Back to Journals » Journal of Multidisciplinary Healthcare » Volume 17
CA125-Associated Activated Partial Thromboplastin Time and Thrombin Time Decrease in Patients with Adenomyosis
Authors Yang F, Wang Q, Ma R, Deng F, Liu J
Received 20 September 2023
Accepted for publication 11 December 2023
Published 16 January 2024 Volume 2024:17 Pages 251—261
DOI https://doi.org/10.2147/JMDH.S435365
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Fanchun Yang, Qingying Wang, Rui Ma, Fangzhen Deng, Jie Liu
Department of Obstetrics and Gynecology, Shanghai Tenth People’s Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China
Correspondence: Jie Liu, Department of Obstetrics and Gynecology, Shanghai Tenth People’s Hospital, Tongji University School of Medicine, No. 301, Yanchang Zhong Road, Shanghai, People’s Republic of China, Email [email protected]
Objective: Adenomyosis patients are in a hypercoagulable state, and studies have shown that carbohydrate antigen125 (CA125) may relate to the hypercoagulability and thrombosis of patients with adenomyosis, but there is still a lack of clarity regarding the changes in CA125-related coagulation indicators. This study was to explore the changes and influencing factors of CA125-related coagulation parameters in patients with adenomyosis.
Methods: Retrospective observational study conducted on 200 patients with adenomyosis (AM group), 240 patients with uterine leiomyoma (LM group) and 81 patients with cervical intraepithelial neoplasia (CIN)-III (control group), of which the coagulation parameters were detected by clinical blood sample collection and statistical method analysis and informed consent was obtained.
Results: The level of CA125 in the AM group was significantly higher than that in the LM group and control group. However, thrombin time (TT) shortened in the AM group when compared with the LM and control group. Activated partial thromboplastin time (APTT) in the AM group was shorter than in the control group. Multivariate logistic regression analysis found that adenomyosis was associated with CA125 level (OR=323.860, 95% CI 90.424– 1159.924, P< 0.001), APTT (OR=1.295, 95% CI 1.050– 1.598, P=0.016), TT (OR=0.642, 95% CI 0.439– 0.938, P=0.022), menorrhagia (OR=7.363, 95% CI 2.544– 21.315, P< 0.001), dysmenorrhea (OR=22.590, 95% CI 8.185– 62.347, P< 0.001). Correlation analysis revealed that APTT (r= − 0.207) and TT (r = − 0.174) were negatively correlated with the level of CA125.
Conclusion: The shortening of CA125-related APTT and TT indicates that it is meaningful to detect coagulation parameters of patients with elevated CA125 levels early, dysmenorrhea and menorrhagia, and maybe further discover the hypercoagulability and prevent the occurrence of thrombus in adenomyosis.
Keywords: adenomyosis, coagulation parameters, hypercoagulability, CA125, APTT, TT
A Letter to the Editor has been published for this article.
Introduction
Adenomyosis is a diffuse or localized lesion of endometrial glands and stroma invading the myometrium, which is a common and benign disease in gynecology.1,2 Menorrhagia and dysmenorrhea are the main symptoms of adenomyosis. However, nearly a third of patients do not suffer from any symptoms.3,4 In recent years, there has been an observed hypercoagulable state in patients with adenomyosis, including changes in D-dimer levels, thrombin spectrum and platelet count (PLT), which may further illustrate the pathogenesis, diagnosis as well as the therapy of adenomyosis.5,6 Previous studies have found the activation of coagulation and a potential risk of infarction and thrombosis in patients with adenomyosis.5 Platelets, as the primary functional cells to maintain the integrity of the vascular wall and early hemostasis, can aggregate in the site of vascular injury in patients with adenomyosis, thus participating in the changes of coagulation, which is associated with abnormal uterine bleeding and hypercoagulability.7 Apart from platelets, activated partial thromboplastin time (APTT), prothrombin time (PT), and thrombin time (TT), as vital coagulation parameters, play an important role in the human body’s coagulation and fibrinolytic system.5,8 In patients with adenomyosis, researchers found that platelets and PT increased, which was associated with anemia.6 In addition, one study also found that APTT and TT significantly shortened in adenomyosis patients with anemia than those without anemia. And hypercoagulability in patients with adenomyosis may be related to the elevated CA125 levels and anemia.9 Patients with adenomyosis often have elevated serum CA125, especially in patients with thrombotic events. There were reports that the level of CA125 was significantly elevated in adenomyosis patients with cerebral infarcts, disseminated intravascular coagulation (DIC) and venous thromboembolism (VTE).10–14 And a recent study about the correlation between hemoglobin (Hb) and coagulation parameters showed that anemia was associated with the increase of PLT and PT in patients with Adenomyosis. However, researchers pointed out that some datas, such as CA125, that may affect coagulation parameters, which need further study.6 Therefore, we speculated that the change of CA125 level may affect the change of coagulation parameters and thus relate to hypercoagulability and even thrombosis in patients with adenomyosis.
In endometriosis, study also found that TT and PT were significantly shorter, and plasma fibrinogen (FIB) levels were significantly higher in patients. And the study found that CA125 combined with fibrinogen had a higher diagnostic value for endometriosis.8 These show that endometriosis patients are in a hypercoagulable state, and CA125 as well as the change of coagulation parameters may be related to a hypercoagulable status.8 Additionally, one study found that the preoperative serum CA125 level was associated with the occurrence of perioperative thromboembolic events in epithelial ovarian cancer.15 CA125, a marker of epithelial ovarian cancer, was significantly elevated in the adenomyosis patients than those diagnosed with uterine fibroids and some other gynecological benign diseases.16,17 And earlier study also demonstrated that CA125 was positively correlated with PLT in adenomyosis.18 Considering that CA125 may be related to the change of coagulation and the differential expression in adenomyosis, uterine fibroids, and other gynecological diseases, we conducted a cross-sectional study to determine the changes in coagulation parameters (PLT, PT, APTT, TT, FIB (plasma fibrinogen) and INR (international normalized ratio)) in patients with adenomyosis as compared to patients with uterine fibroids and patients with cervical intraepithelial neoplasia (CIN)-III who underwent cervical conization or hysterectomy. Previous studies have found no significant difference in coagulation parameters between different degrees of CIN patients and normal women during non-menstrual periods. Therefore, CIN-III patients can be used as a control group for this study.9
Materials and Methods
Patients
The study recruited 1453 patients from January 2018 to December 2022, among which 592 patients with adenomyosis and 580 patients with uterine leiomyoma underwent laparoscopic/laparotomic/Hysteroscope/Focused ultrasound ablation (FUAS) surgery, and 281 patients with CIN-III who underwent laparoscopic hysterectomy and cervical conization at Shanghai Tenth People ‘s Hospital Affiliated to Tongji University. All patients’ general information and clinical data, including age, BMI, gravidity, abortion, menorrhagia, hemoglobin, dysmenorrhea, uterine volume, CA125, CA199, and coagulation parameters, were retrospectively obtained from the medical records of hospitalized patients. Among which, abortion refers to the termination of pregnancy when the pregnancy is less than 28 weeks and the fetal weight is less than 1000g, including spontaneous abortion and induced abortion. This study complied with the Declaration of Helsinki and was approved by the Ethics Committee of Shanghai Tenth People ‘s Hospital and informed consent was obtained from patients. We use preoperative ultrasound, magnetic resonance imaging (MRI)and postoperative histopathology to diagnose patients with adenomyosis, uterine fibroids and CIN-III. There were 392 patients with adenomyosis (AM group), 340 patients with uterine leiomyoma (LM group) and 200 patients with CIN-III (control group) that were excluded from the study. As a result, the remaining 521 patients were finally included in this study, including 200 patients with adenomyosis, 240 patients with uterine fibroids, and 81 patients with CIN-III (Figure 1). None of the patients had a history of diabetes, hypertension, cancer, kidney disease, autoimmune diseases, coagulation disorders and hematological diseases that affect coagulation status. And 6 months before collecting blood samples, all patients did not use anticoagulants, estrogen and progesterone preparations as well as blood and iron supplement therapy.19,20
 |
Figure 1 Study flow diagram. |
Blood Samples Collection and Assays
We collected preoperative peripheral blood samples on the day before surgery from all patients who were hospitalized and underwent surgery during the non-menstrual period. CA125(range:0–35U/mL), CA199(range:0–27U/mL), hemoglobin (Hb, range: 115–150g/L) and platelet count (PLT, range:125–350×109/L), and coagulation parameters, including plasma prothrombin time (PT, range:8.5–14.5s), activated partial thromboplastin time (APTT, range:18.4–38.4s), thrombin time (TT, range: 14.5–20.5s), and fibrinogen (FIB, range:2–4g/L), D-dimer (range:0–0.55g/L), INR (range:1.5–2.8 at anticoagulant therapy) were measured by automatic blood analyzer.
Uterine Volume Calculation
Ultrasound is used to measure the size of the uterus which includes the length, width and thickness of the uterus. And the calculation formula of uterine volume is the length of the uterus multiplied by the width and then multiplied by the thickness, and finally multiplied by 0.5233.
Statistical Analysis
SPSS 22.0 software and MedCalc Statistical Software (MedCalc® Statistical Software version 19.6.1; https://www.medcalc.org) was used for statistical analysis. In this study, we used Shapiro–Wilk test to determine whether the continuous variables conformed to the normal distribution. The continuous variables in this study are non-normal distribution variables, which are shown as the median with an interquartile range [M (P25–75)]. And the intragroup differences were compared by using nonparametric tests. Univariate analysis was performed by χ2 tests. Multivariate analysis was accomplished by logistic regression, and the covariates included were statistically significant variables that were found in univariate analysis. The correlation between coagulation parameters and the level of CA125 was analyzed by Spearman correlation analysis. P < 0.05 was considered statistically significant.
Results
Patients’ Characteristics
Compared with the AM group, the patients in the control group were younger and had smaller BMI. However, there was no significant difference between the AM group and LM groups. The number of gravidity and abortion in the AM group was more than the LM and control groups. And there were more patients suffering from the menorrhagia, anemia and dysmenorrhea in the AM group as compared with the LM group (p<0.001) and control groups (p<0.001). And compared with the LM and control groups, the larger uterine size, the higher level of CA125 and CA199 were found in the AM group (p<0.001). Particularly, the level of CA125 was significantly elevated in the AM group. The detailed information was depicted in Table 1.
 |
Table 1 Patient characteristics among groups |
Comparisons of Coagulation Parameters Between Groups
PLT in the control group was lower than that in the AM group (p<0.001). However, there was no significant difference between the LM group and AM groups. Compared with control group, APTT in the AM group was shorter (p=0.001). Nevertheless, there was no statistical significance between the AM and LM group. There was a significant difference in TT among the three groups (p=0.002). Compared with the LM and control groups, TT in the AM group was shorter. In addition, compared with the control group, the level of D-dimer was elevated in the AM group (p<0.001). But there was no statistical significance between the AM and LM groups. As for the PT and INR, there was no statistical significance between the three groups. The level of FIB in the AM group was higher than that in the LM and control group. However, it was not statistically significant. (Table 2; Figure 2).
 |
Table 2 Comparisons of Coagulation Parameters Between Groups |
The Correlations Between Coagulation Parameters and Adenomyosis
Based on the different characteristics between the three groups, we performed multivariate logistic regression analysis in which the LM group and control group were coded as 0 and the AM group was coded as 1 so that we could further explore whether adenomyosis leads to the change of coagulation. Multivariate logistic regression analysis showed that adenomyosis was associated with the CA125 level (OR=323.860, 95% CI 90.424–1159.924, P<0.001), APTT (OR=1.295, 95% CI 1.050–1.598, P=0.016), TT (OR=0.642, 95% CI 0.439–0.938, P=0.022). However, there was no correlation between CA125 and PT, FIB, D-dimer and INR. And gravidity, abortion, age, BMI, Hb, uterine size and CA199 were not statistically significant. Additionally, menorrhagia (OR=7.363, 95% CI 2.544–21.315, P<0.001), dysmenorrhea (OR=22.590, 95% CI 8.185–62.347, P<0.001) were high-risk factors for adenomyosis (Table 3). And we also found the same results when compared the patients with adenomyosis and patients with uterine leiomyoma by Multivariate logistic regression analysis (Table S1). Furthermore, we used Spearman’s analysis to analyze the potential correlat ons of APTT and TT with menorrhagia and dysmenorrhea in patients with adenomyosis. The results showed that there was no correlation (Table S2). Therefore, we speculated that CA125 may relate to coagulation indicators.
 |
Table 3 Correlations of Coagulation Parameters, Characteristics and Adenomyosis |
Correlation of APTT and TT with CA125 in Patients with Adenomyosis
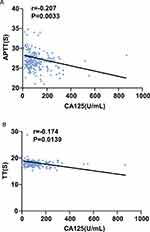
We used Spearman’s analysis to analyze the potential correlations of APTT and TT with CA125 in patients with adenomyosis. The results showed that both APTT (r=−0.207, P<0.05) and TT (r=−0.174, P<0.05) were negatively correlated with the level of CA125 (Figure 3).
Predictive Value of APTT and TT in the Diagnosis of Adenomyosis
It has been found that APTT and TT correlated with adenomyosis. And in 16 patients with adenomyosis who had thrombosis during the same period, the result revealed that APTT and TT shortened (Table S3). Therefore, we used APTT and TT as predictive value in the diagnosis of adenomyosis and further could predict the risk of hypercoagulability and even thrombosis in adenomyosis. The AUC of APTT was 0.601 (95% CI 0.558–0.644) with a sensitivity of 67.50%, and a specificity of 49.84% as well as a cut-off value of 27s. The AUC of TT was 0.587 (95% CI 0.544–0.630) with a sensitivity of 34.5% and a specificity of 80.06%, and a cut-off value of 17.3s (Figure 4). Additionally, we found that the level of CA125 was significantly elevated in 16 patients with thrombosis in adenomyosis (Table S3). In the analysis of the receiver operating characteristic curve, the AUC of CA125 was 0.954 (95% CI 0.932–0.970) with a sensitivity of 86.00%, a specificity of 96.26%, and a cut-off value of 37.6U/mL (Figure S1).
 |
Figure 4 Predictive Value of activated partial thromboplastin time (APTT) and thrombin time (TT) in the Diagnosis of Adenomyosis based on the receiver operating characteristic curve. |
Discussion
The study has found that there is a hypercoagulable state in adenomyosis, involving the participation of important coagulation parameters such as APTT and PT, and this process also involves PLT, CA125 and coagulation factors.7,21 In our study, we found that APTT and TT decreased in patients with adenomyosis, and were associated with the level of CA125. As a vital coagulation parameter, APTT is often used to predict the tendency of bleeding and a hypercoagulable state. Shorter APTT is associated with hypercoagulability, manifested as an increase in thrombin generation and an increased risk of thrombosis as well as the elevated procoagulant factors, such as FV, thrombin cleaves factor VIII (FVIII), FXI and FXII and von Willebrand factor (VWF).22,23 Short APTT represents a procoagulant milieu and has been considered the independent risk factor of venous thromboembolism.24,25 In the study of trauma patients, FVIII was activated and then bound to VWF via VWF-platelet interaction at the site of endothelial inflammation or injury, thus further promoting the release of FVIII/VWF and platelet aggregation, which finally contributed to the activation of APTT and the pathogenesis of venous thrombosis.26 In addition, as a known coagulation factor, FIB is associated with hypercoagulation and the release of tranexamic acid 2(TXA2).27 Thrombin can activate platelets to produce TXA2, thus can activate more platelet and induce plasminogen activator inhibitor-1 (PAI-1) expression in ectopic endometrium,28 which is consistent with the illustration in adenomyosis.29 And all these can activate the coagulation cascade, and then contributing to the higher FIB levels and shorter APTT in adenomyosis. In our study, we also found the shortening of APTT and the increase of FIB level in patients with adenomyosis as compared with the patients with uterine leiomyoma and CIN-III. In particular, in 16 adenomyosis patients with thrombosis, APTT further decreased and the level of FIB further increased.
Apart from APTT, TT reflects the time required for thrombin to convert fibrinogen to fibrin. FIB is the substrate of the process, so its level affects the length of TT.30,31 Based on the activation of FVIII, TT can reflect a thrombin burst, which was synergistic with the generation of FIXa caused by the process that APTT activated the intrinsic pathway, thus contributing to the conversion of FIB to fibrin.31,32 In our study, the results suggested the decrease of TT with accompanying by the increase of FIB. The shorter TT in the adenomyosis patients than in the patients with uterine leiomyoma or the control group may be due to the higher level of FIB in the adenomyosis. These reflected that the hypercoagulable state of adenomyosis might be related to the shortening of APTT and TT. A study of Lin et al found that, compared with the control group, APTT, TT decreased and FIB increased in patients with adenomyosis.6 And another study also showed that compared with patients in uterine leiomyoma with anemia, TT was significantly shorter in adenomyosis with anemia. Anemia can affect the coagulation parameters and increase the risk of thrombus formation.33,34 Similarly, in the study of endometriosis, researchers also found that APTT and TT shortened whereas FIB increased in endometriosis compared with the control group.35 And elevated FIB as well as shortened TT were associated with the state of hypercoagulability in endometriosis.36 Another study also revealed that patients with endometriosis had significantly shortened APTT, which may relate to a potential hypercoagulable status associated with the disease.37 These indicated that shortened APTT and TT may be associated with hypercoagulation in adenomyosis and increased the risk of thrombosis.
CA125, as a mucinous serum marker, expresses in patients with various diseases, which is associated with hypercoagulation status.38,39 There were reports that thrombus occurred in several patients with gynecological diseases and elevated CA125 level was found in these patients, and the increased CA125 level might reflect hypercoagulability.12,40 A recent study also found that adenomyosis can result in cerebral venous sinus thrombosis and found a high CA125 level in these patients.41 And the same results were observed in our study. Our study also found a higher CA125 level in adenomyosis than that in the uterine leiomyoma and the CIN-III patients. In the analysis of the receiver operating characteristic curve, the AUC of CA125 was 0.954 (95% CI 0.932–0.970) with a sensitivity of 86.00%, a specificity of 96.26%. (Figure S1). And in 16 patients with thrombosis in adenomyosis, we found that the level of CA125 was significantly elevated (Table S3). The results showed that CA125 has a significant value in the diagnosis of adenomyosis and in evaluating the risk of thrombosis in patients. Furthermore, our results found that the increase in APTT and TT were negatively correlated with the level of CA125, indicating that the higher the level of CA125, the lower the APTT and the PT as well as the higher risk of hypercoagulability and the formation of thrombus in adenomyosis. Our study firstly revealed that CA125 level was negatively related to the level of APTT and PT, and may relate to the hypercoagulable state and even thrombosis in adenomyosis, which is undoubtedly a very clinically significant thing. Therefore, the early detection of coagulation parameters and the level of CA125 may be helpful for the diagnosis, prevention and treatment of the hypercoagulable state and even the thrombosis in adenomyosis. Additionally, we also found that dysmenorrhea and menorrhagia were high-risk factors for adenomyosis. Menorrhagia can cause anemia, further promote the formation of a hypercoagulable state, and may eventually promote thrombosis.6 These indicated that adenomyosis patients with dysmenorrhea and menorrhagia have a higher risk of thrombosis.
Although a study illustrated that CA125 was positively correlated with FIB and D-dimer in uterine non-bleeding periods and hypercoagulability may be related to enlarged uterine volume, increased serum CA125 and anemia.9 Our results also revealed that CA125 was correlated with hypercoagulability, but was negatively correlated with the level of APTT and TT, and there were not statistically significant between coagulation indicators and anemia as well as uterine volume, which was partly different from that study.9 This maybe attribute to the differences in patients’ baseline characteristics, such as age and BMI, and the differences of research, including uterine non-bleeding periods or bleeding periods, differences in blood sample detection methods, and some other factors. Because we collected preoperative peripheral blood samples on the day before surgery from all patients who were hospitalized and underwent surgery during the non-menstrual period and performed comparative analysis of three groups, which was different from that study. Therefore, we need further stratified analysis, expanded sample size, and even multi-center studies to explore the relationship between anemia, uterine volume, CA125 level and coagulation parameters. Additionally, since this is a retrospective study, some data may affect coagulation parameters, such as menstrual coagulation parameters, adenomyosis type, and type of uterine leiomyoma, which cannot be obtained. Moreover, we only collect data from patients in non-menstrual period with adenomyosis, uterine leiomyoma, and CIN-III in our hospital from 2018 to 2022. Therefore, multi-center, larger-sample studies will be needed in the future, including more factors affecting coagulation parameters and hypercoagulable status, to further confirm the results.
Conclusion
In summary, our results showed that the APTT and TT decreased in women with adenomyosis and negatively correlated with the level of CA125, which may be associated with hypercoagulability and even thrombosis in adenomyosis. And dysmenorrhea and menorrhagia were high-risk factors for adenomyosis. These indicate that for adenomyosis patients with high CA125 levels, dysmenorrhea and menorrhagia, coagulation parameters should be detected in time and early, and closely monitored so that we can detect the hypercoagulability in patients with adenomyosis as soon as possible, and prevent the thrombosis. However, there are some other factors that may affect the coagulation parameters in adenomyosis. Therefore, multi-center, larger-sample studies will be needed in the future.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure
The authors declare that there is no conflict of interest.
References
1. Bulun SE, Yildiz S, Adli M, Wei JJ. Adenomyosis pathogenesis: insights from next-generation sequencing. Human Reprod Update. 2021;27(6):1086–1097. doi:10.1093/humupd/dmab017
2. Donnez J, Donnez O, Dolmans MM. Introduction: uterine adenomyosis, another enigmatic disease of our time. Fertil Sterility. 2018;109(3):369–370. doi:10.1016/j.fertnstert.2018.01.035
3. Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23(2):164–185. doi:10.1016/j.jmig.2015.09.018
4. Gordts S, Grimbizis G, Campo R. Symptoms and classification of uterine adenomyosis, including the place of hysteroscopy in diagnosis. Fertil Sterility. 2018;109(3):380–388.e381. doi:10.1016/j.fertnstert.2018.01.006
5. Yamanaka A, Kimura F, Yoshida T, et al. Dysfunctional coagulation and fibrinolysis systems due to adenomyosis is a possible cause of thrombosis and menorrhagia. Eur J Obstet Gynecol Reprod Biol. 2016;204:99–103. doi:10.1016/j.ejogrb.2016.07.499
6. Lin Q, Li T, Ding S, Yu Q, Zhang X. Anemia-associated platelets and plasma prothrombin time increase in patients with adenomyosis. J Clin Med. 2022;11(15):4382. doi:10.3390/jcm11154382
7. Guo SW. The role of platelets in the pathogenesis and pathophysiology of adenomyosis. J Clin Med. 2023;12(3). doi:10.3390/jcm12030842
8. Ding S, Lin Q, Zhu T, et al. Is there a correlation between inflammatory markers and coagulation parameters in women with advanced ovarian endometriosis? BMC Womens Health. 2019;19(1):169. doi:10.1186/s12905-019-0860-9
9. Zhang HY, Wang AQ, Zhu S, et al. 子宫腺肌症患者凝血功能的变化 [Changes of coagulation function in patients with adenomyosis]. Zhonghua Fu Chan Ke Za Zhi. 2022;57(3):179–189. Chinese. doi:10.3760/cma.j.cn112141-20211229-00759
10. Yin X, Wu J, Song S, Zhang B, Chen Y. Cerebral infarcts associated with adenomyosis: a rare risk factor for stroke in middle-aged women: a case series. BMC Neurol. 2018;18(1):213. doi:10.1186/s12883-018-1213-2
11. Uchino K, Shimizu T, Mizukami H, et al. Nonbacterial thrombotic endocarditis complicated by cerebral infarction in a patient with adenomyosis with high serum CA125 level; a case report. J Stroke Cerebrovasc Dis. 2018;27(3):e42–e45. doi:10.1016/j.jstrokecerebrovasdis.2017.09.064
12. Aiura R, Nakayama S, Yamaga H, Kato Y, Fujishima H. Systemic thromboembolism including multiple cerebral infarctions with middle cerebral artery occlusion caused by the progression of adenomyosis with benign gynecological tumor: a case report. BMC Neurol. 2021;21(1):14. doi:10.1186/s12883-021-02045-7
13. Akira S, Iwasaki N, Ichikawa M, et al. Successful long-term management of adenomyosis associated with deep thrombosis by low-dose gonadotropin-releasing hormone agonist therapy. Clin Exp Obstet Gynecol. 2009;36(2):123–125.
14. Son J, Lee DW, Seong EY, et al. Acute kidney injury due to menstruation-related disseminated intravascular coagulation in an adenomyosis patient: a case report. J Korean Med Sci. 2010;25(9):1372–1374. doi:10.3346/jkms.2010.25.9.1372
15. Zhou Q, Zhu C, Shen Z, et al. Incidence and potential predictors of thromboembolic events in epithelial ovarian carcinoma patients during perioperative period. Eur J Surg Oncol. 2020;46(5):855–861. doi:10.1016/j.ejso.2020.01.026
16. Kil K, Chung J, Pak H, et al. Usefulness of CA125 in the differential diagnosis of uterine adenomyosis and myoma. Eur J Obstet Gynecol Reprod Biol. 2015;185:131–135. doi:10.1016/j.ejogrb.2014.12.008
17. Tang Y, Ming-Tao Y, Xiang R, et al. Preoperative CA125 as a risk factor for symptom recurrence of adenomyosis after ultrasound-guided high-intensity focused ultrasound ablation surgery. Int j Hyperthermia. 2022;39(1):1164–1169. doi:10.1080/02656736.2022.2107716
18. Jiang C, Liu C, Guo J, et al. CA125 modified by PLT and NLR improves the predictive accuracy of adenomyosis-derived pelvic dense adhesion. Medicine. 2017;96(19):e6880.
19. Kannel WB. Oral contraceptive hypertension and thromboembolism. Int J Obstet Gynaecol. 1978;16(6):466–472. doi:10.1002/j.1879-3479.1979.tb00951.x
20. Lim HS, MacFadyen RJ, Bakris G, Lip GY. The role of hyperglycaemia and the hypercoagulable state in the pathogenesis of cardiovascular events in diabetes mellitus: implications for hypertension management. Curr Pharm Des. 2006;12(13):1567–1579. doi:10.2174/138161206776843322
21. Jovin T, Boosupalli V, Zivkovic S, Wechsler L, Gebel JJN. High titers of CA-125 may be associated with recurrent ischemic strokes in patients with cancer. Neurology. 2005;64(11):1944–1945. doi:10.1212/01.WNL.0000163850.07976.63
22. Korte W, Clarke S, Lefkowitz JB. Short activated partial thromboplastin times are related to increased thrombin generation and an increased risk for thromboembolism. Am J Clin Pathol. 2000;113(1):123–127. doi:10.1309/G98J-ANA9-RMNC-XLYU
23. Mina A, Favaloro EJ, Mohammed S, Koutts J. A laboratory evaluation into the short activated partial thromboplastin time. Blood Coagul Fibrinolysis. 2010;21(2):152–157. doi:10.1097/MBC.0b013e3283365770
24. Mina A, Favaloro EJ, Koutts J. Relationship between short activated partial thromboplastin times, thrombin generation, procoagulant factors and procoagulant phospholipid activity. Blood Coagul Fibrinolysis. 2012;23(3):203–207. doi:10.1097/MBC.0b013e32834fa7d6
25. Tripodi A, Chantarangkul V, Martinelli I, Bucciarelli P, Mannucci PM. A shortened activated partial thromboplastin time is associated with the risk of venous thromboembolism. Blood. 2004;104(12):3631–3634. doi:10.1182/blood-2004-03-1042
26. Tanaka K, Terada R, Butt A, Mazzeffi M, McNeil JJA. Factor VIII: a dynamic modulator of hemostasis and thrombosis in Trauma. Anesthesia Analg. 2023;136(5):894–904. doi:10.1213/ANE.0000000000006356
27. Zwaginga JJ, Koomans HA, Sixma JJ, Rabelink TJ. Thrombus formation and platelet-vessel wall interaction in the nephrotic syndrome under flow conditions. J Clin Invest. 1994;93(1):204–211. doi:10.1172/JCI116947
28. Zhang Q, Duan J, Liu X, Guo SW. Platelets drive smooth muscle metaplasia and fibrogenesis in endometriosis through epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation. Mol Cell Endocrinol. 2016;428:1–16. doi:10.1016/j.mce.2016.03.015
29. Yang B, Gu N, Shi S, et al. Immunoreactivity of plasminogen activator inhibitor 1 and its correlation with dysmenorrhea and lesional fibrosis in adenomyosis. Reprod Sci. 2021;28(8):2378–2386. doi:10.1007/s43032-021-00513-6
30. Liu X, Guo SW. Aberrant immunoreactivity of deoxyribonucleic acid methyltransferases in adenomyosis. Gynecol Obstet Invest. 2012;74(2):100–108. doi:10.1159/000337718
31. Wada H, Shiraki K, Matsumoto T, et al. A clot waveform analysis of thrombin time using a small amount of thrombin is useful for evaluating the clotting activity of plasma independent of the presence of emicizumab. J Clin Med. 2022;11(20):6142. doi:10.3390/jcm11206142
32. Wada H, Ichikawa Y, Ezaki M, et al. The reevaluation of thrombin time using a clot waveform analysis. J Clin Med. 2021;10(21):4840. doi:10.3390/jcm10214840
33. Byrnes JR, Wolberg AS. Red blood cells in thrombosis. Blood. 2017;130(16):1795–1799. doi:10.1182/blood-2017-03-745349
34. Faes C, Ilich A, Sotiaux A, et al. Red blood cells modulate structure and dynamics of venous clot formation in sickle cell disease. Blood. 2019;133(23):2529–2541. doi:10.1182/blood.2019000424
35. Ling X, Wang T. Diagnostic and prognostic value of coagulation-related factors in endometriosis. Am J Transl Res. 2022;14(11):7924–7931.
36. Ding D, Liu X, Guo S. Further evidence for hypercoagulability in women with ovarian endometriomas. Reprod Sci. 2018;25(11):1540–1548. doi:10.1177/1933719118799195
37. Viganò P, Ottolina J, Sarais V, Rebonato G, Somigliana E, Candiani M. Coagulation status in women with endometriosis. Reprod Sci. 2018;25(4):559–565. doi:10.1177/1933719117718273
38. Shao B, Wahrenbrock MG, Yao L, et al. Carcinoma mucins trigger reciprocal activation of platelets and neutrophils in a murine model of trousseau syndrome. Blood. 2011;118(15):4015–4023. doi:10.1182/blood-2011-07-368514
39. Akaishi T, Kuroda H, Tateyama M, et al. Recurrent cerebral infarction synchronous with menorrhagia caused by endometrial stromal sarcoma. J Neurol Sci. 2015;358(1–2):509–511. doi:10.1016/j.jns.2015.09.367
40. Yan Y, Zhang X, Zhong D, Wang A, Wu S, Wu B. Adenomyosis-associated ischemic stroke: pathophysiology, detection and management. Brain Sci. 2022;12(10):1410. doi:10.3390/brainsci12101410
41. Li B, Shi K, Jing C, et al. Successful management of cerebral venous sinus thrombosis due to adenomyosis: case reports and literature review. Clin Neurol Neurosurg. 2023;229:107726. doi:10.1016/j.clineuro.2023.107726
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.