Back to Journals » Nature and Science of Sleep » Volume 16
Causal Relationship Between Sleep Traits and Hypothalamic-Pituitary-Target Gland Axis Function: A Mendelian Randomization Study
Authors Ren Z , Long J, Deng W, Jing Y, Qiu J, Ren W, Liu D
Received 27 September 2023
Accepted for publication 4 February 2024
Published 16 February 2024 Volume 2024:16 Pages 155—175
DOI https://doi.org/10.2147/NSS.S442231
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Ahmed BaHammam
Ziyu Ren,1 Jiangchuan Long,1 Wenzhen Deng,1 Yuanyuan Jing,1 Jingwen Qiu,1 Wei Ren,2 Dongfang Liu1
1Department of Endocrinology and Metabolism, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China; 2Department of Endocrinology and Metabolism, The First Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China
Correspondence: Wei Ren, Department of Endocrinology, The First Affiliated Hospital of Chongqing Medical University, No. 1. You-Yi Road, Yuzhong District, Chongqing, 400016, People’s Republic of China, Tel +86-23-89012755, Email [email protected] Dongfang Liu, Department of Endocrinology, The Second Affiliated Hospital of Chongqing Medical University, No. 74, Linjiang Road, Yuzhong District, Chongqing, 400010, People’s Republic of China, Tel +86-23-63832133, Email [email protected]; [email protected]
Background: In recent years, multiple observational studies have confirmed the association between sleep traits and various human physiopathological states. However, the causal relationship between sleep traits and hypothalamic-pituitary-target gland axis (HPTGA) function remains unknown.
Methods: We obtained summary statistics on sleep traits (insomnia, chronotype, and sleep duration (long and short)) from the UK Biobank database. Data related to the HPTGA functions were obtained from the publicly available database. Subsequently, a two-sample Mendelian randomization (MR) analysis was performed to investigate the causal relationship between different sleep traits and the HPTGA function. Reverse MR analysis was conducted to examine the direction of causality.
Results: The MR analysis results suggested that chronotype is associated with decreased levels of six hormones in HPTGA. Sleep duration was causally associated with decreased levels of free thyroxine and progesterone. Both long and short sleep durations are detrimental to the secretion of prolactin-releasing peptide, somatostatin, and plasma cortisol, while short sleep duration can promote progesterone secretion. After gender stratification, we found that female reproductive function is more susceptible to the influence of unfavorable sleep traits.
Conclusion: Our MR analysis indicated a significant causal association between chronotype and suppressed gonadal function in healthy adult humans, with no apparent gender-specific effect. Extreme sleep durations were also found to be detrimental to the maintenance of normal HPTGA secretion function. Compared to males, gonadal function in the female cohort is more susceptible to extreme sleep habits. Subsequent observational studies are urgently needed to confirm the underlying mechanisms.
Keywords: hypothalamo-hypophyseal-target gland axis, sleep traits, causal inference, Mendelian randomization
Introduction
It is widely recognized in modern medicine that sleep is an active physiological process managed by the central nervous system, which specializes in sleep and wakefulness.1 Sleep is essential not only for recovery from physical and mental illnesses but also for a range of neurological functions in the brain, such as neuronal cells and synaptic growth, and the construction of memory functions.2 The hypothalamic-pituitary-target gland axis (HPTGA), as the most complex neuroendocrine regulatory system in the human body, participates in the regulation of a variety of endocrine metabolic processes by virtue of a sophisticated neurohumoral regulatory network. It plays a decisive role in maintaining homeostasis and regulating the function of endocrine organs.3 There are numerous factors affecting the function of the hypothalamo-hypophyseal system,4 but the association between sleep traits and their function is poorly understood and not systematic and is mostly based on observational studies. Whether there is a potential causal relationship between sleep traits and HPTGA function remains unknown.
There is a growing body of convincing evidence linking sleep disorders to the development of endocrine metabolic disorders.5 There are also a few prospective observational studies suggesting that factors such as sleep rhythms, sleep time offsets, and other factors may be involved in influencing HPTGA function,6 while some studies have taken the exact opposite view.7 However, due to the extreme difficulty in obtaining relevant data, coupled with the likelihood that such designs may not fully account for confounding factors and reverse causality bias,8 the causal relationship between sleep traits and HPTGA function remains unproven. To date, no study has explored the potential causal relationship between the two at the genetic level.
MR design is a genetic instrumental variable analysis method using observational data that can be employed to assess causal hypotheses between modifiable risk factors and outcomes such as disease.9 This approach is less susceptible to measurement errors, confounding, and reverse causality compared to traditional multivariable regression methods, thereby significantly increasing the credibility of causal relationship analysis.10 Therefore, it has been widely applied in recent years.
The aim of this study was to investigate whether there are causal associations of these factors on hypothalamic‒pituitary‒adrenal, thyroid and gonadal axis function using single-nucleotide polymorphism (SNP) data of genetic variants closely associated with sleep duration, sleep chronotype and insomnia symptoms from UK Biobank and IEU Open GWAS project databases. The primary and secondary relationships were also explored by bidirectional MR analysis tests.11 Based on the aforementioned studies, we attempt to elucidate the role of sleep traits in maintaining the function of the hypothalamo-hypophyseal system and to provide a basis for future research on novel clinical treatment modalities and drug development.
Methods
Study Populations
The data on sleep traits used in this study were obtained from the UK Biobank database. In brief, over 500,000 UK residents were recruited by the UK Biobank research center between 2006 and 2010 in a prospective study of all people aged between 40 and 69 years, details of which can be found elsewhere.12 A total of 503,325 participants (5.5%) were enrolled in the study cohort, and self-reported baseline data were collected via questionnaire and assessed with anthropometric indices. To avoid confounding effects and close relatives and affinities, individuals of non-White ethnicities (n=48,667, 0.53%) as well as participants’ relatives (n=190,216, 2.1%) were excluded. The data on some relevant hormones and protein secretions of hypothalamic function (thyrotropin-releasing hormone (TRH), prolactin-releasing peptide (PrRP), corticotropin-releasing factor-binding protein (CRFBP), somatostatin (SRIF), gonadotropin-releasing hormone (GnRH)), pituitary function (growth hormone (GH), prolactin (PRL), luteinizing hormone (LH), follicle-stimulating hormone (FSH), thyroid stimulating hormone (TSH), adrenocorticotropic hormone (ACTH), oxytocin-vasopressin 1), and thyroid function (thyroid peroxidase (TPO), thyroglobulin (Tg)) were obtained from the INTERVAL study conducted by England’s National Health Service Blood and Transplant (NHSBT) from 2012 to 2014. The INTERVAL study included a total of 25 independent research centers and recruited approximately 50,000 blood donors. Participants completed an online questionnaire, which included information on demographic characteristics, anthropometric measurements, lifestyle, and dietary habits. Participants were generally in good health, and those with a history of major diseases (for example, hepatitis B or C, myocardial infarction, cancer, stroke, and AIDS) and those with a recent illness or infection were excluded from the cohort. For further SomaLogic testing, two nonoverlapping subgroups in the INTERVAL cohort were randomly selected, with one participant from each pair of close relatives (first- or second-degree) being excluded to eliminate consanguinity. After genetic quality control and sample quality control, including exclusion of sex mismatch, low call rates, duplicate samples, extreme heterozygosity, and non-European ancestry, 3301 participants were enrolled in subsequent plasma protein or protein complex concentration assays and genome-wide association analyses, as described elsewhere.13
Thyroid function-related circulating TSH and free thyroxine (FT4) data were obtained from a large-scale meta-analysis of a genome-wide association study on thyroid function and dysfunction. This analysis included TSH data from 54,288 participants in 22 independent cohorts and FT4 data from 49,269 participants in 19 cohorts. Only subjects with TSH levels within the normal reference range were included in the TSH and FT4 analyses. Individuals of non-European ancestry, on thyroid medication, or who had undergone previous thyroid surgery were excluded. Detailed descriptions of the quality control procedures can be found in.14
Data on the adrenal-related hormone plasma cortisol were obtained from a meta-analysis of a genome-wide association study (GWAS) of plasma cortisol comprising 11 Western European ethnic cohorts, including 12,579 subjects, from the CORtisol NETwork consortium. Inclusion criteria for study subjects were Caucasian adults 17 years of age or older; exclusion criteria were people on glucocorticoids, pregnant or lactating women, and twins (exclusion of one). A detailed description can be found in.15
Data related to gonadal hormones and related protein secretions were obtained from 2 different studies. E2, TT, BT, and sex hormone-binding globulin (SHBG) data were obtained from the UK Biobank Database. Self-identification by questionnaire as being of other than white European ancestry was excluded, as previously described. A maximum of 425,097 UK Biobank participants were available for analysis of genotypic and phenotypic data after application of quality control criteria (425,097 sera for analysis of SHBG, TT, and E2 levels and 382,988 sera for analysis of BT). Association tests were performed using linear mixed models implemented in BOLT-LMM to account for cryptic population structure and relatedness. Only those autosomal genetic variants that were common (minor allele frequency (MAF) >1%), passed quality control in all 106 batches and were present in both genotyping arrays were included in the genetic relationship matrix.16
Data related to progesterone (P4) and aldosterone (ALD) were obtained from the healthy adult cohort at the Leipzig Research Centre for Civilization Diseases research center in Germany. It included 10,000 citizens of Leipzig, Germany, from 2011–2016, and participants were phenotyped in detail for metabolic diseases and cognitive function, among other things. Samples were excluded from hormonal analysis if subjects were taking steroid medication, if quality control of the steroid profile indicated sample confounding, or if there was an underlying disease (eg, hypogonadotropic hypogonadism, androgen excess, congenital adrenal hyperplasia, adrenal insufficiency, or polycystic ovary syndrome), as described in detail here.17
Data on dehydroepiandrosterone (DHEA) sulfate were obtained from The United Kingdom Household Longitudinal Study, which surveyed 40,000 households in England, Scotland, Wales, and Northern Ireland. Participants have been surveyed annually since 2009, with computer-assisted interviews providing information about their socioeconomic status, attitudes, and behaviors. The study includes phenotypic data from a representative sample of participants across a wide range of social and economic indicators, as well as biospecimen collection, including biometric, physiological, biochemical, and blood measures, and self-reported medical history and medication use. A detailed description can be found elsewhere.18
Sleep Traits
All participants underwent a comprehensive questionnaire survey at baseline, where they were asked about their sleep chronotype (preference for morning or evening), average sleep duration, whether their sleep duration was long or short, and any symptoms of insomnia.
Insomnia Measure
At the baseline assessment, study participants self-reported their age, gender, symptoms of insomnia, and medication use using a touchscreen questionnaire. To assess insomnia symptoms, participants were asked, “Do you have difficulty falling asleep or waking up in the middle of the night?” with response options “never/rarely”, “sometimes”, “usually”, or “prefer not to answer”. Participants who responded “prefer not to answer” were defined as missing.
Chronotype Measure
The assessment of chronotype (morning or evening preference) requires participants to answer the question “Do you consider yourself to be?” with six possible response options: “definitely a ‘morning’ person”, “more of a ‘morning’ than ‘evening’ person”, “more of an ‘evening’ than’morning’ person”, “definitely an ‘evening’ person”, “do not know”, or “prefer not to answer”. These responses are encoded as 2, 1, −1, −2, 0, and missing, respectively.
Sleep Duration Measure
The measurement of sleep duration was assessed by asking participants: “On average, how many hours do you sleep in a 24-hour period? (Including naps)” with responses provided in hours. Sleep duration was treated as a continuous variable and was also categorized into short sleep duration (6 hours or less), normal sleep duration (7 or 8 hours), or long sleep duration (9 hours or more). Extreme responses of less than 3 hours or more than 18 hours were excluded, and responses of “do not know” or “prefer not to answer” were considered missing.
Exposure Data
The GWAS summary-level data on the genetic associations with sleep traits were obtained from three different self-reported studies conducted by the UK Biobank project between 2006 and 2010. In the subsequent MR analysis, we included 78 SNPs associated with continuous sleep duration, 26 SNPs associated with short sleep duration, and 10 SNPs associated with long sleep duration.19 Additionally, we incorporated 41 SNPs related to insomnia symptoms20 and 153 SNPs associated with chronotype.21 For further details, please refer to Supplementary Table 1.
Outcome Data
In this study, three types of outcome variables were defined as hypothalamic function, pituitary function, and target gland function based on the physiological anatomical locations of the HPTGA. For hypothalamic function assessment, we measured the secretion levels of TRH, PrRP, CRFBP, SRIF, and GnRH. Pituitary function was assessed by GH, LH, FSH, ACTH, PRL, and oxytocin-neocortin 1 secretion levels. Cortisol, ALD, TSH, FT4, TPO, and Tg are used to assess adrenal and thyroid function. Due to physiological specificity, there are significant sex differences in male and female gonadal function. Therefore, in the present study, we used E2, P4, TT, BT, DHEA, and SHBG as indicators to assess gonadal function. We conducted analyses both within and across sexes (DHEA lacked sex-stratified data). The specific methods for sex stratification have been extensively described elsewhere.22 We downloaded all feature data reported in the UK Biobank database (https://www.nealelab.is/uk-biobank), the IEU Open GWAS project (https://gwas.mrcieu.ac.uk/), the Thyroidomics Consortium (https://transfer.sysepi.medizin.uni-greifswald.de/thyroidomics/) and the Zenodo database (https://zenodo.org/). After excluding duplicate studies, malignancies, and non-European ancestry, we selected all GWAS summary-level data associated with hypothalamo-hypophyseal system functional assessment indicators, as detailed in Supplementary Table 2.
Instrumental Variable Selection
In the present study, sleep traits were used as the exposure, and HPTGA function served as the outcome (with the two being interchanged in a bidirectional MR analysis). To ensure the utmost authenticity and accuracy in assessing the causal relationship between sleep traits and HPTGA function, this study was conducted according to the following quality control criteria to select instrumental variables (IVs): ① SNPs associated with sleep traits with a significance threshold across the locus (P<5.0×10−8) were selected as potential IVs for the most precise analysis. ② The MAF threshold for variant SNPs of interest was 0.01, and SNPs with MAF≤0.01 were excluded. ③ One of the principles of MR is that the IVs included in the analysis should not be in strong linkage disequilibrium (LD) with each other, as the presence of strong LD may lead to biased results. LD between SNPs for each variant of interest was calculated according to the principles of 1000 Genomes project European samples for reference to ensure that R2<0.001 (clumping window size=10,000 kb), and only the SNPs with the lowest P values were retained. ④ To avoid distortion of strand orientation or allele coding, we deleted palindromic SNPs (eg, with A/T or G/C alleles). ⑤ During the harmonization process, we aligned the alleles with the human genome reference sequence (build 37) and removed ambiguous and duplicated SNPs to ensure the accuracy of the results.
Two-Sample MR Analysis
In this study, we conducted two-sample MR analyses between sleep traits and common hypothalamo-hypophyseal system hormones and protein secretions to explore causal associations. The six commonly used MR test methods were used for characterization containing multiple IVs: maximum likelihood (ML) test, MR‒Egger regression, simple median, weighted median, inverse variance weighted (IVW), and weighted model.23–26 It has been reported that IVW, compared to the other five test methods, has the most stringent testing conditions and the most stable and powerful testing efficiency.25 Therefore, in this study, the analysis method primarily relies on IVW results, with other test results used as supplementary evidence. We used the Benjamini-Hochberg method that controls the false discovery rate for multiple testing.27
Sensitivity Analyses
To further assess and correct potential violations of the MR assumptions in the obtained causal estimates, we performed heterogeneity testing using Cochran’s Q statistics for the IVs that satisfy the significance level in at least one test method (IVW). Q statistics significant at a p value (Q-pval)<0.05 can imply the presence of heterogeneity.28,29 MR‒Egger regression is based on the assumption of instrument strength independent of direct effect, which makes it possible to evaluate the existence of pleiotropy with the intercept term. If the intercept term is equal to zero (MR‒Egger regression P>0.05), this indicates that horizontal pleiotropy does not exist.30 For IVs with heterogeneity, radial MR analysis was conducted using modified second-order weights to instrument the exposure to detect and remove outliers.31 The new variants were subjected to reanalysis to minimize the impact of outliers on the overall analysis. In addition, to identify whether the causal outcomes were driven by a single SNP, “leave-one-out” analysis was performed by sequentially omitting each instrumental SNP.32 Only results that met the above criteria were included in the final analysis. To circumvent the fact that reverse causal IVs will bias the MR estimate, we performed the MR Steiger directionality test to examine whether the exposure contributed to the outcome in a directional manner.32 If Steiger_pval<0.05, it was taken to mean that the exposure only affected the outcome in a unidirectional way.
Bi-Directional MR Analysis
To explore whether HPTGA function has any causal effect on identified sleep traits, we also performed a reverse MR analysis (ie, hormone and protein secretions as exposure and identified sleep traits as outcome) using SNPs associated with HPTGA function as IVs to assess all potential reverse causality.
All analyses were performed using R 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria). Two-sample MR analyses were conducted using the “TwoSampleMR”33 and “RadialMR” R package.31
Results
SNP Selection
Based on the aforementioned IV selection criteria and excluding linkage disequilibrium using a 1000-genome reference panel (R2<0.01 and clumping distance=10,000 kb), we identified 41 IVs associated with insomnia, 153 IVs associated with chronotype, 78 IVs associated with sleep duration, 26 IVs associated with short sleep duration, and 10 IVs associated with long sleep duration. Detailed information regarding the selected instrumental variables can be found in Supplementary Table 1. All F-statistic values of all IVs are greater than 10, indicating that there was no significant weak instrumental bias.
We performed MR testing on sleep trait data containing multiple SNPs using six fundamental MR methods. Sensitivity analysis was conducted on the results that had an IVW p value<0.05 based on the aforementioned methods. All analysis results showing heterogeneity were subjected to the radial MR test. Finally, after removing outliers, the data were included in the final analysis.
Causal Effects of Chronotype on the Negative Regulation of Hypothalamic, Pituitary, and Target Gland Functions
As shown in Table 1, Figure 1, Supplementary Figures and Supplementary Tables 3–7, after polytropy correction and outlier removal, at least one MR testing method indicates that chronotype is the only factor among sleep traits that can modulate the function of the tertiary regulatory centers of the HPTGA separately. After IVW assessment, we found that chronotype is causally associated with the secretion of CRFBP in the hypothalamus (beta value=−0.25, 95% CI=−0.47 to −0.03, P=0.03, Q-P value=0.296), the secretion of FSH (beta value=−0.22, 95% CI=−0.45 to 0.01, P=0.050, Q-P value=0.219) and PRL (beta value=−0.49, 95% CI=−0.79 to −0.19, P=0.001, Q-P value=0.629) in the pituitary, adrenal cortisol levels (beta value=−0.22, 95% CI=−0.41 to −0.02, P=0.028, Q-P value=0.113), gonadal (not differentiated by sex) DHEA sulfate levels (beta value=−0.14, 95% CI=−0.27 to −0.02, P=0.022, Q-P value=0.098), TT levels (beta value=−0.03, 95% CI=−0.05 to −0.02, P=3.89E-07, Q-P value=0.896), and bioavailable testosterone (BT) levels (beta value=−0.07, 95% CI=−0.09 to −0.05, P=3.01E-11, Q-P value=0.504).
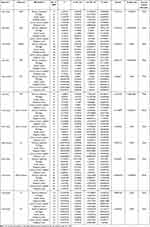 |
Table 1 MR Estimation of Causal Associations Between Sleep Traits and Hypothalamo-Hypophyseal-Target Gland Axis Function |
 |
Figure 1 Forrest plot for the causal association between sleep traits and HPTGA. |
Causal Effects of Sleep Duration on the Negative Regulation of Hypothalamic and Target Gland Functions
As shown in Table 1, Figure 1, Supplementary Figures and Supplementary Tables 3–7, both long sleep and short sleep are causally associated with hypothalamic PrRP secretion. Sleep duration has a causal relationship with thyroid FT4 secretion, and long sleep has a causal relationship with thyroid TPO secretion. Short sleep is causally associated with adrenal cortisol secretion. Both sleep duration and short sleep duration have causal relationships with P4 secretion. Interestingly, short sleep appears to be beneficial for promoting P4 secretion. Sleep duration also has a causal relationship with DHEA secretion. However, after observation, we did not find any evidence supporting a causal effect of sleep duration on pituitary function.
Female Gonadal Function is More Susceptible to Regulation and Influence by Sleep Trait-Related Genotypic Variants
Due to the significant differences in gonadal function among different gender groups, we further stratified the sex hormone secretion data by gender. As seen in Table 2, Figure 2, and Supplementary Table 8, there was a significant causal effect of chronotype on TT (beta value=−0.07, 95% CI=−0.10 to −0.04, P=5.15E-06, Q-P value=0.524) and BT (beta value=−0.06, 95% CI=−0.09 to −0.03, P=1.37E-04, Q-P value=0.522) secretion in males. Female participants showed even more pronounced effects, with significant causal effects of chronotype not only on TT (beta value=−0.05, 95% CI=−0.08 to −0.03, P=1.60E-04, Q-P value=0.701) and BT (beta value=−0.07, 95% CI=−0.09 to −0.05, P=2.18E-08, Q-P value=0.653) levels in females but also exhibited a causal association with estrogen secretion (beta value=−0.12, 95% CI=−0.24 to −0.002, P=4.60E-02, Q-P value=0.316). Furthermore, there was also a significant causal association between sleep duration and BT secretion in females (beta value=0.001, 95% CI=2.54E-04 to 1.76E-03, P=8.81E-03, Q-P value=0.683). In Supplementary Table 8, it can be observed that sleep duration, short sleep, and long sleep traits all show certain trends of impact on the secretion of P4, TT, and SHBG in the female cohort. However, unfortunately, only the ML test yielded a p value of less than 0.05, failing to meet the required statistical significance threshold for this study.
 |
Table 2 MR Estimation of Causal Associations Between Sleep Traits and Gonadal Function (Stratified by Gender) |
 |
Figure 2 Forrest plot for the causal association between sleep traits and gonadal function (stratified by gender). |
Insomnia Does Not Modulate HPTGA Functions
As shown in the Supplemental Table, there is no causal relationship between HPTGA function and genetic susceptibility related to insomnia.
Bidirectional Causal Effects Between HPTGA Function and Sleep Traits
To assess any potential reverse causality effects that may exist, we performed reverse MR analyses using all hypothalamo-hypophyseal system functions for which a causal association existed as exposure and sleep traits as outcome, as detailed in Table 4. We found a bidirectional causal effect between circulating TT (OR=0.97, 95% CI=0.95 to 0.99, P=7.67E-03), BT levels (OR=0.95, 95% CI=0.92 to 0.98, P=3.65E-03) and chronotype in women. Similarly, there was a bidirectional causal effect between BT and sleep duration (OR=1.03, 95% CI=1.01 to 1.05, P=1.62E-02) in females. The above phenomena were observed only in the female cohort, and no reverse causal association was observed between the hypothalamo-hypophyseal system and sleep traits in the male cohort. See Tables 3 and 4 for details.
 |
Table 3 Bi-Directional MR Estimation of the Causal Effects Between Hypothalamic-Pituitary System Function and Sleep Traits |
 |
Table 4 Bi-Directional MR Estimation of the Causal Effects Between Gonadal Function and Sleep Traits (Stratified by Gender) |
Discussion
To the best of our knowledge, the present study is the first investigation using MR analysis to examine the causal relationship between sleep traits and HPTGA function at the genetic level. Based on comprehensive genomic data from over 400,000 European participants, we compared multiple exposure factors (chronotype, insomnia, long sleep, short sleep, and sleep duration) to infer potential causal associations between sleep traits and HPTGA function. Strong evidence suggests that the morning chronotype is associated with decreased circulating TT and BT levels. Extreme sleep durations (either too long or too short) are detrimental to the production and secretion of multiple hormones in the hypothalamo-hypophyseal system.
The hypothalamic-pituitary system comprises three main regulatory axes: the hypothalamic-pituitary-ovarian (HPO) axis, hypothalamic-pituitary-thyroid (HPT) axis, and hypothalamic‒pituitary‒adrenal (HPA) axis. The hypothalamus and pituitary gland, as the highest-level endocrine regulatory centers in the body, are responsible for producing various proteins and peptide fragments to modulate the secretion of hormones in downstream target organs.34 Previous studies have suggested that changes in sleep duration and circadian rhythms can influence hormone levels through humoral and neural pathways. For example, numerous cellular oscillators in the suprachiasmatic nucleus of the hypothalamus are involved in the composition of the body’s circadian rhythm system to feed back to form different sleep characteristics. It periodically regulates its own transcription to maintain homeostasis at the cellular level of organization.35 Unfortunately, there is still a lack of direct observational studies to support the impact of sleep duration on hypothalamic hormone secretion. Through two-sample MR analysis, we identified a strong association between genetic variants related to long sleep, short sleep, and chronotype and hypothalamo-hypophyseal system function. Both excessively long sleep duration (greater than 9 hours) and insufficient short sleep duration (less than 6 hours) were detrimental to the secretion of hypothalamic PrRP. Vas et al’s latest animal study found that PrRP plays a critical role in regulating sleep and affective states, and normal PrRP signaling appears to protect Wistar rat models from stress-induced damage. However, persistent sleep deprivation can lead to overactivation of PrRP cells and depletion of PrRP protein and receptors,36 which is consistent with the findings of this study. Due to the anterior pituitary, PRL secretion is regulated by a variety of other modalities in addition to being stimulated by hypothalamic prolactin-releasing hormone. For example, TRH, vasopressin, and oxytocin are able to stimulate PRL release under certain conditions,37 and considering that the hypothalamus exerts mainly inhibitory effects on PRL, the pituitary may maintain PRL at normal levels through feedback regulation by other circuits. This explains why our study did not observe any evidence of a causal relationship between sleep duration and PRL secretion at the pituitary level.
With increasing age, sleep quality tends to become progressively shallow and fragmented, and the physiological secretion of growth hormone, which is closely related to sleep, also decreases.38 In a prospective study involving seven healthy elderly participants, Ralf-Michael Frieboes’ team administered a random double-blind experimental approach, providing volunteers with hourly infusions of 50 μg of SRIF-14 medication. The results of the study revealed an interesting phenomenon: a significant reduction in total sleep time and rapid eye movement sleep after SRIF administration, suggesting that SRIF causes sleep deterioration in older adults.39 Our study found that prolonged sleep reduces the secretion of growth hormone, which aligns with the above findings. Growth hormone, a regulatory hormone closely related to sleep, is mainly regulated by the relative actions of GH-releasing hormone and SRIF. Pulsatile growth hormone secretion has been confirmed to typically increase during the first half of nighttime sleep, coinciding with the onset of deep sleep.40 Unfortunately, there is no whole genome sequencing data on growth hormone-releasing hormone, so the present study can only be unilaterally verified by the level of growth inhibitory hormone release.
Thirteen years ago, Christoph Randler conducted a study on morning wake-up timings and the cortisol awakening response in adolescent populations and found that individuals with evening types had significantly higher salivary cortisol levels than those with morning types.41 Despite the relatively young age of the study participants (mean age of the adolescents was 14.02 years, SD=0.77, range 13–16, and 23.94 years in adults, SD=3.77, range 20–39), the results are still consistent with the phenomenon observed in our study. Similar results were observed by Imani et al, fractured sleep and sleep deprivation resulted in immediate activation of the organism’s autonomic nerves, which subsequently reduced HPA axis activity.42 We observed that at the level of the upstream center of the HPA axis, the early rise chronotype was detrimental to the secretion of corticotropin-releasing hormone. At the downstream target organ level, cortisol production was similarly inhibited by short sleep and early rise chronotype.
Interestingly, our study found that shorter sleep duration can effectively promote P4 production (regardless of gender), which is in direct contrast to the findings of Nolan et al’s research.43 Nolan concluded that micronized P4 can improve sleep onset latency (effect size=7.10; CI=1.30 to 12.91), increase total sleep duration, and enhance sleep quality and efficiency. To clarify whether there is a reverse causal association between the two, we performed a bidirectional MR analysis, which demonstrated that short sleep duration affects P4 production from a single direction. No potential causal association was observed after sex stratification (see Table 3 for details). We believe the reason for the different results is that Nolan’s study population focused primarily on postmenopausal women with a relatively small sample size, which also had a significant single-sex bias. Coupled with the fact that their study was primarily focused on exploring the biological efficacy of P4 interventions for sleep quality, there was also significant directional specificity, which led to the opposite findings in our study. Therefore, multicenter observational studies with larger sample sizes are still needed to clarify the role of short sleep on P4 secretion.
There is compelling evidence that females appear to be more susceptible to sleep disturbances than males, and sleep disturbances have a greater physiological impact on females.44 However, few prospective observational studies have focused on the effects of sleep traits on gonadal steroid hormones. A prospective follow-up of 259 menstruation-stabilized women by Kara A. Michels’ team indicated that women who suffered sleep deprivation had lower circulating TT levels,45 which is also similar to our findings. However, Michels did not find any differences in average hormone concentrations related to chronotype. This may be due to the blood samples used for the study being collected during menstruation, where physiological fluctuations in estrogen and P4 levels could introduce bias in hormone measurements, making it challenging to fully represent women’s normal circulating hormone levels objectively. Chronotype not only affects testosterone secretion in females but also shows a significant causal relationship with estrogen secretion. An observational study by Beata Peplonska, involving 345 premenopausal and 187 postmenopausal nurses, suggests that among premenopausal nurses, a higher frequency of night shifts per month is associated with lower circulating estrogen levels.46 The similar conclusion that chronotype is detrimental to TT secretion has also been observed in male cohort. In a study involving male participants, Christoph Randler found a significant negative correlation between Composite Scale of Morningness (CSM) scale scores and TT levels (r(s)=−0.220, p=0.023, two-tailed test). However, there was no significant relationship between TT levels and average sleep duration,47 which further supports our research findings. BT, as a binding product of free testosterone and albumin, can effectively reflect the biometabolic status of testosterone in vivo.48 Similar results have also been observed in circulating BT. Our study found that circulating TT and BT levels were decreased in the early-rise chronotype population without showing significant gender specificity. In contrast, the reverse MR analysis suggested that only the female cohort was found to have circulating TT and BT levels that have a reverse effect on chronotype and sleep duration. Given the limited and controversial results from previous studies, further research with a larger sample size is necessary to validate our findings. However, it is undeniable that sleep traits exhibit significant gender specificity, and females’ health status seems to be more vulnerable to the effects of changes in sleep traits. And in the future, MR analysis, as a promising new class of research modality, can be used to explore the relationship between sleep structure and psychiatric and metabolic lineage disorders.49
Admittedly, this study has some limitations. First, in our pursuit of data completeness, the exposure data were sourced from multiple different genome-wide studies, and the differences in study populations may introduce some data bias to our research. Second, our study primarily focused on exploring the expression levels of circulating hormones to investigate the function of the hypothalamo-hypophyseal system. However, relying solely on the secretion capacity of HPTGA may not fully represent its biological functionality. Therefore, further confirmation through animal and clinical studies is warranted. Last, all sleep trait events were self-reported rather than validated by questionnaires or objective measurements, which may lead to exposure misclassification. This aspect deserves careful attention. Future studies could attempt to use actigraphy to pinpoint measurements of sleep.50
Conclusion
In conclusion, our study found a clear causal association between sleep traits at the level of genetic variation and the function of the hypothalamo-hypophyseal system. The early-onset sleep chronotype was causally associated with suppression of gonadotropic function (reduced levels of TT and TB secretion) in adult humans, and this negative potency does not appear to be significantly sex specific. Extreme sleep habits are also detrimental to the maintenance of normal physiological function in the HPTGA, and the female cohort appears to be more susceptible to the effects of unfavorable sleep traits. We did not find significant evidence in our study to support any potential causal association of insomnia in maintaining the function of the hypothalamo-hypophyseal system. Maintaining healthy and regular sleep habits is essential for HPTGA to maintain normal biological functions. The present study also provides new insights into the biological regulatory mechanisms of HPTGA as well as related clinical research, and further large-sample investigations are urgently needed to clarify the potential association between sleep traits and HPTGA.
Abbreviations
MR, Mendelian randomization; CRFBP, corticotropin-releasing factor-binding protein; PRL, prolactin; DHEA, dehydroepiandrosterone; TT, total testosterone; BT, bioavailable testosterone; FT4, free thyroxine; P4, progesterone; PrRP, prolactin-releasing peptide; SRIF, somatostatin; E2, estradiol; HPTGA, hypothalamic-pituitary-target gland axis; SNPs, single-nucleotide polymorphisms; TRH, thyrotropin-releasing hormone; GnRH, gonadotropin-releasing hormone; GH, growth hormone; LH, luteinizing hormone; FSH, follicle-stimulating hormone; TSH, thyroid stimulating hormone; ACTH, adrenocorticotropic hormone; TPO, thyroid peroxidase; Tg, thyroglobulin; NHSBT, England’s National Health Service Blood and Transplant; SHBG, sex hormone-binding globulin; ALD, IVs, aldosterone; instrumental variables; MAF, minor allele frequency; LD, linkage disequilibrium; ML, maximum likelihood; IVW, inverse variance weighted; HPO, hypothalamic-pituitary-ovarian; HPT, hypothalamic-pituitary-thyroid; HPA, hypothalamic‒pituitary‒adrenal.
Data Sharing Statement
The datasets analysed in this study are publicly available summary statistics. Genetic instruments can be obtained from the individual referenced papers.19–21 All summary data of HPTGA can be downloaded from the following website: UK Biobank database (https://www.nealelab.is/uk-biobank), the IEU Open GWAS project (https://gwas.mrcieu.ac.uk/), the Thyroidomics Consortium (https://transfer.sysepi.medizin.uni-greifswald.de/thyroidomics/) and the Zenodo database (https://zenodo.org/).
Ethics Approval and Consent to Participate
All the contents of this study have been reviewed and approved by the Ethics Committee of the second affiliated hospital of Chongqing medical university and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Acknowledgments
All authors of this paper would like to extend their gratitude to all the participants involved in the GWAS cohorts included in this study, as well as to the IEU Open GWAS project, UK Biobank, Thyroidomics Consortium, Zenodo database, CORNET, and other research teams and researchers. We sincerely appreciate the GWAS summary statistics data provided by the aforementioned teams and individuals.
Funding
This research was supported by the science and technology innovation project of Chongqing Municipal Science and Technology Commission (Number: cstc2015shmszx120054); Joint Key Projects of Chongqing Science and Technology Bureau and Health Care Commission (Number: 2022ZDXM004); Talent Program Project of Chongqing Science and Technology Bureau (Number: cstc2021ycjh-hgzxm0050).
Disclosure
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
1. Irwin MR. Sleep and inflammation: partners in sickness and in health. Nat Rev Immunol. 2019;19(11):702–715. doi:10.1038/s41577-019-0190-z
2. Stickgold R, Walker MP. Sleep-dependent memory consolidation and reconsolidation. Sleep Med. 2007;8(4):331–343. doi:10.1016/j.sleep.2007.03.011
3. Alatzoglou KS, Gregory LC, Dattani MT. Development of the pituitary gland. Compr Physiol. 2020;10(2):389–413.
4. Fleseriu M, Hashim IA, Karavitaki N, et al. Hormonal replacement in hypopituitarism in adults: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(11):3888–3921. doi:10.1210/jc.2016-2118
5. Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354(9188):1435–1439. doi:10.1016/S0140-6736(99)01376-8
6. Kecklund G, Axelsson J. Health consequences of shift work and insufficient sleep. BMJ. 2016;355:i5210. doi:10.1136/bmj.i5210
7. van Neijenhof RJGP, van Duijn E, Van Den Berg JF, de Waal MWM, van der Mast RC, Comijs HC. Subjective insomnia symptoms and sleep duration are not related to hypothalamic-pituitary-adrenal axis activity in older adults. J Sleep Res. 2018;27(1):40–46. doi:10.1111/jsr.12570
8. Smith GD, Lawlor DA, Harbord R, Timpson N, Day I, Ebrahim S. Clustered environments and randomized genes: a fundamental distinction between conventional and genetic epidemiology. PLoS Med. 2007;4(12):e352. doi:10.1371/journal.pmed.0040352
9. Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362:k601. doi:10.1136/bmj.k601
10. Smith GD, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32(1):1–22. doi:10.1093/ije/dyg070
11. Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23(R1):R89–R98. doi:10.1093/hmg/ddu328
12. Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. doi:10.1371/journal.pmed.1001779
13. Sun BB, Maranville JC, Peters JE, et al. Genomic atlas of the human plasma proteome. Nature. 2018;558(7708):73–79. doi:10.1038/s41586-018-0175-2
14. Teumer A, Chaker L, Groeneweg S, et al. Genome-wide analyses identify a role for SLC17A4 and AADAT in thyroid hormone regulation. Nat Commun. 2018;9(1):4455. doi:10.1038/s41467-018-06356-1
15. Bolton JL, Hayward C, Direk N, et al. Genome wide association identifies common variants at the SERPINA6/SERPINA1 locus influencing plasma cortisol and corticosteroid binding globulin. PLoS Genet. 2014;10(7):e1004474.
16. Ruth KS, Day FR, Tyrrell J, et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med. 2020;26(2):252–258. doi:10.1038/s41591-020-0751-5
17. Pott J, Horn K, Zeidler R, et al. Sex-specific causal relations between steroid hormones and obesity-A Mendelian randomization study. Metabolites. 2021;11(11):738. doi:10.3390/metabo11110738
18. Prins BP, Kuchenbaecker KB, Bao Y, et al. Genome-wide analysis of health-related biomarkers in the UK Household Longitudinal Study reveals novel associations. Sci Rep. 2017;7(1):11008. doi:10.1038/s41598-017-10812-1
19. Dashti HS, Jones SE, Wood AR, et al. Genome-wide association study identifies genetic loci for self-reported habitual sleep duration supported by accelerometer-derived estimates. Nat Commun. 2019;10(1):1100. doi:10.1038/s41467-019-08917-4
20. Lane JM, Jones SE, Dashti HS, et al. Biological and clinical insights from genetics of insomnia symptoms. Nat Genet. 2019;51(3):387–393. doi:10.1038/s41588-019-0361-7
21. Jones SE, Lane JM, Wood AR, et al. Genome-wide association analyses of chronotype in 697,828 individuals provides insights into circadian rhythms. Nat Commun. 2019;10(1):343. doi:10.1038/s41467-018-08259-7
22. Coviello AD, Haring R, Wellons M, et al. A genome-wide association meta-analysis of circulating sex hormone-binding globulin reveals multiple Loci implicated in sex steroid hormone regulation. PLoS Genet. 2012;8(7):e1002805. doi:10.1371/journal.pgen.1002805
23. Burgess S, Dudbridge F, Thompson SG. Combining information on multiple instrumental variables in Mendelian randomization: comparison of allele score and summarized data methods. Stat Med. 2016;35(11):1880–1906. doi:10.1002/sim.6835
24. Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int J Epidemiol. 2016;45(6):1961–1974. doi:10.1093/ije/dyw220
25. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304–314. doi:10.1002/gepi.21965
26. Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan N, Thompson J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med. 2017;36(11):1783–1802. doi:10.1002/sim.7221
27. Glickman ME, Rao SR, Schultz MR. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J Clin Epidemiol. 2014;67(8):850–857. doi:10.1016/j.jclinepi.2014.03.012
28. Bowden J, Del Greco MF, Minelli C, et al. Improving the accuracy of two-sample summary-data Mendelian randomization: moving beyond the NOME assumption. Int J Epidemiol. 2019;48(3):728–742. doi:10.1093/ije/dyy258
29. Greco MFD, Minelli C, Sheehan NA, Thompson JR. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med. 2015;34(21):2926–2940. doi:10.1002/sim.6522
30. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. 2015;44(2):512–525. doi:10.1093/ije/dyv080
31. Bowden J, Spiller W, Del Greco MF, et al. Improving the visualization, interpretation and analysis of two-sample summary data Mendelian randomization via the Radial plot and Radial regression. Int J Epidemiol. 2018;47(4):1264–1278. doi:10.1093/ije/dyy101
32. Hemani G, Tilling K, Davey Smith G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 2017;13(11):1.
33. Hemani G, Zheng J, Elsworth B, et al. The MR-base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408. doi:10.7554/eLife.34408
34. Plant TM. 60 years of neuroendocrinology: the hypothalamo-pituitary-gonadal axis. J Endocrinol. 2015;226(2):T41–T54. doi:10.1530/JOE-15-0113
35. Rosenwasser AM, Turek FW. Neurobiology of circadian rhythm regulation. Sleep Med Clin. 2015;10(4):403–412. doi:10.1016/j.jsmc.2015.08.003
36. Vas S, Papp RS, Könczöl K, et al. Prolactin-releasing peptide contributes to stress-related mood disorders and inhibits sleep/mood regulatory melanin-concentrating hormone neurons in rats. J Neurosci. 2023;43(5):846–862. doi:10.1523/JNEUROSCI.2139-21.2022
37. Phillipps HR, Yip SH, Grattan DR. Patterns of prolactin secretion. Mol Cell Endocrinol. 2020;502:110679. doi:10.1016/j.mce.2019.110679
38. Aström C, Lindholm J. Growth hormone-deficient young adults have decreased deep sleep. Neuroendocrinology. 1990;51(1):82–84. doi:10.1159/000125320
39. Frieboes RM, Murck H, Schier T, Holsboer F, Steiger A. Somatostatin impairs sleep in elderly human subjects. Neuropsychopharmacology. 1997;16(5):339–345. doi:10.1016/S0893-133X(96)00244-8
40. Van Cauter E, Plat L. Physiology of growth hormone secretion during sleep. J Pediatr. 1996;128(5 Pt 2):S32–S37. doi:10.1016/S0022-3476(96)70008-2
41. Randler C, Schaal S. Morningness-eveningness, habitual sleep-wake variables and cortisol level. Biol Psychol. 2010;85(1):14–18. doi:10.1016/j.biopsycho.2010.04.006
42. Imani MM, Sadeghi M, Khazaie H, et al. Associations between morning salivary and blood cortisol concentrations in individuals with obstructive sleep apnea syndrome: a meta-analysis. Front Endocrinol. 2021;11:568823. doi:10.3389/fendo.2020.568823
43. Nolan BJ, Liang B, Cheung AS. Efficacy of micronized progesterone for sleep: a systematic review and meta-analysis of randomized controlled trial data. J Clin Endocrinol Metab. 2021;106(4):942–951. doi:10.1210/clinem/dgaa873
44. McHenry J, Carrier N, Hull E, Kabbaj M. Sex differences in anxiety and depression: role of testosterone. Front Neuroendocrinol. 2014;35(1):42–57. doi:10.1016/j.yfrne.2013.09.001
45. Michels KA, Mendola P, Schliep KC, et al. The influences of sleep duration, chronotype, and nightwork on the ovarian cycle. Chronobiol Int. 2020;37(2):260–271. doi:10.1080/07420528.2019.1694938
46. Peplonska B, Bukowska A, Lie JA, Gromadzinska J, Zienolddiny S. Night shift work and other determinants of estradiol, testosterone, and dehydroepiandrosterone sulfate among middle-aged nurses and midwives. Scand J Work Environ Health. 2016;42(5):435–446. doi:10.5271/sjweh.3581
47. Randler C, Ebenhöh N, Fischer A, et al. Chronotype but not sleep length is related to salivary testosterone in young adult men. Psychoneuroendocrinology. 2012;37(10):1740–1744. doi:10.1016/j.psyneuen.2012.02.008
48. Manni A, Pardridge WM, Cefalu W, et al. Bioavailability of albumin-bound testosterone. J Clin Endocrinol Metab. 1985;61(4):705–710. doi:10.1210/jcem-61-4-705
49. Sun X, Liu B, Liu S, et al. Sleep disturbance and psychiatric disorders: a bidirectional Mendelian randomisation study. Epidemiol Psychiatr Sci. 2022;31:e26. doi:10.1017/S2045796021000810
50. Liguori C, Mombelli S, Fernandes M, et al. The evolving role of quantitative actigraphy in clinical sleep medicine. Sleep Med Rev. 2023;68:101762. doi:10.1016/j.smrv.2023.101762
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
