Back to Journals » Orthopedic Research and Reviews » Volume 16
Efficacy and Safety of Generic Alendronate for Osteoporosis Treatment
Authors Jarusriwanna A , Malisorn S , Tananoo S , Areewong K, Rasamimongkol S, Laoruengthana A
Received 17 October 2023
Accepted for publication 8 February 2024
Published 22 February 2024 Volume 2024:16 Pages 85—91
DOI https://doi.org/10.2147/ORR.S445202
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Clark Hung
Atthakorn Jarusriwanna,1,* Saran Malisorn,1,* Sirikarn Tananoo,1 Kwanchanok Areewong,2 Supachok Rasamimongkol,1 Artit Laoruengthana1
1Department of Orthopaedics, Faculty of Medicine, Naresuan University, Phitsanulok, Thailand; 2Department of Pharmacy, Naresuan University Hospital, Phitsanulok, Thailand
*These authors contributed equally to this work
Correspondence: Sirikarn Tananoo, Department of Orthopaedics, Faculty of Medicine, Naresuan University, 99 Moo 9, Phitsanulok-Nakhon Sawan Road, Tha Pho, Mueang Phitsanulok, Phitsanulok, 65000, Thailand, Tel +66-5596-5560, Fax +66-5596-5105, Email [email protected]
Background: While osteoporosis increases the risk of fragility fractures, bisphosphonate has been proven to increase bone strength and reduce the risk of vertebral and non-vertebral fractures. In addition to its efficacy, substituting the brand with generic medication is a strategy to optimize healthcare expenditures. This study aimed to evaluate the efficacy of generic alendronate treatment and assess potential adverse events in patients with osteoporosis.
Materials and Methods: A retrospective review was conducted on 120 patients who met the indications for osteoporosis treatment, received weekly generic alendronate (70 mg) for > 1 year, and underwent evaluation through standard axial dual-energy X-ray absorptiometry (DXA). The outcomes of this study were the percent change in bone mineral density (BMD) at the lumbar spine, femoral neck, and total hip after one year of treatment. The major adverse events occurring during medication that led to the discontinuation of drug administration were documented.
Results: Most patients were female (96.7%) with an average age of 69.0 ± 9.3 years. The percent change in BMD increased at all sites after one year of generic alendronate treatment (lumbar spine: 5.6 ± 13.7, p-value < 0.001; femoral neck: 2.3 ± 8.3, p-value = 0.023; total hip: 2.1 ± 6.2, p-value = 0.003), with over 85% of patients experiencing increased or stable BMD. Three patients discontinued the medication due to adverse effects: two had dyspepsia, and one had persistent myalgia.
Conclusion: Generic alendronate may be considered an effective antiresorptive agent for osteoporosis treatment with a low incidence of adverse effects.
Keywords: osteoporosis, fragility fracture, bisphosphonate, alendronate, bone mineral density
Introduction
Osteoporosis is a systemic bone disorder caused by the reduction of bone mass and diminished bone strength.1 This disease stands as one of the most significant risk factors for fragility fractures, which are associated with decreased quality of life, disability, and premature mortality.2,3 Currently, osteoporosis poses a considerable public health concern due to an overall prevalence of 10.3%, with an estimated 27.5 million people in Europe, and 10.2 million in the USA affected.4,5 It is anticipated that half of the women aged over 50 years are likely to be treated for osteoporosis at some point in their lives.6 The ongoing trend of an aging population is expected to further increase the number of patients, and subsequently impact the economic burden on healthcare systems worldwide.7 Projections indicate that this burden may double or even triple by 2040.8
Bisphosphonate is an antiresorptive agent that inhibits osteoclastic activity, reduces bone turnover, and enhances bone strength and bone mass.9 It represents the most prescribed treatment for osteoporosis.10 Alendronate is one of the most common oral bisphosphonates that significantly reduces the risk of fragility fractures, including both vertebral and non-vertebral fractures.11 In addition to the efficacy of alendronate in reducing fragility fractures, the cost-effectiveness of its use in postmenopausal women has become a topic of essential discussion. An analysis revealed that appropriate osteoporosis screening and treatment with alendronate result in significant cost savings when the price is below US$200 per year. However, the cost-effectiveness of preventing fragility fractures and associated morbidity, particularly in low- to middle-income countries, remains unclear.12–14
Alternatively, switching to generic medication is a strategy to optimize healthcare expenditure. Generally, generic drugs are required to demonstrate bioequivalence to the original drugs. Some studies have demonstrated that medication adherence, improvement in bone mineral density (BMD), and reduction in biochemical markers of bone turnover appear similar among brand and generic alendronate.15,16 Although other studies have shown that patients taking generic alendronate are significantly more likely to experience side effects, generic alendronate may still be considered a reasonable option compared to the brand alendronate.17,18
Therefore, this study aimed to evaluate the efficacy and safety of generic alendronate treatment in osteoporosis patients. We hypothesized that using generic alendronate could improve the BMD of patients over a year and maintain a good safety profile.
Materials and Methods
This study was a retrospective chart review of patients who met the indication of osteoporosis treatment and received generic alendronate for at least 1 year. The protocol of this study was approved by the Naresuan University Institutional Review Board (approval no. 447/59). A waiver of consent was endorsed as it would not adversely affect the rights and welfare of the subjects and all patients’ information was kept confidential. All procedures involved in this study were following the ethical standards and compliance with the Declaration of Helsinki. According to the treatment guideline for osteoporosis,19,20 bisphosphonate is considered in high-risk osteoporotic fracture patients, which have one of the following indications: men age more than 50 years or postmenopausal women with either fragility vertebral or hip fracture; bone mineral density (BMD) from a standard axial dual-energy X-ray absorptiometry (DXA) at the lumbar spine, or femoral neck, or total hip with a T-score ≤−2.5; BMD with a T-score between −1 and −2.5 and the 10-year probability of hip fracture ≥3% calculating from the fracture risk assessment tool (FRAX®); BMD with a T-score between −1 and −2.5 and a fragility fracture at either the proximal humerus, pelvis, or distal forearm.
All patients in this study received oral generic alendronate (Bonmax®; Apotex Incorporated, Toronto, Ontario, Canada) at a dose of 70 mg/week, calcium carbonate 1,500 mg/day, and vitamin D2 (ergocalciferol) 20,000 IU/week. Patients attended scheduled follow-ups, including BMD measurements at the spine and hip, one year after initiating generic alendronate treatment (Figure 1). Drug adherence was monitored by reporting the remaining drugs in each follow-up interval and then calculated for the medication possession ratio (MPR).21 Patients without BMD data after one year of treatment or with less than one year of follow-up were excluded from the study. The DXA scans in this study were conducted using the Hologic® Discovery series protocol (Hologic Inc., Waltham, MA, USA).
 |
Figure 1 Flow diagram of patients in this study. |
The outcomes of this study were the mean increase in BMD and the percent change of BMD at the lumbar spine, femoral neck, and total hip after one year of treatment. Patients’ BMD changes were categorized as increased, stable, or decreased based on the percent change in BMD. Specifically, lumbar spine BMD was classified as increased if the gain was ≥3%, stable if the change was between −3% and 3%, and decreased if the loss was ≥3%. For femoral neck and total hip BMD, increased were defined as the gain ≥5%, stability as the change between −5% and 5%, and decreased as the loss ≥5%.15 Additionally, major adverse events occurring during medication that led to discontinuation of drug administration were reported.
The data were presented as mean ± standard deviation (SD) for continuous variables and as percentages (%) for categorical variables. A descriptive analysis was performed for the demographic data. The BMD between baseline and post-treatment was compared using a paired samples t-test, with a p-value <0.05 considered statistically significant. The data analysis was performed using SPSS Statistics version 18 (SPSS Inc., Chicago, IL, USA).
Results
A total of 134 charts of osteoporosis patients who received generic alendronate for at least a one-year duration were reviewed. Out of the initial 134 patients, fourteen patients were excluded from the study. Five patients did not undergo a BMD measurement after one year of treatment, and three patients opted to discontinue medication due to severe adverse effects, including two patients with dyspepsia and one patient with persistent myalgia in the first few weeks. Other 6 patients were lost to follow-up (Figure 1). The demographic data of the remaining 120 patients are briefly summarized in Table 1. The majority of the patients were female (96.7%) with ages ranging from 50 to 95 years (mean 69 ± 9.3). Most of the patients did not have a previous history of vertebral or hip fractures (67.5%), while 27.5% had experienced osteoporotic vertebral compression fractures, and 5% had a history of hip fractures.
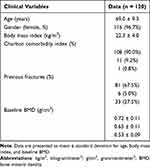 |
Table 1 Patients’ Demographic Data |
The mean BMD increased from baseline to 1-year post-treatment at all sites (Table 2). Overall, the average percent change of BMD increased by 5.6%, 2.3%, and 2.1% at the lumbar spine, femoral neck, and total hip, respectively, and the BMD of all sites reached statistical significance. There were >85% of patients with increased or stable BMD. The majority of patients were classified as having increased BMD in the lumbar spine, with stable BMD in the femoral neck and total hip (Table 3). The average MPR of the patients was 93.4%, which demonstrated good adherence to the treatment. The minor adverse effects that occurred during treatment included 2 patients with myalgia, and 1 patient with fever after taking generic alendronate for the first time; however, these adverse effects improved afterward, and the patients still tolerated the medication.
 |
Table 2 Changes of BMD After One Year of Generic Alendronate Treatment |
 |
Table 3 Patients with Stable, Increased, and Decreased BMD After 1 Year of Generic Alendronate Treatment |
Discussion
Treatment of osteoporosis is important as it can significantly reduce the incidence of fragility fractures and improve patients’ morbidity and mortality. Bisphosphonate, particularly alendronate, is a commonly prescribed antiresorptive agent to prevent those fragility fractures.9–11 Alendronate has been proven to reduce the incidence of spinal and hip fractures by approximately 50% over 3 years in patients with previous vertebral fractures or patients with BMD T-score ≤−2.5 at the hip.22 The cost-effectiveness of treatment has become a topic of debate, leading to strategies like switching from brand to generic medications to optimize healthcare expenditure. Unnanuntana et al15 reported similar efficacy and no significant differences in adverse effects between brand and generic alendronate. In our study, we found that the BMD of patients significantly increased from the baseline at all bone sites after continuing generic alendronate treatment for at least one year. Moreover, more than 85% of patients in our study exhibited increased or stable BMD compared to their baseline level.
Switching from brand to generic drugs offers a cost-effective option to patients, providing greater access to essential medications once the patent protection for the original drug has expired. Nevertheless, generic drugs require rigorous testing and regulation to ensure effectiveness and safety in alternative use to their brand-name counterparts. It is noteworthy that even a 1% improvement in BMD can be a good predictor of treatment efficacy, as the relative risk of fracture decreases by 0.03.23 Therefore, taking generic alendronate could be beneficial in reducing osteoporotic fractures.
An economic evaluation of alendronate for osteoporosis in Chinese postmenopausal women demonstrated that alendronate is more cost-effective than no treatment group, considering both total healthcare costs and the number of fractures.14 A cost-effectiveness analysis in the USA indicated that continuing alendronate for 5 years in postmenopausal women and followed by 5 years of the drug holiday period is the most likely cost-effective approach.24 Typically, brand medications are more expensive than the generic equivalents. At our hospital, generic alendronate costs approximately 70% less than the brand version. Moreover, evidence from cost-utility and budget impact analyses in Thailand supported the cost-effectiveness of alendronate for primary osteoporotic fracture prevention in women aged over 65 years. The generic alendronate had an Incremental Cost-Effectiveness Ratio (ICER) of 85,000 Baht (US$2,413) per Quality-Adjusted Life-Year (QALY) gained, whereas the brand alendronate showed an ICER of 242,000 Baht (US$6,870) per QALY gained.25
There were some adverse effects reported in our study such as myalgia, pyrexia, and dyspepsia. Myalgia and pyrexia were generally mild to moderate in severity and usually resolved within 3 days.26 Dyspepsia might be attributed to slow gastrointestinal (GI) transit time and bioadhesion in generic alendronate.18 To minimize the risk of GI irritation while taking alendronate, it is essential to consume the medication with a full glass of water and remain upright for at least 30 minutes after taking the medication.
Medication adherence is another important issue when considering alendronate treatment, as it can significantly impact the economic benefits of switching medications. Therefore, healthcare providers must follow the treatment guidelines, accurately assess indications, and monitor potential adverse effects. In the present study, we found 3 patients discontinued medication and 6 were lost to follow-up, which accounted for 6.7% of the enrolled patients. Nevertheless, the drug adherence of the remaining patients was at a high level when evaluated by the MPR. A study by Duarte et al27 identified medication effectiveness as the most crucial determinant of drug preference, followed by side effects, medication costs, dosing frequency, and other factors such as formulation, timing on the market, and dosing procedure. Furthermore, holistic care for patients is also crucial. Several studies have demonstrated the association between depression and osteoporosis, as well as caregivers of these patients.28 A study by Chen et al29 demonstrated that patients with osteoporosis had 1.73 times higher rates of depressive symptoms. This might impact physical activities, exercises, nutritional status, and treatment compliance. It would be beneficial for future studies to focus on holistic patient care including mental status.
Many innovative treatment strategies for osteoporosis have gained more focus recently. The imbalance between osteoblasts and osteoclasts, especially the decrease in osteogenic differentiation and increase in osteoclastogenesis, contributes to the existence of osteoporosis. Any targeted therapy to these cells and mechanisms, particularly in those with stem cells, might impact and be a future opportunity for osteoporosis treatment.30,31
This study has several limitations. First, this is a retrospective study, which may introduce inherent biases and lack of some information. The absence of generalized across genders is another limitation of this study, as most patients were female. Nevertheless, this issue demonstrated the characteristics of osteoporosis that occurs mostly in female patients. Lack of disease awareness in males might lead to a lowered number of screening and treatment.32 Second, the study did not investigate biochemical bone markers during treatment, which could provide additional insights into the efficacy of generic alendronate treatment. Third, the duration of treatment evaluation was relatively short. Future research should consider prospective studies with longer periods of generic alendronate continuation and extended follow-up for both bone mineral density (BMD) and biochemical bone markers. Additionally, exploring issues related to cost-effectiveness would be valuable topics for future literature. Finally, data collection on patients’ satisfaction and opinions regarding the treatment is essential to gather feedback about acceptability, convenience, and satisfaction.
Conclusion
Generic alendronate can be considered an effective antiresorptive agent for osteoporosis treatment, with a low incidence of adverse effects. Furthermore, patients with osteoporosis should be encouraged to undergo treatment following a rigorous protocol and monitored carefully for potential adverse effects.
Acknowledgment
The authors gratefully acknowledge Kongpob Reosanguanwong, MD, Panapol Varakornpipat, MD, Bhuwad Chinwatanawongwan, MD, Nattapat Khemworapong, MD, and Pariphat Chompoonutprapa, MD, for their assistance with data collection and technical support.
Funding
This was an unfunded study.
Disclosure
The authors declare no personal or professional conflicts of interest regarding any aspect of this study.
References
1. Drake MT, Clarke BL, Lewiecki EM. The pathophysiology and treatment of osteoporosis. Clin Ther. 2015;37(8):1837–1850. doi:10.1016/j.clinthera.2015.06.006
2. Papaioannou A, Kennedy CC, Ioannidis G, et al. The impact of incident fractures on health-related quality of life: 5 years of data from the Canadian Multicentre Osteoporosis Study. Osteoporos Int. 2009;20(5):703–714. doi:10.1007/s00198-008-0743-7
3. Sattui SE, Saag KG. Fracture mortality: associations with epidemiology and osteoporosis treatment. Nat Rev Endocrinol. 2014;10(10):592–602. doi:10.1038/nrendo.2014.125
4. Hernlund E, Svedbom A, Ivergård M, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos. 2013;8(1):136. doi:10.1007/s11657-013-0136-1
5. Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520–2526. doi:10.1002/jbmr.2269
6. Nelson HD, Helfand M, Woolf SH, Allan JD. Screening for postmenopausal osteoporosis: a review of the evidence for the US preventive services task force. Ann Intern Med. 2002;137(6):529–541. doi:10.7326/0003-4819-137-6-200209170-00015
7. Tarride JE, Hopkins RB, Leslie WD, et al. The burden of illness of osteoporosis in Canada. Osteoporos Int. 2012;23(11):2591–2600. doi:10.1007/s00198-012-1931-z
8. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465–475. doi:10.1359/jbmr.061113
9. Cosman F, Nieves JW, Dempster DW. Treatment sequence matters: anabolic and antiresorptive therapy for osteoporosis. J Bone Miner Res. 2017;32(2):198–202. doi:10.1002/jbmr.3051
10. Black DM, Rosen CJ. clinical practice. Postmenopausal osteoporosis. N Engl J Med. 2016;374(3):254–262. doi:10.1056/NEJMcp1513724
11. Sanderson J, Martyn-St James M, Stevens J, et al. Clinical effectiveness of bisphosphonates for the prevention of fragility fractures: a systematic review and network meta-analysis. Bone. 2016;89:52–58. doi:10.1016/j.bone.2016.05.013
12. Nayak S, Roberts MS, Greenspan SL. Impact of generic alendronate cost on the cost-effectiveness of osteoporosis screening and treatment. PLoS One. 2012;7(3):e32879. doi:10.1371/journal.pone.0032879
13. Cheung EYN, Tan KCB, Cheung CL, Kung AWC. Osteoporosis in East Asia: current issues in assessment and management. Osteoporos Sarcopenia. 2016;2(3):118–133. doi:10.1016/j.afos.2016.07.001
14. You R, Liu Z. Economic evaluation of oral alendronate therapy for osteoporosis in Chinese postmenopausal women: the impact of medication compliance and persistence. Front Pharmacol. 2020;11:575893. doi:10.3389/fphar.2020.575893
15. Unnanuntana A, Jarusriwanna A, Songcharoen P. Randomized clinical trial comparing efficacy and safety of brand versus generic alendronate (Bonmax®) for osteoporosis treatment. PLoS One. 2017;12(7):e0180325. doi:10.1371/journal.pone.0180325
16. Colombo GL, Montecucco CM. Generic vs brand originator alendronate: analysis of persistence and compliance in five Local Healthcare Units in the Lombardy Region of Italy. Clin Cases Miner Bone Metab. 2013;10(3):195–198.
17. Lai PS, Chua SS, Chong YH, Chan SP. The effect of mandatory generic substitution on the safety of alendronate and patients’ adherence. Curr Med Res Opin. 2012;28(8):1347–1355. doi:10.1185/03007995.2012.708326
18. Brown JP, Davison KS, Olszynski WP, Beattie KA, Adachi JD. A critical review of brand and generic alendronate for the treatment of osteoporosis. Springerplus. 2013;2(1):550. doi:10.1186/2193-1801-2-550
19. Charatcharoenwitthaya N, Jaisamrarn U, Songpatanasilp T, et al. Summary of the Thai Osteoporosis Foundation (TOPF) clinical practice guideline on the diagnosis and management of osteoporosis 2021. Osteoporos Sarcopenia. 2023;9(2):45–52. doi:10.1016/j.afos.2023.06.001
20. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists/American College of Endocrinology Clinical Practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr Pract. 2020;26(Suppl 1):1–46.
21. Fatoye F, Smith P, Gebrye T, Yeowell G. Real-world persistence and adherence with oral bisphosphonates for osteoporosis: a systematic review. BMJ Open. 2019;9(4):e027049. doi:10.1136/bmjopen-2018-027049
22. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359–2381. doi:10.1007/s00198-014-2794-2
23. Cummings SR, Karpf DB, Harris F, et al. Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med. 2002;112(4):281–289. doi:10.1016/S0002-9343(01)01124-X
24. Nayak S, Greenspan SL. Cost-effectiveness of five versus ten years of alendronate treatment prior to drug holiday for women with osteoporosis. Osteoporos Int. 2020;31(7):1273–1282. doi:10.1007/s00198-019-05258-2
25. Cost-utility analysis of treatment for prevention of osteoporotic fractures [Internet]. Nonthaburi (TH): Health Intervention and Technology Assessment Program; [cited August 8, 2023]. Available from: https://www.hitap.net/en/research/17703.
26. Orwoll ES, Miller PD, Adachi JD, et al. Efficacy and safety of a once-yearly i.v. infusion of zoledronic acid 5 mg versus a once-weekly 70-mg oral alendronate in the treatment of male osteoporosis: a randomized, multicenter, double-blind, active-controlled study. J Bone Miner Res. 2010;25(10):2239–2250. doi:10.1002/jbmr.119
27. Duarte JW, Bolge SC, Sen SS. An evaluation of patients’ preferences for osteoporosis medications and their attributes: the PREFER-international study. Clin Ther. 2007;29(3):488–503. doi:10.1016/S0149-2918(07)80087-7
28. Sukchokpanich P, Anusitviwat C, Jarusriwanna A, Kitcharanant N, Unnanuntana A. Quality of life and depression status of caregivers of patients with femoral neck or intertrochanteric femoral fractures during the first year after fracture treatment. Orthop Surg. 2023;15(7):1854–1861. doi:10.1111/os.13802
29. Chen K, Wang T, Tong X, et al. Osteoporosis is associated with depression among older adults: a nationwide population-based study in the USA from 2005 to 2020. Public Health. 2023;226:27–31. doi:10.1016/j.puhe.2023.10.022
30. Zeng ZL, Xie H. Mesenchymal stem cell-derived extracellular vesicles: a possible therapeutic strategy for orthopaedic diseases: a narrative review. Biomater Transl. 2022;3(3):175–187. doi:10.12336/biomatertransl.2022.03.002
31. Li X, Wang L, Huang B, et al. Targeting actin-bundling protein L-plastin as an anabolic therapy for bone loss. Sci Adv. 2020;6(47):eabb7135. doi:10.1126/sciadv.abb7135
32. Alswat KA. Gender disparities in osteoporosis. J Clin Med Res. 2017;9(5):382–387. doi:10.14740/jocmr2970w
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
