. Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of sleep medicine and sleep research society. Sleep. 2015;38(6):843–844. doi:10.5665/sleep.4716
20. Chaput JP, Dutil C, Featherstone R, et al. Sleep duration and health in adults: an overview of systematic reviews. Appl Physiol Nutr Metab. 2020;45:10.
21. Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;9:151–161. doi:10.2147/NSS.S134864
22. Irwin MR. Sleep and inflammation: partners in sickness and in health. Nat Rev Immunol. 2019;19(11):702–715. doi:10.1038/s41577-019-0190-z
23. Larson AM, Ryther RC, Jennesson M, et al. Impact of pediatric epilepsy on sleep patterns and behaviors in children and parents. Epilepsia. 2012;53(7):1162–1169. doi:10.1111/j.1528-1167.2012.03515.x
24. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
25. Moola S, Munn Z, Tufanaru C, et al. Chapter 7: systematic Reviews of Etiology and Risk. In: Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis. The Joanna Briggs Institute; 2020.
26. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi:10.1136/bmj.327.7414.557
27. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi:10.1111/j.0006-341X.2000.00455.x
28. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi:10.1136/bmj.315.7109.629
29. Painter E, Rausch JR, Modi AC. Changes in daily activity patterns of caregivers of children with newly diagnosed epilepsy: a case-controlled design. Epilepsy Behav. 2014;31:1–6. doi:10.1016/j.yebeh.2013.11.001
30. Borusiak P, Bast T, Kluger G, et al. A longitudinal, randomized, and prospective study of nocturnal monitoring in children and adolescents with epilepsy: effects on quality of life and sleep. Epilepsy Behav. 2016;61:192–198. doi:10.1016/j.yebeh.2016.05.035
31. Ledet D, Aplin-Kalisz C, Filter M, Dycus P. A pilot study to assess a teaching intervention to improve sleep-wake disturbances in parents of children diagnosed with epilepsy. J Neurosci Nurs. 2016;48(1):2–14. doi:10.1097/JNN.0000000000000179
32. Matthews KA, Patel SR, Pantesco EJ, et al. Similarities and differences in estimates of sleep duration by polysomnography, actigraphy, diary, and self-reported habitual sleep in a community sample. Sleep Health. 2018;4(1):96–103. doi:10.1016/j.sleh.2017.10.011
33. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi:10.1016/0165-1781(89)90047-4
34. Nixon GM, Thompson JM, Han DY, et al. Falling asleep: the determinants of sleep latency. Arch Dis Child. 2009;94(9):686–689. doi:10.1136/adc.2009.157453
35. Williams J, Lange B, Sharp G, et al. Altered sleeping arrangements in pediatric patients with epilepsy. Clin Pediatr. 2000;39(11):635–642. doi:10.1177/000992280003901102
36. Zambrelli E, Turner K, Vignoli A, et al. Sleep disturbances in Italian children and adolescents with epilepsy: a questionnaire study. Epilepsy Behav. 2020;106:107014. doi:10.1016/j.yebeh.2020.107014
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


