Back to Journals » Orthopedic Research and Reviews » Volume 16
High Complication and Revision Rates in Anatomical Total Shoulder Arthroplasty with the Combination of Polyethylene and Cementless Convertible Metal-Backed Glenoid Components: A Retrospective Cohort Study
Authors Hanisch KWJ
Received 18 October 2023
Accepted for publication 8 February 2024
Published 27 February 2024 Volume 2024:16 Pages 93—101
DOI https://doi.org/10.2147/ORR.S442128
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Clark Hung
Klaus WJ Hanisch
The Orthopedic Department, University Hospital South West Denmark, Esbjerg, 6700, Denmark
Correspondence: Klaus WJ Hanisch, Tel +4520535332, Email [email protected]
Background: Historically, Metal-Backed (MB) glenoid components in anatomical total Shoulder arthroplasty (aTSA) are prone to failure primarily due to loosening between the metal and bony surface. However, newer generations of MB glenoid components have performed well in reverse shoulder arthroplasty (RSA), with convertibility being considered to be the most significant benefit of MB components. Theoretically, MB components may be a viable option in “Rotator cuff at risk” cases. The aim of this study is to compare revisions versus revision-free survivorship and highlight problems associated with using convertible MB glenoid components in aTSA.
Methods: Between December 2015 and September 2018, aTSA was performed on 30 patients utilizing 32 implants with convertible MB glenoid (two patients were operated bilaterally). The first investigation was performed at a mean of 55.9 months (43– 76) by search in the national registry for revisions with twelve cases. The second FU on all remaining patients without revisions was conducted at a mean of 54.9 months (46– 71) through physical examination with fourteen patients (sixteen implants), with four patients missing. Demographic data, indications, complications, revisions, and re-operations were recorded for each patient.
Results: High rates of complications led to revisions or re-operation in aTSA in combination with MB (15/32). Seven problems were associated with polyethylene (PE), which included loosening, disengagement, or wear. Eight complications were not directly associated with the MB component. There was one with loosening on the metal–bone interface side. Conversion to RSA was possible in three cases, and secondary cuff failure was seen once. High infection rates (2/32) led to a different strategy for antibiotics and preoperative preparations.
Conclusion: MB glenoid components caused unacceptably high complication and revision rates in aTSA. PE wear, disengagement, or loosening were the main reasons for revisions. Therefore, procedures with MB glenoid components were abandoned in aTSA.
Level of Evidence: Level IV case series, treatment study.
Keywords: shoulder joint, arthroplasty, replacement, metal-backed, uncemented glenoid, revision rate
Introduction
First-generation metal-backed (MB) glenoid components in anatomical total shoulder arthroplasty (aTSA) are prone to failure primarily due to loosening and severe osteolysis,1 which has led practitioners to abandon this strategy. However, a newer generation of this technology with an optimized metal–bone interface showed promising results in a 2010 study,2 with the authors concluding that MB was a viable option for total shoulder replacement. The potential benefits of revision surgery with convertible non-cemented MB were presented by Kany.3 Indications for MB in older patients with thin rotator cuffs were found by Katz.4 There have been previous reports of failure, including polyethylene (PE) disengagement/wear (Lima) or shearing at the metal–PE interface (Zimmer TM glenoid). In late 2015, our department introduced the Eclipse system by Arthrex as a replacement option for patients with osteoarthritis, arthritis, fracture sequelae, or caput necrosis with intact rotator cuffs. In combination with this stemless system, we used an uncemented convertible MB component on the glenoid side with a thin polyethylene (PE) inlay.
The aim of this study is to compare revisions versus revision-free survivorship of MB glenoid components and analyze failures. It highlights the problem of high complication rates with convertible MB glenoid with the PE inlay when used in aTSA.
Earlier modular third-generation glenoid designs by the same design team5 showed favorable results in 115 TSAs, with only two revisions necessary (one subscapularis tendon failure and one PE disengagement) after >12 months of follow-up. When the glenoid baseplate was fixed with a cage screw, catastrophic failures were reported6 with 17 revisions out of 45 implants, of which 13 were related to glenoid loosening (metal–bone/metal–metal) and one involved PE delamination.
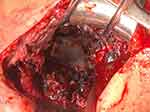
One example of PE wear intraoperative is shown by a picture (Figures 1 and 2) and in X-ray series (Figure 3) with revision to reverse shoulder arthroplasty (RSA) (Figure 4). A case of persistent posterior instability (Walch C) is shown in the CT scan series (Figure 5). Figure 6 shows the survival rate for aTSA with convertible MB glenoid components (Kaplan–Meier).
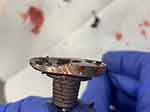 |
Figure 2 Metal defect in the cranial part of the baseplate. |
 |
Figure 4 Status after revision to Univers reverse by Arthrex (Figure 4). |
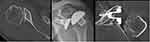 |
Figure 5 Case example 2: X-ray and computerized tomography (CT) show Walch type C, primary posterior luxation X-ray and correction of retroversion (CT) still posterior luxation. |
 |
Figure 6 The survival rate for Arthrex Eclipse with MB glenoid system. |
Materials and Methods
The following results represent a single-center cohort aTSA group receiving a stemless humeral system with a cementless convertible MB glenoid component and PE inlay. All patients gave written consent to participate in this study, ethical approval was not necessary, due to the retrospective character of this study. Based on the local hospital registry and journal system, we retrospectively enrolled all relevant cases from December 2015 to September 2018 of our department (Esbjerg, Denmark). Overall, 30 patients received 32 stemless aTSA implants. Se Flow chart Figure 7. There were thirteen female patients with a median age of 64.8 years (43–75) and seventeen male patients with a median age of 63.3 years (46–78). First, the national joint replacement registry was searched for revisions or reoperations at a mean of 55.9 months (43–76). Twelve cases were found, with the remaining eighteen patients (twenty implants) remaining unknown. For the group without revisions or reoperations, a regular follow-up was conducted at a mean of 54.9 months (46–71). Fourteen patients with sixteen implants were examined. We found three further complications, all of which were revised to RSA.
 |
Figure 7 Investigation by registry found 12/30 revisions. Second FU by examination found further three revisions in 14 patient (16 implants), four missing to second FU. |
Four patients were missing for the second FU: One died (not related to TSA), one had severe medical and psychological complaints and denied further follow-up, and her WOOS at one year was 87. One patient had follow-up at different institutions; they found signs of secondary cuff failure, and his WOOS at one year was only 17.9. No further surgery was suggested. The last patient had been operated because of psoriasis arthritis at the age of 43. At her last FU at our department, three-month post-operative, her WOOS was 94.7 points, and she was very satisfied. She did not respond and was not reachable. Her medical journal reveals no shoulder complaints.
The group with complications consisted of five females with an average age = 67.7 (62–75) and ten males with an average age = 58.1 (53–72). Preoperative Walch classifications were defined by CT/MR type A1 N4, type A2 N2, type B1 N2, type B2 N2, and type C1 N2. Three were missing classifications, with no preoperative CT/MR scan. Indications were mainly primary osteoarthritis N12, two secondary osteoarthritis after fractures, and one caput necrosis.
The group without complications consisted of eight females with nine implants, with an average age of 64.1 (43–72) and seven males with eight implants, with an average age of 65.7 (46–74). Preoperative Walch classifications were type A1 N7, type A2 N2, type B1 N4, type B2 N2, zero type C. Two were missing classifications, and CT/MR scanning was not viable. The indication was primary osteoarthritis in all cases.
Results of Eclipse with metal-backed glenoid system are shown in Table 1.
 |
Table 1 Results of Eclipse with Metal-Backed Glenoid System |
All but one of the primary procedures were performed by the author, who is specially trained and conducts >80 replacements per year and has conducted >500 shoulder replacements in total. A standard deltopectoral approach was used in all cases. Tenotomy of subscapularis tendon (SCT) and the long head of biceps were performed in all cases. The SCT was re-fixated by trans osseous sutures (Fiberwire 5 Arthrex). The MB baseplate was positioned centrally, and if necessary, orientation was adopted by anterior reaming.
Statistical Analysis
For descriptive purposes, chi-square statistics for categorical variable and t-test for numerical variable were utilized; survival rate was shown using Kaplan–Meier plots. Statistical analyses were done in STATA version 17.0.
Results
For the patients receiving aTSA with convertible MB glenoid components, fifteen revisions or re-operations out of 32 implants were necessary (a revision rate of 46.8%). Two re-operations were necessary: one first stage (Girdle stone status) because of infection and one failed attempt to repair subscapularis. Table 2 compares complication group versus non-complication group.
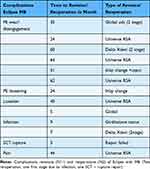 |
Table 2 Complications and Revisions Timetable (Table 2) |
Seven cases had complications due to PE wear, disengagement, or inlay loosening; all received revision surgery. The complications occurred at a mean time of 41.7 months (24–62). Two patients with Walch type C had posterior luxation, though we corrected the retroversion to about 10° in both cases. Two patients suffered from Propionibacterium acne/Cutibacterium acnes or Staphylococcus epidermidis infections. One was pain free after the first stage and did not want further surgery, while the other received the two-stage revision to RSA. Both patients received oxacillin perioperatively, only. By 2019, we changed the perioperative antibiotics to a combination of cephalosporin and penicillin, if tolerated, and routinely used benzoyl peroxide cloth in the preparation. However, no patients in this cohort were involved in this newer regime. One subscapularis rupture occurred five weeks after replacement while the patient was gardening; the patient showed insufficient compliance with post-operative restrictions. An attempt to re-repair the tendon was unsuccessful, and the shoulder remained unstable. However, the patient tolerated his condition. One patient had signs of loosening of the glenoid component on X-rays, and she was revised to RSA.
Table 2 compares complication group versus non-complication group. Most complications occurred in younger males with more severe glenoid deformity. Mean age men 58.1 (53–73) vs. 65.7 (46–74) t-test: p-value 0.09, mean age women 67.7 (62–75) vs 64.1 (43–72) t-test: p-value 0.33. Gender difference did not reach a statistical significance due to the small sample size. 5/ 10 vs 8/9 chis-square-test: p-value 0.22.
Walch classifications were compared in terms of preoperative based on magnetic resonance imaging or CT.
In the group with complications (n = 15), Preoperative Walch classifications were defined by CT/MR type A1 N4, type A2 N2, type B1 N2, type B2 N2, and type C1 N2; three were missing classifications, and CT/MR scanning was not viable.
In the non-complication group (n = 17 implants). Preoperative Walch classifications were type A1 N7, type A2 N2, type B1 N4, type B2 N2, zero type C. Two were missing classifications, and CT/MR scanning was not viable.
WOOS, as clinical outcome at one year post-operative (68.8 vs 77), showed non-clinically relevant difference (MCID 12.3 points). This is not unexpected, because complications typically occur several years after surgery. At last FU in the non-complication group, thirteen answered WOOS. The mean result was 79.9 points.
Figure 6 shows the survival rate for aTSA with convertible MB glenoid components (Kaplan–Meier).
Discussion
Our data showed a high rate of complications that led to revisions or re-operations most often associated with PE wear and disengagement on the convertible, inlay type MB glenoid implant from Arthrex. We primarily used the thin PE version, revising to the thicker plus variant in two cases. The earliest PE complications occurred within two years, with a mean of 41.7 months. Conversely, we experienced good results with the same MB baseplate used in combination with the Universe reverse implant. The thickness of the PE inlay may play a role in PE wear. The use of the thicker versions is probably preferable. On the other hand, there is the risk of overstuffing. The fixation of the PE inlay to the baseplate may be a weak point; disengagement without relevant trauma suggests a potential issue. Uneven load done to persistent instability or malposition seems to be the most important problem on the PE inlay/ MB- side. The potential convertibility from anatomical to reverse in patients with “rotator cuff at risk” -or younger patients with severe glenoid deformity, aimed to avoid reverse replacement in the first surgical treatment were reasons to prefer this type of implant instead of the standard cemented all PE glenoid implants. In Walch C1 type anterior, retaining and correcting the retroversion to about 10 degrees did not solve to problem. Both cases remained unstable and incongruent. Soft tissue balancing was not adequate, and RSA should be used primarily.
Methods to avoid PE-related complications are unclear. Overstuffing or over-tensioning of soft tissue, changes in joint geometry, and instability have been proposed as reasons for failure. Mal positioning is obvious.
Non-implant-related complications could be avoided by optimized patient selection, a different antibiotic strategy, and perioperative preparation to avoid infections. However, insufficient compliance continues to be a concern.
Newer generations of MB glenoid implants show promising results and have the potential benefit of convertibility. A review of newer generations of MB components by Kim7 showed that they had reduced loosening and failure rates versus conventional MB components. However, registry studies from Australia8 and New Zealand9 showed significantly higher revision rates for cementless (17.9%) vs cemented (3.7%) glenoid components within five years. Glenoid wear was found in only seven out of 3159 cementless conventional TSA operations in the Australian registry. The New Zealand Joint Registry showed five times higher revision rates for cementless glenoid components. The trend for choice of glenoid fixation in conventional TSA showed a peak of 46.7% using cementless glenoid components in 2011, declining to 20.1% in 2017. In patients younger than 60 years, Gauci10 found three times higher survival of the cemented PE glenoid vs cementless MB glenoid component ten years after aTSA. One earlier MB glenoid implant with a modular baseplate and cage screw by the same designer team5 failed, mainly due to loosening in the metal/metal or metal/bone interface; only one PE delamination led to revision surgery. This implant has been withdrawn.8 Castagna and Garofalo11 reported disengagement or wear problems in their journey of the glenoid in aTSA utilizing the SMR implant system by Lima.
Cheung12 reported that PE insert exchange was possible in 12 out of 110 revisions, including glenoid components. PE wear or displacement was the primary reason for revision, but rotator cuff tearing (six full and two partial thicknesses) and instability were also found in 8/12 shoulders.
Information from the Danish Shoulder Arthroplasty Registry in April 2022 shows that the use of MB components in aTSA in Denmark has been limited. The registry is mandatory and covers all primary and secondary shoulder replacements in Denmark. The brand most often used in combination with MB components is the Eclipse system from Arthrex. No other revision surgery was found in this cohort.
The concept of MB glenoid in aTSA is of concern; based on our findings we stopped this procedure. Whether there is a space for aTSA with MB in combination with structural bone grafting may still be debatable.
Limitations
The patients included represent a small, single-center cohort, including a single surgeon, except for one instance, individual technical failure, or patient selection may all contribute to the results.
Conclusions
In aTSA, surgical procedures, cementless MB glenoid components have shown high complication, revision, and re-operation rates. In these cases, PE wear and disengagement are the main reasons for revisions. We stopped performing aTSA procedures with MB glenoid components based on these results.
Ethics and Consent Statements
We have obtained written consent from all participants to use and publish data. Ethics approval is not necessary.
Funding
The author did not receive any financial payments or other benefits from any commercial entity related to the subject of this article.
Disclosure
The author reports no conflicts of interest in this work.
References
1. Boileau P, Avidor C, Krishnan SG, Walch G, Kempf J-F, Molé D. Cemented polyethylene versus uncemented metal-backed glenoid components in total shoulder arthroplasty: a prospective, double-blind, randomized study. J Shoulder Elbow Surg. 2002;11(4):351–359. doi:10.1067/mse.2002.125807
2. Castagna A, Randelli M, Garofalo R, Maradei L, Giardella A, Borroni M. Mid-term results of a metal-backed glenoid component in total shoulder replacement. J Bone Joint Surg. 2010;92(10):1410–1415. doi:10.1302/0301-620X.92B10.23578
3. Kany J, Amouyel T, Flamand O, Katz D, Valenti P. A convertible shoulder system: is it useful in total shoulder arthroplasty revisions? Int Orthop. 2015;39(2):299–304. doi:10.1007/s00264-014-2563-z
4. Katz D, Kany J, Valenti P, Sauzières P, Gleyze P, El Kholti K. New design of a cementless glenoid component in unconstrained shoulder arthroplasty: a prospective medium-term analysis of 143 cases. Eur J Orthop Surg Traumatol. 2013;23(1):27–34. doi:10.1007/s00590-012-1109-6
5. Habermayer P, Engel G. Surgical technique for shoulder arthroplasty. Operat Orthop Traumatol. 2004;16:339.
6. Magosch P, Lichtenberg S, Habermeyer P. Survival of stemless humeral head replacement in anatomic shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg. 2021;30(7):e343–e355. doi:10.1016/j.jse.2020.09.034
7. Kim DM, Alabdullatif F, Aldeghaither M, et al. Do modern designs of metal-backed glenoid components show improved clinical results in total shoulder arthroplasty? A systematic review of the literature. Orthop J Sports Med. 2020;8(9):2325967120950307. doi:10.1177/2325967120950307
8. Page RS, Pai V, Eng K, Bain G, Graves S, Lorimer M. Cementless versus cemented glenoid components in conventional total shoulder joint arthroplasty: analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J Shoulder Elbow Surg. 2018;27(10):1859–1865. doi:10.1016/j.jse.2018.03.017
9. Sharplin PK, Frampton CMA, Hirner M. Cemented vs. uncemented glenoid fixation in total shoulder arthroplasty for osteoarthritis: a New Zealand Joint Registry study. J Shoulder Elbow Surg. 2020;29(10):2097–2103. doi:10.1016/j.jse.2020.03.008
10. Gauci MO, Bonnevialle N, Moineau G, Baba M, Walch G, Boileau P. Anatomical total shoulder arthroplasty in young patients with osteoarthritis: all-polyethylene versus metal-backed glenoid. Bone Joint J. 2018;100-B(4):485–492. doi:10.1302/0301-620X.100B4.BJJ-2017-0495.R2
11. Castagna A, Garofalo R. Journey of the glenoid in anatomic total shoulder replacement. Shoulder Elbow. 2019;11(2):140–148. doi:10.1177/1758573218790119
12. Cheung E, Sperling JW, Cofield RH. Polyethylene insert exchange for wear after total shoulder arthroplasty. JSES. 2007;16(1):574–578. doi:10.1016/j.jse.2006.12.009
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.