Back to Journals » International Journal of Nanomedicine » Volume 19
Bladder Cancer in Exosomal Perspective: Unraveling New Regulatory Mechanisms
Authors Yin C, Liufu C , Zhu T, Ye S, Jiang J, Wang M, Wang Y , Shi B
Received 7 January 2024
Accepted for publication 6 April 2024
Published 22 April 2024 Volume 2024:19 Pages 3677—3695
DOI https://doi.org/10.2147/IJN.S458397
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Eng San Thian
Cong Yin,1,2,* Cen Liufu,3,4,* Tao Zhu,3,4 Shuai Ye,1,2 Jiahao Jiang,1,5 Mingxia Wang,3 Yan Wang,3 Bentao Shi1
1Department of Urology, the First Affiliated Hospital of Shenzhen University, Shenzhen Second People’s Hospital, Shenzhen, People’s Republic of China; 2Shenzhen University Health Science Center, Shenzhen, People’s Republic of China; 3Department of Urology, Peking University Shenzhen Hospital, Institute of Urology, Shenzhen PKU-HKUST Medical Center, Shenzhen, People’s Republic of China; 4Shantou University Medical College, Shantou, Guangdong, People’s Republic of China; 5Clinical College of Anhui Medical University, Shenzhen, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Bentao Shi, Department of Urology, the First Affiliated Hospital of Shenzhen University, Shenzhen Second People’s Hospital, Shenzhen, 518035, People’s Republic of China, Email [email protected] Yan Wang, Department of Urology, Peking University Shenzhen Hospital, Institute of Urology, Shenzhen PKU-HKUST Medical Center Shenzhen, Shenzhen, 518036, People’s Republic of China, Email [email protected]
Abstract: Bladder cancer, a prevalent malignant neoplasm of the urinary tract, exhibits escalating morbidity and mortality rates. Current diagnosis standards rely on invasive and costly cystoscopy and histopathology, underscoring the urgency for non-invasive, high-throughput, and cost-effective novel diagnostic techniques to ensure timely detection and standardized treatment. Recent years have witnessed the rise of exosome research in bladder cancer studies. Exosomes contain abundant bioactive molecules that can help elucidate the intricate mechanisms underlying bladder cancer pathogenesis and metastasis. Exosomes hold potential as biomarkers for early bladder cancer diagnosis while also serving as targeted drug delivery vehicles to enhance treatment efficacy and mitigate adverse effects. Furthermore, exosome analyses offer insights into the complex molecular signaling networks implicated in bladder cancer progression, revealing novel therapeutic targets. This review provides a comprehensive overview of prevalent exosome isolation techniques and highlights the promising clinical utility of exosomes in both diagnostic and therapeutic applications in bladder cancer management.
Keywords: exosomes, isolation methods, bladder cancer, biomarkers, treatment
Introduction
Bladder cancer (BCa), originating from the bladder’s epithelial lining, ranks as the 10th most prevalent malignancy globally, according to the GLOBOCAN 2018 estimates. Approximately 570,000 new BCa cases were reported worldwide that year, with Europe and North America exhibiting the highest incidence rates.1 Early detection and intervention of non-muscle invasive BCa are critical for prognosis, as muscle invasion exacerbates outcomes, and chemoresistance heightens recurrence rates while reducing survival.2 Despite its significance, BCa’s early symptoms are often subtle, leading to missed diagnostic and therapeutic opportunities. Moreover, urine obscures bladder lesions, complicating clinical evaluation. While beneficial for early detection, conventional diagnostic cystoscopy and cytology exhibit limited sensitivity and specificity.3,4 Notably, cystoscopy, as an invasive procedure, carries risks of complications, such as stricture, perforation, bleeding, and infection. Therefore, developing more precise, non-invasive BCa diagnostics is an urgent need. Transurethral resection serves as the optimal treatment for early-stage, non-invasive BCa. However, high-grade or metastatic disease often renders chemotherapy and radiotherapy ineffective, leading to adverse effects such as nausea, vomiting, and fatigue.5
Within the current clinical landscape, exosome research has garnered widespread interest for its promising applications in disease diagnosis and treatment. Exosomes are small extracellular vesicles secreted by cells that carry diverse biomolecular cargoes, including proteins, nucleic acids, lipids, and metabolites.6,7 As intercellular messengers, exosomes facilitate the transfer of these molecular components between cells, regulating recipient cell behavior and function under both homeostatic and pathological conditions. Owing to their abundance in biomarkers reflective of parental cell status, exosomes demonstrate significant potential as minimally invasive tools for early disease detection, prognostic stratification, and treatment monitoring. Moreover, the innate encapsulation properties of exosomes, protecting their contents from degradation, have spurred interest in their development as targeted drug delivery systems. The shielded transfer of therapeutics via exosomes to specific tissues or organs may enhance localized drug efficacy while mitigating adverse toxicities. This favorable characteristic has propelled exosomes to the forefront of cancer research.
Wang et al provide an overview of exosomes in BCa, including their biological functions and cutting-edge progress in drug delivery applications.8 The article delves into the precise role of exosomes in BCa and their potential applications, further discussing the isolation techniques, key roles in BCa, and potential advantages in early diagnosis and drug delivery. These comprehensive analyses aim to offer profound insights to researchers and clinicians, making exosomes a potent tool in treating BCa.
Origin of Exosomes and Methods of Isolation
Mechanisms and Biologic Functions of Exosomes
Exosomes, small extracellular vesicles ranging from 30–150 nm in diameter, encapsulate a diverse array of functional biomolecules, including proteins, RNA, DNA, lipids, and metabolites. Originating from multivesicular bodies (MVBs), exosomes are characterized by classical exosomal markers such as CD9, CD81, and CD63 (Table 1). The biogenesis and secretion of exosomes involves a multistep process that first involves the formation of depressions in specific regions of the cell membrane that subsequently encapsulate extracellular material or components of the cell membrane itself to form early endosomes. These early endosomes then undergo a series of biochemical and morphological changes to evolve into late endosomes or multivesicular bodies. During the formation of MVBs, the inner membrane of late endosomes protrudes towards the interior of the vesicle to form multiple intraluminal vesicles (ILVs). This critical step is mainly regulated by the endosomal sorting complexes required for transport (ESCRT)-mediated mechanism, which is responsible for sorting molecules such as proteins, lipids, and RNAs into ILVs, which ultimately become the main body of exosomes.9,10 A critical final stage is the secretion of exosomes from cells, which determines the efficiency of exosomal release. The primary mechanism of exosome secretion occurs through a fusion of MVBs with the plasma membrane, followed by extracellular transport via cytosolization (Figure 1).11,12 However, MVBs may also traffic to lysosomes for degradation through alternate pathways.13 Exosome exocytosis naturally occurs in various biological fluids, including pleural fluid, ascites, amniotic fluid, saliva, blood, urine, and cerebrospinal fluid.14–19
 |
Table 1 Difference Between Exosomes and Microvesicles |
Upon secretion, exosomes interact with neighboring or distant cells through circulation, facilitating cargo transfer to modulate gene expression and signaling pathways in target cells. Extensive evidence indicates that exosomes play essential roles in numerous physiological and pathological processes by mediating intercellular communication. For instance, exosomal microRNAs regulate stem cell differentiation and maintain tissue homeostasis. Moreover, exosomes derived from mesenchymal stem cells promote tissue regeneration by transferring pro-angiogenic factors. In cancer research, tumor cell-derived exosomes reprogram bone marrow progenitor cells to establish pre-metastatic niches. While the mechanisms underlying exosome biogenesis and cargo sorting remain incompletely understood, it is clear that exosomes serve as essential intercellular messengers. Further elucidating these processes will enhance our comprehension of exosomal signaling in health and disease, potentially enabling the development of novel diagnostics and therapeutics.20,21
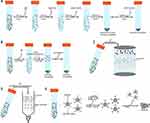
Techniques for Exosome Isolation
Currently, five primary methods of exosome isolation are prevalently utilized (Figure 2). These encompass ultracentrifugation and sedimentation techniques, ultrafiltration, color-blocking exclusion, and immunoaffinity capture, each presenting its own set of unique benefits and drawbacks (Table 2). We give a comprehensive perspective on the existing strategies for exosome isolation by examining the underlying principles, procedures, pros, and cons of each approach.
 |
Table 2 Comparison of Different Isolation Methods for Exosomes |
Ultracentrifugation - The Gold Standard for Exosome Separation
The ultracentrifugation technique is one of the most frequently employed methods for isolating exosomes.22–24 This method involves several stages: first, cells are removed by centrifuging at 300 xg for 10 minutes; then, dead cells are eliminated through 10-minute centrifugation at 2000 xg; subsequent, cellular debris is discarded via centrifugation at 10,000 xg for 30 minutes; following this, exosomes are precipitated and washed through 70-minute centrifugation at 100,000 xg; finally, the exosome precipitates are collected through another round of 70-minute centrifugation at 100,000 xg2.
Polyethylene Glycol (PEG) Sedimentation Method
Previously employed for virus particle isolation, the PEG sedimentation method relies on weak interactions that facilitate the formation of aggregates between PEG and exosomes. By inducing a change in the polymer’s solubility at 4°C, the target particles precipitate along with the polymer. Consequently, exosomes can be centrifuged to the bottom of the centrifuge tube, enabling their separation from the supernatant.25 Although this method yields a high quantity and is operationally straightforward, its major drawback is the compromised purity it offers. Additional purification steps or alternative isolation methods must be considered to obtain purer exosome samples. Selecting the appropriate isolation method should be based on the specific research goals and requirements to ensure optimal results.
Ultrafiltration - A Prospect for Large-Scale Industrialization
Ultrafiltration, a widely used technique for exosome separation, operates on the principle of membrane filtration. Utilizing filters equipped with nano- or micro-pores, this method selectively traps particles larger than a defined pore size, allowing smaller particles to pass into the filtrate. One of the key advantages of this method is its simplicity and speed, typically completing the process in less than an hour.26 Additionally, it does not require advanced equipment and has no volume limitations, making it suitable for processing large samples. However, it’s worth noting that the ultrafiltration method may result in the co-separation of contaminants similar in size to the exosomes, potentially compromising purity.
Exclusion Chromatography - Mitigating Damage During Separation
Exclusion chromatography, a technique that separates components based on molecular size and shape, involves loading the sample onto the stationary phase and eluting it through the mobile phase. Exosomes, typically small in size (30–150 nm), traverse through the pores of the stationary phase while larger cellular debris and proteins are retained. This method can be implemented using a variety of stationary phases, including gel filtration chromatography and size exclusion chromatography. Gel filtration chromatography uses gels with diverse pore sizes to retain larger particles, enabling smaller exosomes to enter the mobile phase. In contrast, size exclusion chromatography employs porous materials, selecting the suitable pore size for exosome separation. Combined with other methods, this technique can yield highly purified exosomes while reducing impurity proteins. Unlike centrifugation and ultrafiltration, exclusion chromatography employs passive gravitational flow, optimizing the preservation of the biological functions of exosomes.27 However, it is important to note that this method has limitations, such as extended separation time, significant sample dilution, limited separation volume, and low throughput. Processing one sample at a time makes it unsuitable for high-throughput applications.
Affinity Capture - Isolating High-Concentration Exosomes
Affinity capture hinges on the specific interaction between a target molecule and its corresponding ligand. Initially, ligands that specifically bind to exosome surface molecules are selected. These ligands could be antibodies, receptors, and affinity tags. For instance, specific antibodies can capture distinct proteins on the exosome surface. Subsequently, the ligand is immobilized on a stationary phase, such as magnetic beads, gels, or porous materials.28 Affinity capture techniques effectively trap target molecules using stationary phases with ample surface area and affinity. Once the sample contacts the stationary phase, the target molecule binds specifically to the ligand. This is followed by an elution step to remove non-specifically bound components. The target molecules in the eluate can then undergo qualitative and quantitative analysis, employing methods like electrophoresis, mass spectrometry, and immunoblotting. The success of the affinity capture method relies on selecting suitable ligands and stationary phases, along with proper optimization and validation. However, this method may be susceptible to non-specific binding and background noise, necessitating appropriate control experiments and optimization steps to ensure the accuracy and purity of the separation and enrichment.29
As scientific research progresses, the exploration of cell line supernatants has broadened to encompass the analysis of various body fluids, including blood, milk, urine, and cerebrospinal fluid. While the fundamental approach to exosome extraction from these fluids mirrors that from cell culture supernatants, the specifics and optimization strategies in practice must be tailored based on sample handling, protein concentration, extraction efficacy, and exosome quality to accommodate diverse biological samples and research objectives.9,30
Regarding precise pre-treatment procedures, for serum samples, it is advisable to draw blood in the morning on an empty stomach and promptly place the blood into EDTA-free tubes. By vortexing or flipping the tube 5 to 10 times, the supernatant is then allowed to clot naturally by resting at room temperature (22°C to 25°C) for 30 minutes or at 4°C for 3 to 4 hours before being moved to a new centrifuge tube. For urinary exosome collection, the first-morning urine (approximately 50 mL) should be collected, ensuring to avoid bacterial contamination and managing diet before collection, notably by refraining from eating or drinking after 22:00 the night before, to maintain sample integrity. Sample purity is vital for exosomal proteomics research. Currently, iodixanol-based density gradient centrifugation is regarded as the most effective purification technique, with differential centrifugation and exclusion chromatography as alternatives.31 For exosomal RNA research, several established exosome isolation kits, like the exoRNeasy kit, offer a practical and efficient method for exosomal RNA isolation.32 Overall, the selection of a suitable exosome isolation method requires a comprehensive consideration of the specific needs of the sample type, volume, viscosity, and desired downstream applications (including protein, small RNA, lncRNA, exosomes subpopulation studies, etc.) to ensure the accuracy and reliability of the study.
Role of Exosomes in Intercellular Communication and Tumor Development
The pivotal role of exosomes in intercellular communication and their significant regulatory influence on physiological and pathological processes are illustrated in this article. Since the first documentation of erythrocytes secreting transferrin receptors via vesicles in 1983,13 the array of substances transported by exosomes has broadened to encompass complex proteins, RNAs, and signaling lipids.33–35 Previous studies have shown in several dimensions that these carryover substances regulate physiological processes, such as cell proliferation, differentiation, and metabolism, by transferring information between cells via exosomes.36–41
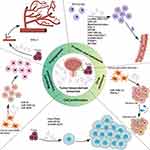
In recent years, exosomes have garnered substantial attention in the field of oncology. Research has highlighted the instrumental role of exosomes derived from cancer cells in tumor growth, progression, immune evasion, angiogenesis, and drug resistance (Figure 3). In comparison to normal cells, cancer cells expel a larger quantity of exosomes, establishing them as integral component of the tumor microenvironment. These exosomes participate in numerous processes related to tumor growth, invasion, and metastasis. For instance, in pancreatic cancer, elevated expression levels of miR-1246, miR-4644, miR-3976, and miR-4306 have been observed in patients’ serum, whereas these miRNAs are less expressed in healthy individuals.42 Similarly, research on breast cancer has disclosed that exosome-carried miR-105 compromises intercellular tight junctions by interfering with tight junction protein 1 (ZO-1). This reduces the barriers’ resistance to cancer cell metastasis. In highly metastatic tumors, the suppression of miR-105 expression mitigates this effect.43 Furthermore, exosomes play a crucial role in enabling cancer cells to evade immune surveillance by carrying immunosuppressive molecules such as FasL and TRAIL, diminishing immune cells activity and promoting tumor growth.44
Beyond communication between cancer cells, exosomes mediate interactions between cancer cells and surrounding stromal cells, shaping a microenvironment conducive to tumor growth. Specifically, tumor cell-derived exosomes carrying vascular endothelial growth factor may play an instrumental role in tumor angiogenesis, contributing to a microenvironment that encourages pre-metastasis of tumor cells.45 Moreover, exosome proteases, such as metalloproteinases, can degrade the extracellular matrix, facilitating tumor cells in breaching tissue barriers and promoting invasion.46,47 Conversely, cancer cells can alter their fate by taking up exosomes released by stromal cells. Recent findings suggest that breast cancer cells can stimulate stromal cells to release RN7SL1-bearing exosomes, which could enhance tumor growth, metastasis, and drug resistance upon uptake by breast cancer cells.48
Interestingly, exosomes function as couriers, directly influencing tumor cells as information sources. For instance, the interaction of exosomes with pancreatic cancer cells can decrease the expression of Notch-1 target genes in the nucleus, inhibiting the Notch-1-dependent cell survival pathway and ultimately triggering apoptosis in pancreatic cancer cells.49 However, in other types of cancers, such as gliomas, exosome mRNAs are translated and expressed upon entering the nucleus, stimulating cell proliferation and contributing to tumor growth. The role of exosomes in either promoting proliferation or inducing apoptosis in tumor cells remains unclear, necessitating further investigation.50
In the BCa microenvironment, exosomes serve as important mediators of information transfer, and attention should also be paid to macrophages, especially tumor-associated macrophages (TAMs). These macrophages predominantly exhibit the M2 phenotype and represent a significant proportion of immune cells. Studies have shown that in certain Bcas, the proportion of these cells can be as high as 30% to 50%.51 The ratio and activity of TAMs may be collectively influenced by the tumor’s biological characteristics, the patient’s immune status, and other factors in the microenvironment. In this context, the interaction between TAMs and tumor cells, especially through extracellular vesicle-mediated intercellular communication, plays a significant role in the development of tumors. These exosomes not only regulate the biological behavior of tumor cells but also influence the polarization state of TAMs, thereby playing a key role in promoting or inhibiting tumor progression.52
In recent years, research has gradually revealed the role of exosomes derived from TAMs in aspects such as tumor growth, invasion, metastasis, angiogenesis, immune response, and chemotherapy resistance. For example, Yin53 found that LncRNA SBF2-AS1 is enriched in the exosomes of M2 macrophages and can be absorbed by pancreatic cancer cells PANC-1, thereby promoting proliferation, invasion, and migration. Additionally, the high expression of LncRNA SBF2-AS1 is associated with poor prognosis in Bca, participates in the polarization of M2 macrophages, and drives tumor growth.54 These findings underscore the importance of research based on Bca macrophage-derived exosomes particularly the further exploration of LncRNA SBF2-AS1. Furthermore, miR-29a-3p from tumor macrophage-derived exosomes can target FOXO3 and inhibit GSK3β activity through the AKT/GSK3β pathway in ovarian cancer cells, enhancing PD-L1 expression, helping tumor cells evade the immune system, and further leading to poor prognosis. This discovery reveals the role of TAM-derived exosomes in tumor immune evasion mechanisms.55 It is worth noting that although much research focuses on the role of TAM-derived exosomes in promoting tumor progression, there is evidence that they can inhibit tumor progression under certain conditions. Specifically, exosomes from M1 macrophages, when combined with the chemotherapy drug gemcitabine, can exert synergistic cytotoxic effects, induce inflammatory damage to Bca cells, and significantly inhibit tumor growth.56 This finding provides new insights for developing novel treatment strategies, especially in leveraging the dual nature of TAMs to combat cancer.
Biological Functions of Exosomes in BCa
Much like other malignancies, the considerable influence of BCa cells on the tumor microenvironment and pivotal tumor processes is manifested through the release of exosomes.2,57 Lladen with diverse protein and RNA molecules. these exosomes not only disrupt communication among cancer cells but also have the potential to disturb the interplay between cancer cells and normal cells. This could potentially induce normal bladder epithelial cells to adopt cancer-like characteristics, fostering tumor formation and progression.58,59 Shielded by membrane vesicles, the protein and RNA molecules within exosomes can evade degradation by external RNA enzymes and proteases in harsh microenvironments. Consequently, when transported to surrounding normal cells through exosomes, these molecules can manipulate biological behaviors such as proliferation, migration, and apoptosis of the recipient cells, thus encouraging tumor growth.60–63
Proteins in Exosomes
Beckham et al64 suggested that exosomes derived from high-grade BCa cells and the urine of patients with high-grade BCa could enhance the angiogenesis and migration of BCa cells and endothelial cells. Silencing EDIL-3 expression in the exosomes resulted in the lost of their angiogenesis and migration-promoting function in uroepithelial and endothelial cells. In addition, levels of EDIL-3 in exosomes purified from the urine of patients with high-grade BCa were significantly higher than those of healthy individuals. The researchers concluded that EDIL-3 operates through the activation of the epidermal growth factor receptor signaling pathway, and inhibiting this pathway could block EDIL-3-induced bladder cell migration. This study suggested the presence of biologically active molecules, such as EDIL-3, in exosomes isolated from the urine of BCa patients. Identifying these components and their associated oncogenic pathways could lead to new therapeutic targets and treatment strategies. In another study, Qiang Song et al found that KRT6B, detected in exosomes of BCa origin, regulates EMT and tumor immunity in BCa.65 This suggests that transferring encapsulated KRT6B to adjacent cells and the surrounding microenvironment could regulate the EMT process and immune response.
Given that exosomes in biological fluids originate from tumor tissues, researchers have found that proteomic analysis of these exosomes effectively identifies potential cancer biomarkers. Surman et al66 used a shotgun nanoLC-MS/MS proteomic approach to analyze the protein content of exosomes released from Bca T-24 cells and normal ureteral epithelial HCV-29 cells in vitro. They also assessed the pro-carcinogenic effects of BCa-derived exosomes on non-invasive cells’ proliferation and migration properties. The study identified 1158 proteins in T-24-derived exosomes, while HCV-29-derived exosomes contained a smaller number of 259 identified proteins. With 938 proteins uniquely present in T-24-derived exosomes, this study suggests their potential use as diagnostic and predictive biomarkers in BCa management.
miRNAs in Exosomes
Cancer is characterized by the loss of intercellular growth inhibition, leading to uncontrolled and continuous cell proliferation.67 Comprehensive studies on exosomal miRNAs have garnered considerable academic interest in recent years. MiRNAs, a category of non-coding RNAs comprising around 22 nucleic acids, suppress gene expression following sequence-specific interactions with the untranslated region (UTR) of corresponding mRNAs. There are stable biomarkers abundantly present in exosomes. Fan Lin et al68 confirmed that BCa cells exhibited elevated levels of miR-21 compared to M0 macrophages. This miRNA, transported via exosomes to M0 macrophage, stimulated STAT3 expression. Inhibiting miR-21 neutralized this effect, fostering a favorable polarized M2 macrophage environment. This insight enhances our comprehension of BCa cell and immune cell interactions. Beyond STAT3 activation, miR-21 remarkably drives cancer progression by activating macrophages’ PI3K/AKT pathway, highlighting its multilevel regulatory function in the immune microenvironment of BCa tissue exosomes and offering new insights for future cancer immunotherapy design.
Gang Shan et al69 has contributed significantly to understanding BCa’s molecular regulatory network, emphasizing the pivotal role of cancer-associated fibroblast (CAF)-derived exosomes in BCa development. Their detailed analysis of miR-148b-3p in exosomes elucidated how this miRNA subtly orchestrates cellular phenotypic changes, driving metastasis, epithelial-mesenchymal transition (EMT) and the development of drug resistance in BCa cells. Exosomal miRNAs also act as cancer suppressors. Jian-Hong Wu’s study70 offered a novel understanding of miR-4792/FOXC1 signaling in BCa development. Exosome-mediated miR-4792 selectively reduces highly expressed FOXC1 level, significantly inhibiting tumor growth by modulating cell growth, aerobic glycolysis, and lactate content. This research enhances our understanding of the molecular-level regulatory mechanisms in BCa.
Similarly, by directly suppressing FZD8 expression, miR-375-3p effectively blocked Wnt/β-catenin pathway signaling and its downstream molecules Cyclin D1 and c-Myc. The effect of miR-375-3p was also evident in the increased expression of caspase 1 and caspase 3, which induced apoptosis in T24 cells.71 Exosome-derived miR-375-3p acts as a repressor in BCa, inhibiting cell proliferation and metastasis, and directing BC cells toward apoptosis. This study validated its potential to inhibit tumor growth through the T24 xenograft mouse model, offering empirical support for future BCa therapeutic strategies.
In serum exosomes, miRNAs gained increasing importance in BCa research. Xinbao Yin et al discovered elevated levels of miR-663b in the plasma exosomes of Bca patients.72 Later, Exosomes containing miR-663b were found to promote cell proliferation and epithelial-mesenchymal transition. Notably, miR-663b acted as a tumor promoter in BC cells by targeting the Ets2 repressor.69 As advancements in detection platform technology continue to unfold, Liang Yan and his team employed a miRNA probe microarray platform to discern eight aberrantly expressed miRNAs in the urinary exosomes of high-grade BCa patients. Their research elucidated that miR-4644 propels the progression of BCa by regulating UBIAD1.73 Collectively, these discoveries underscore the potential of miRNAs in serum exosomes as pivotal diagnostic markers and invaluable resources for exploring the molecular mechanisms underlying BCa and formulating innovative therapeutic strategies.
Exosomal LncRNAs
Research has shown that the role of exosomal components in tumorigenesis and progression extends beyond miRNAs; encapsulated lncRNAs within exosomes also exert significant biological impacts.74 LncRNAs are non-coding RNAs exceeding 200 nucleotides in length.75 They perform essential functions in various life processes, including dosage compensation effects, epigenetic regulation, and cell cycle and differentiation.76–78 Increasingly, studies affirm that exosomes harbor specific lncRNAs intimately linked with the initiation and progression of BCa.79
Urothelial carcinoma-associated 1 (UCA1), an overexpressed lncRNA in BCa tissues, modulates multiple downstream targets or pathways, such as cAMP response element-binding protein (CREB), phosphatidylinositol 3-kinase (PI3K), protein kinase B (AKT), and the Wnt pathway.80–84 Thereby enhancing the proliferation of BCa cells. Intriguingly, UCA1 is also present in exosomes. Research by Xue et al85 demonstrated that exosomal lncRNA-UCA1 was markedly upregulated in the serum of BCa patients compared to healthy controls. A series of rigorous experiments confirmed that exosomal lncRNA-UCA1 fosters the growth and proliferation of BCa cells by modulating the EMT process, particularly in hypoxic conditions. This discovery not only deepens our comprehension of the role of exosomes in BCa development, but also underscores the significance of lncRNA-UCA1 in regulating the tumor microenvironment. Another study discovered that exosomal lncRNA-UCA1 directed the EMT process, migration, and invasion of BCa cells by regulating the hsa-miR-145/ZEB1/2/FSCN1 pathway, revealing the pivotal role of lncRNA-UCA1 in BCa cell behavior, though the molecular mechanism under hypoxic conditions remains not fully elucidated.86
Furthermore, Zheng and colleagues explored PTENP1 in exosomes, identifying it as a promising emerging biomarker for BCa detection.87 Their study unveiled the unique role of exosomes: exosomes originating from normal cells effectively delivered PTENP1 to BCa cells, significantly inhibiting growth and metastasis by inducing cell cycle arrest. This finding emphasizes the crucial role of exosomal PTENP1 in facilitating communication between normal cells and BCa cells during bladder carcinogenesis.
On a related note, lymph node metastasis is a critical process in tumor progression and a vital reference for tumor staging and treatment selection. Previous studies have demonstrated that exosomes not only transfer information between the lymphatic system and BCa, but also have the potential to comprehensively reshape the lymphatic system by transmitting epigenetic and genetic information. Chen et al discovered that tumor-secreted exosomal lncRNAs participate in lymphatic angiogenesis and lymph node metastasis of BCa in a novel, non-VEGF-dependent manner, validated through in vivo and in vitro models.88 Additionally, Zheng et al found that BCYRN1 in exosomes interacted with the VEGF-C/VEGFR3 signaling pathway to enhance the mechanism of BCa-induced lymphatic metastasis synergistically. This innovative finding suggests that BCYRN1 could be a promising therapeutic target to stimulate the therapeutic prospects of BCa.89
To conclude, exosomes hold a critical position in BCa, actively dictating the biological activities of cancer cells and the tumor microenvironment by transporting a broad range of bioactive molecules. These molecules present promising potential as targets and biomarkers for therapeutic interventions and prognostic evaluations of BCa. Nevertheless, the precise mechanisms of action and clinical applications of exosomes in BCa necessitate further investigation and exploration.90
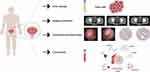
Exosomes as Potential Biomarkers in BCa
Exosomes exhibit immense potential in BCa biomarker research, offering distinct advantages over traditional serological markers. Firstly, exosomal proteins demonstrate enhanced sensitivity. Secondly, given the extensive heterogeneity in tumors, the likelihood of identifying a singular diagnostic biomarker is limited. However, exosomes can be perceived as an amalgamation of multiple potential markers, encompassing potentially oncogenic proteins and mutant DNA and RNA.12 Analyzing proteins within exosomes can help identify novel biomarkers associated with BCa, encompassing aspects like cell proliferation, metastasis, and drug resistance. These markers can mirror the status and functionality of tumor cells, thereby playing a pivotal role in early diagnosis, prognosis evaluation, and personalized treatment of BCa (Figure 4).79,91–93
For noninvasive diagnosis and long-term monitoring, identifying protein biomarkers in urine is crucial. Although the FDA has approved six markers for BCa diagnosis and monitoring, the sensitivity of urine exfoliative cytology remains limited and depends on pathologists’ analytical precision. Consequently, it has yet to be extensively utilized in the urological community.93,94 Exosomes have garnered interest for early-stage disease diagnosis, as exosomal proteins may reflect their cellular origin and aid in cancer detection.95 Joanne L. Welton and colleagues revealed notable differences in the expression of CD36 and CD44 between healthy individuals and BCa patients.96 With the development of extracellular vesicle-based proteomic analysis, significant progress has been made in the field of Bca biomarker screening. While various potential urine biomarkers for Bca have been identified, the focus has been on molecular markers with higher representativeness and diagnostic value (Table 3). For instance, Yi-Ting Chen and colleagues conducted groundbreaking research using iTRAQ-based methods to quantitatively analyze urinary proteins in Bca,97 with APOA1 showing 83.8% sensitivity and 94% specificity for Bca diagnosis. This finding was subsequently validated by Li et al, who further confirmed the validity of APOA1 as a Bca biomarker by analyzing urine samples from 48 Bca patients and 24 healthy controls.98 Additionally, Esteban Orenes-Piñero et al identified Reg-1 as an important biomarker for Bca diagnosis, staging, and prognosis using 2D-PAGE technology.99 In another research direction, the application of miRNA sequencing technology has yielded breakthrough results. Amuran found that miR-19b1-5p, 21–5p, 136–3p, 139–5p, and 210–3p were significantly upregulated in Bca patients’ urine exosomes, distinguishing them from healthy controls with 80% sensitivity and 88% specificity.100 Concurrently, Yazarlou’s study demonstrated the efficient diagnostic capabilities of lncRNA UCA1-201 and lncRNA UCA1-203 in differentiating Bca patients from healthy individuals in urine exosomes.101 Compared to urine extracellular vesicle research, the use of plasma/serum exosomes in Bca studies is relatively limited. This limitation may be related to the high relevance of urine in urological disease research and its lower invasiveness requirements. Furthermore, the broader cellular sources represented by RNA in urine exosomes can effectively mitigate the impact of tumor heterogeneity on a single biopsy point.35
 |
Table 3 Exosomes Serve as Potential Diagnostic Biomarkers for BCa |
Biomarkers serve as invaluable tools in the diagnosis and surveillance of cancer, offering the unique ability for frequent sampling and real-time tracking of the tumor milieu, including microenvironment assessment and metastatic potential.102 The concept of “liquid biopsy” has emerged to facilitate precise, recurrent, and non-invasive sampling. In urine, exosomes secreted by normal and tumor cells, exhibit unique molecular characteristics, positioning them as potential tools for “liquid biopsy”94 Utilizing advanced sequencing technologies for profound molecular property analysis, exosomes derived from blood and urine samples are extensively employed to monitor tumor dynamic evolution. The PredicineBEACON study suggests the potential application of liquid biopsy methods, incorporating blood and urine samples, for individual mutation analysis in diagnostic and therapeutic decision-making processes.103 However, research on BCa exosomes faces challenges related to biomarker consistency, stemming from variations in experimental design, considering the blood and urine environments encompass a multitude of complex solutes, cells, proteins, plasma, and immune components.
Moreover, the presence of exosomes in urine from multiple sources, such as the bladder, kidney, and normal urinary epithelium, may contribute to specific artifactual differences.104 Regardless, exosomes, as representatives of the BCa microenvironment, immune interactions, and cellular communication, hold substantial promise for early diagnosis, treatment, and prognostic evaluation of BCa. With the advancements in histologic technology platforms, we foresee the rapid integration of a new generation of exosome biomarkers into clinical practice.
The Role of Exosomes in BCa Therapy
Presently, a multimodal therapeutic approach is deemed the gold standard in managing BCa, with the selection of which is largely contingent upon the patient’s disease stage. Nevertheless, until now, there is a notable gap in data concerning the employment of exosomes, or extracellular also known as extracellular vesicles (EVs), have been gaining traction in early disease detection and monitoring, attributable to their non-invasive characteristics, thereby emerging as a promising option within the liquid biopsy sector. Their unique biological attributes, including low immunogenicity, specific tissue targeting capabilities, and efficient encapsulation effector molecules, particularly in engineered exosomes, offer superior targeting precision and drug carriage efficiency (Table 4).105–108 This burgeoning field presents diverse strategies worthy of further exploration.
 |
Table 4 Advantages and Disadvantages of Different Drug Delivery Systems |
Interference with Exosome Synthesis and Secretion
The machinery for the production and secretion of exosomes involves key regulators such as heparin-like enzymes, vesicular ATPases, and the Rab family. Heparanase-like enzymes, prevalent in aggressive tumors, facilitate the prolific secretion of exosomes.109 Vesicular ATPases, on the other hand, efficiently catalyze the fusion of multivesicular bodies with the cell membrane for exosome release. Rab27a, a member of the Rab family, when silenced and uncommanded, significantly curtails exosome secretion, thereby impeding tumor metastasis.110
Fortunately, these orchestrators face resistance. Notably, heparanase-like enzymes have adversaries such as PG545 and M402, demonstrating anti-tumor metastatic activity in animal models.111 Utilizing these agents effectively disrupts heparanase-like enzyme function, reducing exosomal secretion and potentially inhibiting BCa progression. Concurrently, inhibitors of vesicular ATPase interfere with exosome release, improving drug resistance.
Modulation of Exosomes by Curcumin
Curcumin, a derivative of the rhizome extract of the Zingiberaceae plant, exhibits anticancer potential across a broad spectrum of recent studies. One of its potential mechanisms of action involves targeting the effects of cancer cell exosomes, which play a pivotal yet detrimental role in fostering cancer proliferation. A study uncovered a unique facet of curcumin: its capacity to package miR-21 into exosomes, yielding an anticancer effect selectively.112 Furthermore, miR-21 has been implicated in numerous stages of tumor progression, including BCa. This discovery lays a robust groundwork for future investigations into curcumin’s therapeutic role in BCa.
Inhibition of Exosome Reception
Inhibiting exosome reception is recognized as an advanced strategy in cancer treatment, possessing potential anti-cancer effects. This approach aims to disrupt the exchange of information between tumor cells and their environment, effectively impeding tumor growth and progression. Upon entry into recipient cells, exosomes bind to the heparan sulfate proteoglycans (HSPG) receptor on the cell surface, facilitating information transfer.113–115 By disrupting this interaction, particularly through the use of heparin, a known competitor for HSPG receptor binding sites, exosome reception can be significantly reduced. This disruption attenuates the activity of tumor cells, positioning heparin as a potent instrument in blocking exosome reception.115
Furthermore, inhibiting exosome reception can impact the immune response within the tumor microenvironment. Exosomes are capable of modulating immune cell function, and may even foster immune evasion by tumors. By disrupting the exosome-mediated immunosuppression mechanism, inhibiting exosome reception could potentially enhance the ability of immune cells to target tumors, thereby improving the efficacy of immunotherapy.
Exosomes as Drug Delivery Vehicles
The field of tumor therapy has seen the emergence of numerous nanodelivery systems, including liposomes, proteins, and recombinant viral vectors.116 However, a shared limitation of these conventional delivery systems is their insufficient ability to penetrate tissues or recipient cells.117 Compared to these traditional drug delivery vehicles, exosomes are rich in various membrane proteins on their surface, some of which are target-specific, such as integrins, selectins, etc. These membrane proteins can bind to corresponding receptors on the surface of tumor cells, thereby achieving targeted recognition and binding of exosomes to tumor cells, offering promising avenues for enhanced tumor therapy (Figure 5).
Significant advancements have been made in the study of engineered exosomes in recent years. For instance, researchers such as Lydia Alvarez-Erviti have successfully used dendritic cells to produce exosomes containing exogenous siRNA targeting BACE1, injecting them intravenously into mice. Results demonstrated that the exosomes could specifically enter neurons in the mouse brain, decreasing BACE1 levels in brain tissues without any non-specific uptake in other tissues. This confirms the potential of exosomes for systemic delivery across the blood-brain barrier.118
Furthermore, the properties of exosome membranes effectively shield endogenous substances from degradation. As a result, various small molecule drugs, including paclitaxel and gemcitabine, have been successfully encapsulated into exosomes for precise transport to target organs and therapeutic effects.119,120 Researchers can also modify exosome properties through engineering methods, tailoring them to different drug delivery modes. The application of this emerging technology offers a novel approach to the in-depth study of BCa pathogenesis, providing new hope for the treatment and prognosis of BCa.
Conclusion and Outlook
Exosomes have emerged as pivotal elements in BCa research, presenting abundant avenues for exploration. Their significance spans from origins and isolation methods to their role in tumor development, with potential applications as biomarkers and drug delivery systems. A deeper understanding of how exosomes influence tumor growth, invasion, and microenvironment regulation could enable precise interventions, thereby advancing therapeutic options for BCa patients.
As potential biomarkers, exosomes could provide a means for early diagnosis and ongoing disease monitoring, potentially improving patient survival rates and quality of life. Moreover, their use as targeted drug delivery systems could enhance the precision and tolerability of treatment, offering new hope to BCa patients.
Exosome have evolved significantly over their nearly 80-year history, transitioning from being perceived as mere “garbage” removal entities to coveted tools for non-invasive diagnostics and drug delivery. However, this is just the beginning. As a new starting point, future research should continue to investigate the mechanisms of exosomes, develop novel detection methods, and enhance drug delivery systems. These efforts will be instrumental in advancing BCa research and treatment, ultimately improving clinical management and therapeutic outcomes for patients.
Acknowledgments
This work was supported by the grant from the Science Technology and Innovation Commission of Shenzhen Municipality (No. JCYJ20220531094217040, No. JCYJ20220531094207017 and No. JCYJ20220530150812027), the Scientific Research Foundation of Peking University Shenzhen Hospital (No. KYQD2023308), the 2022 Hospital Clinical Research Project Fund (No. 20223357025), Shenzhen High-level Hospital Construction Fund and ‘San-ming’ Project of Medicine in Shenzhen (No. SZSM202111007). All the figures were created by Adobe Illustrator.
Disclosure
The authors declare no competing interest in this work.
References
1. Bray FF, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal AJ. Erratum: global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2020;70(4):313. doi:10.3322/caac.21609
2. Nawaz M, Camussi G, Valadi H, et al. The emerging role of extracellular vesicles as biomarkers for urogenital cancers. Nat Rev Urol. 2014;11(12):688–701. doi:10.1038/nrurol.2014.301
3. Grossman HB, Soloway M, Messing E, et al. Surveillance for recurrent bladder cancer using a point-of-care proteomic assay. JAMA. 2006;295(3):299–305. doi:10.1001/jama.295.3.299
4. Lotan Y, Roehrborn CG. Sensitivity and specificity of commonly available bladder tumor markers versus cytology: results of a comprehensive literature review and meta-analyses. Urology. 2003;61(1):109–118. doi:10.1016/S0090-4295(02)02136-2
5. Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med. 2017;376(11):1015–1026. doi:10.1056/NEJMoa1613683
6. He C, Zheng S, Luo Y, Wang B. Exosome Theranostics: biology and Translational Medicine. Theranostics. 2018;8(1):237–255. doi:10.7150/thno.21945
7. Milane L, Singh A, Mattheolabakis G, Suresh M, Amiji MM. Exosome mediated communication within the tumor microenvironment. J Control Release. 2015;219:278–294. doi:10.1016/j.jconrel.2015.06.029
8. Yang Y, Miao L, Lu Y, Sun Y, Wang S. Exosome, the glass slipper for Cinderella of cancer-bladder cancer? J Nanobiotechnology. 2023;21(1):368. doi:10.1186/s12951-023-02130-8
9. Doyle LM, Wang MZ. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells. 2019;8(7):727. doi:10.3390/cells8070727
10. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478). doi:10.1126/science.aau6977
11. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–383. doi:10.1083/jcb.201211138
12. Li A, Zhang T, Zheng M, Liu Y, Chen Z. Exosomal proteins as potential markers of tumor diagnosis. J Hematol Oncol. 2017;10(1):175. doi:10.1186/s13045-017-0542-8
13. Harding C, Heuser J, Stahl P. Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: demonstration of a pathway for receptor shedding. Eur J Cell Biol. 1984;35(2):256–263.
14. Sun Y, Liu S, Qiao Z, et al. Systematic comparison of exosomal proteomes from human saliva and serum for the detection of lung cancer. Anal Chim Acta. 2017;982:84–95. doi:10.1016/j.aca.2017.06.005
15. Zhang X, Bao L, Yu G, Wang H. Exosomal miRNA-profiling of pleural effusion in lung adenocarcinoma and tuberculosis. Front Surg. 2022;9:1050242. doi:10.3389/fsurg.2022.1050242
16. Andre F, Schartz NE, Movassagh M, et al. Malignant effusions and immunogenic tumour-derived exosomes. Lancet. 2002;360(9329):295–305. doi:10.1016/S0140-6736(02)09552-1
17. Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A. 2004;101(36):13368–13373. doi:10.1073/pnas.0403453101
18. Street JM, Barran PE, Mackay CL, et al. Identification and proteomic profiling of exosomes in human cerebrospinal fluid. J Transl Med. 2012;10(1):5. doi:10.1186/1479-5876-10-5
19. Keller S, Rupp C, Stoeck A, et al. CD24 is a marker of exosomes secreted into urine and amniotic fluid. Kidney Int. 2007;72(9):1095–1102. doi:10.1038/sj.ki.5002486
20. Minciacchi VR, Freeman MR, Di Vizio D. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol. 2015;40:41–51. doi:10.1016/j.semcdb.2015.02.010
21. Kalluri R, McAndrews KM. The role of extracellular vesicles in cancer. Cell. 2023;186(8):1610–1626. doi:10.1016/j.cell.2023.03.010
22. Muller L, Hong CS, Stolz DB, Watkins SC, Whiteside TL. Isolation of biologically-active exosomes from human plasma. J Immunol Methods. 2014;411:55–65. doi:10.1016/j.jim.2014.06.007
23. Zarovni N, Corrado A, Guazzi P, et al. Integrated isolation and quantitative analysis of exosome shuttled proteins and nucleic acids using immunocapture approaches. Methods. 2015;87:46–58. doi:10.1016/j.ymeth.2015.05.028
24. Yang D, Zhang W, Zhang H, et al. Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics. Theranostics. 2020;10(8):3684–3707. doi:10.7150/thno.41580
25. Konoshenko MY, Lekchnov EA, Vlassov AV, Laktionov PP. Isolation of Extracellular Vesicles: general Methodologies and Latest Trends. Biomed Res Int. 2018;2018:8545347. doi:10.1155/2018/8545347
26. Yu LL, Zhu J, Liu JX, et al. A Comparison of Traditional and Novel Methods for the Separation of Exosomes from Human Samples. Biomed Res Int. 2018;2018:3634563. doi:10.1155/2018/3634563
27. Gámez-Valero A, Monguió-Tortajada M, Carreras-Planella L, Franquesa M, Beyer K, Borràs FE. Size-Exclusion Chromatography-based isolation minimally alters Extracellular Vesicles’ characteristics compared to precipitating agents. Sci Rep. 2016;6:33641. doi:10.1038/srep33641
28. Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in Exosome Isolation Techniques. Theranostics. 2017;7(3):789–804. doi:10.7150/thno.18133
29. Clayton A, Court J, Navabi H, et al. Analysis of antigen presenting cell derived exosomes, based on immuno-magnetic isolation and flow cytometry. J Immunol Methods. 2001;247(1–2):163–174. doi:10.1016/S0022-1759(00)00321-5
30. Momen-Heravi F, Balaj L, Alian S, et al. Alternative methods for characterization of extracellular vesicles. Front Physiol. 2012;3:354. doi:10.3389/fphys.2012.00354
31. Choi DS, Kim DK, Kim YK, Gho YS. Proteomics of extracellular vesicles: exosomes and ectosomes. Mass Spectrom Rev. 2015;34(4):474–490. doi:10.1002/mas.21420
32. Tang YT, Huang YY, Zheng L, et al. Comparison of isolation methods of exosomes and exosomal RNA from cell culture medium and serum. Int J Mol Med. 2017;40(3):834–844. doi:10.3892/ijmm.2017.3080
33. Tkach M, Théry C. Communication by Extracellular Vesicles: where We Are and Where We Need to Go. Cell. 2016;164(6):1226–1232. doi:10.1016/j.cell.2016.01.043
34. Schey KL, Luther JM, Rose KL. Proteomics characterization of exosome cargo. Methods. 2015;87:75–82. doi:10.1016/j.ymeth.2015.03.018
35. Wang YT, Shi T, Srivastava S, Kagan J, Liu T, Rodland KD. Proteomic Analysis of Exosomes for Discovery of Protein Biomarkers for Prostate and Bladder Cancer. Cancers. 2020;12(9):2335. doi:10.3390/cancers12092335
36. Iraci N, Gaude E, Leonardi T, et al. Extracellular vesicles are independent metabolic units with asparaginase activity. Nat Chem Biol. 2017;13(9):951–955. doi:10.1038/nchembio.2422
37. Li Y, Zheng Q, Bao C, et al. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 2015;25(8):981–984. doi:10.1038/cr.2015.82
38. Yang E, Wang X, Gong Z, Yu M, Wu H, Zhang D. Exosome-mediated metabolic reprogramming: the emerging role in tumor microenvironment remodeling and its influence on cancer progression. Signal Transduct Target Ther. 2020;5(1):242. doi:10.1038/s41392-020-00359-5
39. Marar C, Starich B, Wirtz D. Extracellular vesicles in immunomodulation and tumor progression. Nat Immunol. 2021;22(5):560–570. doi:10.1038/s41590-021-00899-0
40. Kumar B, Garcia M, Weng L, et al. Acute myeloid leukemia transforms the bone marrow niche into a leukemia-permissive microenvironment through exosome secretion. Leukemia. 2018;32(3):575–587. doi:10.1038/leu.2017.259
41. Hu W, Liu C, Bi ZY, et al. Comprehensive landscape of extracellular vesicle-derived RNAs in cancer initiation, progression, metastasis and cancer immunology. Mol Cancer. 2020;19(1):102. doi:10.1186/s12943-020-01199-1
42. Madhavan B, Yue S, Galli U, et al. Combined evaluation of a panel of protein and miRNA serum-exosome biomarkers for pancreatic cancer diagnosis increases sensitivity and specificity. Int, J, Cancer. 2015;136(11):2616–2627. doi:10.1002/ijc.29324
43. Zhou W, Fong MY, Min Y, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25(4):501–515. doi:10.1016/j.ccr.2014.03.007
44. Kugeratski FG, Kalluri R. Exosomes as mediators of immune regulation and immunotherapy in cancer. Febs j. 2021;288(1):10–35. doi:10.1111/febs.15558
45. Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular Vesicles in Cancer: cell-to-Cell Mediators of Metastasis. Cancer Cell. 2016;30(6):836–848. doi:10.1016/j.ccell.2016.10.009
46. Jouida A, O’Callaghan M, Mc Carthy C, Fabre A, Nadarajan P, Keane MP. Exosomes from EGFR-Mutated Adenocarcinoma Induce a Hybrid EMT and MMP9-Dependant Tumor Invasion. Cancers. 2022;14(15):3776. doi:10.3390/cancers14153776
47. Qiu Z, Zhong Z, Zhang Y, Tan H, Deng B, Meng G. Human umbilical cord mesenchymal stem cell-derived exosomal miR-335-5p attenuates the inflammation and tubular epithelial-myofibroblast transdifferentiation of renal tubular epithelial cells by reducing ADAM19 protein levels. Stem Cell Res Ther. 2022;13(1):373. doi:10.1186/s13287-022-03071-z
48. Nabet BY, Qiu Y, Shabason JE, et al. Exosome RNA Unshielding Couples Stromal Activation to Pattern Recognition Receptor Signaling in Cancer. Cell. 2017;170(2):352–366.e313. doi:10.1016/j.cell.2017.06.031
49. Ristorcelli E, Beraud E, Mathieu S, Lombardo D, Verine A. Essential role of Notch signaling in apoptosis of human pancreatic tumoral cells mediated by exosomal nanoparticles. Int, J, Cancer. 2009;125(5):1016–1026. doi:10.1002/ijc.24375
50. Keller S, König AK, Marmé F, et al. Systemic presence and tumor-growth promoting effect of ovarian carcinoma released exosomes. Cancer Lett. 2009;278(1):73–81. doi:10.1016/j.canlet.2008.12.028
51. Franklin RA, Liao W, Sarkar A, et al. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344(6186):921–925. doi:10.1126/science.1252510
52. Chen Q, Li Y, Gao W, Chen L, Xu W, Zhu X. Exosome-Mediated Crosstalk Between Tumor and Tumor-Associated Macrophages. Front Mol Biosci. 2021;8:764222. doi:10.3389/fmolb.2021.764222
53. Yin Z, Zhou Y, Ma T, et al. Down-regulated lncRNA SBF2-AS1 in M2 macrophage-derived exosomes elevates miR-122-5p to restrict XIAP, thereby limiting pancreatic cancer development. J Cell Mol Med. 2020;24(9):5028–5038. doi:10.1111/jcmm.15125
54. Zhang Y, Zhu B, He M, et al. N6-Methylandenosine-Related lncRNAs Predict Prognosis and Immunotherapy Response in Bladder Cancer. Front Oncol. 2021;11:710767. doi:10.3389/fonc.2021.710767
55. Lu L, Ling W, Ruan Z. TAM-derived extracellular vesicles containing microRNA-29a-3p explain the deterioration of ovarian cancer. Mol Ther Nucleic Acids. 2021;25:468–482. doi:10.1016/j.omtn.2021.05.011
56. Tang Z, Tang C, Sun C, Ying X, Shen R. M1 macrophage-derived exosomes synergistically enhance the anti- bladder cancer effect of gemcitabine. Aging (Albany NY). 2022;14(18):7364–7377. doi:10.18632/aging.204200
57. Junker K, Heinzelmann J, Beckham C, Ochiya T, Jenster G. Extracellular Vesicles and Their Role in Urologic Malignancies. Eur Urol. 2016;70(2):323–331. doi:10.1016/j.eururo.2016.02.046
58. Wu CH, Silvers CR, Messing EM, Lee YF. Bladder cancer extracellular vesicles drive tumorigenesis by inducing the unfolded protein response in endoplasmic reticulum of nonmalignant cells. J Biol Chem. 2019;294(9):3207–3218. doi:10.1074/jbc.RA118.006682
59. Ogorevc E, Hudoklin S, Veranič P, Kralj-Iglič V. Extracellular vesicle-mediated transfer of membranous components from the highly malignant T24 urinary carcinoma cell line to the non-malignant RT4 urinary papilloma cell line. Protoplasma. 2014;251(3):699–702. doi:10.1007/s00709-013-0544-5
60. Georgantzoglou N, Pergaris A, Masaoutis C, Theocharis S. Extracellular Vesicles as Biomarkers Carriers in Bladder Cancer: diagnosis, Surveillance, and Treatment. Int J Mol Sci. 2021;22(5):2744. doi:10.3390/ijms22052744
61. Liu YR, Ortiz-Bonilla CJ, Lee YF. Extracellular Vesicles in Bladder Cancer: biomarkers and Beyond. Int J Mol Sci. 2018;19(9). doi:10.3390/ijms19092822
62. Mao W, Wang K, Wu Z, Xu B, Chen M. Current status of research on exosomes in general, and for the diagnosis and treatment of kidney cancer in particular. J Exp Clin Cancer Res. 2021;40(1):305. doi:10.1186/s13046-021-02114-2
63. Wood SL, Knowles MA, Thompson D, Selby PJ, Banks RE. Proteomic studies of urinary biomarkers for prostate, bladder and kidney cancers. Nat Rev Urol. 2013;10(4):206–218. doi:10.1038/nrurol.2013.24
64. Beckham CJ, Olsen J, Yin PN, et al. Bladder cancer exosomes contain EDIL-3/Del1 and facilitate cancer progression. J Urol. 2014;192(2):583–592. doi:10.1016/j.juro.2014.02.035
65. Song Q, Yu H, Cheng Y, et al. Bladder cancer-derived exosomal KRT6B promotes invasion and metastasis by inducing EMT and regulating the immune microenvironment. J Transl Med. 2022;20(1):308. doi:10.1186/s12967-022-03508-2
66. Surman M, Kędracka-Krok S, Jankowska U, Drożdż A, Stępień E, Przybyło M. Proteomic Profiling of Ectosomes Derived from Paired Urothelial Bladder Cancer and Normal Cells Reveals the Presence of Biologically-Relevant Molecules. Int J Mol Sci. 2021;22(13). doi:10.3390/ijms22136816
67. Abercrombie M. Contact inhibition and malignancy. Nature. 1979;281(5729):259–262. doi:10.1038/281259a0
68. Lin F, Yin HB, Li XY, Zhu GM, He WY, Gou X. Bladder cancer cell‑secreted exosomal miR‑21 activates the PI3K/AKT pathway in macrophages to promote cancer progression. Int J Oncol. 2020;56(1):151–164. doi:10.3892/ijo.2019.4933
69. Shan G, Zhou X, Gu J, et al. Downregulated exosomal microRNA-148b-3p in cancer associated fibroblasts enhance chemosensitivity of bladder cancer cells by downregulating the Wnt/β-catenin pathway and upregulating PTEN. Cell Oncol Dordr. 2021;44(1):45–59. doi:10.1007/s13402-020-00500-0
70. Wu JH, Sun KN, Chen ZH, He YJ, Sheng L. Exosome-Mediated miR-4792 Transfer Promotes Bladder Cancer Cell Proliferation via Enhanced FOXC1/c-Myc Signaling and Warburg Effect. J Oncol. 2022;2022:5680353. doi:10.1155/2022/5680353
71. Li Q, Huyan T, Cai S, et al. The role of exosomal miR-375-3p: a potential suppressor in bladder cancer via the Wnt/β-catenin pathway. FASEB j. 2020;34(9):12177–12196. doi:10.1096/fj.202000347R
72. Yin X, Zheng X, Liu M, et al. Exosomal miR-663b targets Ets2-repressor factor to promote proliferation and the epithelial-mesenchymal transition of bladder cancer cells. Cell Biol Int. 2020;44(4):958–965. doi:10.1002/cbin.11292
73. Yan L, Li Q, Sun K, Jiang F. MiR-4644 is upregulated in plasma exosomes of bladder cancer patients and promotes bladder cancer progression by targeting UBIAD1. Am J Transl Res. 2020;12(10):6277–6289.
74. Liu Q. The emerging roles of exosomal long non-coding RNAs in bladder cancer. J Cell Mol Med. 2022;26(4):966–976. doi:10.1111/jcmm.17152
75. Mirzadeh Azad F, Polignano IL, Proserpio V, Oliviero S. Long Noncoding RNAs in Human Stemness and Differentiation. Trends Cell Biol. 2021;31(7):542–555. doi:10.1016/j.tcb.2021.02.002
76. Hu Q, Egranov SD, Lin C, Yang L. Long noncoding RNA loss in immune suppression in cancer. Pharmacol Ther. 2020;213:107591. doi:10.1016/j.pharmthera.2020.107591
77. Huang Z, Zhou JK, Peng Y, He W, Huang C. The role of long noncoding RNAs in hepatocellular carcinoma. Mol Cancer. 2020;19(1):77. doi:10.1186/s12943-020-01188-4
78. Liu K, Gao L, Ma X, et al. Long non-coding RNAs regulate drug resistance in cancer. Mol Cancer. 2020;19(1):54. doi:10.1186/s12943-020-01162-0
79. Tong Y, Liu X, Xia D, et al. Biological Roles and Clinical Significance of Exosome-Derived Noncoding RNAs in Bladder Cancer. Front Oncol. 2021;11:704703. doi:10.3389/fonc.2021.704703
80. Fan Y, Shen B, Tan M, et al. Long non-coding RNA UCA1 increases chemoresistance of bladder cancer cells by regulating Wnt signaling. Febs j. 2014;281(7):1750–1758. doi:10.1111/febs.12737
81. Pan J, Li X, Wu W, et al. Long non-coding RNA UCA1 promotes cisplatin/gemcitabine resistance through CREB modulating miR-196a-5p in bladder cancer cells. Cancer Lett. 2016;382(1):64–76. doi:10.1016/j.canlet.2016.08.015
82. Wang X, Gao Z, Liao J, et al. lncRNA UCA1 inhibits esophageal squamous-cell carcinoma growth by regulating the Wnt signaling pathway. J Toxicol Environ Health A. 2016;79(9–10):407–418. doi:10.1080/15287394.2016.1176617
83. Wu W, Zhang S, Li X, Xue M, Cao S, Chen W. Ets-2 regulates cell apoptosis via the Akt pathway, through the regulation of urothelial cancer associated 1, a long non-coding RNA, in bladder cancer cells. PLoS One. 2013;8(9):e73920. doi:10.1371/journal.pone.0073920
84. Yang C, Li X, Wang Y, Zhao L, Chen W. Long non-coding RNA UCA1 regulated cell cycle distribution via CREB through PI3-K dependent pathway in bladder carcinoma cells. Gene. 2012;496(1):8–16. doi:10.1016/j.gene.2012.01.012
85. Xue M, Chen W, Xiang A, et al. Hypoxic exosomes facilitate bladder tumor growth and development through transferring long non-coding RNA-UCA1. Mol Cancer. 2017;16(1):143. doi:10.1186/s12943-017-0714-8
86. Xue M, Pang H, Li X, Li H, Pan J, Chen W. Long non-coding RNA urothelial cancer-associated 1 promotes bladder cancer cell migration and invasion by way of the hsa-miR-145-ZEB1/2-FSCN1 pathway. Cancer Sci. 2016;107(1):18–27. doi:10.1111/cas.12844
87. Zheng R, Du M, Wang X, et al. Exosome-transmitted long non-coding RNA PTENP1 suppresses bladder cancer progression. Mol Cancer. 2018;17(1):143. doi:10.1186/s12943-018-0880-3
88. Chen C, Luo Y, He W, et al. Exosomal long noncoding RNA LNMAT2 promotes lymphatic metastasis in bladder cancer. J Clin Invest. 2020;130(1):404–421. doi:10.1172/JCI130892
89. Zheng H, Chen C, Luo Y, et al. Tumor-derived exosomal BCYRN1 activates WNT5A/VEGF-C/VEGFR3 feedforward loop to drive lymphatic metastasis of bladder cancer. Clin Transl Med. 2021;11(7):e497. doi:10.1002/ctm2.497
90. Greening DW, Gopal SK, Xu R, Simpson RJ, Chen W. Exosomes and their roles in immune regulation and cancer. Semin Cell Dev Biol. 2015;40:72–81. doi:10.1016/j.semcdb.2015.02.009
91. Lee N, Canagasingham A, Bajaj M, et al. Urine exosomes as biomarkers in bladder cancer diagnosis and prognosis: from functional roles to clinical significance. Front Oncol. 2022;12:1019391. doi:10.3389/fonc.2022.1019391
92. Lopez-Beltran A, Cheng L, Gevaert T, et al. Current and emerging bladder cancer biomarkers with an emphasis on urine biomarkers. Expert Rev Mol Diagn. 2020;20(2):231–243. doi:10.1080/14737159.2020.1699791
93. Ng K, Stenzl A, Sharma A, Vasdev N. Urinary biomarkers in bladder cancer: a review of the current landscape and future directions. Urol Oncol. 2021;39(1):41–51. doi:10.1016/j.urolonc.2020.08.016
94. Matuszczak M, Kiljańczyk A, Salagierski M. A Liquid Biopsy in Bladder Cancer-The Current Landscape in Urinary Biomarkers. Int J Mol Sci. 2022;23(15):8597. doi:10.3390/ijms23158597
95. Kalluri R. The biology and function of exosomes in cancer. J Clin Invest. 2016;126(4):1208–1215. doi:10.1172/JCI81135
96. Welton JL, Khanna S, Giles PJ, et al. Proteomics analysis of bladder cancer exosomes. Mol Cell Proteomics. 2010;9(6):1324–1338. doi:10.1074/mcp.M000063-MCP201
97. Chen YT, Chen CL, Chen HW, et al. Discovery of novel bladder cancer biomarkers by comparative urine proteomics using iTRAQ technology. J Proteome Res. 2010;9(11):5803–5815. doi:10.1021/pr100576x
98. Li H, Li C, Wu H, et al. Identification of Apo-A1 as a biomarker for early diagnosis of bladder transitional cell carcinoma. Proteome Sci. 2011;9(1):21. doi:10.1186/1477-5956-9-21
99. Orenes-Piñero E, Cortón M, González-Peramato P, et al. Searching urinary tumor markers for bladder cancer using a two-dimensional differential gel electrophoresis (2D-DIGE) approach. J Proteome Res. 2007;6(11):4440–4448. doi:10.1021/pr070368w
100. Güllü Amuran G, Tinay I, Filinte D, Ilgin C, Peker Eyüboğlu I, Akkiprik M. Urinary micro-RNA expressions and protein concentrations may differentiate bladder cancer patients from healthy controls. Int Urol Nephrol. 2020;52(3):461–468. doi:10.1007/s11255-019-02328-6
101. Yazarlou F, Modarressi MH, Mowla SJ, et al. Urinary exosomal expression of long non-coding RNAs as diagnostic marker in bladder cancer. Cancer Manag Res. 2018;10:6357–6365. doi:10.2147/CMAR.S186108
102. Shi Y, Mathis BJ, He Y, Yang X. The Current Progress and Future Options of Multiple Therapy and Potential Biomarkers for Muscle-Invasive Bladder Cancer. Biomedicines. 2023;11(2):539. doi:10.3390/biomedicines11020539
103. Yu D, Li Y, Wang M, et al. Exosomes as a new frontier of cancer liquid biopsy. Mol Cancer. 2022;21(1):56. doi:10.1186/s12943-022-01509-9
104. He L, Zhu D, Wang J, Wu X. A highly efficient method for isolating urinary exosomes. Int J Mol Med. 2019;43(1):83–90. doi:10.3892/ijmm.2018.3944
105. Gavas S, Quazi S, Karpiński TM. Nanoparticles for Cancer Therapy: current Progress and Challenges. Nanoscale Res Lett. 2021;16(1):173. doi:10.1186/s11671-021-03628-6
106. Hao Y, Ji Z, Zhou H, et al. Lipid-based nanoparticles as drug delivery systems for cancer immunotherapy. MedComm. 2023;4(4):e339. doi:10.1002/mco2.339
107. Liao Z, Tu L, Li X, Liang XJ, Huo S. Virus-inspired nanosystems for drug delivery. Nanoscale. 2021;13(45):18912–18924. doi:10.1039/D1NR05872J
108. Zhang P, Chen Y, Zeng Y, et al. Virus-mimetic nanovesicles as a versatile antigen-delivery system. Proc Natl Acad Sci U S A. 2015;112(45):E6129–6138. doi:10.1073/pnas.1505799112
109. Thompson CA, Purushothaman A, Ramani VC, Vlodavsky I, Sanderson RD. Heparanase regulates secretion, composition, and function of tumor cell-derived exosomes. J Biol Chem. 2013;288(14):10093–10099. doi:10.1074/jbc.C112.444562
110. Bobrie A, Krumeich S, Reyal F, et al. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res. 2012;72(19):4920–4930. doi:10.1158/0008-5472.CAN-12-0925
111. Ramani VC, Purushothaman A, Stewart MD, et al. The heparanase/syndecan-1 axis in cancer: mechanisms and therapies. Febs j. 2013;280(10):2294–2306. doi:10.1111/febs.12168
112. Taverna S, Giallombardo M, Pucci M, et al. Curcumin inhibits in vitro and in vivo chronic myelogenous leukemia cells growth: a possible role for exosomal disposal of miR-21. Oncotarget. 2015;6(26):21918–21933. doi:10.18632/oncotarget.4204
113. Chen L, Brigstock DR. Integrins and heparan sulfate proteoglycans on hepatic stellate cells (HSC) are novel receptors for HSC-derived exosomes. FEBS Lett. 2016;590(23):4263–4274. doi:10.1002/1873-3468.12448
114. Christianson HC, Svensson KJ, van Kuppevelt TH, Li JP, Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci U S A. 2013;110(43):17380–17385. doi:10.1073/pnas.1304266110
115. Zhang S, Guo M, Guo T, et al. DAL-1/4.1B promotes the uptake of exosomes in lung cancer cells via Heparan Sulfate Proteoglycan 2 (HSPG2). Mol Cell Biochem. 2022;477(1):241–254. doi:10.1007/s11010-021-04268-1
116. Vader P, Mol EA, Pasterkamp G, Schiffelers RM. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev. 2016;106(Pt A):148–156. doi:10.1016/j.addr.2016.02.006
117. Palazzolo S, Memeo L, Hadla M, et al. Cancer Extracellular Vesicles: next-Generation Diagnostic and Drug Delivery Nanotools. Cancers. 2020;12(11):3165. doi:10.3390/cancers12113165
118. Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–345. doi:10.1038/nbt.1807
119. Saari H, Lázaro-Ibáñez E, Viitala T, Vuorimaa-Laukkanen E, Siljander P, Yliperttula M. Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of Paclitaxel in autologous prostate cancer cells. J Control Release. 2015;220(Pt B):727–737. doi:10.1016/j.jconrel.2015.09.031
120. Li YJ, Wu JY, Wang JM, Hu XB, Cai JX, Xiang DX. Gemcitabine loaded autologous exosomes for effective and safe chemotherapy of pancreatic cancer. Acta Biomater. 2020;101:519–530. doi:10.1016/j.actbio.2019.10.022
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.