Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 16
Real-Life Anemia Management Among Patients with Non-Dialysis-Dependent Chronic Kidney Disease in Three European Countries
Authors Fliser D, Mata Lorenzo M, Houghton K, Ainsworth C, Blogg M, González de Antona Sánchez E , Portoles J
Received 4 January 2023
Accepted for publication 18 March 2023
Published 13 April 2023 Volume 2023:16 Pages 115—129
DOI https://doi.org/10.2147/IJNRD.S401598
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pravin Singhal
Danilo Fliser,1 Maria Mata Lorenzo,2 Katherine Houghton,3 Claire Ainsworth,3 Martin Blogg,2 Elena González de Antona Sánchez,4 Jose Portoles5
1Saarland University Medical Center, Homburg, Germany; 2Astellas Pharma Europe Ltd., Addlestone, UK; 3RTI Health Solutions, Manchester, UK; 4Astellas Pharma España S.A., Madrid, Spain; 5Hospital Universitario Puerta de Hierro, Madrid, Spain
Correspondence: Danilo Fliser, Saarland University Medical Center, Homburg, Germany, Tel +49 – 6841 – 16 15040, Fax +49 – 6841 – 16 15454, Email [email protected]
Background: Anemia is prevalent among patients with chronic kidney disease (CKD), yet current evidence indicates that treatment may not adhere to Kidney Disease: Improving Global Outcomes (KDIGO) guidelines. We aimed to document the management of patients with non-dialysis-dependent (NDD)-CKD receiving erythropoiesis-stimulating agent (ESA) therapy in Europe.
Methods: This retrospective, observational study extracted information from medical records in Germany, Spain, and the UK. Eligible patients were adults with NDD-CKD stages 3b– 5 who initiated ESA therapy for anemia between January and December 2015. Anemia was defined as hemoglobin (Hb) < 13.0 g/dL (males) or < 12.0 g/dL (females). Data regarding ESA treatment, treatment response, concomitant iron therapy and blood transfusions were extracted up to 24 months post-ESA initiation, and data on CKD progression until abstraction date.
Results: Eight hundred and forty-eight medical records were abstracted. Approximately 40% received no iron therapy prior to ESA initiation. At ESA initiation, mean ± standard deviation Hb level was 9.8 ± 1.0 g/dL. Most patients received darbepoetin alfa, and switching between ESAs was rare (8.5% of patients). Concomitant intravenous and oral iron therapy was prescribed for 36% and 42% of patients, respectively, during initial ESA therapy. Mean Hb levels reached the target level (10– 12 g/dL) within 3– 6 months of ESA initiation. Hb, transferrin saturation, and ferritin levels were infrequently monitored from 3 months post-ESA initiation. Rates of blood transfusion, dialysis, and diagnosis of end-stage renal disease were 16.4%, 19.3%, and 24.6%, respectively. Rates of kidney transplant and death were 4.8% and 8.8%, respectively.
Conclusion: Among ESA-treated patients, ESA initiation was in accordance with KDIGO guidelines, but subsequent monitoring of Hb and iron deficiency were suboptimal.
Keywords: anemia, chronic kidney disease, clinical care process, erythropoiesis-stimulating agents, iron therapy, real-life management
Graphical Abstract:
Plain Language Summary
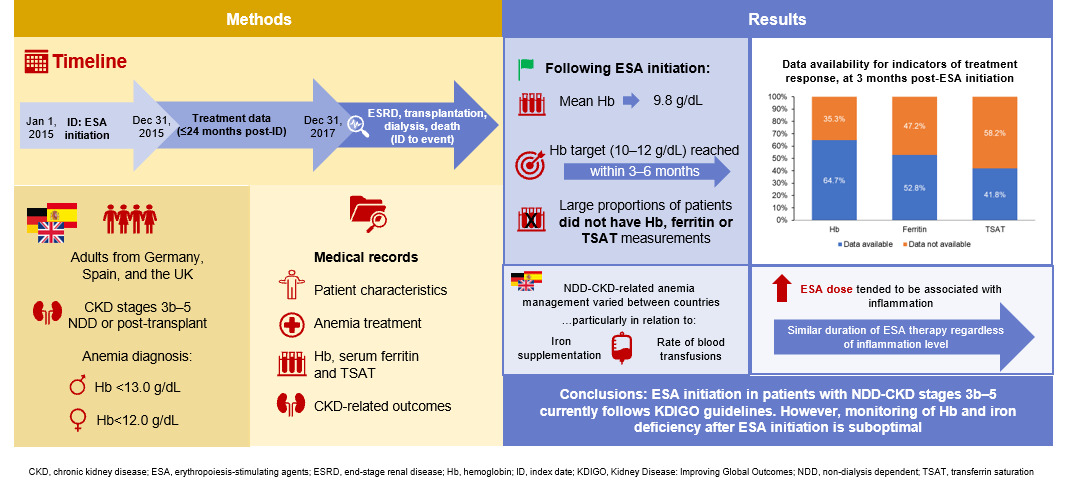
Anemia is common among patients with chronic kidney disease (CKD); treatments include iron therapy and erythropoiesis-stimulating agents (ESAs). Guidelines from the Kidney Disease: Improving Global Outcomes organization recommend the use of ESAs when hemoglobin levels fall below 10 g/dL, to correct any iron deficiency before starting ESA treatment, and to test hemoglobin levels and blood iron status at least every 3 months during ESA treatment. We reviewed 848 medical records from Germany, Spain, and the UK to better understand current anemia treatment practices for adults with moderate-to-advanced CKD who were not on dialysis and were receiving ESA therapy. We found that approximately one-fifth of all patients received dialysis, 16% had a blood transfusion, and 5% received a kidney transplant. ESA treatment was started at an average hemoglobin level of 9.8 g/dL, and the target level (10–12 g/dL) was reached within 3–6 months. However, 40% of patients received no iron therapy before starting ESAs, and hemoglobin levels and iron status were not tested regularly after ESA initiation. Our findings from this sample of European patients highlight that although guidelines for starting ESA treatment appear to be followed, the guidelines for regular subsequent testing may not always be adhered to in clinical practice.
Introduction
Anemia is a common complication of chronic kidney disease (CKD). Its prevalence in non-dialysis-dependent (NDD) patients with CKD stages 3a–5 (estimated glomerular filtration rate [eGFR] <60 mL/min/1.73m2 1) is 40–80%, increasing with CKD stage.2,3 CKD progression is one of the most frequently identified risk factors for the development of anemia.4–6 Furthermore, anemia is associated with increased risk of major adverse cardiac events, all-cause and cardiovascular mortality, hospitalization, and CKD progression.4,5 Treatments for anemia include iron therapy and erythropoiesis-stimulating agents (ESAs).7,8
Iron deficiency is frequently observed in patients with NDD-CKD, affecting approximately half of this population, and rates remain broadly constant across CKD stages.2 Moreover, iron supplementation is vital to ensure adequate iron stores for erythropoiesis and has been shown to improve response to ESA treatment.7 Kidney Disease: Improving Global Outcomes (KDIGO) guidelines recommend the correction of any iron deficiency in patients with NDD-CKD anemia, prior to ESA initiation, and that ESAs only be initiated in patients with hemoglobin (Hb) <10 g/dL.7 It is also recommended that other potential causes of anemia, such as chronic inflammation, be investigated before considering ESA therapy, and to evaluate iron status at least every 3 months during ESA treatment.7
Evidence to date suggests that management of patients with anemia of NDD-CKD is suboptimal. Results from the CKDopps study showed that among NDD-CKD patients with Hb <10 g/dL, 34% in France and 30% in Germany did not receive either iron or ESA treatment in the 3 months following Hb measurement.9 In a follow-up analysis of CKDopps, only 28% of patients with Hb <10 g/dL in Brazil, Germany, and the USA received ESA treatment within 12 months.10 Another study in Italy found that 40% of ESA-eligible patients did not receive ESA treatment.11 To gain further clarity, we aimed to document real-world treatment and management of patients with NDD-CKD stages 3b–5 who received ESA therapy in Germany, Spain, and the UK. Given the differences between countries in reimbursement policies, which make evaluation of combined data difficult, this study presents data per country.
Methods
Ethics
All procedures were performed in accordance with the ethical standards of the relevant institutional review boards (IRBs) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. RTI International’s IRB (Research Triangle Park, North Carolina) determined that this study met all criteria for exemption from ethical considerations (IRB ID: STUDY00020563). In Germany and Spain, the study protocol and its amendments were approved by the Ärztekammer des Saarlandes (ID: 132/19) and the Hospital Universitario Puerta de Hierro Majadahonda (ID: AST-ESA-2019-01), respectively. Under UK Health Research Authority guidelines,12 this study was not considered to be research and was thus exempt from National Health Service Research Ethics Committee review.
Informed consent was not sought due to the nature of the study: all retrieved data were anonymous and were collected as part of routine diagnosis and treatment. Medical professionals abstracting the data were healthcare professionals (HCPs) who had legitimate access to the medical records, and the review had no effect on patient care.
Study Design and Participants
This was a non-interventional, retrospective cohort study of medical records in Germany, Spain, and the UK. A customized data collection form was completed by HCPs using patient medical records.
Participating HCPs were nephrologists, consultants, associate specialists, and specialist nurses who were eligible if they had 3–30 years of experience, were treating at least 10 patients per year for CKD-related anemia, and were actively involved in decision making for the management of these patients. A convenience sampling approach was used to recruit HCPs. For patient selection, a quasi-random method was applied by asking HCPs to select records for patients whose last name began with a randomly generated letter, to reduce the chance of bias.
Eligible patients were adults (≥18 years) with CKD stages 3b–5,1 who initiated ESA treatment for anemia between January 01, 2015 and December 31, 2015 (inclusive). Patients had to have received treatment for CKD continuously in the same clinic following their initial diagnosis, to maximize the probability of a complete data record. Anemia was defined as a record of Hb concentration <13.0 g/dL in males and <12.0 g/dL in females. Patients were excluded if they had received dialysis, a transplant of any kind prior to or upon initiation of ESA treatment, a diagnosis of end-stage renal disease (ESRD), or had participated in an interventional clinical trial upon initiation of or during ESA treatment. The date of first ESA initiation following anemia diagnosis was designated as the index date (Supplementary Figure 1). Absolute iron deficiency was defined as serum ferritin ≤100 ng/mL and transferrin saturation (TSAT) ≤20%.13
Data Extraction and Statistical Analyses
Sociodemographic characteristics and clinical history at the time of ESA initiation were collected. ESA treatment patterns; concomitant intravenous (IV) and oral iron therapy; clinical indicators of response to treatment (Hb level, C-reactive protein [CRP] level, ferritin level, TSAT, and eGFR); and blood transfusions were extracted from the index date until the earliest of the following: 24 months after initiation, the last available medical record, dialysis, ESRD diagnosis, kidney transplant, or death (Supplementary Figure 1). For clinical indicators of response to treatment, physicians were asked to provide the number of measurements from ESA initiation and the dates of each measurement; dates were then aligned according to time since ESA initiation. Data on the incidence of dialysis, ESRD, kidney transplant, and death were collected from ESA initiation until the date of medical record abstraction (Supplementary Figure 1). Methoxy polyethylene glycol-epoetin beta (MPG-EPO; Mircera®) treatment patterns were analyzed separately to those of other darbepoetin alfa due to differences in treatment schedules; the exception was the analysis of the association of patient characteristics or ESA type/dose with time to dialysis or time to ESRD (see below), in which MPG-EPO was grouped with darbepoetin alfa due to the small sample size of the MPG-EPO-treated patient group.
Patient characteristics and outcomes were described for the total population, for each country, by inflammation and diabetes status, and by ESA dose group. Inflammation status was determined by a patient’s documented CRP data from ESA initiation up to 24 months after initiation: consistently inflamed (CRP consistently recorded at ≥5 mg/L), never inflamed (CRP consistently recorded at <5 mg/L), or fluctuating inflammation (CRP levels varied between <5 mg/L and ≥5 mg/L across testing occasions). For the purposes of this study, target Hb level was defined as 10–12 g/dL. Subgroup allocation by ESA dose level was dependent on reported weekly dosing levels throughout the study period. ESA weekly dose groups were defined as follows: Group 1, <5000 international units (IU) short-acting/<5000 IU darbepoetin alfa/<1800 IU MPG-EPO; Group 2, ≥5000 to ≤8000 IU short-acting/≥5000 to <8000 IU darbepoetin alfa/≥1800 to <3000 IU MPG-EPO; Group 3, >8000 to ≤16,000 IU short-acting/≥8000 to ≤16,000 IU darbepoetin alfa/≥3000 to <5400 IU MPG-EPO; Group 4, >16,000 IU short-acting>16,000 IU darbepoetin alfa/≥5400 IU MPG-EPO. Patients included in the dosing range subgroups remained within that dosing range for the duration of the study period; patients who transitioned between dose groups during the study period were categorized into “increasing dosing transition” or “decreasing dosing transition” groups, as appropriate, for analysis.
Data were summarized descriptively. Cox proportional hazards regression models were used to estimate time to dialysis and time to ESRD (separately), with selected patient demographics, patient baseline characteristics, and ESA treatment type/dose included as predictors (Supplementary Table 1). For the Cox analysis, patients receiving stable ESA doses were included in three dose groupings: Group 1, Group 2, and Groups 3+4 combined (see above); due to sample size, Groups 3 and 4 were combined into one group and patients in the transitioning dose groups were not included. The significance of interactions was assessed using the Wald test, and p values <0.05 were considered significant. No imputation methods were applied to missing data.
Results
Patient Characteristics
A total of 848 medical records were abstracted: 211 from Germany, 430 from Spain, and 207 from the UK. On average, patients had had CKD-related anemia for over 5 years (Table 1). Patients in the overall cohort were approximately evenly distributed between inflammation subgroups (Table 1). Prior to ESA initiation, 39.9% of patients had received no iron therapy—this proportion was numerically higher in Spain compared with Germany and the UK (Table 1). The mean length of time between anemia diagnosis and ESA initiation was over 8 months, and the mean Hb level at ESA initiation was 9.8 g/dL (range 5–13 g/dL) (Table 1).
 |
Table 1 Patient Sociodemographic and Clinical Characteristics |
At baseline, 162 (19.1%) patients had absolute iron deficiency (Germany n=31 [14.7%], Spain n=98 [22.8%], and UK n=33 [15.9%]). Of these patients, five had unknown or no documented treatment for NDD-CKD-related anemia prior to ESA therapy (Germany n=3 [9.7%], Spain n=1 [1.0%], and UK n=1 [3.0%]), and 57 (35.2%) received no iron therapy prior to ESA therapy (Germany n=3 [9.7%], Spain n=41 [41.8%], and UK n=13 [39.4%]).
ESA Treatment
During their initial ESA treatment, most patients (67.0%) received darbepoetin alfa; 23.7% and 9.3% received short-acting ESAs and MPG-EPO, respectively (Supplementary Figure 2). This pattern was similar between countries. The majority of patients received their ESA treatment via subcutaneous administration and at home (Table 2). Most patients (776/848, 91.5%) received just one ESA agent continuously from initiation until either treatment discontinuation or the end of the observation period (Table 2; Supplementary Figure 2). Approximately one-third of patients discontinued ESA treatment within 2 years of initiation (Table 2). The primary reason for discontinuation or treatment switch was attainment of Hb target levels.
 |
Table 2 ESA Treatment Course |
The mean ESA dose varied between countries (Table 2). During initial ESA therapy, 312 patients (36.8%) had a dose reduction, and 306 (36.1%) had a dose increase (patients could experience both an increase and a reduction) (Table 2). Inflammation level tended to be associated with ESA dose; however, the range of doses recorded was wide for all ESA types and inflammation subgroups (Table 3). Duration of ESA treatment also appeared similar across inflammation subgroups (mean 38.7–42.4 months) (Table 3).
 |
Table 3 ESA Dose and Duration by Inflammation Subgroup |
Concomitant Iron Therapy
During initial ESA therapy, concomitant IV iron therapy was prescribed for 35.6% of the study population and was more common in the UK than in Germany or Spain (Figure 1A). The overall proportion of patients receiving concomitant oral iron therapy was 41.7% but was much lower in the UK compared with Germany or Spain; UK patients were notably more likely to receive IV rather than oral iron (Figure 1A). In the 162 patients with absolute iron deficiency, concomitant iron therapy during initial ESA therapy was prescribed to 81 (50.0%) patients, with the highest proportional prescriptions in the UK (n=23, 69.7%), compared with Germany (n=18, 58.1%) and Spain (n=40, 40.8%).
Figure 1 Continued.
The highest ESA dose group was associated with lower usage of concomitant iron therapy than the other ESA dose groups (Figure 1B). The proportion of patients receiving concomitant IV or oral iron therapy did not differ significantly between inflammation subgroups (Figure 1C). Notably, the proportion of patients receiving concomitant iron therapy decreased from initial to subsequent ESA therapy courses (Figure 1D). However, few patients (n=72) had more than one ESA therapy course (Supplementary Figure 2), and the number of patients with information available also decreased over this period.
Response to Treatment and Clinical Outcomes
Hemoglobin Levels
Mean Hb levels reached the target level of 10–12 g/dL within 3–6 months of ESA initiation (Table 4). Within 24 months of ESA initiation, 86.7% (735/848) of patients achieved the Hb target level; this percentage was broadly similar across ESA types and across countries (Figure 2). However, the proportion of patients with available Hb measurements declined following ESA initiation: after 3 months, only 64.7% (545/843) of patients had a documented Hb measurement, and this proportion continued to decline over the observation period (Table 4).
 |
Table 4 Clinical Indicators of Response to Treatment Following ESA Initiation |
Among patients who achieved the target Hb levels, approximately three-quarters (74.6%; 548/735) had a documented Hb measurement indicating maintenance of their Hb levels 6 months after target achievement, and less than half (49.4%, 363/735) after 12 months. Many of these patients had no documented Hb levels, and the proportion of missing data following target achievement increased over time (at 6 months: 13.2%, 97/735; at 12 months: 22.9%, 168/735). For these patients, it is unknown whether the Hb target was maintained.
Other Clinical Indicators
Ferritin and TSAT levels tended to increase over the 24 months following ESA initiation, while eGFR decreased (Table 4). After a median TSAT of 20% at ESA initiation, levels increased above 20% for the remainder of the observation period (Table 4). Similar to Hb, ferritin, TSAT, and eGFR data were not documented for many patients, especially after ESA initiation (Table 4). Additionally, one-quarter of all patients had no documented CRP measurements for the entire observation period (Table 1).
Blood Transfusions
Within 24 months of ESA initiation, 16.4% (87/848) of patients required a blood transfusion; this proportion was notably higher in Spain than in the UK and Germany (Figure 3). Patients with consistent or fluctuating inflammation more commonly had blood transfusions than patients with no inflammation (Supplementary Figure 3A). Higher ESA doses were also more commonly associated with blood transfusions (Supplementary Figure 3B).
CKD Progression and Deaths
From ESA initiation until the abstraction date, rates of dialysis and ESRD were 19.3% and 24.6%, respectively, and were similar between countries (Figure 3). The expected time to both dialysis (p=0.002) and ESRD (p=0.020) significantly increased with each year increase in age (Supplementary Table 1). Patient sex, country, time to ESA initiation, and Charlson comorbidity index at baseline were not significantly associated with time to either dialysis or ESRD (Supplementary Table 1). The expected time to both dialysis and ESRD significantly increased with each unit increase in baseline eGFR (p<0.001 for both outcomes); similarly, lower CKD stage at the time of anemia diagnosis was significantly associated with longer time to dialysis (stage 4 versus stage 5, p=0.003), but not ESRD (Supplementary Table 1). Neither the ESA type first initiated nor ESA weekly dose group was significantly associated with time to dialysis or ESRD (Supplementary Table 1).
The rate of kidney transplant was low overall (4.8%; 41/848) and was similar between countries (Figure 3). The rate of death (8.8% overall; 75/848) was also similar between countries (Figure 3). Of all 75 patients who had died at the time of data abstraction, 18 had received a short-acting ESA, 53 had received darbepoetin alfa, and 4 had received MPG-EPO, for their initial ESA treatment. No differences in the rate of death were observed as a function of inflammation or ESA dose group (Supplementary Figure 3A and B).
Discussion
This study provides insights into real-life management of patients with NDD-CKD anemia, including ESA treatment and concomitant iron therapy, in Germany, Spain, and the UK. Across the three countries, the mean and median Hb level at ESA initiation was 9.8 g/dL, which is in accordance with KDIGO guidelines for anemia treatment in patients with NDD-CKD.7 However, the Hb level at initiation ranged from 5 to 13 g/dL, indicating that some patients were initiated on ESAs despite having a Hb level above 10.0 g/dL. In addition, 9% of patients did not have a recorded Hb measurement upon ESA initiation, despite the guideline recommendations. As these patients did have Hb measurements at later dates, we consider the missing data to be evidence that Hb levels were not measured close to ESA initiation for these patients, rather than indicative of incomplete data extraction.
It is also recommended to monitor Hb concentration every 3 months, yet only 65% had an Hb measurement observed at 3 months after ESA initiation, and this proportion continued to decline up to 15 months. The reasons for the missing data are uncertain and may be due to incomplete documentation in the original medial record, and/or CKD progression to dialysis, ESRD, transplant, or death. Our findings are similar to those from the international CKDopps study, in which <50% of patients with an index Hb level <10 g/dL had a Hb measurement in the subsequent 3 months.2,9
KDIGO guidelines recommend a target Hb level of 10 to ≤11.5 g/dL for ESA treatment, while noting that higher Hb targets (11.5 to ≤13 g/dL) may be beneficial for individual patients.7 For the purposes of this study, patients were considered to have met the target Hb level if they had a documented Hb measurement between 10 and 12 g/dL (the higher target level allowed for variability in the guideline recommendations and potential laboratory errors). Within 2 years, the majority of patients achieved this target. However, it is uncertain how many patients maintained their target level: by 12 months after target achievement, <50% of patients had a documented Hb level within the target range, and there was a substantial proportion with missing data.
Prior to ESA initiation, approximately 60% of patients had received either IV or oral iron therapy; this could indicate that iron deficiency had been explicitly investigated and corrected in line with KDIGO guidelines. However, ferritin and TSAT measurements were missing at ESA initiation for 15% and 33% of patients, respectively, so it is not possible to determine whether iron therapy was indicated for these patients. Moreover, only approximately half of patients had documented ferritin and TSAT measurements in the months following ESA initiation, and only 36% and 42% of patients received concomitant IV and/or oral iron therapy, respectively. After ESA initiation, patients did not remain iron deficient over the 2-year observation period: TSAT levels were consistently >20% and ferritin >100 ng/mL, based on available measurements. In the CKDopps study, 26–77% of patients with NDD-CKD stages 3a–5, depending on country and CKD stage, had no TSAT or ferritin measurements within 3 months of their index Hb measurement,9 and only 29–43% of patients with Hb <10 g/dL received iron therapy in the 3 months following their index Hb measurement.9 Other research in Italy found that a majority of patients with iron deficiency (TSAT ≤20% and ferritin ≤100 ng/mL) did not receive iron therapy,11 while a study of nephrology centers in Ireland reported that only 14.1% of patients with iron deficiency received iron therapy.14
Despite availability of global (KDIGO) guidelines, there were differences in the management of these patients between countries. There was a substantially higher rate of blood transfusions in Spain than in Germany or the UK, which may be linked to the lower use of IV iron in Spain compared with the other countries. Conversely, in the UK, there was a markedly higher use of IV versus oral iron, whereas the reverse was seen in the other two countries. The suboptimal management of anemia and iron deficiency, and between-country differences in management, may reflect variability between KDIGO and national or local guidelines, or uncertainty as to their application.8,14 Another underlying cause may be lack of resources or training,15 or country-specific medical administration practices.
A notable finding of our study was the tendency for inflammation to be associated with higher ESA doses, although this was not consistent across all ESA types. This may indicate resistance to ESAs in patients with inflammation, either because of infection16 or because other possible causes of anemia were not sufficiently investigated. This finding is consistent with a study of patients with CKD in Sweden, which found a clear relationship between CRP levels and ESA dose.3 Alternatively, functional iron deficiency could be a consequence of inflammatory status, since inflammation upregulates hepcidin, causing iron to be trapped within cells and making it unavailable for erythropoiesis.3 Moreover, our data show that a numerically higher proportion of patients with either consistent or fluctuating inflammation received a blood transfusion than those with no inflammation. However, due to the nature of this study, statistical analyses were not conducted for these relationships and no firm conclusions can be drawn. CRP levels were unusually high (mean 13.1 mg/L, median 5.0 mg/L)—it is not certain whether this is due to poor data recording or if it is reflective of the real-life situation. Some patients may have had intercurrent bacterial infection or the numbers may reflect a negative selection bias, whereby HCPs were more likely to monitor and record CRP data for patients whose levels were abnormal.
Strengths of this study include its 2-year observation period, which enabled longitudinal follow-up of patient outcomes from ESA treatment. This study was of a retrospective, observational, non-interventional design, in which centers were not selected on the basis of their compliance with clinical best practice. We, therefore, consider it representative of typical clinical practice in the participating countries.
However, the retrospective design and dependence on medical records are also limitations, as it was not possible to follow up on the reasons underlying the observed data. The dependence on medical records may have led to the absence of some patient data, since records require that treating physicians are sufficiently motivated to provide complete information. We also acknowledge that relatively modest numbers of patients from each country were included. In addition, with the exception of the incidence data for dialysis, ESRD, kidney transplant, and death, the last possible date for the 24-month follow-up was December 31, 2017; consequently, the data analyzed here are a minimum of four years old and some changes in clinical practice may have occurred in the intervening period. The study was designed to be descriptive and no hypothesis testing was conducted. Lastly, the convenience sampling strategy for recruiting HCPs and high levels of missing data restrict the conclusions that can be drawn; caution should be taken when extrapolating these findings to other patient populations.
Conclusions
Patients in Germany, Spain, and the UK with anemia of NDD-CKD receive ESAs in accordance with KDIGO guidelines, but subsequent monitoring and management is suboptimal and may be indicative of unmet need. The proportion of patients who remain iron deficient during ESA therapy also signifies a potential treatment gap. Patients who had higher levels of inflammation tended to receive higher ESA doses.
Abbreviations
CKD, chronic kidney disease; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; ESA, erythropoiesis-stimulating agent; ESRD, end-stage renal disease; Hb, hemoglobin; HCP, healthcare professional; IRB, institutional review board; IV, intravenous; KDIGO, Kidney Disease: Improving Global Outcomes; MPG-EPO, methoxy polyethylene glycol-epoetin beta; NDD, non-dialysis-dependent; TSAT, transferrin saturation.
Data Sharing Statement
Researchers may request access to anonymized participant-level data, trial-level data, and protocols from Astellas-sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing, see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
Ethics Approval
RTI International’s IRB (Research Triangle Park, North Carolina) determined that this study met all criteria for exemption from ethical considerations (IRB ID: STUDY00020563). In Germany and Spain, the study protocol and its amendments were approved by the Ärztekammer des Saarlandes (ID: 132/19) and the Hospital Universitario Puerta de Hierro Majadahonda (ID: AST-ESA-2019-01), respectively. Under UK Health Research Authority guidelines,12 this study was not considered to be research and was thus exempt from National Health Service Research Ethics Committee review.
Informed Consent
Due to the nature of this retrospective observational study, patients were not informed that the study is taking place, and consent was not collected. The IRB and ethics committee review packages submitted for this study therefore requested a waiver of informed consent requirements.
Acknowledgments
These data were presented in part as a mini-oral presentation at the 58th ERA-EDTA Congress, June 5–8, 2021; as a poster presentation at the 51st Congress of the Sociedad Española de Nefrología, October 15–18, 2021; as a poster presentation at the 13th annual meeting of the Deutsche Gesellschaft für Nephrologie, September 23–26, 2021; and as a poster presentation at Virtual ISPOR Europe, November 30–December 3, 2021.
Author Contributions
All authors met the following authorship criteria:
- Significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas.
- Drafted or written, or substantially revised or critically reviewed the article.
- Have agreed on the journal to which the article will be submitted.
- Reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage.
- Agree to take responsibility and be accountable for the contents of the article.
Funding
This study was initiated and supported by Astellas Pharma, Inc. Medical writing support was provided by Iona Easthope, DPhil, of Lumanity, funded by Astellas Pharma Inc.
Disclosure
DF has received speaker and consultant fees for Astellas, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Norgine, and Vifor Pharma. MML is an employee of Astellas Pharma, Inc. KH is an employee of RTI Health Solutions, which provides consulting and other research services to pharmaceutical, device, governmental, and non-governmental organizations. KH works with many companies and does not receive payment or honoraria directly from these companies for services rendered. CA was an employee of RTI Health Solutions at the time of this study and its analysis. RTI Health Solutions received funding from Astellas Pharma Inc. for the design and conduct of this study, statistical analysis, and study report preparation. She is currently affiliated with OPEN Health Evidence and Access, Manchester, UK. MB is an employee of Astellas Pharma Europe, Ltd. EGAS is an employee of Astellas Pharma España S.A. JP has received speaker fees for Astellas, Amgen, AstraZeneca, GSK, Vifor Pharma, and advisory fees from Astellas and Vifor Pharma. The authors report no other conflicts of interest in this work.
References
1. Kidney Disease: Improving Global Outcomes. 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney International Supplements; 2013. Available from: https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf.
2. Guedes M, Robinson BM, Obrador G, Tong A, Pisoni RL, Pecoits-Filho R. Management of anemia in nondialysis chronic kidney disease: current recommendations, real-world practice, and patient perspectives. Kidney360. 2020;1(8):855–862. doi:10.34067/kid.0001442020
3. Evans M, Bower H, Cockburn E, Jacobson SH, Barany P, Carrero JJ. Contemporary management of anaemia, erythropoietin resistance and cardiovascular risk in patients with advanced chronic kidney disease: a nationwide analysis. Clin Kidney J. 2020;13(5):821–827. doi:10.1093/ckj/sfaa054
4. Portolés J, Gorriz JL, Rubio E, et al. The development of anemia is associated to poor prognosis in NKF/KDOQI stage 3 chronic kidney disease. BMC Nephrol. 2013;14:2. doi:10.1186/1471-2369-14-2
5. Palaka E, Grandy S, van Haalen H, McEwan P, Darlington O. The impact of CKD anaemia on patients: incidence, risk factors, and clinical outcomes – a systematic literature review. Int J Nephrol. 2020;2020:7692376. doi:10.1155/2020/7692376
6. Alagoz S, Dincer MT, Eren N, et al. Prevalence of anemia in predialysis chronic kidney disease: is the study center a significant factor? PLoS One. 2020;15(4):e0230980. doi:10.1371/journal.pone.0230980
7. Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney International Supplements; 2012. Available from: https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-Anemia-Guideline-English.pdf.
8. Ogden L, Bennett L, Ebah LM. Iron deficiency anaemia in chronic kidney disease: an overview. J Kidney Care. 2018;3(Sup6):S3–S8. doi:10.12968/jokc.2018.3.Sup6.S3
9. Wong MMY, Tu C, Li Y, et al. Anemia and iron deficiency among chronic kidney disease stages 3-5ND patients in the chronic kidney disease outcomes and practice patterns study: often unmeasured, variably treated. Clin Kidney J. 2019;13(4):613–624. doi:10.1093/ckj/sfz091
10. Lopes MB, Tu C, Zee J, et al. A real-world longitudinal study of anemia management in non-dialysis-dependent chronic kidney disease patients: a multinational analysis of CKDopps. Sci Rep. 2021;11(1):1784. doi:10.1038/s41598-020-79254-6
11. Minutolo R, Locatelli F, Gallieni M, et al. Anaemia management in non-dialysis chronic kidney disease (CKD) patients: a multicentre prospective study in renal clinics. Nephrol Dial Transplant. 2013;28(12):3035–3045. doi:10.1093/ndt/gft338
12. UK Health Research Authority. Defining research table; 2017. Available from: http://www.hra-decisiontools.org.uk/research/docs/DefiningResearchTable_Oct2017-1.pdf.
13. Gafter-Gvili A, Schechter A, Rozen-Zvi B. Iron deficiency anemia in chronic kidney disease. Acta Haematol. 2019;142(1):44–50. doi:10.1159/000496492
14. Stack AG, Alghali A, Li X, et al. Quality of care and practice patterns in anaemia management at specialist kidney clinics in Ireland: a national study. Clin Kidney J. 2018;11(1):99–107. doi:10.1093/ckj/sfx060
15. Rainey H. The challenges of managing iron deficiency anaemia in chronic kidney disease: survey results. J Kidney Care. 2018;3(Sup6):S9–S13. doi:10.12968/jokc.2018.3.sup6.s9
16. Kanbay M, Perazella MA, Kasapoglu B, Koroglu M, Covic A. Erythropoiesis stimulatory agent- resistant anemia in dialysis patients: review of causes and management. Blood Purif. 2010;29(1):1–12. doi:10.1159/000245041
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



