Back to Journals » Clinical Ophthalmology » Volume 17
Effect of Repeated Intravitreal Injections in Glaucoma Spectrum Diseases
Authors Vilares-Morgado R , Correia V, Ferreira AM , Alves F, Melo AB, Estrela-Silva S , Araújo J, Tavares-Ferreira J , Silva M, Rocha-Sousa A , Carneiro A, Barbosa-Breda J
Received 16 October 2023
Accepted for publication 7 November 2023
Published 22 November 2023 Volume 2023:17 Pages 3613—3627
DOI https://doi.org/10.2147/OPTH.S441500
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Rodrigo Vilares-Morgado,1,2 Vera Correia,3 Ana Margarida Ferreira,1 Flávio Alves,1 António Benevides Melo,1,3 Sérgio Estrela-Silva,1,3 Joana Araújo,1,3 João Tavares-Ferreira,1,3 Marta Silva,1 Amândio Rocha-Sousa,1,2 Angela Carneiro,1,2 João Barbosa-Breda1,2,4
1Department of Ophthalmology, Centro Hospitalar e Universitário de São João, Porto, Portugal; 2UnIC@RISE, Department of Surgery and Physiology, Faculty of Medicine of the University of Porto, Porto, Portugal; 3Faculty of Medicine, University of Porto, Porto, Portugal; 4KULeuven, Research Group Ophthalmology, Department of Neurosciences, Leuven, Belgium
Correspondence: Rodrigo Vilares-Morgado, Department of Ophthalmology, Centro Hospitalar e Universitário de São João, Alameda Professor Hernâni Monteiro, 4202 – 451, Porto, Portugal, Tel +351 914 471 067, Fax +351 225 512 100, Email [email protected]
Purpose: To evaluate whether repeated intravitreal injections (IVI) with an anti-vascular endothelial growth factor (anti-VEGF) agent are associated with glaucomatous progression in eyes with glaucoma spectrum diseases (GSD).
Methods: Single-center, retrospective, longitudinal study of patients with bilateral and similar GSD who: (1) received ≥ 8 IVI in only one eye during the study period; (2) had ≥ 2 retinal nerve fiber layer thickness (RNFL) measurements obtained by spectral-domain optical coherence tomography (SD-OCT) at least 12 months apart. The primary outcome was the absolute RNFL thickness change, comparing injected and fellow uninjected eyes. Linear mixed effects models were constructed, including a multivariable model.
Results: Sixty-eight eyes from 34 patients were included, 34 injected and 34 fellow uninjected eyes. Average baseline age was 67.68± 21.77 years with a follow-up of 3.66± 1.89 years and 25.12± 14.49 IVI. RNFL thickness decreased significantly from 80.92± 15.78 to 77.20± 17.35 μm (p< 0.001; − 1.18± 1.93 μm/year) in injected eyes and from 79.95± 17.91 to 76.61± 17.97 μm (p< 0.001; − 1.07± 0.98 μm/year) in uninjected eyes. In a multivariable linear mixed model of injected eyes, only higher baseline RNFL thickness (p < 0.001) significantly predicted higher absolute RNFL thickness loss. Neither absolute RNFL thickness variation (p=0.716) nor RNFL rate (p=0.779) was significantly different between paired injected and uninjected eyes. Absolute IOP variation was not significantly different between groups (16.62± 4.77 to 15.09± 4.34 mmHg in injected eyes and 17.68± 5.01 to 14.50± 3.39 mmHg in fellow uninjected eyes; p=0.248). The proportion of eyes receiving glaucoma medical treatment increased significantly in both groups (55.9% to 76.5% in injected eyes; p=0.039; 58.8% to 76.5% in uninjected eyes; p = 0.031). The number of glaucoma medications also increased significantly in both groups (1.03± 1.11 to 1.59± 1.18 glaucoma medications in injected eyes; p=0.003; 1.09± 1.11 to 1.56± 1.19 glaucoma medications in uninjected eyes; p=0.003).
Conclusion: Repeated IVI do not seem to accelerate glaucomatous progression. Future studies with a longer follow-up are needed.
Keywords: glaucoma, intravitreal injections, progression, optical coherence tomography, retinal nerve fiber layer thickness
A Letter to the Editor has been published for this article.
A Response to Letter by Prof. Dr. Budu has been published for this article.
Introduction
Intravitreal injections are currently the mainstay of treatment of various ophthalmological conditions, including the exudative form of age-related macular degeneration (AMD), macular edema secondary to diabetic retinopathy (DME) and central or branch retinal vein occlusion (RVO).1–3 These diseases comprise the majority of retina practice cases, requiring regular injections often over various years so as to maintain an adequate visual function.1–4 As an example, in the UK’s largest ophthalmology hospital (Moorfield’s Eye Hospital), the number of injections increased 11-fold from 2009 to 2019 (44,924 injections delivered in 2019), with a predicted increase to nearly 83000 injections in 2029.5 Most eyes are injected with medications that inhibit the action of vascular endothelial growth factor A (anti-VEGF), while some are injected with corticosteroids.6
However, despite the undeniable beneficial effects, they lead to an immediate, although generally transient, intraocular pressure (IOP) elevation, of which data regarding long-term effects are inconsistent.4,7–10 It is postulated that these repeated pressure spikes, or even sustained ocular hypertension (OHT), may promote glaucomatous damage in susceptible optic nerves, leading to faster retinal nerve fiber layer (RNFL) thinning over time.11 Until recently, most research efforts regarding injection-related optic nerve damage were done in patients with non-glaucomatous eyes, having yielded mixed results.12–23
Glaucoma is the leading cause of irreversible vision loss in the world and patients with concomitant retinal pathology have a twofold increased risk of blindness and low vision.24,25 It is hypothesized that these patients may be more susceptible to additional injury after repeated IOP spikes, as the optic nerve has already sustained an initial insult. Moreover, in a 2016 meta-analysis, the risk of sustained IOP elevation was higher when patients with pre-existing glaucoma were included in the analysis.7
To date, only a limited number of studies have been conducted to evaluate the potentially damaging effects of repeated intravitreal anti-VEGF injections on glaucoma progression of patients with pre-existing glaucoma or ocular hypertension, using imaging metrics such as RNFL thickness.26–32 These have generated conflicting results and several only report the injected eye group, with no control for comparison.28,30,31 Thus, they present questionable methodologies to answer this relevant question. More important than identifying progression (which is inherent to glaucoma), is to identify faster progression, which can be achieved by comparing injected with fellow uninjected eyes with glaucoma. Up to now, only 4 studies presented such methodology,26,27,29,32 but none included only paired eyes with symmetrical GSD or performed a multivariable analysis to adjust for potential confounders. As such, there remains a paucity of high-quality evidence on this topic.
The main purpose of this study is to ascertain if repeated intravitreal anti-VEGF injections lead to faster RNFL thickness changes between injected and fellow uninjected eyes of patients with symmetrical glaucoma spectrum diseases (GSD).
Methods
The study was approved by the Institutional Ethics Review Board of Centro Hospitalar Universitário de São João, Porto, Portugal. The protocol conformed with the canons of the Declaration of Helsinki for research involving human participants, as well as the European Union’s General Data Protection Regulation. Informed consent was waived in view of the retrospective nature of the study. This article was redacted according to the recommendations of The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement.33
Study Design and Setting
This is a retrospective, single center, longitudinal study. Patients followed in Centro Hospitalar Universitário São João, Porto, Portugal, who had at least one glaucoma department consultation from January 1st, 2008, to June 12th, 2021, were cross-referenced with the procedural codes for intravitreal injections, namely the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM)’s code 1479 and International Classification of Diseases, Tenth Revision, Procedure Classification System (ICD-10-PCS)’s code 3E0C3GC. In Portugal, coding is done by trained medical coders based on information routinely reported in medical records and pathology and surgical reports.34 Cross-referencing of retinal or glaucoma diagnosis codes, as well as those from spectral-domain optical coherence tomography (SD-OCT) exams were not conducted, since that would create a source of heterogeneity, as these are not yet properly coded for many patients in this hospital. The medical records of all retrieved patients were manually reviewed for compliance with inclusion criteria.
Study Participants
We included both eyes of patients ≥18 years old, diagnosed with unilateral exudative AMD, RVO or DME, that had received 8 or more intravitreal anti-VEGF injections, were followed in glaucoma medical consultations with a concurrent diagnosis of bilateral OHT, glaucoma suspect (GS) or definite primary open angle glaucoma (POAG) or normal tension glaucoma (NTG) and who had at least 2 SD-OCT RNFL thickness measurements, 12 or more months apart (SPECTRALIS Heidelberg® Retina OCT imaging platform, Heidelberg Engineering, Heidelberg, Germany). The fellow uninjected eye of each patient was also included as a matching control case. The study period for each eye was defined as the interval from first to the most recent available SD-OCT scan with peripapillary RNFL measurements. At least 8 intravitreal anti-VEGF injections had to be given during the study period. Baseline was defined as the time-point of the first RNFL thickness measurement obtained within 6 months of an intravitreal injection (considered the first study period injection for this study’s purpose). In cases where intravitreal injections were started before the first OCT was available, the first SD-OCT performed with a RNFL thickness measurement was used as baseline. Final follow-up was defined by the last RNFL measurement obtained in the 6 months following the administration of the last anti-VEGF injection. Only patients who were injected with an anti-VEGF agent (bevacizumab, ranibizumab or aflibercept) were included. Any patient injected with intravitreal corticosteroids was excluded in order to exclude the potential bias caused by the eventual steroid-induced ocular hypertension. Different anti-VEGF intravitreal drugs were sometimes given in the same eye throughout the study period, at the discretion of the retina specialist (in some cases, bevacizumab had to be switched for aflibercept, for example, as depicted in Table 1). Patients were excluded when the GSD was asymmetrical in severity at baseline (defined as an asymmetry of ≥10 μm in baseline global peripapillary SD-OCT RNFL thickness measurements). Intravitreal corticosteroid injection, poor image quality (defined as a signal strength score inferior to 15 points out of 40 in the SPECTRALIS Heidelberg® scale of image quality/signal strength), significant RNFL segmentation artifacts, secondary and angle-closure glaucoma spectrum diseases and a baseline mean RNFL thickness inferior to 50 μm (since segmentation errors are more likely) were applied as exclusion criteria.35 Two hundred and twenty-one patients were initially identified by cross-referencing simultaneous regular glaucoma ophthalmological consultations with ≥8 total intravitreal injections per eye and having ≥2 SD-OCT scans with peripapillary RNFL measurements. Electronic-based medical records and OCT scans were then reviewed for compliance with inclusion and exclusion criteria. Details of study population selection are depicted in Figure 1.
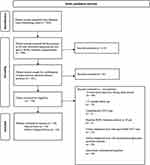 |
Figure 1 Flow diagram of study population selection. |
 |
Table 1 Characterization of Retinal Disease in the Included Eyes |
We compared the evolution of SD-OCT RNFL thickness in injected and fellow uninjected eyes from the same patient. To that end, we included both eyes from the aforementioned patients with bilateral ocular hypertension (OHT), glaucoma suspects (GS) or definite glaucoma (POAG or NTG) who: (1) received ≥8 intravitreal injections in one eye during the study period; (2) did not require intravitreal injections in the fellow eye during the study period; (3) had ≥2 SD-OCT exams at least 12 months apart, throughout the study period.
Data Collection
The following information was extracted for each study eye, based on the patient’s electronic medical records, procedure reports and SPECTRALIS Heidelberg® software database: demographic data, type of GSD, primary retinal disease, number of intravitreal injections (both the total amount each eye received and the number of injections received during the study period), duration of intravitreal treatment, administered intravitreal drugs, prior retinal laser treatment and need for retinal laser treatment during study period, lens status (all patients underwent a complete slit-lamp examination performed by a trained glaucoma specialist), baseline topical and/or oral ocular hypotensive medication, glaucoma medical treatment adjustments during the study period, prior and study time need for glaucoma surgery or laser, best-corrected visual acuity (BCVA) measurements at the earliest and most recent visit (the BCVA was originally determined with ETDRS charts and posteriorly converted to logarithmic minimum angle of resolution (logMAR) units, for statistical analysis), IOP measurements with Goldmann applanation tonometry at the initial and the final visit (using the standard Haag-Streit Goldmann applanation tonometer AT900® (Haag-Streit International, Koeniz, Switzerland)), standard automated perimetry (SAP) measurements (mean deviation (MD), pattern standard deviation (PSD) and foveal sensitivity; 24:2 SITA Standard examinations performed with Zeiss Humphrey® Field Analyser 2, Carl Zeiss Meditec AG, Jena, Germany), SD-OCT scan parameters (signal strength, global RNFL thickness) at the initial and final visit, central corneal thickness (CCT), and duration of follow-up. Unfortunately, due to the adoption of the SPECTRALIS Glaucoma Module Premium Edition® late during the study period, there was an insufficient number of scans to report data on macular ganglion cell layer (GCL) thickness and Bruch’s membrane opening minimum rim width (BMO-MRW) thickness. The dates of the first and last intravitreal injections and SD-OCT scans with RNFL measurements were also collected.
Data Analysis
The primary outcome was the rate and absolute value of RNFL thickness (in microns) change throughout follow-up. To compare categories of subtypes of GSD with a more similar sample size, we decided to group some of these glaucomatous disorders, whenever this was plausible in terms of pathophysiology. Therefore, patients were divided into 2 groups: OHT/GS or definite glaucoma (which included eyes with either POAG or NTG). Secondary outcomes included: absolute change of visual field MD, PSD and foveal sensitivity throughout follow-up, adjustments in glaucoma medical, laser or surgical treatment, BCVA change and IOP absolute change (in mmHg) over the follow-up period.
Statistical Methods
Statistical analysis was performed using IBM-SPSS® for Mac®, version 27.36 Normally distributed data is reported as mean and standard deviation (SD) while non-normally distributed data is reported as median and interquartile range (IQR). Kolmogorov–Smirnov and Shapiro–Wilk tests were used to assess whether each variable followed a normal distribution.
The rate and absolute variation of RNFL thickness throughout follow-up (primary outcome), as well as the absolute variation of BCVA, IOP, visual field MD, PSD and foveal sensitivity, and adjustments in glaucoma medical, laser or surgical treatment throughout follow-up (secondary outcomes) were compared in injected and uninjected eyes with independent samples student’s t, Mann–Whitney-U and Chi-square tests for continuous, ordinal, and categorical variables, respectively. Furthermore, we compared the rate and absolute variation of RNFL thickness throughout follow-up in all injected and fellow uninjected eyes, as well as according to the median number of intravitreal injections during the study period and the presence or absence of established glaucoma (as opposed to eyes with ocular hypertension or glaucoma suspects). A type I error rate (alpha) of 0.05 was established as the criterion for statistical significance.
Linear mixed effects models with best linear unbiased predictors were constructed to estimate rates of change for the included eyes, with random effects applied at the patient level in order to account for patient-specific variations. In this type of analysis, the average rate of change for an outcome variable is described using a linear function of time, and subject-specific deviations from this average rate are introduced by random intercepts and random slopes. The variance-covariance matrix in these models was unstructured. The primary model included time as a fixed effect and a random intercept and was used to evaluate whether included eyes presented a statistically significant absolute decrease of RNFL thickness throughout follow-up and a significant rate of RNFL thinning. The following model included both time and injection status as fixed effects and was used to evaluate whether injected eyes presented a statistically significant higher absolute decrease of RNFL thickness than their fellow eyes. Additional models were applied to injected eyes to evaluate the impact of demographics and ocular factors, such as baseline age, follow-up duration, type of retinal disease, number of total and study time injections, CCT, type of GSD, baseline RNFL thickness, number of baseline and final glaucoma hypotensive medications, baseline IOP and IOP variation throughout follow-up, and baseline MD and MD variation during follow-up. These factors and covariates were included as fixed effects in the univariate models, given the better model fit we obtained. A multivariate model was also constructed including variables with p < 0.10 in the univariate models or those judged as clinically relevant despite the result in the univariate model. Significance in the linear mixed models was assessed using the Wald test. Paired Student’s t-tests and paired Wilcoxon tests were used to compare continuous and ordinal variables, respectively. McNemar’s test was used to compare categorical variables. A type I error rate (alpha) of 0.05 was established as the criterion for statistical significance.
Results
A total of 68 eyes from 34 patients were included (Table 2). Average baseline age (at the time of the first OCT scan) was 67.68 ± 21.77 years, while average final patient age (at the time of the last OCT scan) was 71.35 ± 21.86 years. Eighteen patients (52.9%) were male. The follow-up duration was 3.66 ± 1.89 years, with a range of 1.11 to 7.78 years. Thirty-two eyes (47.1%) presented POAG, 6 eyes (8.8%) presented NTG, 18 eyes (26.5%) were GS and 12 eyes (17.6%) presented OHT. There were 38 eyes of 19 patients with definite glaucoma (POAG or NTG) (55.9% of our sample) and 30 eyes of 15 patients with GS or OHT (44.1% of our sample). For all included eyes (n = 68), average baseline RNFL thickness was 80.43 ± 16.76 μm and average baseline IOP was 17.15 ± 4.89 mmHg. Thirty-nine eyes (57.4%) were under IOP lowering medical treatment at baseline, with a mean number of glaucoma medications of 1.06 ± 1.11. We present the correlations between baseline and final demographic, clinical, tomographic, and functional visual fields’ parameters in Supplemental Table 1.
 |
Table 2 Demographics and Baseline Characteristics of the Patients and Eyes Included in This Study |
Regarding the retinal disease of the injected study eyes (n = 34 eyes), 18 eyes presented exudative AMD, 3 eyes presented DME, 10 eyes presented RVO with macular edema and 3 eyes presented other retinal disorders with indication for regular intravitreal injections, such as myopic choroidal neovascularization (CNV) or CNV secondary to angioid streaks (Table 1). As for the fellow uninjected eyes (n = 34 eyes), 13 eyes presented no retinal disease, 11 eyes presented intermediate AMD, 7 eyes presented late-stage atrophic AMD and 3 eyes presented diabetic retinopathy without DME (Table 1). On average, injected eyes received 25.12 ± 14.49 intravitreal injections throughout the study period and 35.21 ± 21.06 injections in total (if we also included intravitreal injections given prior to the study period). Most eyes received either bevacizumab (91.2%) and/or aflibercept (70.6%) during the study period. 16.6% of the injected eyes underwent retinal laser treatment for their primary retinal disorder during the study period.
Analysing only injected eyes (n = 34 eyes), RNFL thickness decreased significantly between the beginning and the end of the study period, from 80.92 ± 15.78 μm to 77.20 ± 17.35 μm (p<0.001; mean rate of −1.18 ± 1.93 μm/year). There were no significant differences in IOP (16.62 ± 4.77 mmHg to 15.09 ± 4.34 mmHg; mean absolute IOP change of −1.53 ± 5.57 mmHg; p=0.119), BCVA (0.64 ± 0.48 vs 0.71 ± 0.57; p=0.517), visual fields’ MD (−10.50 ± 7.76 vs −11.74 ± 8.42 dB; p=0.209), PSD (p=0.132) or foveal sensitivity (p=0.246) during the study. Linear mixed modeling demonstrated a significant overall average global RNFL thickness loss of −3.72 ± 5.30 μm (p<0.001; mean rate of −1.18±1.93 μm/year) throughout follow-up. There was a significant association between the number of intravitreal injections during the study period and the absolute RNFL thickness change (eyes with a 1 unit higher-than-average number of study period intravitreal injections lost an additional 0.522 μm of RNFL thickness throughout follow-up; p=0.003), as well as an association between the total number of injections (including those provided before the study period) and the absolute RNFL thickness change (eyes with a 1 unit higher-than-average number of total intravitreal injections lost an additional 0.250 μm of RNFL thickness throughout follow-up; p=0.045). RNFL loss was significantly higher in eyes with higher baseline RNFL thickness (p<0.001). Absolute RNFL thickness change was not significantly associated with baseline age (p=0.108), follow-up duration (p = 0.062) or central corneal thickness (p=0.243). The type of retinal disease being treated was not significantly associated with the total RNFL thickness decrease (p=0.134), even when comparing AMD against all other retinal diseases combined (p=0.072). Both baseline IOP (p=0.224) and IOP variation throughout follow-up (p=0.142) were not significantly associated with absolute RNFL thickness change. Naturally, baseline IOP and absolute IOP change throughout follow-up were significantly associated (p<0.001). The number of baseline glaucoma medications (p=0.183) was not significantly associated with absolute RNFL thickness change throughout follow-up. However, the number of final glaucoma medications was (eyes with a 1 unit lower-than-average number of final glaucoma medications lost an additional 4.799 μm of RNFL thickness throughout follow-up; p=0.030). There was a significant association between absolute RNFL thickness change throughout follow-up and the types of GSD (using all different subtypes of glaucomatous disorders in the injected study eyes) of the included injected eyes (p<0.001). When implementing a simplified classification of glaucoma with 2 categories (POAG or NTG; OHT or glaucoma suspects), the association remained significant (p<0.001). Eyes with NTG (−7.12 ± 9.75 μm; mean rate of −2.99±3.43 μm/year) presented the highest total variation and rate of RNFL thinning, followed by POAG eyes (−4.79 ± 5.42 μm; mean rate of −1.56±2.07 μm/year), GS eyes (−2.26 ±2.74 μm; mean rate of −0.54±1.00 μm/year), and finally eyes with OHT (−1.85 ± 5.32 μm; mean rate of −0.39±1.35 μm/year).
Both baseline MD (eyes with a 1 unit more negative than average baseline MD value lost an additional 1.466 μm of RNFL thickness throughout follow-up; p < 0.001) and MD variation (from −10.50 ± 7.76 vs −11.74 ± 8.42 dB; p = 0.012) were significantly associated with absolute RNFL thickness change. For each unit of MD absolute value increase throughout follow-up, absolute RNFL thickness loss increased 0.657 μm throughout follow-up. RNFL thickness variation was not associated with BCVA change (p = 0.553), absolute central visual field foveal sensitivity change (p=0.594) or PSD change (p=0.135) throughout follow-up. As expected, lower central visual field sensitivity was significantly associated with worse BCVA (6.89 dB decrease in central sensitivity for each 1 logMAR unit increase in BCVA throughout follow-up; p=0.010). A multivariable linear mixed model was constructed including baseline age, baseline RNFL thickness, baseline IOP, baseline MD, and number of study period injections as predictors for RNFL thickness variation. In this model, only a higher baseline RNFL thickness (p < 0.001) significantly predicted higher absolute RNFL thickness loss. Baseline age (p=0.888), baseline IOP (p=0.759), baseline MD (p=0.420) and number of study period injections (p = 0.638) were not significant predictors of RNFL thinning.
Regarding uninjected eyes (n = 34 eyes; Table 3), 17 eyes (50%) presented POAG, 3 eyes (8.8%) presented NTG, 9 eyes (26.5%) were glaucoma suspects and 5 eyes (14.7%) presented OHT. Analysing all uninjected eyes (n = 34 eyes), RNFL thickness decreased significantly between the beginning and the end of the study period, from 79.95 ± 17.91 μm to 76.61 ± 17.97 μm (p<0.001; mean rate of −1.07 ± 0.98 μm/year). IOP decreased significantly from 17.68 ± 5.01 mmHg to 14.50 ± 3.39 mmHg (p=0.005). There were no significant differences in BCVA (0.31 ± 0.44 vs 0.39 ± 0.47; p=0.082), visual fields’ MD (−6.72 ± 5.60 vs −7.38 ± 5.84 dB; p=0.285), PSD (p=0.268) or foveal sensitivity (p=0.366) during the study. A significant increase in the proportion of pseudophakic eyes (p=0.031) was registered, from 32.4% to 50%. There was a significant increase in the proportion of eyes under glaucoma medical treatment throughout follow-up (58.8% at baseline and 76.5% at final follow-up; p=0.031) and the average number of glaucoma medications per eye also increased significantly (p=0.003), from 1.09 ± 1.11 medications to 1.56 ± 1.19 medications. In terms of glaucoma laser and surgical treatment, SLT was performed in 3 eyes (8.8%) throughout the study period. No glaucoma surgeries were performed. Nonetheless, there were no significant differences in the proportion of eyes that received glaucoma laser (p=0.250) or surgical treatment (p=1.000) before the beginning of the study and throughout the study period.
Finally, we compared the rate and absolute variation of RNFL thickness, as well as the absolute variation of BCVA, IOP, visual field MD, PSD and foveal sensitivity, and adjustments in glaucoma medical, laser or surgical treatment throughout follow-up in paired injected and uninjected eyes (Table 3). There were no significant differences between groups in baseline RNFL thickness (p=0.814), baseline IOP (p=0.375), baseline MD (p=0.130), baseline PSD (p=0.387), baseline central sensitivity (p=0.125), baseline glaucoma medical treatment (p=0.806) and baseline number of glaucoma medications (p=0.828). Injected eyes presented only a significantly worse baseline BCVA (0.64 ± 48 logMAR units vs 0.31 ± 44 logMAR units in fellow uninjected eyes; p=0.004). Therefore, we concluded that both groups did not present significant differences regarding relevant baseline parameters. Absolute RNFL thickness change was −3.72 ± 5.30 μm in injected eyes (from 80.92 ± 15.78 μm to 77.20 ± 17.35 μm) and −3.34 ± 3.12 μm in uninjected eyes (from 79.95 ± 17.91 μm to 76.61 ± 17.97 μm), while RNFL rate was −1.18 ± 1.93 μm/year in injected eyes and −1.07 ± 0.98 μm/year in fellow uninjected eyes. Neither absolute RNFL thickness change (p=0.716) nor RNFL rate (p=0.779) was significantly different in paired injected and fellow uninjected eyes. Absolute IOP variation was not significantly different between groups (−1.53 ± 5.57 mmHg in injected eyes and −3.18 ± 6.08 mmHg in fellow uninjected eyes; p=0.248). Absolute visual fields’ MD variation was also not significantly different between groups (−1.25 ± 4.28 dB in injected eyes and −0.66 ± 2.68 dB in fellow uninjected eyes; p=0.605). The same applies to the absolute variation in the number of glaucoma medical treatment drugs (+0.56 ± 1.02 drugs in injected eyes and +0.47 ± 0.86 drugs in fellow uninjected eyes; p=0.701). Furthermore, when analysing all 68 eyes, linear mixed modeling demonstrated that having had injections was not significantly associated with absolute RNFL thickness change throughout follow-up (p=0.795). The baseline and final RNFL thicknesses registered throughout follow-up in all study eyes are depicted in Figure 2.
 |
Figure 2 Baseline and final RNFL thickness registered throughout follow-up in all study eyes. |
Discussion
After adjusting for potential confounders, there were no significant differences in the rate of RNFL change or absolute RNFL variation between injected and fellow uninjected eyes, with bilateral symmetric similar glaucoma spectrum disease and similar IOP. These findings suggest that the transient IOP elevations that occur after repeated intravitreal injections might not be sufficient to result in an aggravated absolute RNFL thickness decrease, in eyes with concurrent glaucoma spectrum diseases.
Not many studies have investigated the effect of repeated intravitreal injections in RNFL thickness variation in patients with OHT, GS or patients with definite glaucoma. There are even fewer that provide a control group, which ideally should be the contralateral untreated eye with symmetrical glaucomatous disease. There is biological plausibility that the repetitive and transient IOP elevations after intravitreal injections might further aggravate the glaucomatous optic neuropathy, given the already damaged ganglion cell axons and the dysfunctional mechanisms of IOP regulation that these eyes present.4,7–11 The initial studies that investigated this matter presented conflicting results.
Du et al26 compared 28 regularly injected eyes with bilateral POAG or pseudoexfoliative glaucoma (PXF) glaucoma or NTG or OHT and unilateral exudative AMD or RVO or DME with their fellow uninjected eyes for a period of 3.67 (44 months) years. In their study, there were no significant differences between eyes in the mean rate of global RNFL thickness change (−4.27 μm/year in injected eyes vs −1.17 μm/year in fellow uninjected eyes; p=0.094). In their study, baseline mean global RNFL thickness was not significantly different between groups (81.3 μm; 95% CI, 73.1–89.5 μm in injected eyes and 74.9 μm; 95% CI, 66.7–83.0 μm in fellow uninjected eyes; p=0.175). Despite detecting a faster rate of RNFL thinning in the superior quadrant of injected eyes when compared to their fellow uninjected eyes (p=0.030), the mean baseline RFNL thickness of the superior quadrant in injected eyes was significantly thicker compared with fellow uninjected eyes (p=0.016), which can compromise this superior quadrant RNFL rate comparison. We only studied the global RNFL thickness and did not collect data on the RNFL thickness for each quadrant as we considered a significant difference in global RNFL thickness to be more clinically relevant, compared to a significant difference in one or more quadrants, which could be subject to other biases and confounders, such as the concurrent retinal disease and corresponding treatments. Furthermore, in the study by Du et al,26 average baseline IOP was 16.7 mmHg (95% CI, 15.2–18.2 mmHg) for injected eyes and 17.7 mmHg (95% CI, 16.1–19.2 mmHg) for fellow uninjected eyes (p=0.402). There were no differences in IOP between groups at any time point throughout follow-up (p=0.398). The average number of baseline hypotensive medications was 1.3 for injected eyes and 1.4 for fellow uninjected eyes (p = 0.908) and the proportion of eyes that required the addition of ≥1 hypotensive medication was similar in both groups (32.1% in injected eyes vs 21.4% in fellow uninjected eyes; p = 0.178). Even though the authors state that there was a significantly mean change in IOP compared with baseline at 24 (p=0.017) and 36 months (p=0.021) for uninjected eyes, the mean change in IOP for injected eyes is not reported. Thus, despite stating that absolute global RNFL thickness change throughout follow-up was significant in injected eyes (p=0.001), but not in fellow uninjected eyes (p=0.509), and that injected eyes presented a significantly higher rate of change of visual fields’ MD (p=0.019), the lack of data regarding IOP variation in injected eyes cannot be overlooked, since an absence of significant IOP decrease in injected eyes can result in a more significant RNFL thickness loss and MD negative variation regardless of the anti-VEGF intravitreal injections. Furthermore, the authors did not perform multivariable analysis to control for potential confounders (such as the IOP change throughout follow-up or the different types of GSD included). In our cohort, with a follow-up of 3.66±1.89 years, both injected and fellow uninjected eyes presented a significant absolute RNFL thickness decrease, which was not significantly different between injected and fellow uninjected eyes and was not significantly associated with baseline IOP or IOP variation throughout follow-up, both in univariate and in multivariable analysis. Moreover, we presented stricter exclusion criteria, such as the exclusion of eyes with baseline RNFL thickness <50 μm and the exclusion of eyes with secondary forms of glaucoma.
Lee et al27 compared 16 regularly injected eyes with bilateral POAG and exudative AMD with fellow uninjected eyes with POAG and atrophic AMD throughout 4.8 years (mean patient baseline age of 73.3 ± 7.9 years) and identified a decreased retinal ganglion cell layer (RGCL) thickness and a faster rate of RGCL thinning. Similarly to our study, no significant differences between injected/uninjected eyes were detected regarding RNFL thickness variation. Their average follow-up period was 58.4 ± 25.5 (24 to 98) months, and the mean number of anti-VEGF injections received per eyes was 10.6 ± 10.4 (3 to 40). There were no significant differences regarding mean baseline RNFL thickness between injected (78.2 ± 11.0 μm) and untreated fellow eyes (73.9 ± 12.7 μm; p=0.139). The same was true for mean final RNFL thickness between injected (73.2 ± 6.4 μm) and untreated fellow eyes (70.8 ± 10.3 μm; p=0.476). In their study, there were also no significant differences regarding mean baseline IOP (12.1 ± 2.0 mmHg in injected eyes vs 12.5 ± 1.8 mmHg in fellow eyes; p=0.169) and mean final IOP (11.81 ± 2.51 mmHg in injected eyes with 1.25 ± 0.68 hypotensive medications vs 11.69 ± 2.02 mmHg in fellow eyes with 1.31 ± 0.70 hypotensive medications; p=0.886).27 In our cohort, injected eyes with POAG/NTG and AMD (n = 7 eyes; mean patient baseline age of 77.63 ± 6.68 years) presented a mean baseline RNFL thickness of 71.95 ± 9.75 μm, final RNFL thickness of 69.42 ± 5.59 μm and RNFL thickness change rate of −0.48 ± 1.51 μm/year, which is lower than the one published by Lee et al27 6 of these 7 eyes were under hypotensive medication at final follow-up (mean number of final hypotensive medications was 2.43 ± 1.27), with a mean baseline IOP of 17.57 ± 5.50 mmHg, mean final IOP of 16.43 ± 5.03 mmHg, and an IOP variation throughout follow-up of −1.14 ± 6.67 mmHg. As for their fellow uninjected eyes (n = 7 eyes), RNFL thickness decreased from 76.88 ± 15.04 μm to 72.05 ± 14.55 μm (RNFL thickness change rate of −1.55 ± 1.04 μm/year), while IOP decreased from 19.14 ± 5.79 mmHg to 14.57 ± 3.60 mmHg (absolute IOP change of −4.57 ± 7.30 mmHg). Six of these 7 fellow uninjected eyes with POAG and intermediate or late atrophic AMD were under hypotensive medication at final follow-up (mean number of final hypotensive medications was 2.43 ± 1.27). Our results cannot be directly compared to the ones presented by Lee et al,27 since their study does not present rates of RNFL thickness change for injected or fellow uninjected eyes, the absolute measures of RNFL thickness were obtained with a different OCT (Cirrus high definition OCT (HD-OCT)), eyes with previous glaucoma filtering surgery were excluded, there was no exclusion of eyes with baseline mean RNFL thickness inferior to 50 μm (in which segmentation errors are more likely) and the number of final hypotensive medications was lower than in our cohort. Nonetheless, in both studies, there were no significant differences in absolute RNFL measurements between injected and fellow uninjected eyes, with bilateral symmetric similar glaucoma spectrum disease and similar IOP.
Swaminathan et al29 compared 53 regularly injected eyes with bilateral definite glaucoma or GS and exudative AMD with their fellow uninjected eyes, throughout a period of 3.7 years (mean baseline patient age of 79.0 ± 8.0 years). Similarly to our study, the rate of RNFL thinning was not significantly different between eyes. The baseline RNFL thickness was 81.5 ± 15.0 μm for injected eyes and 81.4 ± 14.2 μm for uninjected eyes (p=0.483), while the baseline IOP was 15.0 ± 4.6 mmHg for injected eyes and 15.3 ± 4.1 mmHg for uninjected eyes (p=0.641). Comparing their study to our sample of eyes with POAG/NTG and exudative AMD, baseline patient age was similar, but our absolute baseline RNFL thickness values were lower and our baseline IOP values were higher, which prevents accurate comparisons regarding the absolute RNFL thickness change or the RNFL thickness rate of change. Furthermore, in their study, uninjected eyes demonstrated a significant rate of RNFL loss of −0.620 μm/year (p=0.029), while injected eyes presented an additional non-significant incremental RNFL loss of −0.385 μm/year (p=0.324), thus, presenting a similar rate of RNFL loss to injected eyes with POAG/NTG and AMD in the study by Kopic et al32 (−1.07 μm/year; mean baseline RNFL thickness of 77.27 ± 12.48 μm), but a higher rate of RNFL thickness loss when compared with our cohort (−0.48 ± 1.51 μm/year; mean baseline RNFL thickness of 71.95 ± 9.75 μm), which could be related to the higher baseline RNFL thickness of their cohorts.29,32 Swaminathan et al measured the post injection IOP elevation and found that it was not correlated with faster RNFL loss.29
Kopic et al32 included 60 patients with bilateral POAG and exudative AMD or DME that required injection in only one eye throughout 1 year of follow-up and reported a greater RNFL thinning only in injected eyes with bilateral POAG and DME (but not AMD), when compared with their fellow eyes. However, there are some methodological issues with this study, mainly concerning the group with concomitant DME. First and foremost, the authors did not compare the absolute RNFL thickness change throughout follow-up in each group. Instead, they performed 2 separate comparisons: average baseline RNFL thickness in paired injected and fellow uninjected eyes; average final RNFL thickness in paired injected and fellow uninjected eyes. These two isolated comparisons are not clinically relevant, as we know that final RNFL thickness will be highly dependent on baseline RNFL thickness and the parameter that is most clinically relevant is the variation in RNFL thickness throughout follow-up in injected eyes versus their fellow uninjected eyes. The only significant finding of this study was that the final RNFL thickness of injected eyes with DME was inferior to the final RNFL thickness of their fellow uninjected eyes (81.10 ± 16.41 μm vs 90.03 ± 13.52 μm; p=0.023). The same asymmetry already existed at baseline, with the baseline RNFL thickness of injected eyes with DME being 84.90 ± 16.41 μm, while the baseline RNFL thickness of their fellow eyes was 92.30 ± 13.60 (p=0.062). Second, this study did not provide information on baseline and/or IOP variation throughout follow-up, neither information regarding glaucoma medical, laser or surgical treatment in injected and/or fellow uninjected eyes. In their study,32 injected eyes with bilateral POAG and exudative AMD presented similar baseline RNFL thicknesses (p=0.832) and similar final RNFL thicknesses (p=0.758). In injected eyes with POAG and AMD (n = 30 eyes; mean baseline age of 67.37 ± 11.93 years), RNFL thickness decreased from 77.27 ± 12.48 μm to 76.20 ±12.53 μm (mean decrease of −1.07 μm/year), while in their fellow uninjected eyes (n = 30 eyes), RNFL thickness decreased from 76.46 ± 16.40 to 75.03 ± 16.39 (mean decrease of −1.43 μm/year), throughout a one-year follow-up period. In the same study, in injected eyes with POAG and DME (n = 30 eyes; mean baseline age of 64.5 ± 11.22 years), RNFL thickness decreased from 84.90 ± 16.41 μm to 81.10 ± 16.08 μm (mean decrease of −3.80 μm/year), while in fellow uninjected eyes (n = 30 eyes), RNFL thickness decreased from 92.30 ± 13.60 μm to 90.03 ± 13.53 μm (mean decrease of −2.27 μm/year). Nonetheless, the authors did not compare these variations between groups. Instead, baseline RNFL thickness values were compared for each group and final RNFL thickness values were separately compared for each group, without taking into account the baseline RNFL thickness and the RNFL variation over time. In our cohort, injected eyes with POAG/NTG and AMD (n = 7 eyes; mean patient baseline age of 77.63 ± 6.68 years) presented a mean baseline RNFL thickness of 71.95 ± 9.75 μm, final RNFL thickness of 69.42 ± 5.59 μm and RNFL thickness change rate of −0.48 ± 1.51 μm/year, which is lower than the one published by Kopic et al.32 We have reported that 6 of these 7 eyes were under hypotensive medication at final follow-up (mean number of final hypotensive medications was 2.43 ± 1.27), with a mean baseline IOP of 17.57 ± 5.50 mmHg, mean final IOP of 16.43 ± 5.03 mmHg, and an IOP variation throughout follow-up of −1.14 ± 6.67 mmHg. No IOP-related data was presented by Kopic et al,32 which prevents accurate comparisons to be made. We only had one injected eye with POAG/NTG and DME in our cohort, which prevents further comparisons to their case series.
As stated previously, 3 out of the 4 studies on this matter which present a comparison of injected and fellow uninjected eyes have demonstrated an absence of significant RNFL decrease when comparing injected eyes with bilateral glaucoma with their fellow uninjected eyes. The only study which presented a difference between injected and fellow uninjected eyes did not compare the variation in RFNL thickness between groups, but instead compared the values of baseline RNFL thickness between eyes and the values of final RNFL thickness between eyes, independently. Nonetheless, the goal of our study was to compare a large sample of injected eyes with concurrent bilaterally similar GSD with their fellow uninjected eyes, using a stricter methodology, with more stringent inclusion and exclusion criteria, data collection on IOP and glaucoma treatment at baseline and throughout follow-up, and accounting for potential confounders with univariable and multivariable linear mixed modeling analysis. We excluded secondary forms of glaucoma, since these may have fundamentally different mechanisms of disease that result in different and often asymmetrical rates of RNFL loss, as well as eyes with a baseline mean RNFL thickness <50 μm (since segmentation errors are more likely to occur). Our sample size was similar to other previously published studies (68 eyes from 34 patients). However, we were extremely meticulous with our inclusion and exclusion criteria, in order to establish a highly homogeneous sample which could limit potential confounders.
Our study limitations include its retrospective nature and the lack of data regarding the macular ganglion cell complex (GCC) thickness, as well as the Bruch’s membrane opening-minimum rim width (BMO-MRW) thickness for included eyes. Moreover, by excluding all patients with a baseline OCT RNFL thickness below 50 µm, we ended up with a very small representation of advanced glaucoma (baseline average MD was −10.50 ± 7.76 dB in injected eyes and −6.72± 5.60 dB in uninjected eyes). We must acknowledge that 12 out of 34 included patients (35.3%) do not have data on visual fields’ MD, which could partially account for the absolute MD values being higher than expected for the average RNFL thickness values and for the absence of a significant variation in MD throughout follow-up. Furthermore, in injected eyes, 11.8% of the included eyes had been previously treated with retinal laser photocoagulation and 16.6% were also treated with retinal laser during the study period. Retinal laser photocoagulation has been associated with decreased peripapillary RNFL thickness due to the thermal damage generated in all retinal layers, inducing retinal ganglion cell death.37 Furthermore, none of the aforementioned studies which included patients with DME or RVO presented data on the proportion of eyes that were treated with retinal laser prior or during the study period, which could impact RNFL measurements and change.26,32
Conclusion
In conclusion, eyes with glaucomatous spectrum disorders who are submitted to repeated intravitreal injections do not seem to have a significantly faster RNFL thickness decline in comparison to their fellow untreated eyes. Despite regular follow-up and an escalation in glaucoma treatment, these eyes may present significant RNFL thinning throughout time. Thus, these patients should be regularly and closely monitored and regular optic nerve evaluations should be made to allow an early detection of glaucoma progression and adequate intervention.
Key Points
- It has been postulated that the immediate (though generally transient) intraocular pressure elevation (IOP) that follows anti-VEGF intravitreal injections (IVI) may promote glaucomatous damage in eyes with glaucoma spectrum diseases (GSD), leading to faster retinal nerve fiber layer (RNFL) thinning over time.
- To date, only a limited number of studies have been conducted on this matter, with conflicting results, and several only report the injected eye group, with no control for comparison.
- The main purpose of this study was to ascertain if repeated intravitreal anti-VEGF IVI lead to faster RNFL thickness changes between injected and fellow uninjected eyes of patients with symmetrical GSD.
- In our study with a follow-up of 3.66±1.89 years, RNFL thickness decreased significantly from 80.92±15.78 to 77.20±17.35 μm (p<0.001; −1.18±1.93 μm/year) in injected eyes and from 79.95±17.91 to 76.61±17.97 μm (p<0.001; −1.07±0.98 μm/year) in fellow uninjected eyes, despite regular follow-up and an escalation in glaucoma treatment. Neither absolute RNFL thickness variation (p=0.716) nor RNFL rate (p=0.779) was significantly different between paired injected and uninjected eyes.
- Repeated IVI do not seem to accelerate glaucomatous progression in eyes with pre-existing GSD. Future studies with a longer follow-up are needed.
Previous Presentation of Interim Findings
The abstract of this paper was presented at the 2022 ARVO meeting, as a poster presentation, with interim findings. The poster’s abstract was published in “Poster Abstracts” in INVESTIGATIVE OPHTHALMOLOGY & VISUAL SCIENCE.
Data Sharing Statement
Access to any supplemental information such as the study protocol or anonymized data can be available upon reasonable request.
Ethics/Ethical Approval
The study was approved by the Institutional Ethics Review Board of Centro Hospitalar Universitário de São João, Porto, Portugal (certificate approval number CES269/2021). The protocol conformed with the canons of the Declaration of Helsinki for research involving human participants, as well as the European Union’s General Data Protection Regulation. Informed consent was waived in view of the retrospective nature of the study. This article was redacted according to the recommendations of The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement.
Acknowledgments
Only the named authors have collaborated in the writing of this paper.
Author Contributions
All authors contributed to the study’s conception and design. Material preparation and data acquisition were performed by Flávio Alves, António Benevides Melo, Sérgio Estrela-Silva, Joana Araújo, João Tavares-Ferreira, Marta Silva, Amândio Rocha-Sousa, Ângela Carneiro and João Barbosa-Breda. Data collection and analysis were performed by Rodrigo Vilares-Morgado, Vera Correia and Ana Margarida Ferreira. The first draft of the manuscript was written by Rodrigo Vilares-Morgado, Vera Correia and João Barbosa-Breda. The remaining authors critically reviewed the article. All authors agreed on the journal to which the article should be submitted. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Thus, all authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
The authors declare that they have no financial ties to declare. No funding or sponsors were undertaken in the preparation of the manuscript.
Disclosure
The authors have no conflicts of interest to declare.
References
1. Solomon SD, Lindsley K, Vedula SS, Krzystolik MG, Hawkins BS. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2019;3:CD005139.
2. Ehlers JP, Yeh S, Maguire MG, et al. Intravitreal pharmacotherapies for diabetic macular edema: a report by the American Academy of Ophthalmology. Ophthalmology. 2022;129(1):88–99.
3. Shalchi Z, Mahroo O, Bunce C, Mitry D. Anti-vascular endothelial growth factor for macular oedema secondary to branch retinal vein occlusion. Cochrane Database Syst Rev. 2020;7:CD009510.
4. Hoguet A, Chen PP, Junk AK, et al. The effect of anti-vascular endothelial growth factor agents on intraocular pressure and glaucoma: a report by the American Academy of Ophthalmology. Ophthalmology. 2019;126(4):611–622. doi:10.1016/j.ophtha.2018.11.019
5. Chopra R, Preston GC, Keenan TDL, et al. Intravitreal injections: past trends and future projections within a UK tertiary hospital. Eye. 2022;36(7):1373–8.
6. Ghanchi F, Bourne R, Downes SM, et al. An update on long-acting therapies in chronic sight-threatening eye diseases of the posterior segment: AMD, DMO, RVO, uveitis and glaucoma. Eye. 2022;36(6):1154–1167. doi:10.1038/s41433-021-01766-w
7. Zhou Y, Zhou M, Xia S, Jing Q, Gao L. Sustained elevation of intraocular pressure associated with intravitreal administration of anti-vascular endothelial growth factor: a systematic review and meta-analysis. Sci Rep. 2016;6(1):39301. doi:10.1038/srep39301
8. Atchison EA, Wood KM, Mattox CG, Barry CN, Lum F, MacCumber MW. The real-world effect of intravitreous anti-vascular endothelial growth factor drugs on intraocular pressure: an analysis using the iris registry. Ophthalmology. 2018;125(5):676–682. doi:10.1016/j.ophtha.2017.11.027
9. de Vries VA, Bassil FL, Ramdas WD. The effects of intravitreal injections on intraocular pressure and retinal nerve fiber layer: a systematic review and meta-analysis. Sci Rep. 2020;10(1):13248. doi:10.1038/s41598-020-70269-7
10. Levin AM, Chaya CJ, Kahook MY, Wirostko BM. Intraocular pressure elevation following intravitreal anti-VEGF injections: short- and long-term considerations. J Glaucoma. 2021;30(12):1019–1026. doi:10.1097/IJG.0000000000001894
11. Nuesi R, Swaminathan SS. Effect of intravitreal injections on retinal imaging metrics in glaucomatous and non-glaucomatous eyes. Curr Ophthalmol Rep. 2020;8(3):111–119. doi:10.1007/s40135-020-00235-z
12. Horsley MB, Mandava N, Maycotte MA, Kahook MY. Retinal nerve fiber layer thickness in patients receiving chronic anti-vascular endothelial growth factor therapy. Am J Ophthalmol. 2010;150(4):558–61 e1. doi:10.1016/j.ajo.2010.04.029
13. Martinez-de-la-Casa JM, Ruiz-Calvo A, Saenz-Frances F, et al. Retinal nerve fiber layer thickness changes in patients with age-related macular degeneration treated with intravitreal ranibizumab. Invest Ophthalmol Vis Sci. 2012;53(10):6214–6218. doi:10.1167/iovs.12-9875
14. Sobaci G, Gungor R, Ozge G. Effects of multiple intravitreal anti-VEGF injections on retinal nerve fiber layer and intraocular pressure: a comparative clinical study. Int J Ophthalmol. 2013;6(2):211–215. doi:10.3980/j.issn.2222-3959.2013.02.20
15. Shin HJ, Shin KC, Chung H, Kim HC. Change of retinal nerve fiber layer thickness in various retinal diseases treated with multiple intravitreal antivascular endothelial growth factor. Invest Ophthalmol Vis Sci. 2014;55(4):2403–2411. doi:10.1167/iovs.13-13769
16. Demirel S, Batioğlu F, Özmert E, Erenler F. The effect of multiple injections of ranibizumab on retinal nerve fiber layer thickness in patients with age-related macular degeneration. Curr Eye Res. 2015;40(1):87–92. doi:10.3109/02713683.2014.917190
17. Parlak M, Oner FH, Saatci AO. The long-term effect of intravitreal ranibizumab on retinal nerve fiber layer thickness in exudative age-related macular degeneration. Int Ophthalmol. 2015;35(4):473–480. doi:10.1007/s10792-014-9972-2
18. Beck M, Munk MR, Ebneter A, Wolf S, Zinkernagel MS. Retinal ganglion cell layer change in patients treated with anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Am J Ophthalmol. 2016;167:10–17. doi:10.1016/j.ajo.2016.04.003
19. Jo YJ, Kim WJ, Shin IH, Kim JY. Longitudinal changes in retinal nerve fiber layer thickness after intravitreal anti-vascular endothelial growth factor therapy. Korean J Ophthalmol. 2016;30(2):114–120. doi:10.3341/kjo.2016.30.2.114
20. Zhang Z, Yang X, Jin H, et al. Changes in retinal nerve fiber layer thickness after multiple injections of novel VEGF decoy receptor conbercept for various retinal diseases. Sci Rep. 2016;6(1):38326. doi:10.1038/srep38326
21. Zucchiatti I, Cicinelli MV, Parodi MB, et al. Effect of intravitreal ranibizumab on ganglion cell complex and peripapillary retinal nerve fiber layer in neovascular age-related macular degeneration using spectral domain optical coherence tomography. Retina. 2017;37(7):1314–1319. doi:10.1097/IAE.0000000000001360
22. Gómez-Mariscal M, Puerto B, Muñoz-Negrete FJ, de Juan V, Rebolleda G. Acute and chronic optic nerve head biomechanics and intraocular pressure changes in patients receiving multiple intravitreal injections of anti-VEGF. Graefes Arch Clin Exp Ophthalmol. 2019;257(10):2221–2231. doi:10.1007/s00417-019-04354-7
23. Valverde-Megías A, Ruiz-Calvo A, Murciano-Cespedosa A, Hernández-Ruiz S, Martínez-de-la-Casa JM, García-Feijoo J. Long-term effect of intravitreal ranibizumab therapy on retinal nerve fiber layer in eyes with exudative age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2019;257(7):1459–1466. doi:10.1007/s00417-019-04325-y
24. Blindness GBD, Vision Impairment C, Briant PS, Vision Loss Expert Group of the Global Burden of Disease S. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the right to sight: an analysis for the global burden of disease study. Lancet Glob Health. 2021;9(2):e144–e60. doi:10.1016/S2214-109X(20)30489-7
25. Griffith JF, Goldberg JL. Prevalence of comorbid retinal disease in patients with glaucoma at an academic medical center. Clin Ophthalmol. 2015;9:1275–1284. doi:10.2147/OPTH.S85851
26. Du J, Patrie JT, Prum BE, Netland PA, Shildkrot YE. Effects of intravitreal anti-VEGF therapy on glaucoma-like progression in susceptible eyes. J Glaucoma. 2019;28(12):1035–1040. doi:10.1097/IJG.0000000000001382
27. Lee WJ, Kim YK, Kim YW, et al. Rate of macular ganglion cell-inner plexiform layer thinning in glaucomatous eyes with vascular endothelial growth factor inhibition. J Glaucoma. 2017;26(11):980–986. doi:10.1097/IJG.0000000000000776
28. Saleh R, Karpe A, Zinkernagel MS, Munk MR. Inner retinal layer change in glaucoma patients receiving anti-VEGF for neovascular age related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2017;255(4):817–824. doi:10.1007/s00417-017-3590-4
29. Swaminathan SS, Kunkler AL, Quan AV, et al. Rates of RNFL thinning in patients with suspected or confirmed glaucoma receiving unilateral intravitreal injections for exudative AMD. Am J Ophthalmol. 2021;226:206–216. doi:10.1016/j.ajo.2020.12.016
30. Park CH, Lee KI, Park HY, Lee JH, Kim IT, Park CK. Changes in the retinal nerve fiber layer after intravitreal injections of bevacizumab in glaucoma patients. J Korean Ophthalmol Soc. 2014;55(5):693–701. doi:10.3341/jkos.2014.55.5.693
31. Rud’ko AS, Budzinskaya MV, Andreeva IV, Karpilova MA, Smirnova TV. Effect of intravitreal injections of ranibizumab and aflibercept on the retinal nerve fiber layer in patients with concomitant neovascular age-related macular degeneration and glaucoma. Vestn Oftalmol. 2019;135(5. Vyp. 2):177–183. doi:10.17116/oftalma2019135052177
32. Kopić A, Biuk D, Barać J, Vinković M, Benašić T, Kopić V. Retinal nerve fiber layer thickness in glaucoma patients treated with multiple intravitreal anti-VEGF (bevacizumab) injections. Acta Clin Croat. 2017;56(3):406–414. doi:10.20471/acc.2017.56.03.07
33. Benchimol EI, Smeeth L, Guttmann A, et al. The reporting of studies conducted using observational routinely-collected health data (record) statement. PLoS Med. 2015;12(10):e1001885. doi:10.1371/journal.pmed.1001885
34. Alonso V, Santos JV, Pinto M, et al. Health records as the basis of clinical coding: is the quality adequate? A qualitative study of medical coders’ perceptions. Health Inf Manag. 2020;49(1):28–37. doi:10.1177/1833358319826351
35. Mwanza JC, Budenz DL, Warren JL, et al. Retinal nerve fibre layer thickness floor and corresponding functional loss in glaucoma. Br J Ophthalmol. 2015;99(6):732–737. doi:10.1136/bjophthalmol-2014-305745
36. Corp. I. IBM SPSS Statistics for Mac. In: Armonk. 27.0 ed. Armonk, NY: IBM Corp; 2020.
37. Park YR, Jee D. Changes in peripapillary retinal nerve fiber layer thickness after pattern scanning laser photocoagulation in patients with diabetic retinopathy. Korean J Ophthalmol. 2014;28(3):220–225. doi:10.3341/kjo.2014.28.3.220
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

