Back to Journals » International Journal of Nanomedicine » Volume 19
Emerging Drug Delivery Vectors: Engineering of Plant-Derived Nanovesicles and Their Applications in Biomedicine
Authors Yang LY, Li CQ , Zhang YL, Ma MW, Cheng W, Zhang GJ
Received 13 December 2023
Accepted for publication 28 February 2024
Published 13 March 2024 Volume 2024:19 Pages 2591—2610
DOI https://doi.org/10.2147/IJN.S454794
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor R.D.K. Misra
Lu-Yao Yang,1,* Chao-Qing Li,1,2,* Yu-Lin Zhang,1,2 Meng-Wen Ma,3 Wan Cheng,3 Guo-Jun Zhang1,2
1School of Laboratory Medicine, Hubei University of Chinese Medicine, Wuhan, 430065, People’s Republic of China; 2Hubei Shizhen Laboratory, Wuhan, 430065, People’s Republic of China; 3Britton Chance Center for Biomedical Photonics at Wuhan National Laboratory for Optoelectronics - Hubei Bioinformatics & Molecular Imaging Key Laboratory, Department of Biomedical Engineering, College of Life Science and Technology, Huazhong University of Science and Technology, Wuhan, 430074, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guo-Jun Zhang, School of Laboratory Medicine, Hubei University of Chinese Medicine, Wuhan, People’s Republic of China, Email [email protected]
Abstract: Extracellular vesicles can transmit intercellular information and transport biomolecules to recipient cells during various pathophysiological processes in the organism. Animal cell exosomes have been identified as potential nanodrugs delivery vehicles, yet they have some shortcomings such as high immunogenicity, high cytotoxicity, and complicated preparation procedures. In addition to exosomes, plant-derived extracellular vesicles (PDVs), which carry a variety of active substances, are another promising nano-transport vehicles emerging in recent years due to their stable physicochemical properties, wide source, and low cost. This work briefly introduces the collection and characterization of PDVs, then focuses on the application of PDVs as natural or engineered drug carriers in biomedicine, and finally discusses the development and challenges of PDVs in future applications.
Keywords: plant-derived vesicles, activated biomaterials, nanodrugs delivery systems, biomedicine applications
Introduction
Biofilms have subdivided the intracellular space into different regions so that various biochemical reactions can be carried out independently in different spaces, thus enabling the synergistic and functionalization of cellular activities. In addition to the intracellular membrane system, extracellular membrane structures called extracellular vesicles (EVs) also exist outside the cell, which usually include exosomes, microvesicles, and apoptotic vesicles, and their diameters are usually in the range of 40–1000 nm.1 In mammalian cells, the most representative EVs are exosomes, which were first discovered in 1983.2 Studies have shown that almost all living cells could secrete exosomes, including animals, plants, and microorganisms.1 EVs could mediate signal transduction and material transfer between donor and recipient cells directly through a variety of mechanisms including fusion with the recipient cell membrane, receptor-ligand interactions, lattice-mediated cytokinesis, and receptor-mediated cytokinesis to alter the behavior of target cells. It plays an important role in cellular communication such as cell proliferation, tumor angiogenesis, inflammation, and cancer development.3 EVs also have excellent physicochemical properties, such as high stability, low immunogenicity, and long circulation time, and they can carry bioactive molecules such as nucleic acids and proteins for trans-spatial transfer. So EVs are prepared in large quantities for drug molecule delivery carriers.4 Wood was the first to inject siRNA-carrying EVs intravenously into mice, and the EVs were able to reach the mouse brain and exert therapeutic effects.5 Zhuang et al used EVs loaded with anti-inflammatory drugs and successfully crossed the blood-brain barrier after intranasal administration.6
Even though plant-derived vesicles predated the discovery of animal cell exosomes by about 15 years, research on PDVs has been less successful. Researchers are once skeptical that plant cells could produce EVs because of the vast structural differences between plant and animal cells, such as the fact that plant cells have cell walls. As early as 1967, Halperin et al observed by transmission electron microscopy that multivesicular structures arising in carrot cells were able to fuse with the plasma membrane and subsequently release secondary vesicles containing contents into the extracellular space.7 Subsequently, an increasing number of studies have focused on these plant-derived nanovesicles. Table 1 shows the comparison of plant-derived vesicles with animal-derived exosomes. Regente et al obtained extracellular fluid from sunflower seeds and detected the presence of phospholipid components. In the following year, Laura de la canal et al first isolated EVs from sunflower seeds by vacuum permeation-centrifugation.8,9 Since then, more and more researchers have discovered PDVs in different plants such as Arabidopsis, tobacco, and wisteria.10,11 The normal size distribution of PDVs ranges from 30 to 1000 nm. Structures smaller than 30 nm are excluded from consideration because of the difficulty of packing lipids inside a strongly curved geometry. Their drug-loading capacity is also correspondingly low.12 Generally, PDVs display negative zeta potential value ranging from −100 to around 0 mV, illustrating their mutual repulsion and lacking aggregation tendency.13 PDVs carried a large number of active substances that could affect the bioactive processes of target cells and have been used in antibacterial, anti-inflammatory, and antitumor treatments. In 2013, Zhang et al extracted EVs from grapes, loaded them with drugs, and targeted intestinal stem cells after oral administration, thus effectively alleviating dextran sodium sulfate (DSS)-induced intestinal inflammatory responses.14 PDVs expressed some lipids and cell adhesion molecules, such as phosphatidic acid (PA), which could facilitate the binding of PDVs to specific receptor cells.15 Therefore, in addition to their use as therapeutic or regulatory drugs by themselves, PDVs were increasingly prepared by researchers as engineered nanovesicles to improve the utilization of nanodrugs and reduce drug toxicities. In this review, we focus on the current applications of PDVs as natural or engineered drug delivery systems in biomedicine, aiming at providing researchers with new ideas and directions in the construction of engineered nanovesicle delivery systems and discussing the current developments and challenges of engineered nanovesicles (Figure 1).
 |
Table 1 Comparison of PDVs with MEVs |
 |
Figure 1 Extraction and purification of plant vesicles and their biomedical applications. |
Isolation and Preparation of PDVs
Considering the great potential of mammalian cell exosomes in the biomedical field, PDVs have also attracted a lot of attentions. Comparatively, PDVs are more widely and abundantly sourced, less expensive to obtain, and highly biocompatible.23–25 However, similar to exosomes, the first issue to be addressed is how to reduce the cost of obtaining the product and scale up the production process while capturing the balance of yield and purity. For example, simple cleaning, crushing, and juicing of the active parts of the plant are required before isolating PDVs, and these processes might affect the composition and activity of the product. Differential centrifugation is currently the most widely used separation and purification method. The separation principle is based on the fact that particles of different sizes have different settling rates under the action of centrifugal force. This method is cost-effective, simple to operate, and suitable for large-scale sample processing.26 However, it should be aware that multiple centrifugations might damage the structure of PDVs. In this case, a high-density isotonic solution could be added to the bottom of the centrifuge tube as a buffer layer. Therefore, differential centrifugation is often combined with density gradient centrifugation (Figure 2), and commonly used gradient media include sucrose, iodophor, and cesium chloride, many of which have been commercialized.13,27–29
 |
Figure 2 (a) Isolation and preparation of PDVs; (b) Three bands were formed after sucrose gradient ultracentrifugation. PDVs from the 30%/45% interface were visualized by electron microscopy (TEM) and the size distribution of the particles was determined using the Zetasizer Nano ZS. Reprinted with permission from Mu J, Zhuang X, Wang Q, et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol Nutr Food Res. 2014;58(7):1561–1573. © 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.13 |
Ultrafiltration is another commonly used separation method that relies on fluid pressure to drive small molecules through polymeric membranes, migrating while retaining large molecules.30 There are typically two membrane separation modes, direct flow filtration (DFF) and tangential flow filtration (TFF), where the flow channel is perpendicular or parallel to the membrane surface. In contrast, the TFF mode inhibits the formation of a restrictive layer, allowing sample components to pass smoothly through the membrane pores, and the advantages of the TFF mode in terms of separation speed and efficiency allow for large-scale product processing.31,32 Co-precipitation is an attractive method, which is simple to operate and highly profitable Polyethylene glycol is used as a co-precipitating agent by reducing the solubility of PDVs.33 In addition, some positively charged molecules, such as fish sperm protein and sodium acetate, have been used to precipitate negatively charged PDVs.34 Precipitation-based kits have been used commercially, but low purity and high cost severely limit their development, so some impurities are also present in the product precipitates, making subsequent operations difficult.35 Since all methods have advantages and disadvantages, the combined use of different methods could effectively overcome these problems. Table 2 displays pros and cons of different methods, including pre-processing and purification methods. For example, by combining centrifugation and ultrafiltration, low-speed centrifugation is used to initially remove impurities, which avoids subsequent contamination of the membrane and the problem of difficult dispersion of the precipitate after direct ultra-high-speed centrifugation. Passing the centrifuged product through a small pore size membrane could concentrate the filtrate and achieve a sterilization effect. Woith et al combined differential centrifugation with agarose gel electrophoresis to effectively remove soluble protein impurities.10 Kirbs et al used the aqueous two-phase systems (ATPS)-based protocol to separate non-protein material from Punica granatum-derived vesicles.36 Yang et al combined electrophoresis with combined dialysis and isolated lemon vesicles similar in size and number to the standard ultracentrifugation method.37 Table 3 presents an overview of PDVs separation techniques and physical characterizations.
 |
Table 2 The Main Separation Methods of PDVs and Their Advantages and Limitations |
 |
Table 3 Preparation and Physical Characterization of PDVs |
Engineering of PDVs
PDVs can be considered superior to other important carriers due to their excellent biocompatibility, oral safety, and ability to cross biological barriers. They have significant potential in delivering poorly soluble drugs and natural biomolecules. While the functionalization of artificial nanovesicle structures has been extensively studied, the engineering of PDVs may present a new challenge as well as an intriguing opportunity for the development of personalized therapeutic vectors.71,72 In this section, we summarize the strategies that have been adopted to enhance the active targeting of PVDs with drugs. The functionalization of PDVs aims to modify their surfaces or introduce bioactive molecules or imaging tracers in order to achieve high loading efficiency of target bioactive compounds for specific delivery purposes.
Surface Modification of PDVs
First-generation passive targeting strategies with surface polyethylene glycolization modification of nanomaterials have been developed for many years to prolong in vivo circulation time and improve biocompatibility by changing the physicochemical properties of materials. However, due to incomplete surface modification and non-selectivity for targets, drugs are susceptible to clearance by the immune system and indiscriminate attack on systemic tissues.73 Second-generation active targeting strategy modified with specific targeting molecules is the key point for the time being. For example, Wicki et al coupled anti-EGFR antibodies with DOX to construct targeted polyethylene glycol liposomes for the evaluation of patients with advanced solid tumors.74 Surface engineering of nanomedicines has entered different clinical stages, and again, the efficiency and cost of such strategy could not be ignored.3 Although PDVs express some lipid and protein adhesion molecules, such as PA, that promote binding to specific cellular receptors. The in vivo positioning of most PDVs is achieved by their natural distribution pattern, which is often difficult to meet the precise therapeutic role for diseases.15 The abundant active substances on the surface of PDVs provide a large number of reactive sites for surface modification. Based on this, the specific bioactive molecules can be immobilized on the surface of PDVs to develop an active targeting drug delivery system based on PVDs. For example, targeting ligands such as peptides and small molecules are bound to the surface of nanocarriers for selective recognition of targeting receptors overexpressed on the surface of tumor cells, thus efficiently killing tumor cells.75–77 Taking advantage of this simple surface modification of PDVs, Li et al loaded ginger-derived nanovesicles with pRNA-3W and functionalized folic acid to construct targeted RNA drug delivery systems and used them for the direct delivery of anticancer drugs.78 Fan’s group modified functionalized heparin onto the surface of lemon-derived nanovesicles, and then loaded DOX to construct a nanodrug bionic drug delivery system (HRED) (Figure 3).79 The cells consume a large amount of energy while up taking HRED, ie, the ATP content decreases, which reduces the cellular efflux of the drug and ultimately reduces cellular drug resistance. In addition, Fan’s research group also programmed the self-assembly of fruit-derived EVs modified with the tumor-targeting peptide cRGD on the DOX@squalene-PBS interface, allowing structured droplet drugs designed based on EVs to amplify macropinocytosis through deformation and membrane fusion for flexible delivery, and to efficiently cross the BBB/BBTB and penetrate deeply into glioblastoma tissue (Figure 4).80 Besides, the immobilization of ligands on the surface of PDVs could be used for bioimaging. For example, Zhuang et al reported the use of lipophilic carbocyanine dyes to label grapefruit-derived nanocarriers and track the distribution in vivo by fluorescence.81
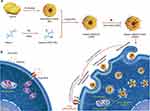 |
Figure 3 (a) Schematic illustration of lemon-derived extracellular vesicle (EV) nanodrugs for overcoming cancer multidrug resistance. The lemon derived EV nanodrugs (marked with heparin-cRGD-EVs-doxorubicin (HRED)) were fabricated by modifying heparin-cRGD (HR) onto the surface of EVs and then by loading with doxorubicin (DOX). The HRED nanodrugs enabled to effectively enter DOX-resistant cancer cells by caveolin-mediated endocytosis, macropinocytosis, and clathrin-mediated endocytosis, exhibiting excellent cellular uptake capacity. They diversified endocytosis capacity enabled to dissipate the intracellular energy. Meanwhile, guided by caveolin-mediated endocytosis, they could also further down-regulate the expression of intracellular caveolin-1 (CAV-1) to reduce ATP production and increase reactive oxygen species (ROS) level. Thus, combing with the endocytosis-triggered energy dissipation and ATP production reduction, our HRED nanodrugs would greatly reduce drug efflux, ensuing efficiently overcoming cancer multidrug resistance. (b) Schematic illustration of free DOX could be effectively pumped out from cancer cells by utilizing the P-glycoprotein (P-gp) and the ATP hydrolysis energy. Reprinted with permission from Li Z, Wang H, Yin H, Bennett C, Zhang HG, Guo P. Arrowtail RNA for ligand display on ginger exosome-like nanovesicles to systemic deliver siRNA for cancer suppression. Sci Rep. 2018;8(1):14644. Creative Commons.78 |
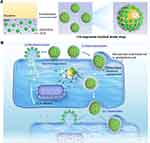 |
Figure 4 Schematic illustration of fruit-derived EVs engineered structural droplet drugs for glioblastoma chemotherapy. (a) Schematic illustration of the fabrication of EVs engineered structural droplet drugs. The fruit-derived EVs-engineered structural droplet drugs were fabricated by programming the self-assembly of fruit-derived EVs at the DOX@squalene-PBS interface. (b) Schematic illustration of interfacial interaction between EVs-engineered structural droplet drugs and neovascular endothelial cells or glioblastoma cells. The obtained EVs-engineered structural droplet drugs exhibited superior flexibility, enabling them to deform to enhance interfacial contact area with cells for efficiently wrapping via macropinocytosis. Beyond that, a large number of EVs onto the interface of droplet drugs would further amplify the transport capability by synchronous membrane fusion. Thus, the obtained EVs-engineered structural droplet drugs would greatly enhance their delivery capability in crossing BBB/BBTB by receptor-mediated macropinocytosis and membrane fusion. In the downstream delivery, they also exhibited excellent transcytosis, affording them deep penetration into glioblastoma tissues and thereby enhancing the antitumor efficacy against glioblastoma. Reprinted with permission from Xiao Q, Zhao W, Wu C, et al. Lemon-derived extracellular vesicles nanodrugs enable to efficiently overcome cancer multidrug resistance by endocytosis-triggered energy dissipation and energy production reduction. Adv Sci. 2022;9(20):e2105274. © 2022 The Authors. Advanced Science published by Wiley-VCH GmbH. Creative Commons.79 |
PDVs as Drug Carriers
In addition to the long-distance transfer of cargo between cells in the same species via PDVs, they could also enable cross-species delivery of substances, and their unique design and transport capabilities reflect their potential for drug transport. A growing number of studies have demonstrated the advantages of PDVs-based drug transport platforms over animal EVs and synthetic carriers. Firstly, animal EVs as drug delivery platforms require critical scrutiny because their innate and derived biological functions play a key role in immunogenicity. For example, EVs derived from mammalian cancer cells have the risk of transmitting precancerous features to the receptor.72,82 Although it is possible to synthesize artificial liposomes with similar structure and composition as PDVs through highly advanced nanotechnology, such as similar membrane structures, both of which could transport hydrophilic and hydrophobic drug molecules.83,84 Differences between the two are also clear, especially in terms of low immunogenicity, strong cellular uptake, and high in vivo environmental stability, while PDVs perform better than synthetic nanoparticles.55 In addition, PDVs offer simpler preparation methods, such as membrane extrusion and microemulsification, avoiding the complex preparation processes of artificial liposomes. Another point is that artificial liposomes only provide drug-delivery vehicles without any therapeutic function.85,86 Therefore, PDVs have been used by more researchers to try to load them with exogenous therapeutic molecules in addition to delivering their endogenous therapeutic molecules, such as nucleic acids, proteins, and other functional small molecules.14,58,87 For example, Zeng et al used vesicles extracted from Aloe vera to encapsulate both the photosensitizer indocyanine green (ICG) and the small molecule chemotherapeutic agent doxorubicin (DOX), and this delivery system combined with phototherapy and chemotherapy showed effective inhibition of breast cancer cells.88 Del Pozo-Acebo et al isolated EVs from broccoli, loaded them with exogenous miRNAs with biological effector capabilities, and then co-incubated them with Caco-2 cells. The results showed that the miRNA was taken up by this intestinal cell line and produced cytotoxicity, indicating that broccoli EVs have the potential to improve the biostability of therapeutic RNA and resist RNase degradation and gastrointestinal digestion.57 In addition to the use of PDVs for encapsulation of small molecule drugs, their surface modification of inorganic nanoparticles has also shown powerful advantages. Mao et al extracted ginger EVs and used them to encapsulate nanocarriers, and the encapsulation of natural plant membranes improved the biocompatibility and blood circulation, and reduced the immunogenicity of the nanocarriers.89 In another study, the investigators extracted grapefruit-derived lipid nanovesicles and modified activated leukocyte surface inflammation-associated membrane protein receptors (IGFDNs) on their surface, and this composite carrier was used for targeted delivery of the anticancer drug DOX to sites of inflammation.90 The authors subsequently detected the release of the drug using a spectrophotometer at 497 nm, and this vesicle coating improved the stability and detectability of the drug. IGFDNs could not only be loaded with anti-cancer drugs, but also be with anti-inflammatory drugs such as curcumin. Then the authors constructed different mouse models of inflammatory disorders for experiments, and the engineered vesicles modified with abundant membrane receptors had better targeting compared to natural vesicles as carriers, ie, the IGFDNs improved the efficiency of the drugs to reach the inflammatory sites.
Therapeutic Potential of PDVs
In addition to being a drug delivery carrier, the application of PDVs as a biological therapeutic agent in biomedicine has also attracted wide attention.19 PDVs usually carry a large number of active substances, showing similar pharmacological functions as the original plants. In this respect, PVDs are significantly superior to single-component therapeutic agents, which might be caused by the synergistic effect of the multi-component in PDVs and the high bioavailability of the nanocrystalline properties of the nanovesicles themselves. Currently, PDVs have played a huge role in the diagnosis and treatment of various diseases, such as cancer, inflammation, intestinal diseases, wound healing, and bone regeneration (Table 4).
 |
Table 4 Therapeutic Potential of Different PDVs |
PDVs for Anti-Cancer
Many types of PDVs have been studied regarding their ability to inhibit cancer cell growth through different mechanisms.98 It has been shown that PDVs could suppress cancer cell proliferation and promote cancer cell death, while not showing toxicity to normal cells (Figure 5). For example, Raimondo et al extracted citrus-derived nanovesicles of size 50–70 nm,68 which could inhibit the growth of different types of tumor cells in blood and solids (mainly A549, SW480, and LAMA84) in a time- and dose-dependent manner in vitro; and chronic myeloid leukemia xenograft tumors in vivo. No significant cell damage was observed in normal cell lines under the same drug treatment conditions. Lemon-derived nanovesicles increased the levels of encoded pro-apoptotic proteins (eg Bad and Bax) while decreasing the expression of anti-apoptotic genes (eg survivin and Bcl-xl), and Trail and its receptor DR5 were observed to be upregulated in vesicle-treated tumor cells.37 These studies suggested that lemon-derived nanovesicles caused cancer cell death through activation of Trail-mediated apoptosis, which was further confirmed in an in vivo LAMA84 xenograft tumor mouse model. The role of citrus-derived nanovesicles in antitumor activity was also investigated by Stanley et al.99 They extracted nanovesicles from different plants (including orange, lemon, and grapefruit) and demonstrated that these micro-nano vesicles specifically inhibited the proliferation of lung, skin, and breast cancer cells, while not inhibiting the proliferation of non-cancerous cells. Further studies revealed that grapefruit-derived nanovesicles inhibited the cell cycle G0/G1 and G2/M transitions, promoted the upregulation of the cytostatic p21, and decreased the expression of melanoma invasion and migration-associated genes. Chen et al extracted extracellular exosome-like vesicles (TFENs) with a size of about 131 nm and a negatively charged surface from edible tea flowers. TFENs also contained high amounts of polyphenols, flavonoids, functional proteins and lipids. The experimental results showed that TFENs could increase intracellular reactive oxygen species content, and the increased reactive oxygen species subsequently triggered mitochondrial damage and inhibited cell cycle, thus resisting the proliferation, migration and invasive activity of breast cancer cells. The results of in vivo experiments indicated that TFENs could accumulate in breast tumor sites and lung metastasis sites after intravenous or oral administration, inhibit the growth and metastasis of breast cancer cells, and regulate the balance of intestinal flora.100 Yang et al combined electrophoresis technique and dialysis for the isolation and purification of lemon vesicles, a time-saving method that did not require special equipment and could filter out a large amount of extra-vesicular hetero proteins and lipid material. The extracted vesicles could mediate the generation of reactive oxygen species, thus causing s-phase block in gastric cancer cells and inducing apoptosis, while vesicles left in the gastrointestinal organs were safe for the organism.37 Tumor-associated macrophages (TAMs) were important components of the tumor microenvironment. TAMs could be polarized into the M1 type, which inhibits tumor growth. While, the M2 type promotes tumor growth. Because most types of tumor microenvironment have a low M1/M2 ratio, stimulation of TAMs to the M1 type helps to inhibit cancer cell growth.101 Cao et al extracted approximately 300 nm of ginseng-derived nanovesicles,62 which inhibited melanoma cell growth by polarizing macrophages to the M1 type after incubation with cells. It is shown that compared to the control group, the M1/M2 of the incubated ginseng-derived nanovesicles were significantly improved at day 12 in the B16F10 xenograft mouse model, and the tumor weight of the experimental group was significantly reduced. The experimental results indicated that the inhibition of macrophage M2-type polarization was associated with the interaction of nanovesicle surface ligands and cell surface Toll-like receptors, and activated the skeletal differentiation antigen 88 (MyD88)-dependent pathway.
 |
Figure 5 Potential applications of PDVs in cancer therapy. Reprinted with permission from Priglinger E, Strasser J, Buchroithner B, et al. Label-free characterization of an extracellular vesicle-based therapeutic. J Extracell Vesicles. 2021;10(12):e12156.32 |
PDVs for Inflammation and Immune Regulation
Inflammation is part of the innate immune machinery, and if left unchecked, is likely to evolve into acute or chronic inflammatory diseases and to induce other diseases, such as cancer.102 Indeed, when taken up by host cells, PDVs trigger a large number of intracellular cascade signals that exert anti-inflammatory effects by modulating host immune activity (Figure 6b).58,64,91,103 In a study, it is shown that PDVs carrying active miRNAs could modulate cross-border communication between the intestinal flora and host immune cells, subsequently mediating homeostatic balance between the immune system and the intestinal flora.58 In another study, nanovesicles from ginger, carrot, grape, and grapefruit were absorbed via F4/80+ from intestinal macrophages and via Lgr5+ from intestinal stem cells after 6 h of oral administration to mice. However, studies on RAW264.7 macrophages in vitro showed that after 24 h of incubation (1 µg/mL),13 only ginger-derived vesicles significantly enhanced the expression of heme oxygenase-1 (HO-1) and IL-10 involved in the control of oxidative stress and inflammation, and ginger-derived nanovesicles also induced the expression of the pro-inflammatory cytokine IL-6.64 These results suggested on one hand that ginger-derived nanovesicles played an important role in the regulation of intestinal inflammation, and on the other hand that vesicles of different plant origins had different biological activities. Recent research also explored the application of plant-derived nanovesicles as new chemotherapeutic immunomodulators. Ou et al extracted exosome-like nanovesicles from Catharanthus Roseus (L.) Don) leaves (CLDEN). CLDEN exhibited excellent stability, which could withstand multiple enzymatic digestions, resist extreme pH environments, and remain stable in gastrointestinal tract stimulation fluids. Biodistribution experiments showed that CLDENs had immune organ targeting after intraperitoneal injection. CLDENs strongly stimulated the secretion of TNF-α in vitro and in vivo, activated the NF-κB signaling pathway, and increased the expression of hematopoiesis-related transcription factor PU.1.93 Wang et al also showed that ginger, grapefruit, and broccoli increased antioxidant and anti-inflammatory mediators in macrophages while downregulating the expression of pro-inflammatory cytokines such as TNF-α and IL-1β (Figure 6c). 94,104 Lemon-derived extracellular vesicles have been shown to transport from the intestine to the kidneys and restore calcium homeostasis and mitochondrial function by regulating the endoplasmic reticulum stress response, thereby alleviating the progression of kidney stones represented by calcium oxalate (CaOx) type in rats (Figure 6a).105 Although researchers have done extensive work on the regulation of inflammation mediated by PDVs, the underlying molecular mechanisms remain unclear so far. Lipids, but not proteins and RNAs, were the main bioactive molecules that inhibited NLRP3 inflammatory vesicles. Since nanovesicles inhibited the activation and assembly of NLRP3 inflammatory vesicles, it is implied that these plant nanovesicles represented a new promising inhibitor of NLRP3 inflammatory vesicles.103 Nuclear translocation of nuclear factor (erythroid-derived 2)-like Nrf2 activated a pleiotropic cytoprotective defense process to avoid inflammatory diseases by inhibiting oxidative stress-mediated tissue damage.106,107 Grapefruit and ginger-derived nanovesicles promoted Nrf2 translocation into the nucleus of macrophages and exerted its cytoprotective effects after 24 h incubation of macrophages. Treatment of primary hepatocytes with the above vesicles significantly increased nuclear translocation of Nrf2, and reduced the production of reactive oxygen species.108,109
 |
Figure 6 PDVs for inflammation and immune regulation. (a) Schematic illustration of CaOx stone-forming mechanism in kidneys and trans-systemic transport and intervention of LEVNs. Reprinted with permission from Zhang L, Li S, Cong M, et al. Lemon-derived extracellular vesicle-like nanoparticles block the progression of kidney stones by antagonizing endoplasmic reticulum stress in renal tubular cells. Nano lett. 2023;23(4):1555–1563. Copyright 2023 American Chemical Society.105 (b) G-ELN inhibited NLRP3 inflammasome activation. Reprinted with permission from Chen X, Zhou Y, Yu J. Exosome-like nanoparticles from ginger rhizomes inhibited NLRP3 inflammasome activation. Mol Pharm. 2019;16(6):2690–2699. Copyright © 2019 American Chemical Society. Creative Commons.91 (c) TDNPs prevent LPS-induced macrophage inflammation. Reprinted with permission from Liu C, Yan X, Zhang Y, et al. Oral administration of turmeric-derived exosome-like nanovesicles with anti-inflammatory and pro-resolving bioactions for murine colitis therapy. J Nanobiotechnol. 2022;20(1):206. Creative Commons.104 |
PDVs for Intestinal Regulation
The human intestines have been exposed to billions of plant-derived nanovesicles every day. These edible plants or herbal-derived vesicles have had a profound effect on the body’s intestine, which could explain why current plant-derived nanovesicles have low immunogenicity.94 In particular, various in vivo and in vitro studies have been done on the effects of vesicles from edible plants or Chinese herbal sources on the intestinal tract of the body. For example, PDVs from grapes, grapefruit, and ginger have been shown to contribute to the maintenance of normal intestinal function. Ju et al gavaged grape-derived EVs to mice and detected intact vesicles in intestinal stem cells at different times. Further studies showed that grape-derived nano-vesicles could directly promote the proliferation of intestinal stem cells and accelerate their formation from individual stem cells into micro-organoids, suggesting that PDVs had good resistance to the gastric acid environment and might penetrate the intestinal mucus barrier to act on intestinal cells.64 Another study also showed that PDVs extracted from grapefruit were resistant to a variety of digestive enzymes, such as pepsin and pancreatic enzymes, and reached the intestine intact and were selectively absorbed by macrophages in the intestine after oral administration.58 These nanovesicles resisted the degree of shortening of the colon in mice with DSS-induced colitis and well prevented weight loss and reduced local lymphocyte infiltration in mice. PDVs derived from broccoli might also help improve colitis and prevent it into colon cancer. It is shown that broccoli-derived nanovesicles could activate adenosine monophosphate-activated protein kinase (AMPK) in dendritic cells and regulate intestinal environmental homeostasis.9 Man et al extracted ginger-derived EVs and investigated their intestinal absorption in rats. The vesicles were disc-shaped with a particle size of about 70 nm and a surface potential of about −27 mV. The absorption kinetics and sites of absorption of ginger vesicles in rats were investigated by the in situ single-pass intestinal perfusion method. The findings showed that the vesicles were absorbed by the small intestine in the concentration range of 15–60 mg/mL, and the absorption capacity of different intestinal segments was duodenum > jejunum > ileum.110 Dou et al studied vesicles of grapefruit origin, in which the key component, brassica, could be hydrolyzed by the intestinal microbiota to an active metabolite, naringenin, which has antitumor effects.111 The researchers successfully transferred si-RNA-CD98 to intestinal epithelial tissue via ginger-derived nanocarriers, and the results showed that these oral nanoplatforms were well retained in the upper colon and ileum, reducing CD98 gene expression, and inhibiting ulcerative colitis induced inflammation while preventing colitis-associated cancers.112
PDVs for Anti-Oxidant
Plants produce a range of non-enzymatic antioxidants, such as ascorbic acid, glutathione, alpha-tocopherol, flavonoids, carotenoids, proline, and phenolic acids. They also have enzymatic antioxidant defense systems, including superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase, to mitigate the toxicity induced by reactive oxygen species (ROS).113,114 Consistent with this, several studies have confirmed the antioxidant effects of PDVs. Perut et al discovered that strawberry-derived EVs were abundant in anthocyanins, folic acid, flavonols, and vitamin C. They also found that they did not exhibit cytotoxic effects on human mesenchymal stromal cells (MSCs) and could dose-dependently prevent oxidative stress in MSCs.115 Nanovesicles derived from citrus lemon also exhibit antioxidant activity and protect human MSCs from H2O2-induced oxidative stress. The vitamin content in the nanovesicles is approximately 0.416 nM vitamin C/μg.69 Grapefruit and ginger-derived EVs can enhance the translocation of Nrf2, a crucial transcription factor that controls cellular antioxidant responses, into the nucleus of macrophages (RAW 264.7 cells) after a 24-hour incubation time, where the transcription factor exerts its cytoprotective effects.13 Similarly, nanovesicles derived from carrots inhibit the production of ROS and apoptosis in cardiomyoblasts and neuroblastoma cells by upregulating the expression of Nrf2, HO-1, and NQO-1 genes.59 In addition, turmeric-derived EVs can inhibit the expression of pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β), while increasing the expression of antioxidant genes such as heme oxygenase 1 (HO-1). It has been demonstrated that TDNPs had the potential to reduce destructive factors and promote protective factors.104 Nanovesicles derived from aloe vera bark have shown to decrease ROS levels in H2O2-treated HaCaT cells and demonstrate antioxidant activity by upregulating the Nrf2 gene and its downstream genes HO-1 and catalase.116 Further studies are needed to determine the in vivo antioxidant effects of PDVs.
PDVs for Regenerative Activities
Wound healing and inflammatory responses are closely linked. Studies have shown that plant extracts and their natural compounds exhibited high activity in wound healing in different conditions, such as inhibition of pro-inflammatory cytokine production, downregulation of oxidative factors, enhancement of antioxidant enzyme activity, and promotion of angiogenesis.117,118 Preclinical studies have shown that products derived from plants could be used to regulate the proliferation and differentiation of mesenchymal stem cells.119 Scratching experiments showed that wheat-derived nanovesicles had higher migration rates of HDF, HUVEC, and HaCaT cells after 48 hours of incubation.
In the field of biological tissue engineering, plant extracts or compounds of plant origin could be classified as biologically active materials for controlling drug release or as biomaterials for cell transplantation.120 Preclinical studies have confirmed that PDVs could inhibit the production of pro-inflammatory cytokines, reduce oxidative factors, enhance antioxidant enzymes, promote the formation of new blood vessels, and regulate the proliferation and differentiation of mesenchymal stem cells and osteoblasts. Therefore, it shows great potential in wound healing, skin diseases, and joint diseases.117 Wheat plant extracts are frequently utilized as natural remedies in traditional medicine. Wheat exosomes have been researched for their potential in treating skin wound healing processes.96 Scratch experiments demonstrated that wheat-derived nanovesicles had higher migration rates on HDF, HUVEC, and HaCaT cells after 48 hours of incubation. They also increased the formation of tubular structures in endothelial cells and enhanced the expression of genes related to wound healing. These findings indicate that the cell migration-enhancing properties of wheat exosomes play a significant role in skin wound healing. Ginseng-derived nanoparticles (GDNP) have also been shown to regulate skin cell proliferation, promoting wound healing and reducing inflammation.121 Another study found that ginseng-derived EVs could serve as a carrier to transfer miRNA to bone marrow-derived mesenchymal stem cells (BMSC), potentially promoting neural differentiation and sensory function recovery of BMSCs by regulating PI3K signaling and cell transcription. This is a breakthrough. The application of neural cell-derived EVs to promote neural stem cell (NSC) differentiation is constrained by low yield, high immune side effects, and expensive manufacturing costs.87 In addition, some researchers isolated EVs from the leaves and stems of Dendropanax morbifera. They found that both leaf- and stem-derived EVs could reduce the melanin content and tyrosinase activity of mouse melanoma cells in a concentration-dependent manner (TYR) activity to achieve skin whitening effect.44 Yam-derived nanovesicles (YNV) can activate the BMP-2/p-p38-dependent Runx2 pathway to enhance osteoblast differentiation and mineralization in bone regeneration in ovariectomy (OVX)-induced osteoporotic mice.95 Pueraria lobata-derived exosome-like nanovesicles (PELN) enhance autophagy by degrading trimethylamine-N-oxide (TMAO), a metabolite produced by intestinal microbiota, in primary rats with OVX-induced osteoporosis. They also promote the differentiation and mineralization of human bone mesenchymal stem cells (hBMSC).97 Therefore, YNV and PELN can be used as safe and orally effective medications for the treatment of osteoporosis.
Biosecurity of PDVs
The ideal nanocarrier or nanomedicine must be guaranteed to have minimal toxicity, non-immunogenicity and side effects in vivo and in vitro.122,123 Not much work has been done to explore the biosafety of PDVs in the organisms because it is a large and lengthy project. Nevertheless, because of their natural origin and close connection with human daily life, people have been more inclined to accept this biomaterial with great potential for application. At least so far, PDVs have shown no worrisome toxic side effects.124 In a study by Zhang et al on the potential effects of grapefruit-derived nanovesicles on cytotoxicity in mice,14 they quantified pro-inflammatory cytokines and serum liver enzyme levels, such as markers of alanine aminotransferase (ALT) and aspartate aminotransferase (AST). The levels of ALT, AST, and pro-inflammatory cytokines were significantly elevated in mice treated with liposomes, while none of the indicators changed significantly in mice injected with grapefruit nanovesicles. No pathological changes or necrosis were observed in the histological samples of the major organs of the corresponding groups of mice, including liver, kidney, spleen, and lung. Zhang and his group determined the effect of grapefruit vesicles on cellular activity in vitro using the MTT method, and the effect of grapefruit vesicles on the cell viability of RAW 264.7 cells and colon cell lines was much less compared to the liposome-treated group.112 These results suggested that synthetic nanoplatforms were susceptible to host immune responses, implying that in vivo these artificial carriers were filtered by organs and that clearance was enhanced. In contrast, the current study has shown that PDVs are significantly non-immunogenic and highly immunomodulatory, and are able to maintain tissue homeostasis and contribute to biological health. Many studies have also shown that one of the key benefits of using PDVs for drug delivery is their ability to reduce the side effects of drugs used on experimental subjects.94
Challenges and Prospects
Research on PDVs has been still in its early stages. Although extracellular vesicle structures were observed in plants in the 1960s, PDVs have gradually gained attention in the last decade. Research on PDVs has also grown and has become more productive, but there is still a long way to go before they are actually used in clinical trials. For any nanoplatform used in vivo, biocompatibility is the most important indicator to be concerned about. So, for the extraction and purification of PDVs, the source, species, and isolation and purification process of the plant must be strictly considered. For example, PDVs should be extracted directly from the extracellular fluid rather than subjecting the sample to harsh treatments, such as grinding, in order to avoid damaging the structure and function of PDVs. Various technical modifications should be made depending on the plant species and laboratory specific conditions, especially for studies using active components within vesicles, the source of the components must be clearly identified to avoid misleading non-vesicular structures. Current research advances suggest that PDVs have a variety of important activities in the regulation of immune responses, cell differentiation and proliferation, anticancer, and tissue microenvironment regulation.23,125 PDVs have been important transmitters of information between homozygous and heterozygous cells, regulating the phenotype and function of recipient cells by transmitting various proteins, functional RNAs, and genetic information. However, there is still no clear definition as to whether the communication between cells is specific or stochastic. In addition, the interaction between the naturally occurring active substances contained in PDVs for regulating target cell activity and the loaded foreign drugs remains unclear. Therefore, this requires more extensive and in-depth studies. The naturally occurring active substances within the vesicles could be removed prior to drug loading, but it is important that this process should not damage the vesicle surface morphology and biological function.
Especially in the field of targeted cancer therapies, drug delivery systems have faced challenges such as targeting effectiveness, side effects, and rapid clearance. The in vivo distribution of PDVs has been mostly dependent on the natural distribution of natural properties without active targeting. Thus researchers might consider to engineer PDVs in different ways to make them specifically targeted and aggregated at the target sites, while at the same time taking care not to cause any immunogenicity. Compared to other engineered nanoparticles, such as artificial liposomes and animal exosomes, PDVs have relatively been newly studied and could cross the blood-brain barrier when used as drug carriers, and they could be considered as vaccine delivery nanocarriers for transdermal administration due to their structural similarity to liposomes with the ability to penetrate the skin.126 PDVs have many advantages when used as therapeutic agents and drug carriers, including i) plant sources for mass production,78 ii) edible plants with minimal cytotoxicity and minimal immunogenicity,58 and iii) high overall biocompatibility.60 Therefore, designing different delivery strategies to accommodate different drugs and different modes of delivery while minimizing systemic hazards is the current focus of research. And, when choosing a retrofit strategy, one must understand the complexity of the system in terms of cargo, end application, and other relevant factors. As seen in the emerging field, a multidisciplinary approach by integrating biological sciences, engineered drug delivery systems, nanoengineering, and technology would help guide the development of PDVs-based therapeutic agents and nano-delivery platforms.
Acknowledgments
The authors are indebted to many scientists whose research results are cited here. This work was supported by the project funded by China Postdoctoral Science Foundation (2023M731042) and Knowledge Innovation Project in Wuhan, Hubei Province, China (2023020201020472).
Disclosure
The authors report no conflicts of interest in this work.
References
1. van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Bio. 2018;19(4):213–228. doi:10.1038/nrm.2017.125
2. Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262(19):9412–9420.
3. Zhao M, Liu S, Wang C, et al. Mesenchymal stem cell-derived extracellular vesicles attenuate mitochondrial damage and inflammation by stabilizing mitochondrial DNA. ACS nano. 2021;15(1):1519–1538. doi:10.1021/acsnano.0c08947
4. Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. 2021;16(7):748–759. doi:10.1038/s41565-021-00931-2
5. Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–345. doi:10.1038/nbt.1807
6. Zhuang X, Xiang X, Grizzle W, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19(10):1769–1779. doi:10.1038/mt.2011.164
7. Halperin W, Jensen WA. Ultrastructural changes during growth and embryogenesis in carrot cell cultures. J Ultrastruct Res. 1967;18(3):428–443. doi:10.1016/s0022-5320(67)80128-x
8. Regente M, Corti-Monzón G, Maldonado AM, Pinedo M, Jorrín J, de la Canal L. Vesicular fractions of sunflower apoplastic fluids are associated with potential exosome marker proteins. FEBS Lett. 2009;583(20):3363–3366. doi:10.1016/j.febslet.2009.09.041
9. Regente M, Pinedo M, San Clemente H, Balliau T, Jamet E, de la Canal L. Plant extracellular vesicles are incorporated by a fungal pathogen and inhibit its growth. J Exp Bot. 2017;68(20):5485–5495. doi:10.1093/jxb/erx355
10. Cai Q, Qiao L, Wang M, et al. Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science. 2018;360(6393):1126–1129. doi:10.1126/science.aar4142
11. Woith E, Melzig MF. Extracellular vesicles from fresh and dried plants-simultaneous purification and visualization using Gel electrophoresis. Int J Mol Sci. 2019;20(2):357. doi:10.3390/ijms20020357
12. Milcovich G, Lettieri S, Antunes FE, et al. Recent advances in smart biotechnology: hydrogels and nanocarriers for tailored bioactive molecules depot. Adv Colloid Interface Sci. 2017;249:163–180. doi:10.1016/j.cis.2017.05.009
13. Mu J, Zhuang X, Wang Q, et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol Nutr Food Res. 2014;58(7):1561–1573. doi:10.1002/mnfr.201300729
14. Wang Q, Zhuang X, Mu J, et al. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat Commun. 2013;4:1867. doi:10.1038/ncomms2886
15. Wang X, Devaiah SP, Zhang W, Welti R. Signaling functions of phosphatidic acid. Prog Lipid Res. 2006;45(3):250–278. doi:10.1016/j.plipres.2006.01.005
16. Di Gioia S, Hossain MN, Conese M. Biological properties and therapeutic effects of plant-derived nanovesicles. Open Med. 2020;15(1):1096–1122. doi:10.1515/med-2020-0160
17. Butreddy A, Kommineni N, Dudhipala N. Exosomes as naturally occurring vehicles for delivery of biopharmaceuticals: insights from drug delivery to clinical perspectives. Nanomaterials. 2021;11(6):1481. doi:10.3390/nano11061481
18. Meng W, He C, Hao Y, Wang L, Li L, Zhu G. Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug Deliv. 2020;27(1):585–598. doi:10.1080/10717544.2020.1748758
19. Cong M, Tan S, Li S, et al. Technology insight: plant-derived vesicles-how far from the clinical biotherapeutics and therapeutic drug carriers? Adv Drug Deliv Rev. 2022;182:114108. doi:10.1016/j.addr.2021.114108
20. Sharma S, Masud MK, Kaneti YV, et al. Extracellular vesicle nanoarchitectonics for novel drug delivery applications. Small. 2021;17(42):e2102220. doi:10.1002/smll.202102220
21. Johnson J, Wu YW, Blyth C, Lichtfuss G, Goubran H, Burnouf T. Prospective therapeutic applications of platelet extracellular vesicles. Trends Biotechnol. 2021;39(6):598–612. doi:10.1016/j.tibtech.2020.10.004
22. Wiklander OPB, Brennan M, Lötvall J, Breakefield XO, El Andaloussi S. Advances in therapeutic applications of extracellular vesicles. Sci Transl Med. 2019;11(492). doi:10.1126/scitranslmed.aav8521
23. Yang C, Zhang M, Merlin D. Advances in plant-derived edible nanoparticle-based lipid nano-drug delivery systems as therapeutic nanomedicines. J Mater Chem B. 2018;6(9):1312–1321. doi:10.1039/c7tb03207b
24. Quesenberry PJ, Aliotta J, Camussi G, et al. Potential functional applications of extracellular vesicles: a report by the NIH common fund extracellular RNA communication consortium. J Extracell Vesicles. 2015;4:27575. doi:10.3402/jev.v4.27575
25. Zhang M, Viennois E, Prasad M, et al. Edible ginger-derived nanoparticles: a novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials. 2016;101:321–340. doi:10.1016/j.biomaterials.2016.06.018
26. Shao H, Im H, Castro CM, Breakefield X, Weissleder R, Lee H. New technologies for analysis of extracellular vesicles. Chem Rev. 2018;118(4):1917–1950. doi:10.1021/acs.chemrev.7b00534
27. Iwai K, Minamisawa T, Suga K, Yajima Y, Shiba K. Isolation of human salivary extracellular vesicles by iodixanol density gradient ultracentrifugation and their characterizations. J Extracell Vesicles. 2016;5:30829. doi:10.3402/jev.v5.30829
28. Kowal J, Arras G, Colombo M, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. P Natl Acad Sci. 2016;113(8):E968–77. doi:10.1073/pnas.1521230113
29. Vaillancourt M, Hubert A, Subra C, et al. Velocity gradient separation reveals a new extracellular vesicle population enriched in miR-155 and mitochondrial DNA. Pathogens. 2021;10(5):526. doi:10.3390/pathogens10050526
30. Nordin JZ, Lee Y, Vader P, et al. Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomed Nanotechnol Biol Med. 2015;11(4):879–883. doi:10.1016/j.nano.2015.01.003
31. Cheruvanky A, Zhou H, Pisitkun T, et al. Rapid isolation of urinary exosomal biomarkers using a nanomembrane ultrafiltration concentrator. Am J Physiol Renal Physiol. 2007;292(5):F1657–61. doi:10.1152/ajprenal.00434.2006
32. Priglinger E, Strasser J, Buchroithner B, et al. Label-free characterization of an extracellular vesicle-based therapeutic. J Extracell Vesicles. 2021;10(12):e12156. doi:10.1002/jev2.12156
33. Rider MA, Hurwitz SN, Meckes DG. ExtraPEG: a polyethylene glycol-based method for enrichment of extracellular vesicles. Sci Rep. 2016;6:23978. doi:10.1038/srep23978
34. Brownlee Z, Lynn KD, Thorpe PE, Schroit AJ. A novel ”salting-out” procedure for the isolation of tumor-derived exosomes. J Immunol Methods. 2014;407:120–126. doi:10.1016/j.jim.2014.04.003
35. Gardiner C, Di Vizio D, Sahoo S, et al. Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. J Extracell Vesicles. 2016;5:32945. doi:10.3402/jev.v5.32945
36. Kırbaş OK, Bozkurt BT, Asutay AB, et al. Optimized isolation of extracellular vesicles from various organic sources using aqueous two-phase system. Sci Rep. 2019;9(1):19159. doi:10.1038/s41598-019-55477-0
37. Yang M, Liu X, Luo Q, Xu L, Chen F. An efficient method to isolate lemon derived extracellular vesicles for gastric cancer therapy. J Nanobiotechnol. 2020;18(1):100. doi:10.1186/s12951-020-00656-9
38. Huang Y, Wang S, Cai Q, Jin H. Effective methods for isolation and purification of extracellular vesicles from plants. J Integr Plant Biol. 2021;63(12):2020–2030. doi:10.1111/jipb.13181
39. Duong P, Chung A, Bouchareychas L, Raffai RL. Cushioned-density gradient ultracentrifugation (C-DGUC) improves the isolation efficiency of extracellular vesicles. PLoS One. 2019;14(4):e0215324. doi:10.1371/journal.pone.0215324
40. Hopiavuori BR, Masser DR, Wilkerson JL, et al. Isolation of neuronal synaptic membranes by sucrose gradient centrifugation. Methods Mol Biol. 2017;1609:33–41. doi:10.1007/978-1-4939-6996-8_4
41. Dellê H, Saito MH, Yoshimoto PM, Noronha IL. The use of iodixanol for the purification of rat pancreatic islets. Transplant Proc. 2007;39(2):467–469. doi:10.1016/j.transproceed.2007.01.039
42. Xu F, Mu J, Teng Y, et al. Restoring oat nanoparticles mediated brain memory function of mice fed alcohol by sorting inflammatory dectin-1 complex into microglial exosomes. Small. 2022;18(6):e2105385. doi:10.1002/smll.202105385
43. Liu NJ, Wang N, Bao JJ, Zhu HX, Wang LJ, Chen XY. Lipidomic analysis reveals the importance of GIPCs in Arabidopsis leaf extracellular vesicles. Mol Plant. 2020;13(10):1523–1532. doi:10.1016/j.molp.2020.07.016
44. Lee R, Ko HJ, Kim K, et al. Anti-melanogenic effects of extracellular vesicles derived from plant leaves and stems in mouse melanoma cells and human healthy skin. J Extracell Vesicles. 2020;9(1):1703480. doi:10.1080/20013078.2019.1703480
45. Liang Y, Lehrich BM, Zheng S, Lu M. Emerging methods in biomarker identification for extracellular vesicle-based liquid biopsy. J Extracell Vesicles. 2021;10(7):e12090. doi:10.1002/jev2.12090
46. Sharma P, Ludwig S, Muller L, et al. Immunoaffinity-based isolation of melanoma cell-derived exosomes from plasma of patients with melanoma. J Extracell Vesicles. 2018;7(1):1435138. doi:10.1080/20013078.2018.1435138
47. Kang YT, Hadlock T, Lo TW, et al. Dual-Isolation and profiling of circulating tumor cells and cancer exosomes from blood samples with melanoma using immunoaffinity-based microfluidic interfaces. Adv Sci. 2020;7(19):2001581. doi:10.1002/advs.202001581
48. Berger E, Colosetti P, Jalabert A, et al. Use of nanovesicles from Orange juice to reverse diet-induced gut modifications in diet-induced obese mice. Mol Ther Methods Clin Dev. 2020;18:880–892. doi:10.1016/j.omtm.2020.08.009
49. You JY, Kang SJ, Rhee WJ. Isolation of cabbage exosome-like nanovesicles and investigation of their biological activities in human cells. Bioact Mater. 2021;6(12):4321–4332. doi:10.1016/j.bioactmat.2021.04.023
50. Böing AN, van der Pol E, Grootemaat AE, Coumans FA, Sturk A, Nieuwland R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J Extracell Vesicles. 2014;3. doi:10.3402/jev.v3.23430
51. Sitar S, Kejžar A, Pahovnik D, et al. Size characterization and quantification of exosomes by asymmetrical-flow field-flow fractionation. Analy Chem. 2015;87(18):9225–9233. doi:10.1021/acs.analchem.5b01636
52. Zhang H, Lyden D. Asymmetric-flow field-flow fractionation technology for exomere and small extracellular vesicle separation and characterization. Nat Protoc. 2019;14(4):1027–1053. doi:10.1038/s41596-019-0126-x
53. Zeng L, Wang H, Shi W, et al. Aloe derived nanovesicle as a functional carrier for indocyanine green encapsulation and phototherapy. J Nanobiotechnology. 2021;19(1):439. doi:10.1186/s12951-021-01195-7
54. Trentini M, Zanotti F, Tiengo E, et al. An apple a day keeps the doctor away: potential role of miRNA 146 on macrophages treated with exosomes derived from apples. Biomedicines. 2022;10(2). doi:10.3390/biomedicines10020415
55. Fujita D, Arai T, Komori H, et al. Apple-derived nanoparticles modulate expression of organic-anion-transporting polypeptide (OATP) 2B1 in Caco-2 Cells. Mol Pharm. 2018;15(12):5772–5780. doi:10.1021/acs.molpharmaceut.8b00921
56. He B, Cai Q, Qiao L, et al. RNA-binding proteins contribute to small RNA loading in plant extracellular vesicles. Nat Plants. 2021;7(3):342–352. doi:10.1038/s41477-021-00863-8
57. Del Pozo-Acebo L, López de Las Hazas MC, Tomé-Carneiro J, et al. Therapeutic potential of broccoli-derived extracellular vesicles as nanocarriers of exogenous miRNAs. Pharmacol Res. 2022;185:106472. doi:10.1016/j.phrs.2022.106472
58. Deng Z, Rong Y, Teng Y, et al. Broccoli-derived nanoparticle inhibits mouse colitis by activating dendritic cell AMP-activated protein kinase. Mol Ther. 2017;25(7):1641–1654. doi:10.1016/j.ymthe.2017.01.025
59. Kim DK, Rhee WJ. Antioxidative effects of carrot-derived nanovesicles in cardio myoblast and neuroblastoma cells. Pharmaceutics. 2021;13(8):1203. doi:10.3390/pharmaceutics13081203
60. Teng Y, Ren Y, Sayed M, et al. Plant-derived exosomal microRNAs shape the gut microbiota. Cell Host Microbe. 2018;24(5):637–652. doi:10.1016/j.chom.2018.10.001
61. Teng Y, Xu F, Zhang X, et al. Plant-derived exosomal microRNAs inhibit lung inflammation induced by exosomes SARS-CoV-2 Nsp12. Mol Ther. 2021;29(8):2424–2440. doi:10.1016/j.ymthe.2021.05.005
62. Cao M, Yan H, Han X, et al. Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. J Immunother Cancer. 2019;7(1):326. doi:10.1186/s40425-019-0817-4
63. Cho EG, Choi SY, Kim H, et al. Panax ginseng-derived extracellular vesicles facilitate anti-senescence effects in human skin cells: an eco-friendly and sustainable way to use ginseng substances. Cells. 2021;10(3). doi:10.3390/cells10030486
64. Ju S, Mu J, Dokland T, et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol Ther. 2013;21(7):1345–1357. doi:10.1038/mt.2013.64
65. Kalarikkal SP, Sundaram GM. Edible plant-derived exosomal microRNAs: exploiting a cross-kingdom regulatory mechanism for targeting SARS-CoV-2. Toxicol Appl Pharmacol. 2021;414:115425. doi:10.1016/j.taap.2021.115425
66. Stanly C, Alfieri M, Ambrosone A, Leone A, Fiume I, Pocsfalvi G. Grapefruit-derived micro and nanovesicles show distinct metabolome profiles and anticancer activities in the A375 human melanoma cell line. Cells. 2020;9(12):2722. doi:10.3390/cells9122722
67. Savcı Y, Kırbaş OK, Bozkurt BT, et al. Grapefruit-derived extracellular vesicles as a promising cell-free therapeutic tool for wound healing. Food Funct. 2021;12(11):5144–5156. doi:10.1039/d0fo02953j
68. Raimondo S, Naselli F, Fontana S, et al. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget. 2015;6(23):19514–19527. doi:10.18632/oncotarget.4004
69. Baldini N, Torreggiani E, Roncuzzi L, Perut F, Zini N, Avnet S. Exosome-like nanovesicles isolated from citrus limon L. exert antioxidative effect. Curr Pharm Biotechnol. 2018;19(11):877–885. doi:10.2174/1389201019666181017115755
70. Lei C, Teng Y, He L, et al. Lemon exosome-like nanoparticles enhance stress survival of gut bacteria by RNase P-mediated specific tRNA decay. iScience. 2021;24(6):102511. doi:10.1016/j.isci.2021.102511
71. Vabbilisetty P, Sun XL. Liposome surface functionalization based on different anchoring lipids via Staudinger ligation. Org Biomol Chem. 2014;12(8):1237–1244. doi:10.1039/c3ob41721b
72. Lin YY, Chen CY, Ma DL, Leung CH, Chang CY, Wang HD. Cell-derived artificial nanovesicle as a drug delivery system for malignant melanoma treatment. Biomed Pharmacother. 2022;147:112586. doi:10.1016/j.biopha.2021.112586
73. Zhang L, He F, Gao L, et al. Engineering exosome-like nanovesicles derived from asparagus cochinchinensis can inhibit the proliferation of hepatocellular carcinoma cells with better safety profile. Int J Nanomed. 2021;16:1575–1586. doi:10.2147/ijn.S293067
74. Wicki A, Witzigmann D, Balasubramanian V, Huwyler J. Nanomedicine in cancer therapy: challenges, opportunities, and clinical applications. J Control Release. 2015;200:138–157. doi:10.1016/j.jconrel.2014.12.030
75. Armstrong JP, Holme MN, Stevens MM. Re-engineering extracellular vesicles as smart nanoscale therapeutics. ACS nano. 2017;11(1):69–83. doi:10.1021/acsnano.6b07607
76. Narmani A, Rezvani M, Farhood B, et al. Folic acid functionalized nanoparticles as pharmaceutical carriers in drug delivery systems. Drug Dev Res. 2019;80(4):404–424. doi:10.1002/ddr.21545
77. Mansoori B, Mohammadi A, Abedi-Gaballu F, et al. Hyaluronic acid-decorated liposomal nanoparticles for targeted delivery of 5-fluorouracil into HT-29 colorectal cancer cells. J Cell Physiol. 2020;235(10):6817–6830. doi:10.1002/jcp.29576
78. Li Z, Wang H, Yin H, Bennett C, Zhang HG, Guo P. Arrowtail RNA for ligand display on ginger exosome-like nanovesicles to systemic deliver siRNA for cancer suppression. Sci Rep. 2018;8(1):14644. doi:10.1038/s41598-018-32953-7
79. Xiao Q, Zhao W, Wu C, et al. Lemon-derived extracellular vesicles nanodrugs enable to efficiently overcome cancer multidrug resistance by endocytosis-triggered energy dissipation and energy production reduction. Adv Sci. 2022;9(20):e2105274. doi:10.1002/advs.202105274
80. Chen J, Pan J, Liu S, et al. Fruit-derived extracellular vesicles engineered structural droplet drugs for enhanced glioblastoma chemotherapy. Adv Mater. 2023;35(45):e2304187. doi:10.1002/adma.202304187
81. Zhuang X, Teng Y, Samykutty A, et al. Grapefruit-derived nanovectors delivering therapeutic miR17 through an intranasal route inhibit brain tumor progression. Mol Ther. 2016;24(1):96–105. doi:10.1038/mt.2015.188
82. Schillaci O, Fontana S, Monteleone F, et al. Exosomes from metastatic cancer cells transfer amoeboid phenotype to non-metastatic cells and increase endothelial permeability: their emerging role in tumor heterogeneity. Sci Rep. 2017;7(1):4711. doi:10.1038/s41598-017-05002-y
83. Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 2015;6:286. doi:10.3389/fphar.2015.00286
84. Wang MZ, Niu J, Ma HJ, et al. Transdermal siRNA delivery by pH-switchable micelles with targeting effect suppress skin melanoma progression. J Control Release. 2020;322:95–107. doi:10.1016/j.jconrel.2020.03.023
85. Garay RP, El-Gewely R, Armstrong JK, Garratty G, Richette P. Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agents. Expert Opin Drug Deliv. 2012;9(11):1319–1323. doi:10.1517/17425247.2012.720969
86. Abu Lila AS, Kiwada H, Ishida T. The accelerated blood clearance (ABC) phenomenon: clinical challenge and approaches to manage. J Control Release. 2013;172(1):38–47. doi:10.1016/j.jconrel.2013.07.026
87. Xu XH, Yuan TJ, Dad HA, et al. Plant exosomes as novel nanoplatforms for microRNA transfer stimulate neural differentiation of stem cells in vitro and in vivo. Nano Lett. 2021;21(19):8151–8159. doi:10.1021/acs.nanolett.1c02530
88. Zeng L, Shi W, Wang H, et al. Codelivery of π-π stacked dual anticancer drugs based on aloe-derived nanovesicles for breast cancer therapy. ACS Appl. Mater. Interfaces. 2022;14(24):27686–27702. doi:10.1021/acsami.2c06546
89. Mao Y, Han M, Chen C, et al. A biomimetic nanocomposite made of a ginger-derived exosome and an inorganic framework for high-performance delivery of oral antibodies. Nanoscale. 2021;13(47):20157–20169. doi:10.1039/d1nr06015e
90. Wang Q, Ren Y, Mu J, et al. Grapefruit-derived nanovectors use an activated leukocyte trafficking pathway to deliver therapeutic agents to inflammatory tumor sites. Cancer Res. 2015;75(12):2520–2529. doi:10.1158/0008-5472.Can-14-3095
91. Chen X, Zhou Y, Yu J. Exosome-like nanoparticles from ginger rhizomes inhibited NLRP3 inflammasome activation. Mol Pharm. 2019;16(6):2690–2699. doi:10.1021/acs.molpharmaceut.9b00246
92. Urzì O, Cafora M, Ganji NR, et al. Lemon-derived nanovesicles achieve antioxidant and anti-inflammatory effects activating the AhR/Nrf2 signaling pathway. iScience. 2023;26(7):107041. doi:10.1016/j.isci.2023.107041
93. Ou X, Wang H, Tie H, et al. Novel plant-derived exosome-like nanovesicles from Catharanthus roseus: preparation, characterization, and immunostimulatory effect via TNF-α/NF-κB/PU.1 axis. J Nanobiotechnol. 2023;21(1):160. doi:10.1186/s12951-023-01919-x
94. Wang B, Zhuang X, Deng ZB, et al. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol Ther. 2014;22(3):522–534. doi:10.1038/mt.2013.190
95. Hwang JH, Park YS, Kim HS, et al. Yam-derived exosome-like nanovesicles stimulate osteoblast formation and prevent osteoporosis in mice. J Control Release. 2023;355:184–198. doi:10.1016/j.jconrel.2023.01.071
96. Şahin F, Koçak P, Güneş MY, Özkan İ, Yıldırım E, Kala EY. In vitro wound healing activity of wheat-derived nanovesicles. Appl Biochem Biotechnol. 2019;188(2):381–394. doi:10.1007/s12010-018-2913-1
97. Zhan W, Deng M, Huang X, et al. Pueraria lobata-derived exosome-like nanovesicles alleviate osteoporosis by enhancing autophagy. J Control Release. 2023;364:644–653. doi:10.1016/j.jconrel.2023.11.020
98. Ly NP, Han HS, Kim M, Park JH, Choi KY. Plant-derived nanovesicles: current understanding and applications for cancer therapy. Bioact Mater. 2023;22:365–383. doi:10.1016/j.bioactmat.2022.10.005
99. Skotland T, Sandvig K, Llorente A. Lipids in exosomes: current knowledge and the way forward. Prog Lipid Res. 2017;66:30–41. doi:10.1016/j.plipres.2017.03.001
100. Chen Q, Li Q, Liang Y, et al. Natural exosome-like nanovesicles from edible tea flowers suppress metastatic breast cancer via ROS generation and microbiota modulation. Acta Pharm Sin B. 2022;12(2):907–923. doi:10.1016/j.apsb.2021.08.016
101. Goswami KK, Ghosh T, Ghosh S, et al. Tumor promoting role of anti-tumor macrophages in tumor microenvironment. Cell Immunol. 2017;316:1–10. doi:10.1016/j.cellimm.2017.04.005
102. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. doi:10.1126/science.aau6977
103. Liu B, Lu Y, Chen X, et al. Protective role of shiitake mushroom-derived exosome-like nanoparticles in D-galactosamine and lipopolysaccharide-induced acute liver injury in mice. Nutrients. 2020;12(2):477. doi:10.3390/nu12020477
104. Liu C, Yan X, Zhang Y, et al. Oral administration of turmeric-derived exosome-like nanovesicles with anti-inflammatory and pro-resolving bioactions for murine colitis therapy. J Nanobiotechnol. 2022;20(1):206. doi:10.1186/s12951-022-01421-w
105. Zhang L, Li S, Cong M, et al. Lemon-derived extracellular vesicle-like nanoparticles block the progression of kidney stones by antagonizing endoplasmic reticulum stress in renal tubular cells. Nano lett. 2023;23(4):1555–1563. doi:10.1021/acs.nanolett.2c05099
106. Wu KC, Cui JY, Klaassen CD. Effect of graded Nrf2 activation on phase-I and -II drug metabolizing enzymes and transporters in mouse liver. PLoS One. 2012;7(7):e39006. doi:10.1371/journal.pone.0039006
107. Wang R, Paul VJ, Luesch H. Seaweed extracts and unsaturated fatty acid constituents from the green alga Ulva lactuca as activators of the cytoprotective Nrf2-ARE pathway. Biol Med. 2013;57:141–153. doi:10.1016/j.freeradbiomed.2012.12.019
108. Bak MJ, Ok S, Jun M, Jeong WS. 6-shogaol-rich extract from ginger up-regulates the antioxidant defense systems in cells and mice. Molecules. 2012;17(7):8037–8055. doi:10.3390/molecules17078037
109. Zhuang X, Deng ZB, Mu J, et al. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J Extracell Vesicles. 2015;4:28713. doi:10.3402/jev.v4.28713
110. Man F, Meng C, Liu Y, et al. The study of ginger-derived extracellular vesicles as a natural nanoscale drug carrier and their intestinal absorption in Rats. AAPS Pharm Sci Tech. 2021;22(6):206. doi:10.1208/s12249-021-02087-7
111. Dou W, Zhang J, Sun A, et al. Protective effect of naringenin against experimental colitis via suppression of Toll-like receptor 4/NF-κB signaling. Br J Nutr. 2013;110(4):599–608. doi:10.1017/s0007114512005594
112. Zhang M, Wang X, Han MK, Collins JF, Merlin D. Oral administration of ginger-derived nanolipids loaded with siRNA as a novel approach for efficient siRNA drug delivery to treat ulcerative colitis. Nanomedicine. 2017;12(16):1927–1943. doi:10.2217/nnm-2017-0196
113. Hasanuzzaman M, Bhuyan M, Zulfiqar F, et al. Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants. 2020;9(8):681. doi:10.3390/antiox9080681
114. Kasote DM, Katyare SS, Hegde MV, Bae H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int J Biol Sci. 2015;11(8):982–991. doi:10.7150/ijbs.12096
115. Perut F, Roncuzzi L, Avnet S, et al. Strawberry-derived exosome-like nanoparticles prevent oxidative stress in human mesenchymal stromal cells. Biomolecules. 2021;11(1):87. doi:10.3390/biom11010087
116. Kim MK, Choi YC, Cho SH, Choi JS, Cho YW. The antioxidant effect of small extracellular vesicles derived from aloe vera peels for wound healing. Tissue Eng Regen Med. 2021;18(4):561–571. doi:10.1007/s13770-021-00367-8
117. Hajialyani M, Tewari D, Sobarzo-Sánchez E, Nabavi SM, Farzaei MH, Abdollahi M. Natural product-based nanomedicines for wound healing purposes: therapeutic targets and drug delivery systems. Int J Nanomed. 2018;13:5023–5043. doi:10.2147/ijn.S174072
118. Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: a cellular perspective. Physiol Rev. 2019;99(1):665–706. doi:10.1152/physrev.00067.2017
119. Xue W, Yu J, Chen W. Plants and their bioactive constituents in mesenchymal stem cell-based periodontal regeneration: a novel prospective. BioMed Res Int. 2018;2018:7571363. doi:10.1155/2018/7571363
120. Klinger-Strobel M, Lautenschläger C, Fischer D, et al. Aspects of pulmonary drug delivery strategies for infections in cystic fibrosis--where do we stand? Expert Opin. Drug Deliv. 2015;12(8):1351–1374. doi:10.1517/17425247.2015.1007949
121. Yang S, Lu S, Ren L, et al. Ginseng-derived nanoparticles induce skin cell proliferation and promote wound healing. J Ginseng Res. 2023;47(1):133–143. doi:10.1016/j.jgr.2022.07.005
122. Ettlinger R, Lächelt U, Gref R, et al. Toxicity of metal-organic framework nanoparticles: from essential analyses to potential applications. Chem Soc Rev. 2022;51(2):464–484. doi:10.1039/d1cs00918d
123. Truskewycz A, Yin H, Halberg N, et al. Carbon dot therapeutic platforms: administration, distribution, metabolism, excretion, toxicity, and therapeutic potential. Small. 2022;18(16):e2106342. doi:10.1002/smll.202106342
124. Dad HA, Gu TW, Zhu AQ, Huang LQ, Peng LH. Plant exosome-like nanovesicles: emerging therapeutics and drug delivery nanoplatforms. Mol Ther. 2021;29(1):13–31. doi:10.1016/j.ymthe.2020.11.030
125. Li X, Liang Z, Du J, et al. Herbal decoctosome is a novel form of medicine. Sci China Life Sci. 2019;62(3):333–348. doi:10.1007/s11427-018-9508-0
126. Li N, Peng LH, Chen X, Nakagawa S, Gao JQ. Transcutaneous vaccines: novel advances in technology and delivery for overcoming the barriers. Vaccine. 2011;29(37):6179–6190. doi:10.1016/j.vaccine.2011.06.086
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
