Back to Journals » Nature and Science of Sleep » Volume 16
Factors Associated with Persistent Obstructive Sleep Apnea After Bariatric Surgery: A Narrative Review
Authors Demaeyer N, Bruyneel M
Received 5 November 2023
Accepted for publication 31 January 2024
Published 7 February 2024 Volume 2024:16 Pages 111—123
DOI https://doi.org/10.2147/NSS.S448346
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sarah L Appleton
Nathalie Demaeyer,1 Marie Bruyneel1,2
1Department of Pneumology, CHU Saint-Pierre, Université Libre de Bruxelles, Brussels, Belgium; 2Department of Pneumology, CHU Brugmann, Université Libre de Bruxelles, Brussels, Belgium
Correspondence: Marie Bruyneel, Department of Pneumology, CHU Saint-Pierre, 322 rue haute, Brussels, 1000, Belgium, Tel +3225354219, Fax +3225353362, Email [email protected]
Abstract: The prevalence of obstructive sleep apnea (OSA) among the bariatric surgery population is estimated to be 45– 70%. However, weight loss obtained by bariatric surgery is not always associated with full remission of OSA, suggesting that other confounding factors are present. This article aims to review the current literature, focusing on factors that could predict the persistence of OSA after bariatric surgery. For this purpose, relevant studies of more than 50 patients that assessed pre- and post-operative presence and severity of OSA detected by poly(somno)graphy (PG/PSG) in bariatric populations were collected. Six retrospective and prospective studies were evaluated that included 1302 OSA patients, with a BMI range of 42.6 to 56 kg/m2, age range of 44.8 to 50.7 years, and percentage of women ranging from 45% to 91%. The studies were very heterogeneous regarding type of bariatric surgery, diagnostic criteria for OSA and OSA remission, and delay of OSA reassessment. OSA remission was observed in 26% to 76% of patients at 11– 12 months post-surgery. Loss to follow-up was high in all studies, leading to a potential underestimation of OSA remission. Based on this limited sample of bariatric patients, age, pre-operative OSA severity, proportion of weight loss, and type 2 diabetes (T2D) were identified as factors associated with OSA persistence but the results were inconsistent between studies regarding the impact of age and the magnitude of weight loss. Several other factors may potentially lead to OSA persistence in the bariatric surgery population, such as fat distribution, ethnicity, anatomical predisposition, pathophysiological traits, supine position, and REM-predominant hypopnea and apnea. Further well-conducted multicentric prospective studies are needed to document the importance of these factors to achieve a better understanding of OSA persistence after bariatric surgery in obese patients.
Keywords: bariatric surgery, gastric sleeve, gastric bypass, obstructive sleep apnea, polygraphy, polysomnography
Introduction
Obstructive sleep apnea (OSA) results from repeated decrease or cessation of upper airway (UA) flow during sleep.1 This condition, if left untreated, is associated with cardiovascular comorbidities (eg, myocardial infarction, diabetes, stroke, hypertension (HT)), increased risk of motor vehicle accidents, and premature mortality.2 OSA is a heterogenous disease with varied predisposing factors, pathophysiological mechanisms, and multiple clinical manifestations (eg, excessive daytime sleepiness (EDS), fatigue, choking, gasping) while a significant proportion of patients who exhibit OSA on a sleep record are asymptomatic.3–7 The prevalence of OSA syndrome (OSAS; ie, OSA associated with symptoms and/or comorbidities) is estimated to be 3–7% in adult men and 2–5% in adult women in the general population1 and this is likely to grow over time due to the obesity epidemic.
In order to characterize the severity of OSA, obstructive apnea/hypopnea index (AHI) values of 5–14, 15–29, and ≥30 are used to define mild, moderate, and severe OSA, respectively. However, AHI is poorly correlated with the severity of clinical symptoms7 despite the fact that there is a linear relationship between AHI and HT2 and AHI correlates with overall mortality in OSA patients.8 The non-modifiable risk factors for OSA are aging, ethnicity, male gender, menopause, and anatomic features.3,5,9,10
OSA is also associated with endocrine disorders (eg, acromegaly, hypothyroidism, type 2 diabetes (T2D)), heart failure, and renal insufficiency and, in these cases, the treatment of the underlying disorder can improve OSA. However, the main modifiable risk factor for OSA remains obesity3,4,11,12 while sedentarity and lifestyle factors (eg, use of sedatives and hypnotics, alcohol and tobacco) also play a significant role.13–15 Obesity is a strong risk factor for OSA, resulting in reduction of lung volume, related to an increase in abdominal fat, and narrowing of the UA due to fat deposition in the oropharyngeal muscle and the surrounding tissues such as the tongue, soft palate, uvula, and lateral pharyngeal wall and increase in neck circumference.4,6,12 Weight loss is thus an efficient treatment in OSA but weight loss takes time and can be only be proposed as the sole treatment in mild-to-moderate OSA patients. In addition, the majority of patients are not able to maintain weight loss over the long-term, and gaining weight is associated with OSA reoccurrence/aggravation.12 Therefore, bariatric surgery is a common strategy used to obtain a sustained and significant weight loss in obese patients.16–20 The prevalence of OSA among the bariatric population is very high compared to the general population and is estimated to be 45%–70%.16,17,21 A recent meta-analysis based on 32 studies that included 2310 patients, reported an OSA remission rate of 65%. Remission was associated with improvements in sleep quality and EDS.19
Nevertheless, weight loss obtained by bariatric surgery is not always associated with full remission of OSA, suggesting that other factors can contribute to OSA. Indeed, craniofacial disharmony is also a risk factor for OSA, related to reduction of pharyngeal airway space, an inferiorly placed hyoid bone, or increased anterior facial height.9 Reduced pharyngeal airway space is associated with the position of tongue, soft palate, maxilla, and mandibula.9 An inferiorly placed hyoid bone increases the risk of pharyngeal collapse. The co-existence of bi-maxillary retrusion and acute cranial base angle may reduce the antero-posterior dimension of the pharyngeal airway. Increases in soft palate thickness and length can also contribute to OSA. Furthermore, increased area and length of the tongue among OSA patients suggests they have a more posterior position of the tongue.9 In addition, people of African descent have a significantly larger tongue that could make them more susceptible to OSA.22
In addition to anatomic factors, the pathophysiological PALM classification has been developed to explain the dynamic factors associated with OSA.6 Four components contribute to OSA. The first is anatomic collapsibility, reflected by the critical occlusion pressure (Pcrit). Fat deposition in the UA and abdominal area (reduced lung volume) increase anatomic collapsibility. Snoring and repeated pharyngeal depression during obstructive events lead to chronic inflammation of the soft tissue, reducing the diameter of the UA and increasing anatomic collapsibility. Moreover, a supine position with rising of the diaphragm, and backdrop of the jaw and tongue also increases anatomic collapsibility. Pcrit can be measured by reduction of continuous positive airway pressure (CPAP) levels, during sleep, determining the pressure at which the pharyngeal airway occludes.6
The second component in the PALM classification is the muscular upper airway gain (UAG). Different muscles are responsible for UA patency: muscles that regulate the position of the hyoid bone, tongue base muscles and pharyngeal constrictors, and muscles of the soft palate.
The interaction between anatomic collapsibility and neuromuscular compensation determines the extent of OSA. Either the neuromuscular compensation offsets the anatomic collapsibility (in all positions and in all sleep stages) or not.6
The third aspect of the PALM system is the loop gain (LG), including 3 components: the control component, via chemoreceptors, the exchange component, via the lungs, and the connection component, regulated by the circulation. LG > 1 reflects a (hyper-)sensitive system leading to excessive ventilatory response (periodic breathing), while LG < 1 is associated with a more stable ventilatory system.6
The last component of the PALM classification is the arousal threshold (AT), defined as the level of inspiratory effort at which obstructive events terminate with an arousal from sleep. If all the mechanisms successfully achieve UA patency and sustainable ventilation (VE), an arousal from sleep may not be required to support ventilation. The threshold required to achieve UA reopening (associated with sustainable VE) is defined as the threshold of effective recruitment (TER). The arousal at the end of an obstructive event occurs when the AT < TER or when hyperventilation follows UA reopening (stimulation of the arousal center). A low AT causes sleep fragmentation, and ventilatory and pharyngeal muscular instability, promoting obstructive events recurrence and EDS.6
Approximately 20% of OSA patients have high anatomic collapsibility and 80% have a combination of anatomic disharmonies and anomalies in AT/UAG/LG.6
PALM 1 (23%) represents very high UA collapsibility (Pcrit > 2.5 cm H2O). In this case, weight loss, positional therapy, oral appliance (OA), CPAP, and UA surgery are the first-line treatments (anatomical treatments).6
PALM 2 (57%) represents intermediate collapsibility (Pcrit between +2.5 and −2.5 cm H2O).6 Patients in subgroup 2a (no major non-anatomical impairment) are candidates for anatomical treatment. Patients in subgroup 2b (one or more non-anatomical impairments) are candidates for a combination of anatomical and non-anatomical treatments.6
PALM 3 (19%) represents low UA collapsibility (Pcrit < −2.5 cm H2O). The treatment for these patients would be non-CPAP treatment options: weight loss, OA, oxygen, and drugs targeting the LG or the AT (eg, acetazolamide, Z-drugs).6,23
A therapeutic level of CPAP ≤ 8 cm H2O corresponds to the PALM 3 group of patients.6 From a pathophysiological point of view, patients exhibiting a PALM 1 and PALM 2a pattern are expected to improve with weight loss. Finally, aging is associated with an increased prevalence of OSA in both sexes, but remains higher in males.24
The multiple factors implicated in OSA physiopathology may explain the potential persistence of OSA after bariatric surgery in obese patients. The aim of the present narrative review was to identify factors associated with persistence of OSA after bariatric surgery.
Methods
For this analysis, relevant studies assessing pre- and post-operative presence and severity of OSA detected by PG/PSG in bariatric populations were selected.
Eligibility Criteria of the Studies
Inclusion criteria: Prospective and retrospective studies assessing pre- and post-operative presence and severity of OSA by polygraphy (PG) or polysomnography (PSG) in adults undergoing bariatric surgery. Full text available in English, in PubMed central and Scopus databases. Relevant keywords: bariatric, gastric sleeve, gastric bypass, sleep apnea, hypopnea, polygraphy, polysomnography.
Exclusion criteria: the studies with a sample size less than 50 patients were excluded. Indeed, 45 participants would have to be included in the sample to show a AHI reduction of −25/h after bariatric surgery,25 with a power at 80%, Type I error (α)=0,05, Type II error (β)=0,2. If primary outcome was mean difference of BMI of – 13,2 (Type I error (α)=0.05 Type II error (β)=0.2 Power = 0.8), a number of 66 patients was required.25 Based on these two hypotheses, it seems relevant that studies with a final sample size of < 45–50 patients were excluded due to high risk of bias.
Results
Six studies of interest that met inclusion criteria were selected, after exclusion of 23 studies with sample size between 8 and 46 patients (median: 24 patients), totalizing 574 patients.
The six studies are summarized in Table 1. Yanari et al17 and Morong et al26 were retrospective studies. The other four studies, de Raaff et al,27 Peromaa-Havisto et al,20 Haines et al,28 and Ravesloot et al,29 were prospective studies. The only multicentric study was Peromaa-Havisto et al.20 These studies included 1302 OSA patients at inclusion (PG/PSG confirmed OSA cases among patients who are going to undergo bariatric surgery) and 690 patients in the final study samples. The flow chart of patient inclusion is shown in Figure 1.
 |
Table 1 Summary of the Six Included Studies Selected from the Literature Review Assessing Pre- and Post-Operative Presence and Severity of OSA by PG or PSG in Adults Undergoing Bariatric Surgery |
Pre-op mean body mass index (BMI) ranged from 42.6 to 56 kg/m2 and mean age varied from 44.8 to 50.7 years. Comorbidities, if reported, were frequent among these patients, with a high prevalence of T2D (from 42% to 67.2%)17,20,27 and HT (from 57.6% to 62%).20,27 The majority of patients were females, ranging from 45% to 91% of the study population.
Patients were assessed by PG and/or PSG in all studies pre- and post-operatively. OSA severity was assessed using AHI calculated during PG/PSG or respiratory disturbance index (RDI), that included respiratory-related arousals, with different severity cut-offs (Haines et al28) and according to different American Academy of Sleep Medicine (AASM) criteria.
Surgical procedures were Roux-en-Y gastric bypass (RYGB) for de Raaff et al,27 Peromaa-Havisto et al,20 and Haines et al,28 sleeve gastrectomy (SG) for Yanari et al,17 and Ravesloot et al29 compared laparoscopic gastric banding (LAGB) vs RYGB vs SG. In one study, the applied surgery was not reported (Morong et al26).
Six-to-nine month post-surgery reassessment was available in 2 studies (de Raaff et al27 and Morong et al26). Post-operative reevaluation was at 6 months and one year in 2 studies (Yanari et al17 and Ravesloot et al29). Changes at 11 to 12 months were assessed in 2 studies (Peromaa-Havisto et al20 and Haines et al28).
During the postoperative OSA reassessment, sleep tests were repeated, but it is important to note that in de Raaff et al27 and in Ravesloot et al29 some patients had PG instead of PSG and the reverse. Post-operative mean BMI decreased significantly in all studies with a decrease ranging between 9.1 and 18 kg/m2. The remission rate for T2D ranged between 45% and 66.7%.17,20 For HT, this was 73% but was only reported by Peromaa et al.20 OSA remission rates exhibited quite a bit of variation among the studies, at between 24% and 74%, but the definition of remission varied between studies: AHI< 5, AHI < 15, or “no more need of CPAP/BiPAP”.
Regarding factors associated with persistence of OSA after bariatric surgery, several analyses were performed. Ravesloot et al29 observed that preoperative BMI was not related to OSA remission. However, De Raaff et al27 determined that excess weight loss (EWL) (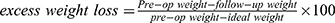 ) < 60% was a predictive factor of OSA persistence when Ravesloot et al29 showed no linear relationship between the extent of weight loss and improvement in OSA. Pre- and post-operative BMI were relatively similar in both studies.
) < 60% was a predictive factor of OSA persistence when Ravesloot et al29 showed no linear relationship between the extent of weight loss and improvement in OSA. Pre- and post-operative BMI were relatively similar in both studies.
de Raaff et al27 concluded that severe apnea (AHI ≥ 30) before surgery was a predictive factor of OSA persistence. Yanari et al4 also observed that pre-operative AHI was an independent prognostic factor of OSA persistence. In Ravesloot et al,29 age was not a predictive factor of OSA remission, unlike the results from de Raaff et al27 in which older age predicted OSA persistence. Regarding comorbidities, Yanari et al17 found that high glycated hemoglobin (HbA1c) post-surgery was an independent prognostic factor for OSA persistence. HT was also identified as a predictive factor of OSA persistence by de Raaff et al.27
Discussion
This narrative review highlights the rate of remission of OSA one year after bariatric surgery. This review was strengthened by having selected studies with a significant sample size at the time of follow-up and a homogeneous and accurate assessment of pre- and post-operative OSA. The lack of well-conducted studies in the field is quite surprising considering the volume of bariatric surgery performed each year. Indeed, there were 18,909 procedures in Germany in 2022.30 As obesity is the strongest risk factor for OSA, among the bariatric population, OSA prevalence reaches 45%–70%.16,17,21
Difficulties Related to the Selection of Studies
When collecting literature on cure rates in OSA after bariatric surgery, several difficulties were faced in terms of selection of high-quality studies. Studies on the topic are highly heterogeneous, some were prospective, others were retrospective, and no randomized controlled trials with an adequate sample size were available. There was also high variability in the assessment of OSA patients among the bariatric surgery population, with vague definitions of OSA (eg, self-declaration of suffering from the disorder or self-reported use of CPAP/bilevel positive airway pressure [BiPAP]). A large number of studies were excluded for insufficient number of patients in the final study sample. The “lost to follow-up” category was high in most of the studies. This is also observed in other settings, as seen in a longitudinal study exploring remission of gastroesophageal reflux after a median of 77 months post-Nissen fundoplication, the “lost to follow-up” group was as high as 14%.31 The long timing of the post-surgery period and the inconvenience of coming back for PSG could be a possible explanation for the loss of patients. The patients with fewer or no symptoms of OSA after surgery were probably more reluctant to come to the follow-up appointments. Indeed, the self-reported discontinuation of CPAP/BiPAP is an inaccurate way to evaluate OSA remission as 70% of the patients are non-compliant with CPAP after bariatric surgery.27
Heterogeneity of the Definition of OSA and OSA Remission
Among the selected studies for the present review, OSA definition was also based on two different sleep recordings, PG or PSG, for evaluating AHI. This could be a source of bias for OSA prevalence as diagnosis based on PG can lead to underestimation of OSA severity or false negative results. Indeed, patients diagnosed using PG are likely to have a 30% lower AHI compared to patients undergoing diagnosis by PSG.32 Moreover, 14% of patients would be missed for a severe OSA diagnosis with PG instead of PSG.32 The use of different AASM criteria (1999, 2007) can also lead to variability in AHI, especially regarding the number of scored hypopneas, as 2007 criteria are more severe.33 For OSA reassessment after surgery, some study teams have used PSG instead of PG, and this can lead to the reverse, an underestimation of remission rate. Of note, variability of remission rate definition AHI < 5, AHI < 15 or “no more need of CPAP/BiPAP” can also explain the large variation in remission rates among the studies.
Another concern was related to CPAP wash-out in CPAP users before performing post-surgery PG/PSG. This essential requirement was not applied in any of the studies. The absence of a wash-out period overestimates the real OSA remission rate. When stopping CPAP use, OSA reoccurs after a mean of one week, associated with a sympathetic activity increase, blood pressure increase, relevant cerebral hypoxia, and disturbed cardiac repolarization but without an increased risk of myocardial infarction.22,23 CPAP withdrawal can, however, increase EDS and reduce driving performance, representing an ethical problem for CPAP/BiPAP withdrawal for the study.34,35 Of note, only 45% of OSA patients suffer from EDS, such that the risk of stopping CPAP one week is low.36 Moreover, for the other patients, they can just be asked to avoid driving during the washout period.
Influence of Age and Sex
In all the included studies, except that of Yanari et al,17 there were more women than men in the final study sample. Indeed, the prevalence of obesity is 15% for women and 11% for men.11 Moreover, women are more inclined to undergo bariatric surgery.11 According to one study, among 61,708 patients over 10 years, 22% of bariatric surgeries were performed on men and 78% on women.37 The predominance of women included in the studies could be a factor related to reduced persistence of OSA after surgery as OSA is mainly related to visceral obesity, typically found in males.38 Women are more likely to develop OSA after menopause, due to hormonal changes and increases in visceral deposition of fat.38 In the 6 studies selected, the mean age was 47 years and, according to the World Health Organization, most women experience menopause between the age of 45 and 55 years, however, we do not know the menopausal status of these patients and its potential implication in reported OSA remission rates.10
Choice of Surgical Techniques and Post-Operative Reassessment Period
The heterogeneity of the studies is also due to the different bariatric surgeries used and the variable post-surgery timing evaluation for persistence/remission of OSA. In a cohort study over 10 years, among 1787 obese patients undergoing RYGB, the patients lost 31% of their baseline weight at one year and 28% at 10 years compared to their nonsurgical matches.39,40 For SG, the patients lost 23% of their baseline at one year and for LAGB, the patients lost 13% of their baseline weight at one year.39,40 The result at 10 years for SG/LAGB was not available since the patients lost to follow-up was too high after four years. After four years, weight loss was higher with RYGB than SG, which was higher than LAGB.39,40 A prospective cohort study reported that among 1406 participants undergoing RYGB, the maximal weight loss (37.4% of their baseline) was achieved after a median of two post-operative years. The highest rate of weight regain was during the first year after their nadir weight.39 Table 2 summarizes the factors possibly linked to variable weight loss after bariatric surgery.
 |
Figure 1 Flow chart of patients included in the six relevant studies. Number at inclusion: total of PSG/PG confirmed OSA patients (AHI>5) that have undergone bariatric surgery. Final number: total of OSA patients with analyzable post-operative PSG/PG. Deaths were reported only in 2 studies (De Raaf et al27 and Haines et al28). Abbreviations: AHI, apnea-hypopnea index; OSA, obstructive sleep apnea; PG, polygraphy; PSG, polysomnography. |
 |
Table 2 Factors Possibly Linked to Variable Weight Loss After Bariatric Surgery |
According to a randomized controlled trial that followed patients who underwent RYGB vs SG for over 10 years, there were no statistically significant differences in T2D, dyslipidemia (DL), or OSA remission but HT remission rates were higher with RYGB.42 Most T2D remission was achieved in two years but no data was presented concerning the best timing for DL/OSA/HTA remission.40 According to this literature review, %EWL was not associated with higher/lower OSA remission rate.27,29,43 We hypothesize that the highest OSA remission rate is associated with maximal weight loss, therefore, after one year. In any case, waiting longer for postoperative evaluation of OSA remission rate increases the risk of a higher number of lost to follow-up patients.
Factors Associated with Persistence of OSA After Bariatric Surgery
In the current study, factors associated with persistence of OSA after bariatric surgery included EWL < 60% (de Raaff et al27), but no relationship was identified between pre-operative BMI and the magnitude of weight loss in Ravesloot et al.29 These differences may be related to the sample size of the studies. Ravesloot et al28 included fewer patients (n = 171 vs 437) with a high loss to follow-up rate at 12 months of 70%. Other factors, such as pre-operative AHI (de Raaff et al27 and Yanari et al17) and aging (de Raaff et al27), were also not identified by Ravesloot et al.29 However, these risk factors seem to be related to OSA persistence as OSA rate increases with age44 and as the reduction of AHI is linearly associated with BMI, such that if AHI is higher at baseline, the magnitude of the decrease will not be higher than that for less severe OSA.45 The fact that these factors were not identified in the study by Ravesloot et al29 may also be related to the use of post-operative PG rather than PSG and an underestimation of post-operative AHI.
Regarding comorbidities, Yanari et al17 reported that high HbA1c post-surgery was an independent prognostic factor for OSA persistence. A meta-analysis of studies that followed patients up to 24 months indicated that the remission rate of T2D was 63.5%–82%.46 Since T2D is a risk factor for OSA,4 persistence of T2D could be an explanation for OSA persistence after bariatric surgery.
HT was identified as a predictive factor of OSA persistence by de Raaff et al.27 The incidence of HT is associated with severe OSA, potentially explaining the remaining presence of post-operative sleep apnea in this specific population.33
One of the lowest remission rates (35%) was observed in Morong et al.26 In this study, they used a more restrictive definition of remission: PSG with AHI < 5. Their hypothesis was that positional OSA (POSA) was underestimated among patients after bariatric surgery suggesting that the habit of sleeping in a supine position with CPAP/BiPAP should explain the larger proportion of POSA after surgery. Morong et al26 noticed that only low AHI was a significant independent predictor for POSA and that 44% of those who were originally diagnosed with OSA developed POSA after surgery. POSA is associated with backward positioning of the lower jaw, smaller lower facial height, smaller craniofacial volume, and smaller volume of lateral pharyngeal wall soft tissue.
Finally, the lost to follow-up rates in the studies included in this literature review were broadly elevated, from 12% (Peromaa et al20) to 70% (Ravesloot et al29), introducing a possible selection bias.
Conclusions
In conclusion, we identified, from this limited sample of bariatric patients, that age, pre-operative OSA severity, proportion of weight loss, and T2D may be factors related to OSA persistence after bariatric surgery but the reported results were inconsistent between studies for age and the magnitude of weight loss. However, several other factors influencing the persistence of OSA should be prospectively explored in adequately powered studies that include strategies to standardize OSA diagnosis and limit loss to follow-up. These include fat distribution, anatomical predispositions and pathophysiological traits, ethnicity, supine position, and rapid eye movement (REM)-predominant hypopnea and apnea.
This could be accomplished by setting up a multicentric prospective study, ideally a randomized controlled trial. Obese patients (BMI > 40), candidates for RYGB, would be randomized in two arms, surgery or behavioral intervention with OSA resolution as primary aim. We would choose RYGB since it is associated with a higher weight loss.39,40 Baseline assessments should include home-PSG before surgery/behavioral intervention, sleep endoscopy, 3-D cone beam computed tomography for extensive anthropometric measurements, waist and neck circumference and fat distribution with free-fat mass component (assessed by dual-energy X-ray absorptiometry). Additional measurements assessing OSA pathophysiological phenotypes, such as Pcrit (H2O) and other PSG ventilatory analysis could be performed in patients with confirmed OSA during attended PSG. The better post-operative timing to re-assess patients would be one year because maximal weight loss is observed at this point in long-term prospective studies.39,40 Thus, one year after surgery/behavioral intervention, home-PSG should be repeated (after one-week wash-out of CPAP, if CPAP-treated34–36) together with 3-D cone beam computed tomography, waist and neck circumference, fat distribution with free-fat mass component to understand factors associated with OSA resolution or persistence. Using home-PSG would bring an added value to assessment of sleep position, REM, and supine OSA in the patients’ usual sleep environment. To obtain a population sample that would allow sufficient representation of all the factors possibly associated with the persistence of OSA post-operatively, based on the prevalence of these factors as followed: menopausal women, 20%, ethnicity other than Caucasians, 30%,47 craniofacial anomalies, 30%,48 REM-predominant OSA, 30%,49 T2D, 40% in a bariatric population, the sample size should be 300 patients in each arm (surgery/behavioral intervention). To obtain this population of 600 OSA patients eligible for RYGB, 1200 patients would have to be screened. This type of study could make a major contribution to understanding the evolution of OSA after bariatric surgery in obese patients.
Abbreviations
AASM, American Academy of Sleep Medicine; AHI, apnea-hypopnea index; AT, arousal threshold; BiPAP, bilevel positive airway pressure; BMI, body mass index; CPAP, continuous positive airway pressure; DL, dyslipidemia; EDS, excessive daytime sleepiness; EWL, 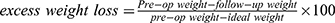 ; HbA1c, glycated hemoglobin; HT, hypertension; LAGB, laparoscopic gastric banding; LG, loop gain; OA, oral appliance; OAGB, one anastomosis gastric bypass; OSA, obstructive sleep apnea; OSAS, OSA-associated with symptoms and/or comorbidities; PG, polygraphy; PSG, polysomnography; RDI, respiratory disturbance index; REM, rapid eye movement; RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy; T2D, type 2 diabetes; TER, threshold of effective recruitment; UA, upper airway; UAG, muscular upper airway gain; VE, ventilation.
; HbA1c, glycated hemoglobin; HT, hypertension; LAGB, laparoscopic gastric banding; LG, loop gain; OA, oral appliance; OAGB, one anastomosis gastric bypass; OSA, obstructive sleep apnea; OSAS, OSA-associated with symptoms and/or comorbidities; PG, polygraphy; PSG, polysomnography; RDI, respiratory disturbance index; REM, rapid eye movement; RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy; T2D, type 2 diabetes; TER, threshold of effective recruitment; UA, upper airway; UAG, muscular upper airway gain; VE, ventilation.
Ethics Approval and Informed Consent
This review did not require any ethics and informed consent.
Consent for Publication
Authors give consent for the publication of the present material.
Acknowledgments
The authors acknowledge the contribution of a medical writer, Sandy Field, PhD, for English language editing and formatting of the manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors have no competing interests to declare for this work.
References
1. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165(9):1217–1239. doi:10.1164/rccm.2109080
2. Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. doi:10.1056/NEJM200005113421901
3. Zinchuk A, Yaggi HK. Phenotypic subtypes of OSA: a challenge and opportunity for precision medicine. Chest. 2020;157(2):403–420. doi:10.1016/j.chest.2019.09.002
4. Akset M, Poppe KG, Kleynen P, et al. Endocrine disorders in obstructive sleep apnoea syndrome: a bidirectional relationship. Clin Endocrinol. 2023;98(1):3–13. doi:10.1111/cen.14685
5. Andreozzi F, Van Overstraeten C, Ben Youssef S, et al. African ethnicity is associated with a higher prevalence of diabetes in obstructive sleep apnea patients: results of a retrospective analysis. Sleep Breath. 2020;24:857–864. doi:10.1007/s11325-019-01912-5
6. Bosi M, De Vito A, Eckert D, et al. Qualitative phenotyping of obstructive sleep apnea and its clinical usefulness for the sleep specialist. Int J Environ Res Public Health. 2020;17(6):2058. doi:10.3390/ijerph17062058
7. Pevernagie DA, Gnidovec-Strazisar B, Grote L, et al. On the rise and fall of the apnea-hypopnea index: a historical review and critical appraisal. J Sleep Res. 2020;29:e13066. doi:10.1111/jsr.13066
8. Kendzerska T, Mollayeva T, Gershon AS, et al. Untreated obstructive sleep apnea and the risk for serious long-term adverse outcomes: a systematic review. Sleep Med Rev. 2014;18(1):
9. Neelapu BC, Kharbanda OP, Sardana HK, et al. Craniofacial and upper airway morphology in adult obstructive sleep apnea patients: a systematic review and meta-analysis of cephalometric studies. Sleep Med Rev. 2017;31:79–90. doi:10.1016/j.smrv.2016.01.007
10. Menopause [Internet]; 2023. Available from: https://www.who.int/news-room/fact-sheets/detail/menopause.
11. Cooper AJ, Gupta SR, Moustafa AF, et al. Sex/gender differences in obesity prevalence, comorbidities, and treatment. Curr Obes Rep. 2021;10(4):
12. Romero-Corral A, Caples SM, Lopez-Jimenez F, et al. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest. 2010;137(3):711–719. doi:10.1378/chest.09-0360
13. Zeng X, Ren Y, Wu K, et al. Association between smoking behavior and obstructive sleep apnea: a systematic review and meta-analysis. Nicotine Tob Res. 2023;25(3):364–371. doi:10.1093/ntr/ntac126
14. Liu Y, Yang L, Stampfer MJ, et al. Physical activity, sedentary behaviour and incidence of obstructive sleep apnoea in three prospective US cohorts. Eur Respir J. 2022;59(2):2100606. doi:10.1183/13993003.00606-2021
15. Kolla BP, Foroughi M, Saeidifard F, et al. The impact of alcohol on breathing parameters during sleep: a systematic review and meta-analysis. Sleep Med Rev. 2018;42:59–67. doi:10.1016/j.smrv.2018.05.007
16. Hariri K, Kini SU, Herron DM, et al. Resolution of symptomatic obstructive sleep apnea not impacted by preoperative body mass index, choice of operation between sleeve gastrectomy and roux-en-Y gastric bypass surgery, or severity. Obes Surg. 2018;28(5):1402–1407. doi:10.1007/s11695-017-3042-6
17. Yanari S, Sasaki A, Umemura A, et al. Therapeutic effect of laparoscopic sleeve gastrectomy on obstructive sleep apnea and relationship of type 2 diabetes in Japanese patients with severe obesity. J Diabetes Investig. 2022;13(6):1073–1085. doi:10.1111/jdi.13755
18. Sarkhosh K, Switzer NJ, El-Hadi M, et al. The impact of bariatric surgery on obstructive sleep apnea: a systematic review. Obes Surg. 2013;23(3):414–423. doi:10.1007/s11695-012-0862-2
19. Al Oweidat K, Toubasi AA, Tawileh RBA, et al. Bariatric surgery and obstructive sleep apnea: a systematic review and meta-analysis. Sleep Breath. 2023;27(6):2283–2294. doi:10.1007/s11325-023-02840-1
20. Peromaa-Haavisto P, Tuomilehto H, Kössi J, et al. Obstructive sleep apnea: the effect of bariatric surgery after 12 months. A prospective multicenter trial. Sleep Med. 2017;35:85–90. doi:10.1016/j.sleep.2016.12.017
21. Timmerman M, Basille D, Basille-Fantinato A, et al. Short-term assessment of obstructive sleep apnea syndrome remission rate after sleeve gastrectomy: a cohort study. Obes Surg. 2019;29(11):3690–3697. doi:10.1007/s11695-019-04110-0
22. Davidson TM, Sedgh J, Tran D, et al. The anatomic basis for the acquisition of speech and obstructive sleep apnea: evidence from cephalometric analysis supports the great leap forward hypothesis. Sleep Med. 2005;6(6):497–505. doi:10.1016/j.sleep.2005.03.007
23. Bruyneel M. Obstructive sleep apnea phenotypes eligible for pharmacological treatment. Front Sleep. 2023;2. doi:10.3389/frsle.2023.1261276
24. Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. 2017;34:70–81. doi:10.1016/j.smrv.2016.07.002
25. Wong A-M, Barnes HN, Joosten SA, et al. The effect of surgical weight loss on obstructive sleep apnoea: a systematic review and meta-analysis. Sleep Med Rev. 2018;42:85–99. doi:10.1016/j.smrv.2018.06.001
26. Morong S, Benoist LBL, Ravesloot MJL, et al. The effect of weight loss on OSA severity and position dependence in the bariatric population. Sleep Breath. 2014;18(4):851–856. doi:10.1007/s11325-014-0955-3
27. de Raaff CAL, Coblijn UK, Ravesloot MJL, et al. Persistent moderate or severe obstructive sleep apnea after laparoscopic roux-en-Y gastric bypass: which patients? Surg Obes Relat Dis. 2016;12(10):1866–1872. doi:10.1016/j.soard.2016.03.014
28. Haines KL, Nelson LG, Gonzalez R, et al. Objective evidence that bariatric surgery improves obesity-related obstructive sleep apnea. Surgery. 2007;141(3):354–358. doi:10.1016/j.surg.2006.08.012
29. Ravesloot MJL, Hilgevoord AJ, van Wagensveld BA, et al. Assessment of the effect of bariatric surgery on obstructive sleep apnea at two postoperative intervals. Obes Surg. 2014;24(1):22–31. doi:10.1007/s11695-013-1023-y
30. Germany bariatric surgery procedures count by segments and forecast to 2030 [Internet]. Market Research Reports & Consulting | GlobalData UK Ltd. Available from: https://www.globaldata.com/store/report/germany-bariatric-surgery-procedures-analysis/.
31. Luostarinen M. Nissen fundoplication for reflux esophagitis long-term clinical and endoscopie results in 109 of 127 consecutive patients. Ann Surg. 1993;217(4):329–337. doi:10.1097/00000658-199304000-00004
32. Escourrou P, Grote L, Penzel T, et al. The diagnostic method has a strong influence on classification of obstructive sleep apnea. J Sleep Res. 2015;24(6):730–738. doi:10.1111/jsr.12318
33. Hirotsu C, Haba-Rubio J, Andries D, et al. Effect of three hypopnea scoring criteria on OSA prevalence and associated comorbidities in the general population. J Clin Sleep Med. 2019;15(02):183–194. doi:10.5664/jcsm.7612
34. Schwarz EI, Stradling JR, Kohler M. Physiological consequences of CPAP therapy withdrawal in patients with obstructive sleep apnoea—an opportunity for an efficient experimental model. J Thorac Dis. 2018;10:S24–S32.
35. Kohler M, Stoewhas A-C, Ayers L, et al. Effects of continuous positive airway pressure therapy withdrawal in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2011;184(10):1192–1199. doi:10.1164/rccm.201106-0964OC
36. Ulander M, Hedner J, Stillberg G, et al. Correlates of excessive daytime sleepiness in obstructive sleep apnea: results from the nationwide SESAR cohort including 34,684 patients. J Sleep Res. 2022;31:e13690.
37. Kochkodan J, Telem DA, Ghaferi AA. Physiologic and psychological gender differences in bariatric surgery. Surg Endosc. 2018;32(3):1382–1388. doi:10.1007/s00464-017-5819-z
38. Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol. 2015;402:113–119. doi:10.1016/j.mce.2014.11.029
39. Cho Y-H, Lee Y, Choi JI, et al. Weight loss maintenance after bariatric surgery. World J Clin Cases. 2023;11(18):4241–4250. doi:10.12998/wjcc.v11.i18.4241
40. Maciejewski ML, Arterburn DE, Van Scoyoc L, et al. Bariatric surgery and long-term durability of weight loss. JAMA Surgery. 2016;151(11):1046. doi:10.1001/jamasurg.2016.2317
41. Courcoulas AP, King WC, Belle SH, et al. Seven-year weight trajectories and health outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) Study. JAMA Surg. 2018;153(5):427–434. doi:10.1001/jamasurg.2017.5025
42. Salminen P, Grönroos S, Helmiö M, et al. Effect of laparoscopic sleeve gastrectomy vs roux-en-Y gastric bypass on weight loss, comorbidities, and reflux at 10 years in adult patients with obesity: the SLEEVEPASS randomized clinical trial. JAMA Surg. 2022;157(8):656. doi:10.1001/jamasurg.2022.2229
43. Tahrani AA, Morton J. Benefits of weight loss of 10% or more in patients with overweight or obesity: a review. Obesity. 2022;30(4):802–840. doi:10.1002/oby.23371
44. Launois SH, Pépin J-L, Lévy P. Sleep apnea in the elderly: a specific entity? Sleep Med Rev. 2007;11(2):87–97. doi:10.1016/j.smrv.2006.08.005
45. Mitchell LJ, Davidson ZE, Bonham M, et al. Weight loss from lifestyle interventions and severity of sleep apnoea: a systematic review and meta-analysis. Sleep Med. 2014;15(10):1173–1183. doi:10.1016/j.sleep.2014.05.012
46. Sheng B, Truong K, Spitler H, et al. The long-term effects of bariatric surgery on type 2 diabetes remission, microvascular and macrovascular complications, and mortality: a systematic review and meta-analysis. Obes Surg. 2017;27(10):2724–2732. doi:10.1007/s11695-017-2866-4
47. Van Overstraeten C, Andreozzi F, Youssef SB, et al. Obstructive sleep apnea syndrome phenotyping by cluster analysis: typical sleepy, obese middle-aged men with desaturating events are a minority of patients in a multi-ethnic cohort of 33% women. Curr Med Sci. 2021;41(4):729–736. doi:10.1007/s11596-021-2388-0
48. Kim S-J, Alnakhli WM, Alfaraj AS, et al. Multi-perspective clustering of obstructive sleep apnea towards precision therapeutic decision including craniofacial intervention. Sleep Breath. 2021;25(1):85–94. doi:10.1007/s11325-020-02062-9
49. Qanash S, Mufti H, Alhejaili F, et al. The prevalence of rapid eye movement-related obstructive sleep apnea in a sample of Saudi population. Ann Thorac Med. 2023;18(2):90–97. doi:10.4103/atm.atm_388_22
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
