Back to Journals » Infection and Drug Resistance » Volume 17
Reduced Viral Shedding Time in High-Risk COVID-19 Patients Infected by Omicron and Treated with Paxlovid: A Real-World Study from China
Authors Yang W, Peng Y, Wang C , Cai H, Zhang L, Xu J, Wang Y, Wang M, Zhao M, Yu K
Received 24 October 2023
Accepted for publication 23 March 2024
Published 29 March 2024 Volume 2024:17 Pages 1267—1279
DOI https://doi.org/10.2147/IDR.S443574
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Sandip Patil
Wei Yang,1,* Yahui Peng,1,* Changsong Wang,1,* Hongliu Cai,2 Lina Zhang,3 Jun Xu,2 Yongjie Wang,4 Maonan Wang,4 Mingyan Zhao,1 Kaijiang Yu1
1Department of Critical Care Medicine, the First Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang Province, People’s Republic of China; 2Department of Critical Care Medicine, the First Affiliated Hospital, Zhejiang University, Hangzhou, People’s Republic of China; 3Department of Critical Care Medicine, Xiangya Hospital Central South University, Changsha, Hunan Province, People’s Republic of China; 4Department of Critical Care Medicine, Jilin Province People’s Hospital, Changchun, Jilin Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Kaijiang Yu; Mingyan Zhao, Departments of Critical Care Medicine, the First Affiliated Hospital of Harbin Medical University, Harbin Medical University, Harbin, Heilongjiang, 150001, People’s Republic of China, Email [email protected]; [email protected]
Introduction: The purpose of this study was to compare the viral shedding time in patients infected with the Omicron variant during Paxlovid therapy and conventional therapy and to analyze the effects of Paxlovid on patients infected with COVID-19.
Methods: In this study, the demographic and clinical characteristics and laboratory data of 3159 patients infected with the SARS-CoV-2 Omicron variant treated at Jilin Province People’s Hospital were collected and analyzed. A total of 362 patients received Paxlovid therapy, and 2797 patients received conventional therapy. After propensity score matching (PSM), 1086 patients were obtained.
Results: The difference in platelet (PLT) count between the two groups was statistically significant but within the normal range (P < 0.05). CT value revealed that the nucleic acid test results became negative more quickly in the Paxlovid therapy group. Analysis of the Paxlovid therapy group showed that IgG and IgM levels were increased after Paxlovid therapy administration.
Conclusion: The CT value of the Paxlovid therapy group became negative more quickly. This finding suggests that Paxlovid treatment after early diagnosis of the Omicron variant may achieve good therapeutic efficacy.
Keywords: Paxlovid, Omicron variant, a retrospective study, N-CT
Introduction
At the end of 2019, an outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) occurred in China and spread rapidly worldwide.1–3 On March 11, 2020, the World Health Organization (WHO) designated coronavirus disease 2019 (COVID-19) a global pandemic.4 As of May 5, 2022, approximately 515 million cases of COVID-19 have been reported, resulting in 6.26 million deaths (https://www.worldometers.info/coronavirus/). The COVID-19 pandemic has caused enormous mortality and severe morbidity in developed and developing countries. Currently, 5 variants, Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2) and Omicron (B.1.1.529), have been reported.5 After replacing Delta, Omicron variants became the main variant worldwide, with a significantly greater spread than other variants.6,7
The prevalence of Omicron variants poses more significant challenges for pandemic prevention and control. Omicron has three lineages, BA.1 (B.1.1.529.1), BA.2 (B.1.1.529.2), and BA.3 (B.1.1.529.3), first discovered in South Africa in November 2021.8 The high transmissibility of Omicron variants is a major cause of global concern. Since the advent of Omicron, it has rapidly replaced Delta as the dominant strain worldwide. In the US, Delta accounted for 99% of the new cases on December 4, 2021. However, Omicron accounted for more than 95% of cases by January 8, 2022.9 The basic reproductive number (R0) of the Delta variant was between 6 and 7.10 Omicron is 3.2 times more transmissible than delta and has a 3-day doubling time.11 In contrast, although Omicron is remarkably more frequent than Delta and previous Omicron variants, the virulence of Omicron was markedly lower than that of Delta and reinfections during the Omicron transmission period were clinically milder.12,13
The oral antiviral drug candidate Paxlovid (PF-07321332+ritonavir), has only recently been developed by Pfizer, Inc. Paxlovid provides new hope for treating patients at risk of progressing to severe COVID-19 in the era of the Omicron variant.14 Paxlovid is a therapeutic combination of two compounds: PF-07321332, an oral covalent 3CL protease inhibitor of SARS-CoV-2, and ritonavir, an inhibitor of HIV-1 and HIV-2. Ritonavir is also an inhibitor of cytochrome P450 3A and CYP2D6, thus inhibiting the metabolism of PF-07321332 and allowing the use of lower doses of this substance.15 In one clinical trial, compared with a placebo, a 5-day course of Paxlovid administered within three days of symptom onset reduced the risk of hospitalization and death by 89% over 28 days. Hospitalizations and deaths were 0.8% in the Paxlovid group and 7% in the placebo group. Similar favorable results were observed in patients who started treatment within five days of symptom onset.16 The results of an observational study showed that, compared to controls, patients treated with Paxlovid were more effective at preventing hospital admission and mortality attributable to COVID-19.17
In China’s Jilin Province, the pandemic situation has been well controlled since the outbreak began in early March 2022. Since then, BA.2 has been the predominant Omicron sublineage in Jilin Province, and some of the patients were treated with the Pfizer drug Paxlovid. Therefore, the aim of this study was to compare the viral shedding time of Omicron patients treated with Paxlovid therapy and that of patients treated with conventional therapy and to analyze the side effects of this therapy.
Materials and Methods
Study Subjects
We performed a retrospective study to analyze the effects of Paxlovid on patients infected with the Omicron variant. The study subjects were patients treated at Jilin Province People’s Hospital from March 2022 to April 2022. All procedures in the studies involving human participants were performed under the ethical standards of the institutional research committee and complied with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the Ethics Committee of Jilin Province People’s Hospital (2022059). Informed consent was obtained from all patients before the start of the study.
The Inclusion Criteria
Mild and moderate type: (1) ≥12 years old; (2) confirmed COVID-19 patient; (3) at least one COVID-19 symptom or sign on the day of medication; (4) at least one high-risk factor for developing a severe illness: ≥60 years old; body mass index > 25 kg/m2; heavy smoking (over 400 cigarettes/year); immune-suppressive disease or long-term iatrogenic immunosuppression; chronic pulmonary, cardiovascular, renal disease, or sickle cell disease; hypertension; glycuresis; cancer; neurodevelopmental disorders or other complex medical conditions; and long-term reliance on organ support (such as long-term dialysis patients) or long-term hospitalization.
Severe type: (1) ≥12 years old, (2) confirmed COVID-19 patient, and (3) conformed to the ninth edition of the severe type diagnostic criteria on the day of medication
The Exclusion Criteria
- previously confirmed SARS-CoV-2 infection;
- prior receipt of convalescent COVID-19 plasma, neutralizing antibodies, or specific immunoglobulin therapy;
- received any of the following antiviral drugs: interferon, albidol, ribavirin, hydroxychloroquine, fraveravir, remdesivir, etc.;
- Pregnancy or lactation; history of active liver disease; moderate to severe renal damage; known HIV virus (viral load> 400 copies/mL); or suspected/confirmed active systemic infection.
Clinical Data Collection
Demographic and clinical characteristics and laboratory findings were collected and recorded on the first day of admission. The data, which included hematologic, liver, and renal function data; IgG and IgM levels; and viral load, were collected on Day 1 (baseline) and at 3, 5, 10, and 14 days. Nasopharyngeal or nasal swabs were collected each day from each patient until the first negative test result was obtained.
Viral RNA Detection via RT‒PCR
Nasopharyngeal swabs were collected by well-trained medical staff at the same hospital, who strictly followed standardized procedures. The specimens were kept in virus-containing media. Viral RNA was extracted within 2 h with a Nucleic Acid Isolation Kit according to the manufacturer’s instructions. RT‒PCR was performed by using an RNA Detection Kit for SARS‒CoV-2. RT‒PCR was conducted with primers and probes targeting the N genes and a positive reference gene. The detection limit of the cycle threshold (Ct) was set to 40 (500 copies/mL). Samples with a Ct value less than 40 were considered positive. All tests were performed under strict biosafety conditions and standard operating procedures.
Treatment Method
One group of patients received Paxlovid therapy, and the other group received conventional therapy.
Paxlovid therapy involved administering Paxlovid orally twice a day for 5 days. The recommended dose is 300 mg of nirmatrelvir Tablets (150 mg, 2 tablets) combined with 100 mg of Ritonavir Tablets (100 mg, 1 tablet), which are administered orally every 12 hours for 5 consecutive days. Treatment began as soon as possible within the diagnosis of COVID-19 and within 5 days after the patient’s onset of symptoms. If the patient missed one dose of treatment but did not exceed 8 hours beyond the correct time, the dose was taken as soon as possible to continue following the normal dosing regimen. If the patient failed to adhere to the treatment schedule and exceeded 8 hours, the patient did not take the missed dose but took the next dose at the prescribed time. Double doses were not administered.
Conventional therapy is a general treatment. 1) Patients were required to rest in bed, strengthen supportive treatment, ensure adequate energy and nutritional intake, pay attention to the balance of water and electrolytes, and maintain internal environmental stability. 2) Patients were closely monitored for vital signs, especially resting and finger oxygen saturation, after activity. 3) Blood routine, urine routine, CRP, biochemical indicators (liver, enzymes, myocardial enzymes, renal function, etc.), blood coagulation function, arterial blood gas analysis, chest imaging, etc., were monitored. 4) Standardized and effective oxygen therapy measures were provided according to the patient’s condition, including nasal catheterization, mask oxygen administration and transnasal high-flow oxygen therapy. 5) Antimicrobial therapy: Patients avoided blind or inappropriate use of antibiotics, especially in combination with broad-spectrum antibacterial drugs. Drugs were given according to the actual condition of each patient, and the detailed treatment plan followed the COVID-19 diagnosis and treatment protocol (Trial edition 9) (http://www.nhc.gov.cn/cms-search/downFiles/ef09aa40704620b010951b088b8a27.pdf).
Statistical Analysis
All the statistical analyses were performed using SAS 9.4 software, and the quantitative normally distributed data are presented as the means ± standard deviations (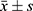 ). Two independent sample t-tests (t values) were used for comparisons between two groups, and paired t-tests were used for comparisons of preoperative and postoperative data (t values were used for statistical analysis). Quantitative data with a skewed distribution are presented as the median and interquartile range (M(P25, P75)), the Wilcoxon rank-sum test was used for comparisons between two groups (the Z value was used for statistical analysis), and the paired rank-sum test was used for comparisons between groups (the S value was used for statistical analysis). Qualitative data are presented as the frequency (percentage), and the χ2 test was used to compare the compositions of two groups (for statistical analysis, the χ2 value was used). Repeated-measures ANOVA was used to compare the differences between repeated-measures quantitative index groups and time points (because of missing values, the SAS program uses proc mixed for analysis, and lsmeans contains both factors and their interaction; therefore, there was no need to adjust the P value of the analysis results). Differences were considered significant if p < 0.05.
). Two independent sample t-tests (t values) were used for comparisons between two groups, and paired t-tests were used for comparisons of preoperative and postoperative data (t values were used for statistical analysis). Quantitative data with a skewed distribution are presented as the median and interquartile range (M(P25, P75)), the Wilcoxon rank-sum test was used for comparisons between two groups (the Z value was used for statistical analysis), and the paired rank-sum test was used for comparisons between groups (the S value was used for statistical analysis). Qualitative data are presented as the frequency (percentage), and the χ2 test was used to compare the compositions of two groups (for statistical analysis, the χ2 value was used). Repeated-measures ANOVA was used to compare the differences between repeated-measures quantitative index groups and time points (because of missing values, the SAS program uses proc mixed for analysis, and lsmeans contains both factors and their interaction; therefore, there was no need to adjust the P value of the analysis results). Differences were considered significant if p < 0.05.
The effectiveness of the Paxlovid therapy was determined by the following two proportions: rate of events in the experimental arm (EER) = number of events/number of patients in the experimental arm; rate of events in the control arm (CER) = number of events/number of patients in the control arm.
The absolute risk increase [(ARI) = (EER − CER)] was accompanied by a 95% CI (95% CI). The risk of death events was greater in the Paxlovid therapy-treated group than in the control group. The sign of ARI is positive when EER > CER and negative otherwise. The number needed to treat [(NNT) = (1/ARI)] expresses the expected number of patients required to obtain one beneficial outcome event, accompanied by the 95% CI.
Logistic regression was used to determine the odds ratio (OR) with a 95% confidence interval (95% CI). OR is the ratio of the probability of the event in the treatment arm against the probability of the event in the control group; it is expressed in decimal values. OR >1.00 or <1.00 indicate a beneficial or a detrimental effect, respectively, of the treatment.
Cox regression (or proportional hazards regression) was used to determine the hazard ratio (HR) with a 95% confidence interval (95% CI). The hazard ratio (HR) was used to determine the effect of the treatment on the time until the first negative antigenic swab test was obtained. Since this was a beneficial intervention (because treatment stopped viral shedding), a positive HR indicates a protective effect of the associated variable.
Result
Clinical Characteristics of Patients in Paxlovid Therapy Group and Conventional Therapy Group
Between March 2022 and April 2022, 3159 patients were enrolled in this study. A total of 362 patients received Paxlovid therapy, and 2797 patients received conventional therapy (Figure 1). The baseline demographic and clinical data of the patients with the Omicron cohort are summarized in Table 1. The results showed that the age, white blood cell count (WBC), hemoglobin (HGB), platelet (PLT), creatinine (Cr), blood urea nitrogen (BUN) and pH were significantly different between the two groups of patients (P <0.05). Patients in the conventional therapy group were younger than those in the Paxlovid therapy group. The changes in WBC, HGB, PLT, Cr, BUN and PH between admission and discharge were smaller in the conventional therapy group. The results of repeated-measures ANOVA of the patients in the two groups showed that there were statistically significant differences of N-CT in viral shedding time of therapy and the interaction between group and viral shedding time of therapy (P<0.05) (Table 2).
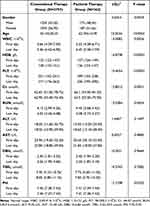 |
Table 1 Comparison of Demographic Data and Clinical Trial Indicators |
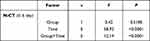 |
Table 2 Results of ANOVA for Repeated Measures CT Values in Two Groups |
 |
Figure 1 Flow chart. |
Table 3 shows the distribution of negative swab tests by number of days after COVID-19 diagnosis among patients treated with Paxlovid therapy and controls. ARI had a positive sign and OR was significantly (OR=1.672; 95% CI: 1.175–2.381), indicating that the treatment was beneficial. While the respective crude odds ratio (OR) was 1672 (95% CI: 1.175–2.381), the corresponding crude hazard ratio (HR) was nonsignificant (HR =0.958; 95% CI: 0.83; 1–106).
 |
Table 3 The Distribution of Negative Swab Tests by Number of Days After COVID-19 Diagnosis Among Patients Treated with Paxlovid Therapy versus Controls |
Table 4 shows the results of regression analysis for the days since COVID-19 diagnosis until first negative swab test. According to the multinomial logistic regression model, patients treated with Paxlovid therapy were more likely to turn negative (OR=2.255; 95% CI=1.566–3.248) than controls were. Age was a risk factor (OR=0.978; 95% CI=0.973–0.984). According to the multivariate Cox regression analysis, the negativization rate was not significantly greater (HR=1.036; 95% CI=0.891–1.203) in patients treated with Paxlovid therapy. Age was also a risk factor (OR=0.995; 95% CI=0.992–0.998).
 |
Table 4 Multinomial Logistic Regression Analysis for Negativization Rates |
Comparison Between the Paxlovid Therapy Group and Conventional Therapy Group After Propensity Score Matching
Due to the significant difference in baseline age, the two groups of patients were matched 1:2 by age via propensity score matching (PSM). After matching, 1086 patients (including 362 in the Paxlovid therapy group and 724 in the conventional therapy group) were obtained. A comparison of demographic data and clinical trial data revealed that the difference in the PLT between the two groups was statistically significant (P<0.05) (Table 5). The change in the PLT between admission and discharge was smaller in the conventional therapy group than in the control group but within the normal range.
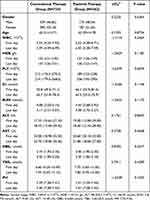 |
Table 5 Comparison of Demographic Data and Clinical Trial Indicators (After PSM) |
The results of repeated-measures variance analysis of CT values of the two groups of patients showed that N-CT had substantial differences in group factor, time factor and interaction between groups and time (P<0.05) (Tables 6 and 7) (Figure 2). Figure 2 shows that the nucleic acid test results becomes negative more quickly in the Paxlovid therapy group than in the control group.
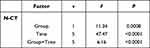 |
Table 6 Results of ANOVA for Repeated Measures CT Values in Two Groups (Proc Mixed) |
 |
Table 7 Repeated Measures Results at Different Time Points in Paxlovid Therapy Group and Conventional Therapy Group |
 |
Figure 2 The trend of N-CT between the two groups. |
Table 8 shows the distribution of negative swab tests by number of days after COVID-19 diagnosis among patients treated with Paxlovid therapy and controls according to propensity score matching. The ARI had a positive sign and OR was significant (OR=2.43; 95% CI=1.648–3.582), indicating that the treatment was beneficial. While the respective crude odds ratio (OR) was 2.43 (95% CI: 1.648–3.582), the corresponding crude hazard ratio (HR) was nonsignificant (HR =1.017; 95% CI: 0.855–1.21).
 |
Table 8 The Distribution of Negative Swab Tests by Number of Days After COVID-19 Diagnosis Among Patients Treated with Paxlovid Therapy versus Controls (After PSM) |
According to the multinomial logistic regression model, patients treated with Paxlovid therapy were more likely to turn negative after propensity score matching (OR=2.437; 95% CI=1.651–3.597) than the controls were. According to the multivariate Cox regression analysis, the normalization rate was not significantly greater (hazard ratio (HR)=1.005; 95% CI=0.845–1.196) (Table 4).
Repeated Measures ANOVA Comparing Differences at Different Time Points in the Same Group
In the Paxlovid therapy group, N-CT significantly differed between Day 1 and Day 2, Day 3, Day 4, Day 5 and Day 6, respectively (P<0.05). There were statistically significant differences between Day 2 and Day 3, Day 4, Day 5 and Day 6 (P<0.05) (Table 9).
 |
Table 9 The Differences of Pairwise Comparison of Different Time in the Same Group |
In the conventional therapy group, the performance of N-CT on Day 1 was significantly different from that on Day 2, Day 3, Day 4, Day 5 and Day 6; Day 2 was quite different from Day 3 (P<0.05) (Table 9).
Repeated Measures ANOVA Results of Pairwise Comparisons in Different Groups at the Same Time Points
N-CT revealed that the difference between the two groups was statistically significant on Days 1, 4 and 5. On Day 1, the N-CT was lower in the Paxlovid therapy group than in the control group, but on Days 4 and 5, the N-CT was greater in the Paxlovid therapy group (Table 10) (Figure 3).
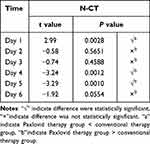 |
Table 10 Results of Pairwise Comparisons of Two Groups at the Same Time Point |
 |
Figure 3 N-CT of pairwise comparisons in two groups at the same time points. *Stands for P < 0.05. |
Changes in Clinical Indicators Before and After Drug Administration in the Paxlovid Therapy Group
We observed the effect of Paxlovid therapy on clinical indicators in the same group of patients. Analysis of clinical indicators before and after treatment in the Paxlovid therapy group revealed significant differences in IgG, IgM, WBC, HGB, PLT, Cr, BUN, ALT, AST, DBIL, and PH before and after treatment (P < 0.05) (Table 11). IgG and IgM levels increased after Paxlovid therapy administration. Before treatment group was clinical indicators without treatment in the first day of Paxlovid therapy. After treatment group was clinical indicators of Paxlovid therapy in fifth day.
 |
Table 11 Comparison of Clinical Indicators Before and After Treatment in Paxlovid Therapy Group |
Discussion
Main Findings
In this retrospective study, we compared the viral shedding time in patients infected with the Omicron variant and treated with Paxlovid therapy and conventional therapy and explored the druggable effects of the Paxlovid on patients infected with the COVID-19 Omicron variant BA.2. We found that, compared with those in the conventional therapy group, the patients in the Paxlovid therapy group exhibited a faster negative CT and greater increase in IgG and IgM.
Interpretations of Findings
In the original two groups, the mean age varied widely. Therefore, we matched the age to the same baseline by using the PSM method. Before matching, the two groups had significant differences in WBC, HGB, PLT, Cr, BUN, and PH. After matching, only the PLT differed. However, these findings were within the normal range and not clinically significant, which may be due to the influence of age. The differences in clinical indices between the Paxlovid treatment group and the conventional therapy group were not obvious. These findings showed that Paxlovid therapy and conventional therapy had little impact on the clinical indicators of patients.
The results showed that the logistic regression of the OR was significant (OR=2.43; 95% CI: 1.648–3.582), but the Cox regression (or proportional hazards regression) of the HR was nonsignificant (HR =1.017; 95% CI: 0.855–1.21) both before and after propensity score matching. The viral loads at baseline differed on the first day. Patients in the two groups had different disease severities, and patients in the Paxlovid therapy group had a greater risk. However, even when the disease was more severe on the first day in the Paxlovid therapy group, the viral load decreased on Day 4. Therefore, we believe that Paxlovid therapy may achieve excellent therapeutic efficacy.
Although significantly different before and after treatment in the Paxlovid group, the WBC, HGB, PLT, Cr, BUN, ALT, AST, DBIL, and pH were within the normal range. Therefore, Paxlovid therapy had no side effects on these patients. In the Paxlovid therapy group, IgG and IgM levels were significantly increased after Paxlovid therapy, which may indicate that Paxlovid therapy has curative effects on Omicron.
Hammond et al18 showed that using nimarrevir and ritonavir in the early stages of COVID-19 can slow disease progression and rapidly reduce the SARS-CoV-2 viral load. Patients who started treatment within 3 and 5 days of symptom onset had 88.9% and 87.8% lower relative risk of hospitalization and death from any cause, respectively. Other observational studies have shown that Paxlovid reduces viral shedding in patients treated with and accelerates the clearance of SARS-CoV-2. Furthermore, previous vaccination and antiviral treatment synergize to reduce the time to negativity even when the effect of COVID-19 vaccination is stronger.17,19 In our results, the CT value of the Paxlovid therapy group compared to the conventional therapy group turned negative faster, suggesting that we may obtain a good therapeutic effect with Paxlovid therapy after the early diagnosis of the Omicron variant.
Limitations
There are several limitations of our study. First, because of the retrospective nature of the study, clinical data were missing or incomplete in some patients, which limited our analysis. For example, we have no information regarding the vaccination status of patients, so we could not further analyze the relationship between viral shedding time and vaccination status. Second, there was a large difference in the initial age of the patients between the two groups, and age may have many complex effects. Therefore, we can minimize the effect of only age. Third, hospitalization and mortality could not be assessed because all patients were hospitalized and did not die. Fourth, because of the difference in initial disease severity between the two groups, the viral load was not at the same baseline level, and we cannot exclude this influencing factor. Finally, because of the lack of raw data for IgG and IgM in the conventional therapy group, we did not compare IgG and IgM between the two groups. We compared IgG and IgM levels in the Paxlovid therapy group between the first day and the last day.
Conclusions
Our data show that the CT value of the Paxlovid therapy group became negative faster than that of the control group, suggesting that the use of the Paxlovid in Omicron variant may achieve excellent therapeutic efficacy after early diagnosis. Moreover, Paxlovid should be used with caution and only in high-risk patients.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Novel Coronavirus Pneumonia Emergency Treatment and Diagnosis Technology Research Project of the Heilongjiang Provincial Science and Technology Department (GA20C001), Natural Science Foundation for Distinguished Young Scholars of Heilongjiang Province (JQ2021H003), the Key R&D project in Heilongjiang Province of Heilongjiang Province (2022ZX01A30), Basic research business fees for provincial higher education institutions in Heilongjiang Province (2018-KYYWF-0491), Research Project of Health and Health Commission of Heilongjiang Province (2018088), Research Innovation Fund of the First Affiliated Hospital of Harbin Medical University (2021M26) and National Multidisciplinary Innovation Team Project of Traditional Chinese Medicine (ZYYCXTD-D-202201).
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. New Engl J Med. 2020;382(8):727–733. doi:10.1056/NEJMoa2001017
2. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. New Engl J Med. 2020;382(13):1199–1207. doi:10.1056/NEJMoa2001316
3. Li X, Li L, Li X, Zhang Z, Ma X. Clinical characteristics and analysis of factors associated with severe COVID-19 patients in Liaoning, China: a multicenter retrospective study. J Transl Crit Care Med. 2020;2(4):90–95. doi:10.4103/jtccm.jtccm_7_21
4. World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19; 2020. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-The-media-briefing-on-covid-19---11-march-2020.
5. World Health Organization. Tracking SARS-CoV-2 variants. Available from: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants.
6. Viana R, Moyo S, Amoako DG, et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in Southern Africa. Nature. 2022;603(7902):679–686. doi:10.1038/s41586-022-04411-y
7. Adam D. What scientists know about new, fast-spreading coronavirus variants. Nature. 2021;594(7861):19–20. doi:10.1038/d41586-021-01390-4
8. Centers for Disease Control and Prevention. COVID data tracker: variant pro-portions; 2022. Available from: https://covid.cdc.gov/covid-data-tracker/#variant-proportions.
9. Chen J, Gao K, Wang R, Wei GW. Prediction and mitigation of mutation threats to COVID-19 vaccines and antibody therapies. Chem. Sci. 2021;12(20):6929–6948. doi:10.1039/D1SC01203G
10. Liu Y, Rocklöv J. The reproductive number of the Delta variant of SARS-CoV-2 is far higher compared to the ancestral SARS-CoV-2 virus. J Travel Med. 2021;28(7). doi:10.1093/jtm/taab124
11. Long B, Carius BM, Chavez S, et al. Clinical update on COVID-19 for the emergency clinician: presentation and evaluation. Am J Emergency Med. 2022;54:46–57. doi:10.1016/j.ajem.2022.01.028
12. Cegolon L, Magnano G, Negro C, Larese Filon F. SARS-CoV-2 reinfections in health-care workers, 1 March 2020–31 January 2023. Viruses. 2023;15(7):1551. doi:10.3390/v15071551
13. Cegolon L, Negro C, Mastrangelo G, Filon FL. Primary SARS-CoV-2 infections, re-infections and vaccine effectiveness during the Omicron transmission period in healthcare workers of Trieste and Gorizia (Northeast Italy), 1 December 2021–31 May 2022. Viruses. 2022;14(12):2688. doi:10.3390/v14122688
14. Owen DR, Allerton CMN, Anderson AS, et al. An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19. Science. 2021;374(6575):1586–1593. doi:10.1126/science.abl4784
15. Mahase E. Covid-19: UK becomes first country to authorise antiviral molnupiravir. BMJ. 2021;375:n2697.
16. Mahase E. Covid-19: pfizer’s paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ. 2021;375:n2713.
17. Cegolon L, Pol R, Simonetti O, Larese Filon F, Luzzati R. Molnupiravir, nirmatrelvir/ritonavir, or sotrovimab for high-risk COVID-19 patients infected by the Omicron variant: hospitalization, mortality, and time until negative swab test in real life. Pharmaceuticals. 2023;16(5):721. doi:10.3390/ph16050721
18. Hammond J, Leister-Tebbe H, Gardner A, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. New Engl J Med. 2022;386(15):1397–1408. doi:10.1056/NEJMoa2118542
19. De Vito A, Moi G, Saderi L, et al. Vaccination and antiviral treatment reduce the time to negative SARS-CoV-2 Swab: a real-life study. Viruses. 2023;15(11):2180. doi:10.3390/v15112180
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
