Back to Journals » Journal of Inflammation Research » Volume 15
Seminal Levels of Omentin-1/ITLN1 in Inflammatory Conditions Related to Male Infertility and Localization in Spermatozoa and Tissues of Male Reproductive System
Authors Moretti E , Signorini C , Noto D , Tripodi SA , Menchiari A, Sorrentino E , Collodel G
Received 18 November 2021
Accepted for publication 2 March 2022
Published 26 March 2022 Volume 2022:15 Pages 2019—2031
DOI https://doi.org/10.2147/JIR.S339515
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Elena Moretti,1 Cinzia Signorini,1 Daria Noto,1 Sergio Antonio Tripodi,2 Andrea Menchiari,3 Ester Sorrentino,2 Giulia Collodel1
1Department of Molecular and Developmental Medicine, University of Siena, Siena, Italy; 2Department of Pathology Unit, AOUS Siena, Siena, Italy; 3Department of Business and Law, University of Siena, Siena, Italy
Correspondence: Elena Moretti, Department of Molecular and Developmental Medicine, University of Siena, Siena, 53100, Italy, Tel +39 0577 232451, Email [email protected]
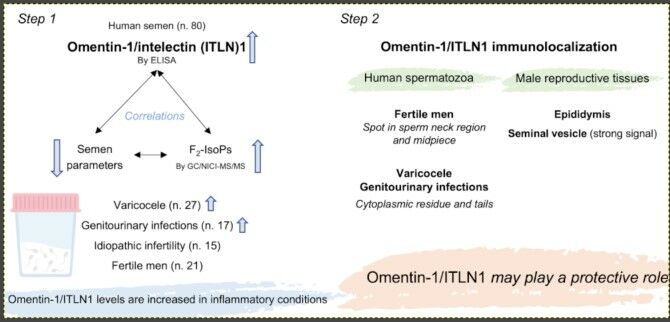
Purpose: Omentin-1/intelectin (ITLN)1 is an adipocytokine with both anti-inflammatory and anti-oxidative stress properties, and little is known about its role in male reproduction. This study was aimed at exploring the relationships among omentin-1/ITLN1, semen parameters and F2-isoprostanes (F2-IsoPs), a maker of oxidative stress, in groups of patients affected by different pathologies. In addition, omentin-1/ITLN1 immunolocalization was assessed in ejaculated spermatozoa and in tissues of male reproductive system.
Patients and Methods: Semen samples of infertile patients with varicocele (n = 27), genitourinary infections (n = 17), idiopathic infertility (n = 15) and fertile men (n = 21) were analyzed following WHO guidelines, and seminal plasma were used to determine omentin-1/ITLN1 by ELISA and F2-IsoP levels by gas chromatography/negative-ion chemical ionization tandem mass spectrometry. Omentin-1/ITLN1 was localized in human sperm and in the tissue of male reproductive system.
Results: Considering all participants, F2-IsoP and omentin-1/ITLN1 levels were positively correlated (p = 0.000), and both these indices were negatively correlated with sperm parameters. Infertile patients showed lower sperm parameters than fertile ones; varicocele and infection groups had significantly increased levels of F2-IsoPs (both p = 0.000) and omentin-1/ITLN1 (p = 0.000 and p = 0.001, respectively). Omentin-1/ITLN1 signal was located as a spot in the connecting piece (in 43.5% of cases midpiece was also labeled) of sperm from fertile men and in cytoplasmic residue and in the entire tail in sperm of patients with varicocele and genitourinary infections. A focal omentin-1/ITLN1 immunolabelling was evident in the basal area of epididymal tubule, and a diffuse signal was present in the seminal vesicle epithelium.
Conclusion: Semen omentin-1/ITLN1 originates from seminal vesicles, its levels increase in inflammatory conditions and are negatively correlated with sperm parameters. For this reason, a sort of protective role of omentin-1/ITLN1 can be postulated, as this adipokine shows anti-inflammatory properties also in many other biological systems.
Keywords: adipocytokines, F2-isoprostanes, human sperm, immunocytochemistry, epididymis, seminal vesicles
Graphical Abstract:
Introduction
The adipose tissue is not only a lipid storage but also an endocrine tissue that secretes more than 600 mediators called adipokines including cytokines, hormones, chemokines and different proteins involved in many functions such as lipid metabolism, glucose homeostasis, inflammation and others.1 The relationship between energy balance and reproductive function has long been recognized, however the pathways involved in both processes are still poorly understood. Gut hormones and adipokines have recently emerged as potential regulators of both energy homeostasis and male and female fertility2–4 and their role in the development of some reproductive disorder has been suggested. The most studied adipokines in the control of reproductive axis are leptin and adiponectin.4–7 However, a rising interest in novel adipokines such as visfatin, chemerin, resistin, apelin is growing up.3,8–11
Estienne et al4 revised the role and the expression of chemerin, visfatin, resistin and apelin in male and female reproduction in humans and animal models, demonstrating that these compounds act all along the hypothalamo-pituitary-gonadal axis. Among the novel adipokines, omentin-1, also known as intelectin-1 (ITLN1), was recently identified12 from human omental adipose tissue. Omentin-1 is expressed in visceral fat as well as mesothelial cells, vascular cells, airway goblet cells, small intestine, colon, ovary, and plasma. It exerts a favorable effect on energy homeostasis, glucose metabolism, cardiovascular protection and acts as anti-inflammatory agent and against oxidative stress (OS).13 Current data suggest that the intracellular signaling pathway of omentin-1 involves the activation of Akt/AMPK/NF-κB and mitogen-activated protein kinases such as ERK, JNK and p38 as well as by binding to lactoferrin resulting in a range of effects in various cell types.2,12–14
Limited data on the reproductive effects of omentin are available and are mainly related to female system since omentin-1 is expressed in placenta and ovary.15 High omentin levels are detected in follicular fluid and granulosa cells of women with polycystic ovarian syndrome.16 Circulating omentin levels were decreased in gestational diabetes mellitus patients17 and increased in women with spontaneous miscarriage compared to those with healthy pregnancies.18
The involvement of omentin-1 in inflammation is well described in literature,2,13 however little is known about the role of omentin-1 in male reproductive system. Male infertility can be caused by situations or pathologies that share an inflammatory base such as varicocele, infections, smoking habit, leukocytospermia and others19–22 with the consequent production of reactive oxygen species (ROS) that cause OS. Spermatozoa are vulnerable to OS due to the high content of polyunsaturated fatty acids (PUFAs) in their plasma membrane, however also DNA and proteins can be severely damaged.23 Recently, F2-isoprostanes (F2-IsoPs), products of arachidonic acid oxidation, are considered a valuable marker of lipid peroxidation (LPO) and can be measured to evaluate the oxidative status in many human pathologies including male infertility.24,25
This study was aimed at quantifying omentin-1/ITLN1 levels in human semen, and at studying its relationship with semen parameters and seminal F2-IsoPs, in different groups of infertile patients and in a group of fertile men. In addition, the localization of omentin-1/ITLN1 in human spermatozoa, testis, epididymis, prostate gland and seminal vesicles was explored.
Patients and Methods
Patient’s Selection
Semen samples were obtained from 59 Caucasian infertile patients (25 to 39 year-olds) who attended the Department of Molecular and Developmental Medicine, University of Siena.
Infertile patients did not achieve pregnancy after two years of unprotected sexual intercourses; the female factor was absent.
The cases enrolled in this study fulfilled the following inclusion criteria: non-azoospermic men, absence of systematic sperm defects, BMI <25 kg/m2, no history of diabetes, metabolic syndrome, radiotherapy, chemotherapy, chronic illness or medication. In addition, the study participants were non-smokers, they did not use drugs, alcohol or dietary supplements. Patients with leukocytospermia were excluded.
A complete clinical history of patients was obtained. Among the medical tests, hormone levels and bacteriological analyses were part of the clinical workup and assessed in a dedicated laboratory. In the population studied, the concentrations of follicle-stimulating hormone (FSH), luteinizing hormone (LH) and testosterone (T) were normal. All patients provided bacteriological analyses of their semen samples. Despite the presence of pathogens in semen, the patients had not symptoms of genital infections.
Physical examinations and scrotal Eco-color Doppler were performed to detect the possible presence of varicocele. Subclinical varicocele was not considered in this study.
The 59 patients were grouped according to clinical diagnosis:
- group with varicocele (n= 27),
- group with genitourinary infections (n= 17),
- group with idiopathic infertility (n= 15)
Twenty-one fertile men (24–36 year-olds) with BMI <25 kg/m2 not affected by diabetes, metabolic disorders, anatomical problems and/or infections and who fathered a child in the last three years, were recruited as controls.
At Siena University this type of study, for no treatment of analyzed patient does not need ethics approval; the participants signed an informed written consent before participating in this research, accepting that their semen samples and the clinical data they supplied might be used for scientific purposes.
Semen Analysis
Semen analysis was performed as reported in WHO guidelines26 by assessing, after liquefaction for 30 min at 37°C, semen volume, pH, sperm concentration and motility. Sperm morphology was evaluated using pre-stained Testsimplets slides (Origio, Italy) and scoring 200 spermatozoa. Sperm vitality was assessed using eosin Y (CI 45380) staining26 and spermatozoa were examined by light microscope scoring red stained cells (dead) and unstained cells (vital). More than 300 sperm per sample were analyzed.
After the analysis, samples were spun at 400 g for 15 min. Seminal plasma was recovered, observed under microscope ensuring the absence of sperm cells and then, stored at −80 °C until use. Omentin-1/ITLN1 and F2-IsoP levels were assayed in seminal plasma.
Omentin-1/ITLN1 Determination
The determination of seminal human omentin-1/ITLN1 was performed by sandwich quantitative enzyme-linked immunosorbent assay (Human Intelectin1/Omentin, ELISA kit, Abbexa Ltd, Cambridge, UK). Spectrometric detection of color intensity at 450 nm allowed the determination of seminal omentin-1/ITLN1 amounts by comparing the optical density of each seminal samples to the standard curve (omentin standard amounts ranging from 0.625 ng/mL to 40 ng/mL). In all experiments, omentin-1/ITLN1 measure was performed in duplicate in each sample.
Total F2-Isoprostane Determination
As previously reported,25,27,28 total (ie unesterified plus esterified to cellular lipids) seminal F2-IsoPs were analyses by gas chromatography/negative-ion chemical ionization tandem mass spectrometry (GC/NICI-MS/MS). Briefly, the seminal plasma samples were added with the antioxidant butylated hydroxytoluene and were treated with a basic hydrolysis. Subsequently, they were acidified to pH 3 with HCl 1N, and were finally spiked with tetradeuterated derivative of prostaglandin F2α. After that, two solid phase extractions were carried out and the final eluates were derivatized. The measured ion was m/z 299 produced from 15-F2t-IsoP (ie, 8-iso-PGF2α, the most represented isomer for F2-IsoP detection).27,28 The quantitation of total F2-IsoPs was referred to the calibration curve (Cayman Chemical, Item No. 16350).
Immunolocalization of Omentin-1/ITLN1 in Ejaculated Spermatozoa
Immunolocalization of omentin-1/ITLN1 was performed in spermatozoa from 10 fertile and 10 infertile individuals with varicocele and positive semen culture as reported in Micheli et al28 with few modifications. Briefly, smeared spermatozoa were fixed in 4% paraformaldehyde in PBS for 15 min and then treated overnight at 4°C with rabbit omentin-1/ITLN1 (Abcam, Cambridge, UK) diluted 1:100. The reaction was revealed by an anti-rabbit antibody raised in goat Alexa Fluor® 488 conjugate (Invitrogen, Thermo Fisher Scientific, Carlsbad, California, USA), diluted at 1:100. Incubation in primary antibody was omitted in control samples (Supplementary Figure 2E and F).
Nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI) solution (Vysis, Downers Grove, IL). Slides were observed with Leica DMI 6000 Fluorescence Microscope (Leica Microsystems, Germany), and the images were acquired by Leica AF6500 Integrated System for Imaging and Analysis (Leica Microsystems, Germany). At least 300 spermatozoa were analyzed for each sample and the different localization of omentin-1/ITLN1 was recorded.
Tissue Samples and Immunohistochemistry
Tissue sections from adult human testis, epididymis, seminal vesicles and prostate gland were collected from the archives of the Pathology Department of the University of Siena. Testicular specimens were obtained from three patients undergoing orchiectomy for testicular seminoma; prostate gland and seminal vesicle specimens from three patients undergoing radical prostatectomy for prostate cancer. Specimens were immediately fixed in 10% neutral buffered formalin for 12 hours and embedded in paraffin. Immunohistochemistry was performed by using the automated Ventana BenchMark ULTRA IHC/ISC (Ventana Co., Roche diagnostic, Monza, Italy). Briefly, tissue sections were deparaffinized with EZ Prep Volume Adjust (Ventana Co., Tucson, AZ, USA) at 75°C. The antigen retrieval was performed using Heat Induced Epitope Retrieval (HIER) in Tris-EDTA buffer pH 7.8 at 38°C for 44 min in the Ventana BenchMark ULTRA (Ventana Co., Roche diagnostic, Monza, Italy).
The sections were incubated with the omentin-1/ITLN1 antibody (Abcam, Cambridge, UK) diluted 1:100 for 1h at 70°C. Then, Ventana standard signal amplification was performed following the automated protocol. UltraView Universal Alkaline Phosphatase Red Detection Kit (Ventana Co., Roche diagnostic, Monza, Italy) allows to display the specific targeted antigen as a red signal.
A double staining using omentin-1/ITLN1 antibody and anti PAX8 monoclonal antibody (MRQ-50, Cell Marque, Rocklin, CA, USA), a specific nuclear marker of seminal vesicle epithelium,29 was carried out. For the use of anti-PAX8, the manufacturer instructions related to Ventana Roche detection kits and automated slide stainers were followed.
PAX8 localization was visualized with hydrogen peroxidase substrate and DAB chromogen, producing a brown precipitate (UltraView Universal DAB Detection Kit from Ventana, Roche diagnostic, Monza, Italy). Samples were counterstained with hematoxylin and analyzed. The positive control of primary antibody was performed on human small intestine and omentum (Supplementary Figure 1A and B). The negative control was carried out by incubating the samples with secondary antibody omitting anti-omentin-1/ITLN1 antibody (Supplementary Figure 2A–D). In each slide, the expression of omentin-1/ITLN1 was examined in the area of histologically non-neoplastic tissue.
Statistical Analysis
Statistical analysis was performed with the SPSS version 23.0 for Windows software package (SPSS Inc, Chicago, IL, USA).
The Spearman’s Rank Correlation Coefficient (rho) was used to discover the relation existing between the variables investigated. The Kolmogorov–Smirnov test was used to verify normality of distribution of the variables. The Levene test was performed to test homoscedasticity and in order to determine the appropriate post-hoc test. Because some variables showed a non-normal distribution, the comparison between groups (fertile, idiopathic infertility, varicocele, genitourinary infections) was performed using the non-parametric tests: Kruskal–Wallis test and the Wilcoxon test. Then, a post-hoc analysis using the Dunnett test or Tukey’s test was applied. Mann–Whitney U-test was used to compare the omentin-1/ITLN1 different labelling patters of sperm from fertile and infertile patients. Data were reported as median (interquartile range [IQR]). P<0.05 was considered significant.
Results
We examined the semen samples of 59 patients; 15 out of 59 patients showed idiopathic infertility, 27 had II and III grade varicocele and 17 showed positive semen cultures (infections). Nine out of 17 semen cultures were positive for Escherichia coli, 5 for Enterococcus faecalis, 2 for Ureaplasma urealyticum, 1 for Morganella morganii. Twenty-one semen samples from fertile men were used as controls.
The median and 25th/75th centiles of the considered variables in all participants (No. 80 cases) included in the study, are reported in Table 1.
 |
Table 1 Median, 25th and 75th Centiles of the Considered Variables in the Group of 80 Cases Included in This Study |
The correlations between the measured variables assessed in the total group of the participants are reported in Table 2.
 |
Table 2 Correlations (Rho Spearman’s Coefficient) Between All the Considered Variables in 80 Individuals |
Regarding sperm parameters, sperm concentration, progressive motility, normal morphology and vitality showed positive correlations (p= 0.000).
F2-IsoP levels were negatively correlated with sperm progressive motility (p= 0.000), normal morphology (p= 0.000) and vitality (p= 0.002). Omentin-1/ITLN1 levels showed negative correlations with sperm concentration (p= 0.027), progressive motility (Figure 1A; p= 0.000), normal morphology (Figure 1B; p= 0.000) and vitality (Figure 1C; p= 0.000) and positive correlation with F2-IsoPs (Figure 1D; p= 0.000).
The considered variables were then compared in the four groups in order to explore if the relationship among sperm parameters, omentin-1/ITLN1 and F2-IsoPs could be related to different pathologies, common causes of human male infertility (Table 3).
 |
Table 3 Median (IQR: 25th and 75th Centile) of the Considered Variables in 59 Subjects, Grouped According to Pathologies, and in 21 Fertile Men; Statistics are Also Reported |
Sperm concentration, progressive motility, normal morphology, and vitality were significantly increased in the group of fertile men compared to those observed in genitourinary infections, varicocele and idiopathic infertility groups (Table 3).
Sperm concentration was significantly increased in samples of men affected by varicocele than that observed in patients with idiopathic infertility (p= 0.005) and genitourinary infections (p= 0.047). Normal sperm morphology and sperm vitality were both significantly higher in varicocele group than in infection group (p= 0.001).
Fertile group showed significantly lower F2-IsoP levels than varicocele (p= 0.000) and infection (p= 0.000) groups. Idiopathic infertility group showed lower F2-IsoP levels than both varicocele and infection groups (p= 0.000).
The omentin-1/ITLN1 concentration (Table 3) was significantly increased in varicocele and infection groups versus fertile group (p= 0.000 and p= 0.001, respectively), in infection group versus varicocele group (p= 0.024) and idiopathic infertility group (p= 0.003).
Immunofluorescence enabled to localize omentin-1/ITLN1 in human spermatozoa. The omentin-1/ITLN1 labeling was present in different areas of human spermatozoa, depending on the semen quality.
Spermatozoa showing the omentin-1/ITLN1 signal as a spot in the neck region, with or without midpiece (Figure 2A and B), were present in a significantly higher percentage in samples of fertile men than in those of patients with varicocele and genitourinary infections (p= 0.000; Figure 3).
The percentages of spermatozoa with the signal in the cytoplasmic residue and along the tail (Figure 2C and D) were significantly higher in varicocele and genitourinary infection specimens than in fertile men samples (p = 0.002 and p = 0.000, respectively, Figure 3).
Finally, the evaluation of omentin-1/ITLN1 cellular localization was performed in sections of human seminiferous epithelium, epididymis, seminal vesicles and prostate gland.
In the seminiferous epithelium of the three analyzed samples, a normal spermatogenetic process was observed. In the germinal epithelium and in the prostate gland tissue the immunoreactivity for omentin-1/ITLN1 was absent (Supplementary Figure 1C and D). A focal cytoplasmic omentin-1/ITLN1 immunolabeling was evident in the basal area of the epididymal tubule (Figure 4A and B). The other cells and, in particular, the principal cells with long stereocilia were negative. A wide signal was present in the cytoplasm of the columnar cells of seminal vesicle epithelium (Figure 4C–E). The double immunostaining clearly confirms the cytoplasmic epithelial immunoexpression of omentin-1/ITLN1 and the nuclear expression (brown) of PAX8 (Figure 4F).
Discussion
The data presented in this paper showed a relationship among seminal omentin-1/ITLN1, sperm parameters and F2-IsoPs, a valuable maker of LPO due to OS. Epididymis and, in particular, seminal vesicles are conceivably the site of production/storage of omentin-1/ITLN1 that has been found in the seminal fluid and immunolocalized in spermatozoa.
The most frequently studied adipokines such as leptin, adiponectin and resistin play important roles in the regulation of different pathophysiologic processes, however their role in reproductive system is still under investigation.5,30 Among new emerging adipokines, omentin-1/ITLN1 is considered a sort of “pleiotropic adipocytokine”13,15,31–33 being effective in all major systems of the human body.
Overall, data concerning the localization and putative functions of omentin-1/ITLN1 in the reproductive system are still limited and mostly focused on female, whereas studies on male reproductive function are scarce.
First, we explored the potential relationships between omentin-1/ITLN1, LPO and semen parameters in the whole group of men included in this study, and then we grouped the cases according to the pathologies as varicocele, infection and idiopathic infertility that are the most common causes of male infertility.34
Varicocele, genitourinary infections and other pathologies are conditions associated with OS.19–22,34 In this research, we dosed seminal levels of F2-IsoP, a by-product of LPO, sensitive enough in assessing oxidative status in human semen.24,25,35,36
The obtained results indicated that an increase in both omentin-1/ITLN1 and F2-IsoP levels is concomitant with a decrease in sperm parameters. Omentin-1/ITLN1 and F2-IsoP levels were significantly higher in semen of patients with varicocele and genitourinary infections than in those of fertile men and patients affected by idiopathic infertility.
Analyzing the behavior of omentin-1/ITLN1, sperm parameters and pathologies causing infertility, two hypotheses can be supported:
- omentin-1/ITLN1 is an adipocytokine that plays a detrimental effect on sperm quality, as observed for resistin and chemerin.3,8,37
- omentin-1/ITLN1 has anti-inflammatory properties,13,38 and its levels can increase to counteract an inflammatory status, caused by varicocele and infections, detrimental for sperm parameters.
The most elevated levels of seminal omentin-1/ITLN1 were detected in patients with genitourinary infections, indicating that this adipocytokine may represent an inflammatory biomarker for this pathology. At this purpose, Canarella et al,39 revising papers on seminal proteomic biomarkers of OS, quoted a study by Wang et al38 in which the overexpression of intelectin 1 was reported in asthenozoospermic patients with OS, thus suggesting the possible presence of genital tract infections in these patients. In these researches intelectin 1 was classified as an infection-induced antimicrobial protein. This last observation supports our second hypothesis that is further validated by the extensive protective effects that omentin-1/ITLN1 exerts in multiple physiological and pathological processes by inhibiting inflammation.1,13,40,41
In patients with idiopathic infertility, despite a reduced sperm quality, both omentin-1/ITLN1 and F2-IsoP levels were similar to those of controls. In several cases, OS has been implicated in idiopathic infertility;42 the low omentin-1/ITLN1 and F2-IsoP levels can be explained considering that idiopathic infertile men show non homogeneous characteristics since other genetic, epigenetic, or environmental unknown causes could be supposed.43
A recent research reported that seminal omentin-1/ITLN1 was increased in fertile men and decreased in case of varicocele, leukocytospermia and smoking habit and it was positively correlated with semen parameters.44 These results were very discordant with our findings. To clarify this issue, we studied for the first time the localization of omentin-1/ITLN1 in human spermatozoa and in male reproductive system. Omentin-1/ITLN1 labelling was absent in the testicular and prostate gland tissues indicating that this adipokine is not involved in the regulation of either system.
On the contrary, the immunoreactivity for omentin-1/ITLN1 was evident in the basal area of epididymal tissue and, in particular, in the columnar cells of the seminal vesicles as demonstrated using the nuclear marker PAX8.29 The epididymis is a crucial region for the maturation of sperm. It is plausible that omentin-1/ITLN1 may influence several processes of post-gonadal sperm modifications, such as motility, egg binding and penetration45 with still unknown mechanisms.
It is well known that seminal vesicles produce the majority of seminal plasma with high concentration of fructose that provides energy for sperm function.46
It is then conceivable that the omentin-1/ITLN1 amount, detected in human semen samples by ELISA experiments, originates from seminal vesicles where it can be produced. Little is known about the relationship between seminal vesicles and adipocytokines and the literature reports deal only with leptin. Leptin and leptin receptor may be involved in the autocrine-paracrine functional regulation of the epithelial cells of adult rat seminal vesicles and prostate gland.47 These studies demonstrated that adipocytokines have a relevant role in the pathophysiology of the reproductive system that deserves attention and further dedicated investigations.
The increased levels of omentin-1/ITLN1 in semen samples of patients affected by varicocele and infections can be explained by the inflammatory status caused by these pathologies that can probably stimulate the omentin-1/ITLN1 production/storage by seminal vesicles.
Finally, omentin-1/ITLN1 was detected by immunofluorescence in spermatozoa and the immunolabelling was very different depending on semen quality. In particular, spermatozoa from patients with varicocele and infection/inflammation showed the signal in the cytoplasmic residue and all along the tail, and sperm from fertile men in the midpiece and in a peculiar spot in the connecting piece. The cytoplasmic residue, in which the localization of the omentin-1/ITLN1 was detected, is one of the most common characteristics of sperm immaturity and an index of alteration in spermatogenetic process.48
These novel findings suggested that human spermatozoa likely have receptors for omentin-1/ITLN1 although the specific receptor has not been identified so far.13
Human intelectin, also known as omentin, is a calcium-dependent galactose-binding lectin and its homologue was found in Xenopus laevis oocyte where participates in the formation of the fertilization envelope that prevents polyspermy.32 It is well known that the sperm glycocalyx is rich in galactose residues and is modified during epididymal transit;49 in addition, recently, Desantis et al demonstrated that the seminal plasma affects the surface glycoprofile of animal spermatozoa.50 From these observations, we can hypothesize that inflammation caused by varicocele and genitourinary infections can be a trigger for omentin-1/ITLN1 secretion and, potentially, for overexpression of galactose residues in the sperm glycocalyx that could explain the diffuse omentin-1/ITLN1 signal detectable in spermatozoa of patients with these pathologies.
Conclusion
In this study, omentin-1/ITLN1 levels are increased in inflammatory conditions as varicocele and infections and are negatively correlated with sperm parameters. For this reason, a sort of protective role of omentin-1/ITLN1 can be postulated, as this adipokine shows anti-inflammatory properties in many other biological systems. Since the studies on omentin-1/ITLN1 and male reproductive system are absent, the present research represents a starting point for other investigations to define the exact role of omentin-1/ITLN1 in the physiology of male reproductive system.
Data Sharing Statement
The data generated and analysed during this study are included in this published article and are available from the corresponding author.
Ethics Approval and Informed Consent
Not applicable. At Siena University this type of study, for no treatment of analyzed patient does not need ethics approval, however, it does require signed informed consents by the patients, by which they accept the possibility to use their semen samples for scientific purposes.
Consent to Participate
All authors read and agreed with the content of the manuscript and all authors gave consent to participate in the study.
Consent for Publication
All authors gave consent for publication of this research.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Dupont J, Pollet-Villard X, Reverchon M, et al. Adipokines in human reproduction. Horm Mol Biol Clin Investig. 2015;24(1):11–24. doi:10.1515/hmbci-2015-0034
2. Comninos AN, Jayasena CN, Dhillo WS. The relationship between gut and adipose hormones, and reproduction. Hum Reprod Update. 2014;20(2):153–174. doi:10.1093/humupd/dmt033
3. Elfassy Y, Bastard JP, McAvoy C, et al. Adipokines in semen: physiopathology and effects on spermatozoas. Int J Endocrinol. 2018;2018:3906490. doi:10.1155/2018/3906490
4. Estienne A, Bongrani A, Reverchon M, et al. Involvement of novel Adipokines, Chemerin, Visfatin, Resistin And Apelin in reproductive functions in normal and pathological conditions in humans and animal models. Int J Mol Sci. 2019;20(18):4431. doi:10.3390/ijms20184431
5. Barbe A, Bongrani A, Mellouk N, et al. Mechanisms of adiponectin action in fertility: an overview from gametogenesis to gestation in humans and animal models in normal and pathological conditions. Int J Mol Sci. 2019;20(7):1526. doi:10.3390/ijms20071526
6. Kawwass JF, Summer R, Kallen CB. Direct effects of leptin and adiponectin on peripheral reproductive tissues: a critical review. Mol Hum Reprod. 2015;21(8):617–632. doi:10.1093/molehr/gav025
7. Fang H, Judd RL. Adiponectin regulation and function. Compr Physiol. 2018;8:1031–1063.
8. Moretti E, Micheli L, Noto D, et al. Resistin in human seminal plasma: relationship with lipid peroxidation, CAT activity, GSH/GSSG ratio, and semen parameters. Oxid Med Cell Longev. 2019;2019:2192093. doi:10.1155/2019/2192093
9. Thomas S, Kratzsch D, Schaab M, et al. Seminal plasma adipokine levels are correlated with functional characteristics of spermatozoa. Fertil Steril. 2013;99(5):1256–1263. doi:10.1016/j.fertnstert.2012.12.022
10. Recinella L, Orlando G, Ferrante C, et al. Adipokines: new potential therapeutic target for obesity and metabolic, rheumatic, and cardiovascular diseases. Front Physiol. 2020;11:578966. doi:10.3389/fphys.2020.578966
11. Gutaj P, Sibiak R, Jankowski M, et al. The role of the adipokines in the most common gestational complications. Int J Mol Sci. 2020;21(24):9408. doi:10.3390/ijms21249408
12. Yang RZ, Lee MJ, Hu H, et al. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metab. 2006;290(6):E1253–1261. doi:10.1152/ajpendo.00572.2004
13. Watanabe T, Watanabe-Kominato K, Takahashi Y, et al. Adipose tissue-derived Omentin-1 function and regulation. Compr Physiol. 2017;7:765–781.
14. Maruyama S, Shibata R, Kikuchi R, et al. Fat-derived factor omentin stimulates endothelial cell function and ischemia-induced revascularization via endothelial nitric oxide synthase-dependent mechanism. J Biol Chem. 2012;287(1):408–417. doi:10.1074/jbc.M111.261818
15. Schaffler A, Neumeier M, Herfarth H, et al. Genomic structure of human omentin, a new adipocytokine expressed in omental adipose tissue. Biochim Biophys Acta. 2005;1732(1–3):96–102. doi:10.1016/j.bbaexp.2005.11.005
16. Bongrani A, Mellouk N, Rame C, et al. Ovarian expression of adipokines in polycystic ovary syndrome: a role for Chemerin, Omentin, and Apelin in follicular growth arrest and ovulatory dysfunction? Int J Mol Sci. 2019;20(15):3778. doi:10.3390/ijms20153778
17. Sun J, Ren J, Zuo C, et al. Circulating apelin, chemerin and omentin levels in patients with gestational diabetes mellitus: a systematic review and meta-analysis. Lipids Health Dis. 2020;19(1):26. doi:10.1186/s12944-020-01209-7
18. Tok A, Ozer A, Kanat-Pektas M, et al. The role of omentin in early pregnancy losses. J Obstet Gynaecol. 2020;40(1):107–110. doi:10.1080/01443615.2019.1606179
19. Collodel G, Moretti E, Micheli L, et al. Semen characteristics and malondialdehyde levels in men with different reproductive problems. Andrology. 2015;3(2):280–286. doi:10.1111/andr.297
20. Fraczek M, Hryhorowicz M, Gill K, et al. The effect of bacteriospermia and leukocytospermia on conventional and nonconventional semen parameters in healthy young normozoospermic males. J Reprod Immunol. 2016;118:18–27. doi:10.1016/j.jri.2016.08.006
21. Agarwal A, Rana M, Qiu E, et al. Role of oxidative stress, infection and inflammation in male infertility. Andrologia. 2018;50(11):e13126. doi:10.1111/and.13126
22. Hassanin AM, Ahmed HH, Kaddah AN. A global view of the pathophysiology of varicocele. Andrology. 2018;6(5):654–661. doi:10.1111/andr.12511
23. Dutta S, Majzoub A, Agarwal A. Oxidative stress and sperm function: a systematic review on evaluation and management. Arab J Urol. 2019;17(2):87–97. doi:10.1080/2090598X.2019.1599624
24. Signorini C, Moretti E, Collodel G. Role of isoprostanes in human male infertility. Syst Biol Reprod Med. 2020;66(5):291–299. doi:10.1080/19396368.2020.1793032
25. Collodel G, Moretti E, Noto D, et al. Fatty acid profile and metabolism are related to human sperm parameters and are relevant in idiopathic infertility and varicocele. Mediators Inflamm. 2020;2020:3640450. doi:10.1155/2020/3640450
26. World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen.
27. Signorini C, Comporti M, Giorgi G. Ion trap tandem mass spectrometric determination of F2-isoprostanes. J Mass Spectrom. 2003;38(10):1067–1074. doi:10.1002/jms.520
28. Micheli L, Collodel G, Moretti E, et al. Redox imbalance induced by docetaxel in the neuroblastoma SH-SY5Y cells: a study of docetaxel-induced neuronal damage. Redox Rep. 2021;26(1):18–28. doi:10.1080/13510002.2021.1884802
29. Ozcan A, Shen SS, Hamilton C, et al. PAX 8 expression in non-neoplastic tissues, primary tumors, and metastatic tumors: a comprehensive immunohistochemical study. Mod Pathol. 2011;24(6):751–764. doi:10.1038/modpathol.2011.3
30. Sarmento-Cabral A, L-López F, Luque RM. Adipokines and their receptors are widely expressed and distinctly regulated by the metabolic environment in the prostate of male mice: direct role under normal and tumoral conditions. Endocrinology. 2017;158(10):3540–3552. doi:10.1210/en.2017-00370
31. Jaikanth C, Gurumurthy P, Cherian KM, et al. Emergence of omentin as a pleiotropic adipocytokine. Exp Clin Endocrinol Diabetes. 2013;121:377–383. doi:10.1055/s-0033-1345123
32. Tsuji S, Uehori J, Matsumoto M, et al. Human intelectin is a novel soluble lectin that recognizes galactofuranose in carbohydrate chains of bacterial cell wall. J Biol Chem. 2001;276(26):23456–23463. doi:10.1074/jbc.M103162200
33. Fain JN, Sacks HS, Buehrer B, et al. Identification of omentin mRNA in human epicardial adipose tissue: comparison to omentin in subcutaneous, internal mammary artery periadventitial and visceral abdominal depots. Int J Obes. 2008;32(5):810–815. doi:10.1038/sj.ijo.0803790
34. Henkel R, Offor U, Fisher D. The role of infections and leukocytes in male infertility. Andrologia. 2021;53(1):e13743. doi:10.1111/and.13743
35. Longini M, Moretti E, Signorini C, et al. Relevance of seminal F2-dihomo-IsoPs, F2-IsoPs and F4-NeuroPs in idiopathic infertility and varicocele. Prostaglandins Other Lipid Mediat. 2020;149:106448. doi:10.1016/j.prostaglandins.2020.106448
36. Collodel G, Signorini C, Nerucci F, et al. Semen biochemical components in varicocele, leukocytospermia, and idiopathic infertility. Reprod Sci. 2021;28(1):91–101. doi:10.1007/s43032-020-00260-0
37. Moretti E, Collodel G, Mazzi L, et al. Resistin, interleukin-6, tumor necrosis factor-alpha, and human semen parameters in the presence of leukocytospermia, smoking habit, and varicocele. Fertil Steril. 2014;102(2):354–360. doi:10.1016/j.fertnstert.2014.04.017
38. Wang J, Wang J, Zhang HR, et al. Proteomic analysis of seminal plasma from asthenozoospermia patients reveals proteins that affect oxidative stress responses and semen quality. Asian J Androl. 2009;11(4):484–491. doi:10.1038/aja.2009.26
39. Cannarella R, Crafa A, Barbagallo F, et al. Seminal plasma proteomic biomarkers of oxidative stress. Int J Mol Sci. 2020;21(23):9113. doi:10.3390/ijms21239113
40. Yamawaki H, Kuramoto J, Kameshima S, et al. Omentin, a novel adipocytokine inhibits TNF-induced vascular inflammation in human endothelial cells. Biochem Biophys Res Commun. 2011;408(2):339–343. doi:10.1016/j.bbrc.2011.04.039
41. Zhong X, Li X, Liu F, et al. Omentin inhibits TNF-α-induced expression of adhesion molecules in endothelial cells via ERK/NF-κB pathway. Biochem Biophys Res Commun. 2012;425(2):401–406. doi:10.1016/j.bbrc.2012.07.110
42. Ritchie C, Ko EY. Oxidative stress in the pathophysiology of male infertility. Andrologia. 2021;53(1):e13581. doi:10.1111/and.13581
43. Bracke A, Peeters K, Punjabi U, et al. A search for molecular mechanisms underlying male idiopathic infertility. Reprod Biomed Online. 2018;36(3):327–339. doi:10.1016/j.rbmo.2017.12.005
44. Ismail SA, Mahran AM, Mosaad E, et al. Omentin-1 in serum and seminal plasma correlate with semen quality. Hum Androl. 2017;7(4):120–126. doi:10.21608/ha.2017.1554.1012
45. Dacheux JL, Belleannee C, Guyonnet B, et al. The contribution of proteomics to understanding epididymal maturation of mammalian spermatozoa. Syst Biol Reprod Med. 2012;58(4):197–210. doi:10.3109/19396368.2012.663233
46. Du Plessis SS, Gokul S, Agarwal A. Semen hyperviscosity: causes, consequences, and cures. Front Biosci. 2013;5(1):224–231. doi:10.2741/e610
47. Malendowicz W, Rucinski M, Macchi C, et al. Leptin and leptin receptors in the prostate and seminal vesicles of the adult rat. Int J Mol Med. 2006;18(4):615–618.
48. Said TM, Agarwal A, Sharma RK, Thomas AJ
49. Tecle E, Gagneux P. Sugar-coated sperm: unraveling the functions of the mammalian sperm glycocalyx. Mol Reprod Dev. 2015;82(9):635–650. doi:10.1002/mrd.22500
50. Desantis S, Lacalandra GM, Batista M, Amann O, Antonelli D, Monaco D. Seminal plasma alters surface glycoprofile of dromedary camel cryopreserved epididymal spermatozoa. Theriogenology. 2021;167:77–84. doi:10.1016/j.theriogenology.2021.03.008
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




