Back to Journals » International Journal of Nanomedicine » Volume 9 » Issue 1
Quantum dots immunofluorescence histochemical detection of EGFR gene mutations in the non-small cell lung cancers using mutation-specific antibodies
Authors Qu Y, Zhang Q, Pan Q, Zhao X, Huang Y, Chen F, Chen H
Received 17 July 2014
Accepted for publication 29 September 2014
Published 9 December 2014 Volume 2014:9(1) Pages 5771—5778
DOI https://doi.org/10.2147/IJN.S71310
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Lei Yang
Yan-Gang Qu,1 Qian Zhang,2 Qi Pan,3 Xian-Da Zhao,4 Yan-Hua Huang,2 Fu-Chun Chen,3 Hong-Lei Chen4
1Department of Pathology, The Central Hospital of Enshi Autonomous Prefecture, Enshi, 2Department of Molecular Pathology, Wuhan Nano Tumor Diagnosis Engineering Research Center, Wuhan, Hubei, People’s Republic of China; 3Department of Thoracosurgery, Traditional Chinese Medical Hospital of Wenling, Wenling, Zhejiang, People’s Republic of China; 4Department of Pathology, School of Basic Medical Science, Wuhan University, Wuhan, Hubei, People’s Republic of China
Background: Epidermal growth factor receptor (EGFR) mutation status plays an important role in therapeutic decision making for non-small cell lung cancer (NSCLC) patients. Since EGFR mutation-specific antibodies (E746-A750del and L858R) have been developed, EGFR mutation detection by immunohistochemistry (IHC) is a suitable screening test. On this basis, we want to establish a new screening test, quantum dots immunofluorescence histochemistry (QDs-IHC), to assess EGFR gene mutation in NSCLC tissues, and we compared it to traditional IHC and amplification refractory mutation system (ARMS).
Materials and methods: EGFR gene mutations were detected by QDs-IHC, IHC, and ADx-ARMS in 65 cases of NSCLC composed of 55 formalin-fixed, paraffin-embedded specimens and ten pleural effusion cell blocks, including 13 squamous cell carcinomas, two adenosquamous carcinomas, and 50 adenocarcinomas.
Results: Positive rates of EGFR gene mutations detected by QDs-IHC, IHC, and ADx-ARMS were 40.0%, 36.9%, and 46.2%, respectively, in 65 cases of NSCLC patients. The sensitivity of QDs-IHC when detecting EGFR mutations, as compared to ADx-ARMS, was 86.7% (26/30); the specificity for both antibodies was 100.0% (26/26). IHC sensitivity was 80.0% (24/30) and the specificity was 92.31% (24/26). When detecting EGFR mutations, QDs-IHC and ADx-ARMS had perfect consistency (κ=0.882; P<0.01). Excellent agreement was observed between IHC and ADx-ARMS when detecting EGFR mutations (κ=0.826; P<0.01).
Conclusion: QDs-IHC is a simple and standardized method to detect EGFR mutations with its high sensitivity and specificity, as compared with real-time polymerase chain reaction. In addition, the development of specific antibodies against EGFR mutation proteins might be useful for the diagnosis and treatment of lung cancer.
Keywords: quantum dots, lung cancer, EGFR, gene mutation, real-time PCR, immunohistochemistry
Introduction
With the aggravation of environmental pollution, lung cancer is almost the most malignant tumor in the world with its high morbidity and mortality.1 The most common histologic subtype is non-small cell lung cancer (NSCLC), which accounts for 80% of all lung cancers.2 Although much progress has been made in the treatment of lung cancer, early diagnosis is difficult and the majority of patients has progressed to an advanced stage when diagnosed. The median survival rate for these patients is only 8–11 months.3
In 2004, a landmark discovery had been made in that somatic mutations in the epidermal growth factor receptor (EGFR) were associated with sensitivity to EGFR tyrosine kinase (TK) inhibitors (TKIs) (EGFR-TKI).4,5 In subsequent large-scale randomized clinical trials, the relationship between EGFR mutation status and efficacy of the EGFR-TKI drug was clearly explained.6–8 Based on these findings, EGFR mutation status in the TK domain can determine the treatment of advanced NSCLC. Patients with EGFR-activating mutations can benefit from EGFR-TKI treatment. Mutations associated with enhanced sensitivity to EGFR-TKIs are found in exons 18–21 of the TK domain of EGFR; in particular, del E746-A750 in exon 19 and the L858R point mutation in exon 21 account for nearly 90% of all the mutations in EGFR in lung cancer.7,9,10 Nowadays, the detection of EGFR mutation status in NSCLC patients has become an expert consensus.11 Different methodologies have been developed for molecular testing, such as the amplified refractory mutation system (ARMS), high-resolution melting, DNA direct sequencing, and next-generation sequencing (NGS). DNA direct sequencing is considered the “gold standard” for the assessment of EGFR mutation status in NSCLC; however, it is time consuming and laborious. The ARMS method is widely used in the clinical testing; however, the commercial assay kit for EGFR is very expensive, and the experiment needs to be done under good experimental conditions with sophisticated real-time polymerase chain reaction (PCR) instruments.12
Immunohistochemistry (IHC) is a well-established method that is widely applied in conventional pathological diagnosis. EGFR mutation-specific rabbit monoclonal antibodies against E746-A750 deletion and L858R (Cell Signaling Technology, Inc., Danvers, MA, USA) have been applied in IHC application. This provides a simple and rapid screening method for assessing EGFR mutation status.13–15
A nanofluorescent material, fluorescent semiconductor nanocrystal quantum dots (QDs), have been widely used in labeling some molecules, such as streptavidin and antibodies, through carbodiimide chemistry, optionally using EDAC (1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide). QD (605 nm)-labeled streptavidin emits bright red fluorescence with 605 nm as the maximum emission wavelength while being stimulated by an excitation light source <580 nm, which is different from green background autofluorescence. Those labeled materials can be successfully applied to biological imaging and IHC detection of gene mutations, such as with human epidermal growth factor receptor 2 (HER 2) amplification in breast cancer.16,17 Moreover, multiple markers can be visualized on one cell for in vitro multiplexed imaging; for example, clinically significant tumor biomarkers including HER2, EGFR, progesterone receptor, estrogen receptor, and mammalian target of rapamycin can be detected quantitatively and simultaneously in breast cancer cells using multicolor QDs.18 QD-based immunofluorescence histochemistry (QDs-IHC) is an established method; it has been validated in many published papers.17,19,20 The staining signal detected by QDs-IHC is much stronger with a lower background when compared with IHC.20 Based on the application of EGFR mutation-specific antibodies, this study was designed to develop a QD-based immunofluorescent approach for EGFR mutation detection, which is a simple, quick, and highly sensitive molecular method for diagnosing EGFR mutation status in NSCLC samples.
Materials and methods
Patients and samples
Fifty-five cases of formalin-fixed, paraffin-embedded (FFPE) NSCLC specimens and ten cases of pleural effusion cell blocks from patients with lung adenocarcinoma were collected, which were provided by the Central Hospital of Enshi Autonomous Prefecture and the Hubei Cancer Hospital from January 2013–August 2014. The cohort consisted of 13 squamous cell carcinomas, 50 adenocarcinomas, and two adenosquamous carcinomas. For each case, the hematoxylin and eosin sections were reviewed by at least two pathologists (YGQ and HLC). This study was approved by the Institutional Ethics Committee of the Central Hospital of Enshi Autonomous Prefecture.
QD immunofluorescence histochemistry staining
NSCLC tissue sections (4 μm thick) were deparaffinized in xylene and rehydrated in a graded ethanol series. QDs-IHC was performed according to the manufacturer's instructions (Wuhan Jiayuan Quantum Dots Co., Ltd., Wuhan, People's Republic of China). Antigen retrieval was performed in ethylene diamine tetraacetic acid (EDTA) (10 mM; pH 9.0) at 100°C for 3 minutes, followed by cooling at room temperature for 30 minutes. For antibody bindings, sections were first incubated in 2% bovine serum albumin (BSA) buffer (Sigma-Aldrich Co., St Louis, MO, USA) at 37°C for 30 minutes, and then three primary antibodies (total EGFR monoclonal antibody [D38B1], EGFR del E746-A750 mutation-specific monoclonal antibody [6B6], and L858R mutation-specific monoclonal antibody [43B2]; Cell Signaling Technology, Inc.) were diluted separately at 1:100 and manually applied to the sections. Specimens were incubated at 4°C overnight with those three primary antibodies, respectively. After that, the slides were then washed three times with Tris-buffered saline (TBS) with Tween® (TBS-T) (0.5% Tween, 0.1 M Tris-base, 0.9% NaCl, and pH 7.6) for 5 minutes each time, and incubated in biotinylated goat antirabbit immunoglobulin G (1:300 dilution; Jackson ImmunoResearch Inc., West Grove, PA, USA) at 37°C for 30 minutes.
Finally, QD (605 nm)-labeled streptavidin (1:400 dilution in 2% BSA; Wuhan Jiayuan Quantum Dots Co., Ltd.) was added to the sections and incubated at 37°C for 30 minutes. After rinsing for three times, sections were sealed with 90% glycerine (Sigma-Aldrich Co.). During the process, negative control samples were performed in parallel, but the primary antibody was replaced with TBS buffer. Total EGFR was used as a positive control.
Immunohistochemistry to detect EGFR mutation
Resected tumor specimens were stained simultaneously using these three antibodies according to the manufacturer's instructions. Antigen retrieval was the same as for the QDs-IHC method. Intrinsic peroxidase activity was blocked using 3% hydrogen peroxide for 10 minutes. After washing the sections with TBS, diluted primary antibodies (1:100) were applied to cover the specimen. Sections were incubated at 4°C overnight. After three washes in TBS for 3 minutes each, the slides were incubated for 30 minutes at room temperature with labeled polymer-horseradish peroxidase antirabbit secondary antibody (EnVision™ kit; Dako Denmark A/S, Glostrup, Denmark). Following three washes in TBS, color was developed by the diaminobenzidine reaction. The sections were counterstained by hematoxylin for 2 minutes.
QDs-IHC and IHC scoring
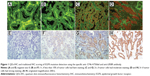
The QD staining signals were detected using Olympus BX51 fluorescence microscopy equipped with an Olympus Micro DP72 camera (Olympus Corporation, Tokyo, Japan). The EGFR-positive signal detected by QDs-IHC was red, target-specific, bright, and photostable. The EGFR-positive signal detected by traditional IHC was brown–yellow or brown. EGFR protein expression was located at the tumor cell membrane and/or cytoplasm. Immunostaining was evaluated by two different pathologists (YGQ and XDZ) using criteria based on published cutoffs. The intensity of cytoplasmic and/or membrane staining, as well as the percentage of positive cells, was recorded. Staining intensity was scored from 0 to 3+, as follows:21,22 0 if tumor cells had a complete absence of staining or faint staining intensity in <10%; 1+ if ≥10% of the tumor cells had faint staining; 2+ if the tumor cells had moderate staining; and 3+ if tumor cells had strong staining (Figure 1). Accordingly, we classified scores of 0 and 1+ as negative and scores of 2+ and 3+ as positive. To assess the sensitivity and specificity of QDs-IHC, we compared these results with those of real-time quantitative PCR.
DNA extraction from NSCLC FFPE tissues and cell blocks
Extraction of genomic DNA from FFPE NSCLC tissue sections was performed using cobas® Sample Preparation Kits (Hoffman-La Roche Ltd., Basel, Switzerland) according to the manufacturer's instructions. The DNA quality and purity were assessed using Varioskan™ Flash (Thermo Fisher Scientific, Waltham, MA, USA).23
Detection of EGFR mutations in exon 18–21 by ARMS
ARMS is a highly sensitive method; it is a real-time PCR-based test. The AmoyDx EGFR Mutation Test Kit (Amoy Diagnostics Co., Ltd., Xiamen, People's Republic of China) has been widely used in the clinical laboratory. We chose this kit to detect 29 EGFR mutation hotspots in exon 18–21. The assay was carried out according to the manufacturer's protocol for the kit with the LightCycler® 480 II real-time PCR system (Hoffman-La Roche Ltd.). Upon completion, the results were analyzed according to the criteria defined by the manufacturer's instructions.
Statistical analysis
Statistical analysis was performed using the statistical software SPSS version 17.0 (IBM Corporation, Armonk, NY, USA). Cohen's kappa was used to determine intraobserver agreement and the agreement between QDs-IHC, IHC, and ARMS. A kappa value between 0.81 and 1.0 was defined as a nearly perfect agreement, between 0.41 and 0.80 as a moderate agreement, between 0.21 and 0.40 as a fair agreement, and between 0.00 and 0.20 as a slight agreement. All tests were two-sided, and a P-value of <0.05 was considered statistically significant.
Results
EGFR mutation detected by QDs-IHC and IHC using mutation-specific antibodies in NSCLC
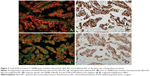
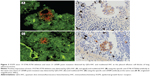
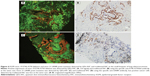
Tissue sections from small biopsies, pleural effusion cell blocks, and surgery were successfully stained by EGFR mutation-specific antibodies, and these antibodies showed distinct immunoreactivity (red signals) for the tumor cells, as presented in Figure 1. A positive signal of the total EGFR protein detected by QDs-IHC and IHC was moderate to strong in all 65 samples, which was regarded as the positive control (Figure 2). The positive rates for the EGFR mutation detected by QDs-IHC and IHC were 40.0% (26/65) and 36.9% (24/65), respectively. In the QDs-IHC method, 12 (46.2%) patients showed E746-A750-specific staining and 14 (53.8%) patients were L858R mutant-specific (Table 1). Figure 2 shows the representative images of the same surgical case of cancer, which carried the L858R mutation and total EGFR. The cancer cells were strongly stained by the total EGFR antibody (Figures 2A and B); moreover, the cancer cells were positively stained for the anti-L858R antibody (Figures 2C and D) and negative for the del E746-A750 deletion. Simultaneously, EGFR mutations were also detected by QDs-IHC and IHC using mutation-specific antibodies at the pleural effusion cell blocks (Figures 3A–D) and small biopsies (Figures 4A–D). EGFR mutations detected by QDs-IHC and IHC exhibited significant difference between squamous cell carcinoma and adenocarcinoma (P<0.05).
EGFR mutation detected by ADx-ARMS
In all the 65 NSCLC specimens, the mutation rates for EGFR detected by ADx-ARMS was 46.15% (30/65), 52.00% (26/50) for adenocarcinoma, 15.38% (2/13) for squamous cell carcinoma, and 100% (2/2) for adenosquamous carcinoma. A significant difference in EGFR mutations was observed between adenocarcinoma and squamous cell carcinoma (P=0.018). ADx-ARMS could detect eleven cases of exon 19 deletion, 14 cases of exon 21 L858R mutations, two cases of exon 20 point mutations (S768I), and one case of exon 20 insertion. Both exon changes such as exon 19 deletion and exon 20 T790M mutations, or exon 18 G719X and exon 20 S768I mutations could be detected simultaneously in the same case (Table 1). Thirty-five wild-type EGFRs were also noted, and there were two invalid results, mainly due to the fact that there was little tissue available; as such, the concentration of extracted DNA was very low (2.54 ng/μL and 2.04 ng/μL).
Comparison of EGFR mutation detection by QDs-IHC, IHC, and ADx-ARMS
We then compared the mutation status between ADx-ARMS and immunostaining-based EGFR. The EGFR mutations identified by these two methods are summarized in Table 1. Of the 26 patients with positive QDs-IHC staining, all of the EGFR mutations (exon 19 deletion and exon 21 L858R mutation) were detected by ADx-ARMS; however, ADx-ARMS could detect four cases of exon 18 or exon 20 changes. If the ADx-ARMS results were true, the sensitivity of QDs-IHC in detecting EGFR mutations, as compared with ADx-ARMS, was 86.7% (26/30); the specific for both antibodies was 100.0% (26/26). Two cases with the exon 21 L858R point mutation, as identified by QDs-IHC, were negatively stained by IHC using the EGFR mutation-specific antibody. IHC sensitivity was relatively low (80.0%; 24/30) and the specificity was 92.31% (24/26). When detecting EGFR mutations, QDs-IHC and ADx-ARMS demonstrated perfect consistency (κ=0.882; P<0.01). Excellent agreement was observed between IHC and ADx-ARMS when detecting EGFR mutations (κ=0.826; P<0.01).
Discussion
Fluorescent semiconductor QDs have attracted tremendous attention over the last decade. The superior optical and electronic properties of QDs over conventional organic dyes, such as high brightness, high photostability, continuous absorption, narrow emission bandwidth, and the ability to simultaneously excite multiple fluorescent colors, make them attractive labels for the development of QDs-IHC imaging for multiplexing cancer biomarker detection on FFPE tissues.24,25 In our study, we confirmed that QDs-IHC is a simple and standardized method for detecting EGFR mutations, and it has high sensitivity and specificity when compared with real-time PCR. In addition, the development of specific antibodies against EGFR mutation proteins might be useful for the diagnosis and treatment of lung cancer.
In general, patients with advanced NSCLC (stage IIIB) do not benefit from surgery alone and are best managed by initial chemotherapy, chemotherapy plus radiation therapy, or radiation therapy alone. EGFR mutation status plays a critical role in the therapeutic decision making for these patients. Nowadays, EGFR-TKIs have been recommended as a first-line therapy in NSCLC patients with activating mutations of EGFR, including gefitinib, erlotinib, afatinib, and so on. Several clinical studies have recently shown that EGFR-TKIs are superior to chemotherapy in NSCLC patients with an EGFR-activating mutation.26,27 Therefore, it is highly important to evaluate the EGFR mutation status in advanced NSCLC patients, especially before any clinical therapy decision is undertaken. Exon 19 del E746-A750 and exon 21 L858R point mutations represent the majority of EGFR mutations.27,28 Analysis of EGFR mutations has become an important tool for targeted therapy in lung cancer, and recently, many efforts have been made to find a more specific and sensitive method (including ARMS and NGS) to detect them.29,30 When compared with conventional DNA direct sequencing, targeted NGS provides a more accurate and clinically useful molecular classification method for lung adenocarcinoma.30 However, these techniques are relatively expensive for routine use in clinical laboratories, and they depend on the quality of the samples.
The goal of our study was to evaluate the accuracy and sensitivity of QDs-IHC in detecting EGFR mutations in NSCLC when compared with traditional IHC and ARMS. Our results showed that EGFR mutations in 40.0% (26/65) of NSCLC patients were detected by QDs-IHC, 12 (46.2%) cases showed E746-A750-specific staining, and 14 (53.8%) patients were L858R mutant-specific. We observed nearly perfect consistency between the positive immunostaining results of QDs-IHC and ADx-ARMS when detecting EGFR mutations status. The sensitivity of QDs-IHC when detecting EGFR mutations, as compared with ADx-ARMS, was 86.7% (26/30); both demonstrated antibody specificity of 100.0% (26/26). However, IHC sensitivity was relatively low (80.0%; 24/30) and the specificity was 92.31% (24/26). Despite the small number of cases in our study, we also identified that QDs-IHC combined with EGFR mutation-specific antibodies to detect an EGFR mutation has a high specificity and sensitivity in NSCLC patients. Furthermore, when compared with ADx-ARMS and traditional IHC, QDs-IHC for EGFR mutation detection can be performed on very small biopsy specimens and pleural effusion cell blocks. This is especially the case in instances when the number of tumor cells of these precious biopsy samples could not reach DNA or RNA extraction requirements. According to our knowledge, this was the first report on the detection of EGFR mutations using QDs-IHC in NSCLC patients.
Our study also showed that these methods could precisely detect EGFR exon 19 deletion or exon 21 L858R mutation status; ADx-ARMS had the best sensitivity, which could also detect exon 18 point mutation and exon 20 insertion. Nevertheless, ADx-ARMS was more expensive and required strict conditions for routine use in clinical laboratories, which is difficult to carry out and popularize in a basic hospital setting. Lung adenocarcinoma showed higher EGFR mutations than squamous cell carcinoma; this mutation was identified by three methods, and it was found that adenocarcinoma exhibits different biological behaviors and requires a different therapeutic strategy.
Conclusion
In the present study, we found that QDs exhibit excellent photostability, a broad excitation spectrum, and a long fluorescence lifetime.20,31 QDs-IHC could accurately detect EGFR mutation protein localization in NSCLC. Taken together, the QDs-IHC technique achieves levels of sensitivity and specificity that are sufficient for detecting EGFR mutation signals in FFPE surgery, biopsy, and cell block specimens, while minimizing costs and optimizing therapeutic options. Combining this method with ADx-ARMS is recommended for the development of improved personalized EGFR-targeted therapeutics.
Acknowledgment
This research is supported by the Natural Science Foundation of China (number 30900652).
Disclosure
The authors report no conflicts of interest in this work.
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. | ||
Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24(28):4539–4544. | ||
Schiller JH, Harrington D, Belani CP, et al; Eastern Cooperative Oncology Group. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346(2):92–98. | ||
Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–2139. | ||
Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–1500. | ||
Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. | ||
Mitsudomi T, Morita S, Yatabe Y, et al; West Japan Oncology Group. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–128. | ||
Tamura K, Okamoto I, Kashii T, et al; West Japan Thoracic Oncology Group. Multicentre prospective phase II trial of gefitinib for advanced non-small cell lung cancer with epidermal growth factor receptor mutations: results of the West Japan Thoracic Oncology Group trial (WJTOG0403). Br J Cancer. 2008;98(5):907–914. | ||
Ono M, Kuwano M. Molecular mechanisms of epidermal growth factor receptor (EGFR) activation and response to gefitinib and other EGFR-targeting drugs. Clin Cancer Res. 2006;12(24):7242–7251. | ||
Mitsudomi T, Kosaka T, Endoh H, et al. Mutations of the epidermal growth factor receptor gene predict prolonged survival after gefitinib treatment in patients with non-small-cell lung cancer with postoperative recurrence. J Clin Oncol. 2005;23(11):2513–2520. | ||
Pirker R, Herth FJ, Kerr KM, et al; European EGFR Workshop Group. Consensus for EGFR mutation testing in non-small cell lung cancer: results from a European workshop. J Thorac Oncol. 2010;5(10):1706–1713. | ||
Zhou S, Zhou M, Peng H, Zeng A, Yu Q, Song X. Comparison of ARMS and direct sequencing for detection of EGFR mutation and prediction of EGFR-TKI efficacy between surgery and biopsy tumor tissues in NSCLC patients. Med Oncol. 2014;31(5):926. | ||
Yu J, Kane S, Wu J, et al. Mutation-specific antibodies for the detection of EGFR mutations in non-small-cell lung cancer. Clin Cancer Res. 2009;15(9):3023–3028. | ||
Brevet M, Arcila M, Ladanyi M. Assessment of EGFR mutation status in lung adenocarcinoma by immunohistochemistry using antibodies specific to the two major forms of mutant EGFR. J Mol Diagn. 2010;12(2):169–176. | ||
Kitamura A, Hosoda W, Sasaki E, Mitsudomi T, Yatabe Y. Immunohistochemical detection of EGFR mutation using mutation-specific antibodies in lung cancer. Clin Cancer Res. 2010;16(13):3349–3355. | ||
Chen C, Xia HS, Gong YP, et al. The quantitative detection of total HER2 load by quantum dots and the identification of a new subtype of breast cancer with different 5-year prognosis. Biomaterials. 2010;31(33):8818–8825. | ||
Chen C, Peng J, Xia HS, et al. Quantum dots-based immunofluorescence technology for the quantitative determination of HER2 expression in breast cancer. Biomaterials. 2009;30(15):2912–2918. | ||
Yezhelyev MV, Al-Hajj A, Morris C, et al. In situ molecular profiling of breast cancer biomarkers with multicolor quantum dots. Adv Mater. 2007;19(20):3146–3151. | ||
Zhao X, He Y, Gao J, et al. Caveolin-1 expression level in cancer associated fibroblasts predicts outcome in gastric cancer. PLoS One. 2013;8(3):e59102. | ||
Chen H, Xue J, Zhang Y, Zhu X, Gao J, Yu B. Comparison of quantum dots immunofluorescence histochemistry and conventional immunohistochemistry for the detection of caveolin-1 and PCNA in the lung cancer tissue microarray. J Mol Histol. 2009;40(4):261–268. | ||
Angulo B, Conde E, Suárez-Gauthier A, et al. A comparison of EGFR mutation testing methods in lung carcinoma: direct sequencing, real-time PCR and immunohistochemistry. PLoS One. 2012;7(8):e43842. | ||
Xiong Y, Bai Y, Leong N, et al. Immunohistochemical detection of mutations in the epidermal growth factor receptor gene in lung adenocarcinomas using mutation-specific antibodies. Diagn Pathol. 2013;8:27. | ||
Hu YC, Zhang Q, Huang YH, Liu YF, Chen HL. Comparison of two methods to extract DNA from formalin-fixed, paraffin-embedded tissues and their impact on EGFR mutation detection in non-small cell lung carcinoma. Asian Pac J Cancer Prev. 2014;15(6):2733–2737. | ||
Xu H, Xu J, Wang X, Wu D, Chen ZG, Wang AY. Quantum dot-based, quantitative, and multiplexed assay for tissue staining. ACS Appl Mater Interfaces. 2013;5(8):2901–2907. | ||
Zhu Y, Hong H, Xu ZP, Li Z, Cai W. Quantum dot-based nanoprobes for in vivo targeted imaging. Curr Mol Med. 2013;13(10):1549–1567. | ||
Hirsch F, Camidge RD, Kabbinavar F, et al. Biomarker status correlates with clinical benefit: phase 2 study of single-agent erlotinib (E) or E intercalated with carboplatin and paclitaxel (ECP) in an EGFR biomarker selected NSCLC population. J Thorac Oncol. 2008;Suppl 4:S267. | ||
Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7(3):169–181. | ||
Riely GJ, Pao W, Pham D, et al. Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12(3 Pt 1):839–844. | ||
Ohnishi H, Ohtsuka K, Ooide A, Matsushima S, Goya T, Watanabe T. A simple and sensitive method for detecting major mutations within the tyrosine kinase domain of the epidermal growth factor receptor gene in non-small-cell lung carcinoma. Diagn Mol Pathol. 2006;15(2):101–108. | ||
Han JY, Kim SH, Lee YS, et al. Comparison of targeted next-generation sequencing with conventional sequencing for predicting the responsiveness to epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) therapy in never-smokers with lung adenocarcinoma. Lung Cancer. 2014;85(2):161–167. | ||
Akhtar RS, Latham CB, Siniscalco D, Fuccio C, Roth KA. Immunohistochemical detection with quantum dots. Methods Mol Biol. 2007;374:11–28. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.