Back to Journals » Psychology Research and Behavior Management » Volume 17
Association of Retinal Neurovascular Impairment with Disease Severity in Patients with Major Depressive Disorder: An Optical Coherence Tomography Angiography Study
Authors Wang Y, Li C, Liu L, Yang Y, He X, Li G, Zheng X, Ren Y, Zhao H, Du Z, Jiang J, Kuang Y, Jia F, Yu H, Yang X
Received 4 October 2023
Accepted for publication 27 February 2024
Published 9 April 2024 Volume 2024:17 Pages 1573—1585
DOI https://doi.org/10.2147/PRBM.S443146
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Igor Elman
Yan Wang,1,2,* Cong Li,1,2,* Lei Liu,1,* Yuan Yang,3,* Xue He,1,2 Gang Li,3 Xianzhen Zheng,3 Yun Ren,1,4 Hanpeng Zhao,1,5 Zhenchao Du,1,4 Jianrong Jiang,1,5 Yu Kuang,1 Fujun Jia,3 Honghua Yu,1 Xiaohong Yang1,2
1Guangdong Eye Institute, Department of Ophthalmology, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou, People’s Republic of China; 2School of Medicine, South China University of Technology, Guangzhou, People’s Republic of China; 3Guangdong Mental Health Center, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou, People’s Republic of China; 4Shantou University Medical College, Shantou, People’s Republic of China; 5The Second School of Clinical Medicine, Southern Medical University, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaohong Yang; Honghua Yu, Email [email protected]; [email protected]
Background: Identifying the fundus objective biomarkers for the major depressive disorders (MDD) may help promote mental health. The aim of this study was to evaluate retinal neurovascular changes and further investigate their association with disease severity in MDD.
Methods: This cross-sectional study conducted in the hospital enrolled patients with MDD and healthy controls.The retinal neurovascular parameters for all subjects, including vessel density (VD), thickness of ganglion cell complex (GCC) and retinal nerve fiber layer (RNFL), and optic nerve head (ONH) eg are automatically calculated by the software in optical coherence tomography angiography (OCTA). The severity of MDD including depressive symptoms, anxiety, cognition, and insomnia was assessed by Hamilton Depression Rating Scale (HAMD), Hamilton Anxiety Scale (HAMA), Montreal Cognitive Assessment (MoCA), and Insomnia Severity Index (ISI) respectively.
Results: This study included 74 MDD patients (n=74 eyes) and 60 healthy controls (HCs) (n=60 eyes). MDD patients showed significantly decreased VD of superficial and deep capillary plexus, thickness of GCC and RNFL, and volume of ONH (all p< 0.05) and increased vertical cup-to-disc ratio and global loss volume (GLV) (all p< 0.05) compared to HCs. Positive associations were found between HAMD scores and cup area (r=0.30, p=0.035), cup volume (r=0.31, p=0.029), and disc area (r=0.33, p=0.020) as well as ISI scores and RNFL thickness (r=0.34, p=0.047).
Conclusion: We found the retinal neurovascular impairment and its association with disease severity in MDD patients. OCTA showed promise as a potential complementary assessment tool for MDD.
Keywords: major depressive disorder, retinal neurovasculature, optical coherence tomography angiography, disease severity
Introduction
Major depressive disorder (MDD) is one of the most common mental disorders, which seriously limits psychosocial functioning and diminishes quality of life. World Health Organization (WHO) ranked major depression as the third cause of burden of disease worldwide and estimates that the disease will rank first by 2030.1 In China, depressive disorders are projected to be the second leading cause of years lived with disability.2 Currently, the diagnosis of MDD relies heavily on interviews and questionnaires, which are highly subjective and poorly reproducible.3 Consequently, developing reliable and objective biomarkers to assist in the diagnosis of MDD is needed. Studies have revealed significant structural and functional damage in the brain of patients with MDD through magnetic resonance imaging (MRI),4 such as decreased brain volume, white matter atrophy and hippocampal volume, and impaired neuronal linkages. However, MRI is expensive, time-consuming, and has an insufficient resolution, making it difficult to facilitate in the early diagnosis of MDD, particularly in populations with limited medical resources. Therefore, further noninvasive, objective and convenient approaches are warranted in the early diagnosis of MDD.
The eye is an extension of the central nervous system and has a similar cellular composition, functional receptors, and blood supply to the brain.5 As the fundus can be assessed non-invasively, quickly, and accurately, the retina could provide an ideal window into the microcirculation and neurodegeneration of the brain. Optical coherence tomography angiography (OCTA) can provide high resolution retinal images repeatedly and quantify the retinal vessel density (VD) and thickness of each nerve layer.6 Previous studies have demonstrated neural structural damage of the retina in MDD patients, such as decreased ganglion cell layer (GCL) and inner plexiform layer (IPL) volumes,7 reduced retinal nerve fiber layer (RNFL) and macular thickness, and enlarged disc area and cup volume.8 In the animal model, LeGates et al found that ganglion cells in the retinal neural structure are the main sensory channel driving the regulation of mood.9,10 Also, Fernandez et al demonstrated that the ganglion cells - perihabenular (PHb) pathway drives the light-mediated mood alterations.11 Additionally, Huang et al demonstrated that activation of the retina- ventral lateral geniculate nucleus and intergeniculate leaflet (vLGN/IGL)- lateral habenula (LHb) pathway could decrease depressive-like behaviors.12 Thus, it is reasonable to speculate that retinal neurovascular damage may have a correlation with the severity of depression. However, there is very limited literature comprehensively investigating the retinal vascular structure in MDD, and the association between these retinal neural structure changes and disease severity of MDD remains to be established.
The aim of this study was to explore the retinal neurovascular alteration in MDD patients, and further investigate the association between OCTA indicators and the severity of disease (including depressive symptoms, anxiety, cognition, and insomnia).
Method
Participants
This cross-sectional study included patients diagnosed with MDD aged 18 years or older at the psychiatric department of Guangdong Provincial People’s Hospital from January to December 2022. The psychiatrists invited in patients with depressive disorders from the clinic and explained to them the goal of the study and the items on the assessment that they needed to complete. The MDD was diagnosed by professional psychiatrists utilizing the Diagnostic and International Classification of Diseases, 10th edition (ICD-10).
Healthy controls were recruited by distributing leaflets in the community. Ophthalmologists measured the demographic traits of the subjects and conducted examinations of the eyes. Weight, height, and blood pressure were measured. Body mass index (BMI) was calculated as weight (kg) divided by height in meters squared (m2). Additionally, all participants completed comprehensive ocular examinations, including intraocular pressure (IOP), best corrected visual acuity (BCVA), and fundus photography. IOP was measured using air-puff non-contact tonometer (NCT). The BCVA was tested by the Snellen charts. Furthermore, all participants underwent fundus photography with naturally dilated pupils in a dark room. Participants with any co-morbid psychiatric diagnoses (eg bipolar disorder, schizophrenia, and substance dependence) were excluded. Other exclusion criteria were as follows: a history of (1) hypertension, diabetes, cardiovascular disease, and other systemic diseases, (2) eye diseases such as glaucoma, macular degeneration, retinal occlusions, ocular trauma, etc., (3) intraocular surgery; (4) current pregnancy/breastfeeding, (5) head injury with neurological sequelae, (6) intellectual disability, or neurologic disorders.
The study was conducted to comply the principles of the Declaration of Helsinki and approved by the institutional review board of Guangdong Provincial People’s Hospital (grant number: KY-Q-2022-145-03). All subjects have agreed to participate in the study and signed the written informed consent.
Questionnaire Evaluation
A professionally trained physician as a psychiatrist completed the questionnaire evaluation including the Hamilton Depression Rating Scale (HAMD), the Hamilton Anxiety Scale (HAMA), the Chinese version of the Montreal Cognitive Assessment (MoCA) and the Insomnia Severity Index (ISI) through structured interviews.
The 24-item HAMD13 was used to assess each patient’s depressive symptoms severity. The severity rating for the HAMD are as follows: no depression (0–7); mild depression (8–19); moderate depression (20–34); and severe depression (≥35).
The 14-question HAMA14 was used to assess MDD patient’s anxiety symptoms severity. There are 5 questions in each. Scores for responses are 0 (never), 1 (mild), 2 (moderate), 3 (severe), or 4 (very severe). The overall HAMA score is divided into three categories: no anxiety (0–6), mild and moderate anxiety (7–13), and severe anxiety (≥14).
The MoCA is a screening instrument that evaluates cognitive symptoms of MDD patients on a single page and scores range from 0 to 30. The domains are: visuospatial/executive functions, naming, verbal memory registration and learning, attention, abstraction, and 5-minute delayed verbal memory. To distinguish mild cognitive impairment (MCI) from healthy controls, a cut-off of 26 (+1 for 12 years of education)15 was used.
The insomnia severity of patients was assessed by ISI,16 which is a brief self-report instrument measuring the patient’s perception of his or her insomnia. The ISI comprises 7 items assessing the severity of sleep-onset and sleep maintenance difficulties, satisfaction with current sleep pattern, interference with daily functioning, noticeability of impairment attributed to the sleep problem, and degree of distress or concern caused by the sleep problem. The overall score can be between 0 and 28, and each item is graded on a 0 to 4 scale. A higher score denotes more severe insomnia.
OCTA
An OCTA (Version 2017.1.0.151; RTVue-XR Avanti; Optovue, Fremont, California) instrument was used to capture retinal images with patients’ pupils dilated in a dark room. With the OCTA scans, we utilized HD modes for the optical disc (4.5 x 4.5 mm2) and macula (6 x 6 mm2). Based on the percentage of the area filled by vessels, retinal blood VD was assessed using the split-spectrum amplitude-decorrelation-angiography (SSADA) software algorithm.17 The capillary density was assessed using built-in software that automatically filters out vessels larger than 33 mm in diameter. Superficial capillary plexus (SCP) refers to the ILM to 10 μm above the inner plexiform layer (IPL). Deep capillary plexus (DCP) is defined as 10 μm above the IPL to 10 μm below the outer plexiform layer (OPL). In the macular region, SCP and DCP were automatically generated by the software. The VD of four areas in the SCP and DCP (whole image, fovea, parafovea, and perifovea) was available for analysis. The foveal area of the macula refers to the inner circle with a diameter of 1 mm, the parafoveal area refers to the annular area between the concentric circles with a diameter of 1 and 3 mm, and the perifoveal area refers to the annular area between the concentric circles with a diameter of 3 and 6 mm.
GCC scan mode is used to acquire parameters pertaining to GCC. The inner retinal thickness from ILM to IPL is represented by the ganglion cell complex thickness (GCC-t). Focal Loss Volume (FLV), which expresses the amount of significant ganglion cell loss as a percentage of the map area, quantifies the amount of GCC loss (by volume). The average GCC loss throughout the whole GCC map is quantified by the term “Global Loss Volume” (GLV). For a percent loss of GCC thickness, GLV is the total number of pixels where the fractional deviation map value is less than 0, multiplied by the total map area. The AngioVue program automatically calculated FLV and GLV. By using the ONH scanning mode, the pertinent parameters of the optic nerve head (ONH) and the thickness of the retinal nerve fiber layer (RNFLt) around the disc were acquired. We measured RNFLt by scanning with a circle scanner 3.45 mm in diameter, which was automatically divided into superior and inferior hemifields. The photos having an overall quality index (QI) of 6 or higher were eligible for the subsequent quantitative analysis.
Statistical Analysis
The demographic and clinical characteristics were compared between these two groups. Continuous variables were presented as mean (SD) or median (interquartile range, IQR). The Shapiro–Wilk test was used to determine whether the data were normally distributed. The independent-sample t-test was used to compare quantitative variables with normal distributions between the two groups. Mann–Whitney test was used to compare differences between non-normally distributed quantitative data. Categorical variables were presented as numbers and percentages and compared with the χ2 test. After controlling for confounding factors, multiple linear regression models were used to compare retinal neurovascular differences between the two groups. Considering the influence of antidepressants, nonparametric test was used to compare retinal neurovascular parameters among different duration of drug use. Pearson’s correlation analyses were performed to investigate the relationships between retinal structural parameters and questionnaire scores. Refraction values were converted to spherical equivalent (SE), calculated as spherical dioptric power plus one-half of the cylindrical dioptric power. Only the eye with the better image quality and meeting the overall QI was used for analysis for each participant.
All data were analyzed by using SPSS (IBM SPSS, Version 26.0, IBM Corporation, Armonk, New York) software. P <0.05 was regarded as statistically significant, and all p values were provided based on two-tailed statistics.
Results
Demographics
This research sequentially enrolled 60 HCs (n=60 eyes) and 74 MDD patients (n=74 eyes). Table 1 showed the demographic, clinical, and ocular characteristics of participants. There were no significant differences between the MDD and HCs for age, diastolic blood pressure (DBP), visual acuity, spherical equivalent, the BCVA, and IOP. In comparison to HCs, MDD patients were more likely to be females (p<0.05) and have lower systolic blood pressure (SBP) (p<0.05) and body mass index (p<0.05).
 |
Table 1 Demographic, Clinical, and Ocular Characteristics of Healthy Controls and Major Depressive Disorder Groups |
Comparison of Retinal Neurovascular Parameters
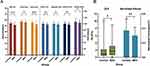
The comparison of retinal neurovascular parameters of these two groups was shown in Table 2 and Figure 1. Compared with HCs, the MDD group demonstrated significantly lower SCP VD of PeriFovea (p<0.05), DCP VD of the whole (p<0.001), ParaFovea (p<0.05), PeriFovea (p<0.001), and lower overall QI (p<0.001) of retinal VD image. The MDD group had a significant decrease in the GCC-t of the mean (p<0.05), superior-hemifield (p<0.05), and inferior-hemifield (p<0.05) sectors, as well as an increase in GCC-GLV (p<0.05) compared to the HCs. The MDD group had a lower SSI (p<0.001) than the HCs group. Furthermore, compared to the HCs, MDD patients had a larger vertical cup-to-disc ratio (p<0.05) and reduced rim area (p<0.05), optic nerve head volume (p<0.05), and inferior-hemifield RNFL (p<0.05).
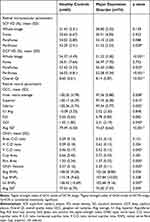 |
Table 2 Comparison of Retinal Neurovascular Parameters Between Healthy Controls and Major Depressive Disorder Groups |
After adjustment for age, sex, SBP, BMI, and image quality, we found that DCP VD of the Whole (β=−2.501, p=0.004), ParaFovea (β=−1.56, p=0.017) and PeriFovea (β=−2.65, p=0.004), GCCt of the mean (β=−4.00, p=0.006), superior-hemifield (β=−4.34, p=0.006) and inferior-hemifield (β=−3.73, p=0.010), GCC-GLV (β=1.22, p=0.012), Optic nerve head Volume (β=−0.07, p=0.027), average (β=−4.95, p=0.018) and inferior-hemifield (β=−5.55, p=0.018) RNFL are significantly associated with MDD (Table 3). Nevertheless, after controlling for the confounding variables above, there were no significant correlations between SCP VD of PeriFovea, vertical C-D ratio, and rim area with MDD (all p>0.05) (Table 3).
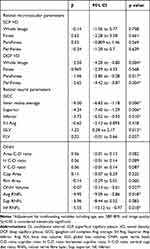 |
Table 3 Comparison of Retinal Neurovascular Parameters of Healthy Controls and Major Depressive Disorder Groups After Adjusting for Confounding Variablesa |
Correlations Between Neurovascular Parameters and Questionnaire Scores
We further investigated the associations between retinal neurovascular parameters and the disease severity of MDD. The results showed that cup area (r=0.30, p=0.035), cup volume (r=0.31, p=0.029), and disc area (r=0.33, p=0.020) are significantly positively correlated with HAMD scores. A positive correlation was also found between RNFL thickness and ISI scores (r=0.34, p=0.047) (Figure 2). However, we did not find significant correlations between retinal neurovascular parameters with MoCA and HAMA scores (Table S1).
Subgroup Analysis
In the female subgroup, MDD was significantly associated with DCP VD of the whole (β=−2.61, p=0.029), ParaFovea (β=−2.02, p=0.018) and PeriFovea (β=−2.99, p=0.018), superior GCC (β=−3.79, p=0.046), nerve head volume (β=−0.072, p=0.027) and GLV (β=1.36, p=0.042) after adjusting age, SBP, BMI, and image quality. However, in the male subgroup, there was no significant correlations between retinal neurovascular parameters with MDD (all p>0.05) (Tables 4 and S2)
 |
Table 4 Comparison of Retinal Neurovascular Parameters in Female and Male Subjects Separately After Adjusting for Confounding Variablesa |
In the subjects aged ≤ 40 years, MDD was significantly associated with DCP VD of the Whole (β=−2.36, p=0.025) and PeriFovea (β=−2.51, p=0.024) and GLV (β=1.20, p=0.013) after controlling for age, sex, SBP, BMI, and image quality. Moreover, after correcting for confounding factors, rim area (β=−0.34, p=0.025) and RNFL (β=−9.95, p=0.013) significantly associated with MDD in the aged >40 years subgroup (Tables 5 and S3).
 |
Table 5 Comparison of Retinal Neurovascular Parameters in Aged ≤40 Years and >40 Years Subjects Separately After Adjusting for Confounding Variablesa |
MDD patients were separated into three groups based on the duration of antidepressant medication. There were no appreciable differences in the neurovascular parameters among these three subgroups (all p>0.05) (Table 6)
 |
Table 6 Comparison of Retinal Neurovascular Parameters of MDD Patients with Different Years of Use for Antidepressants |
Discussion
This study showed that patients with MDD had reduced retinal VD and nerve head volume and thinner GCC and RNFL. Interestingly, we further discovered that the severity of depressive symptoms and insomnia correlate with optic nerve head structures. Moreover, we found impairment of retinal neurovasculature was more significant in female MDD patients. The retinal microcirculation damage was more pronounced in MDD patients aged ≤40 years, but atrophy of retinal nerve structures was more evident in patients aged>40 years. These findings suggest the neurovascular impairment of the retina is anticipated to be a novel objective biomarker of MDD and may partially reflect the disease severity.
Our research confirms earlier findings by discovering decreased retinal VD and RNFL thickness in MDD patients. According to Liu et al,18 MDD patients had thinner subfoveal choroidal thickness and less retinal VD density. Studies7,8,19 have revealed that RNFL thickness was significantly reduced in MDD patients. There is no definitive explanation for the reduction in retinal VD and thinning of the retinal nerve layer in MDD. The following are some potential theories. First, studies20,21 have shown that microvascular damage occurs in MDD patients due to oxidative stress and endothelial dysfunction. As a result, excessive oxidative stress22–24 in MDD patients may cause damage to the retinal vascular structures. Second, there is evidence that MDD is linked to reduced cerebrovascular reactivity25 and features of cerebral small-vessel disease.26 Previous researches27,28 have shown that changes in the retinal microvascular are linked to a higher chance of cerebral small-vessel disease symptoms. Therefore, retinal microvascular damage in MDD patients may be an ocular manifestation of their cerebrovascular dysfunction. However, to further elucidate this issue, both the cerebral and retinal vessels in MDD patients are supposed to be observed concurrently. Regarding the thinner nerve layer observed in MDD patients, on the one hand, this could be a retinal indicator of the neurodegeneration,4,29 because the retinal nerve layer is structurally and functionally comparable to brain neurons. On the other hand, impaired retinal microvascular in MDD patients may result in a deficiency of nutrients in the retinal nerve layer.
As for the impaired retinal neurovascular structure of female while not in male of MDD patients in this study, it may be due to sex differences in some biological variables in MDD.30 For example, Birur et al31 reported higher levels of the cytokines interleukin (IL)-8 and interferon-γ in female patients with depression but not in male compared with healthy individuals. And as mentioned above, inflammatory factors can damage the neurovascular structures of the retina, so it could help explain female patients with MDD have impaired retinal neurovascular structure, whereas males do not. Intriguingly, we found the atrophy of retinal nerve structures was more evident in MDD patients aged >40 years than those aged ≤40 years, this is consistent with the notion that MDD affects neural structures to different degrees from early to late adulthood. For instance, Myoraku et al32 showed that individuals with MDD generally presented with greater gray matter volume and cortical thickness during young adult and early middle-age years, but often smaller gray matter volume and cortical thickness than HCs predominantly starting at later middle-age years.
Notably, we found a substantial positive correlation between the depressive symptoms severity of MDD and the optic nerve head structure parameters, and intensity of insomnia and the RNFL thickness. Previous research also found that damage to retinal nerve structures correlated with disease severity in MDD. For example, Heide et al33 discovered that decreased RNFL thickness was associated with a higher incidence of clinically relevant depressive symptoms as well as an increase in depressive symptoms with time. Kalenderoglu et al7 discovered substantial negative relationships between GCL and IPL volumes and the severity of MDD. According to Liu et al,8 RNFL and macular thickness were correlated with sleep quality and the depressive symptoms severity. The retinal neural structure parameters associated with the disease severity of MDD in this study are differ from previous studies, which may be related to the different means of assessing MDD. For instance, compared with the Liu et al,8 we did not discover RNFL in patients with MDD linked with the depressive symptoms severity. Maybe it was because we assessed the depressive symptoms severity using HAMD, whereas Liu et al8 used Patient Health Questionnaire-9 (PHQ-9). However, in general, this study and previous studies support an association between retinal neural structure and the disease severity of MDD, and future larger studies are needed to draw more accurate conclusions using a consistent assessment of MDD. The connections between cup area, cup volume, and disc area with depressive symptoms severity found in this study could be supported by the research8 which demonstrated the cup-volume enlargement in both eyes as well as disc-area enlargement of the right eye in patients with MDD. Studies34,35 have previously demonstrated a substantial comorbidity rate between glaucoma and depression. Because the cup area and cup volume are positively correlated with the depressive symptoms severity in this study, we postulated that the morphological changes in the optic nerve head of MDD patients may represent their vulnerability to glaucoma. Aside from that, people who use selective serotonin reuptake inhibitors (SSRIs) are more likely to develop acute angle-closure glaucoma.36 Patients in this study with more depressive symptoms are more likely to use more doses of antidepressants, resulting in a more prominent effect on optic nerve morphology. Contrary to what we anticipated, we discovered a slightly positive connection between the severity of insomnia and RNFL thickness. This might be connected to the patient’s retinal inflammation at the time of the disease’s onset. According to a study,37 MDD individuals suffer increased insomnia during attacks. It’s consistent with Yildiz et al38 who discovered that the severity of depression was weakly positively linked with RNFL thickness.
This study has comprehensively evaluated the retinal neurovascular changes in MDD patients and confirmed their association with disease severity. Our findings suggest that the potential of the retinal neurovascular structure in providing a non-invasive, rapid, and inexpensive objective biomarker for the diagnosis and assessment of disease severity of MDD. Nevertheless, the results of the study must be viewed in light of its limitations: (1) This cross-sectional study is difficult to illustrate how the retinal neurovascular changes as depressive disorder progress. (2) The sample size of this study was relatively small. (3) The confounding effect on the outcomes of antidepressants cannot be ignored, even though no differences in neurovascular parameters were found between patients with different duration of antidepressant medication. In the future, the confounding effects of antidepressants could be studied by resetting the subgroups. For example, retinal neurovascular parameters could be compared in MDD patients who have taken antidepressants to those who have not, via conducting cross-sectional study with larger sample sizes, or in MDD patients before and after medication use via conducting a longitudinal study. (4) The results of this research, which only included outpatients, cannot be easily generalizable to all MDD patients. But impaired retinal neurovascular in MDD outpatients in the study indicates that OCTA showed promise as a potential complementary assessment tool for depressive disorders, and further cohort studies are needed to explore the value of OCTA in the investigation of depressive disorders. (5) Given the time constraints and the high consistency of the questionnaire between the two physicians in the pilot study, the questionnaire was finally scored only by a professionally trained physician to obtain comprehensive data and an appropriate sample size. Based on the findings of this study, future research could focus on HAMD, with two psychologists performing independent assessments to improve the reliability of the questionnaire assessment. At last, to better explain the pathophysiology of MDD, further researches to investigate the molecular mechanisms of retina neurovascular injury using animal studies are needed.
In conclusion, our study showed retinal neurovascular damage in MDD patients correlates with disease severity. Assessment in retinal structure could provide a non-invasive, easy, and cost-effective approach for investigating biomarkers and disease severity in MDD.
Acknowledgments
Yan Wang, Cong Li, Lei Liu, and Yuan Yang are co-first authors for this study. The authors thank the Statistics Office, Information and Statistics Center, Guangdong Provincial People’s Hospital, and Guangdong Academy of Medical Sciences for their assistance in statistical analyses; And thank the staff of the Department of Psychiatry for their assistance in the recruitment of participants.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Ethics Statement
The studies involving human participants were reviewed and approved by the Research Ethics Committee of Guangdong Provincial People’s Hospital [grant number: KY-Q-2022-145-03]. The patients/participants provided their written informed consent to participate in this study.
Funding
This work was supported by the National Natural Science Foundation of China (82271125 and 82171075), the Special Fund Project of Technology Achievement Transformation in Life and Health Innovation of the Greater Bay Area (GBALH202308), the launch fund of Guangdong Provincial People’s Hospital for NSFC (8217040449, 8227040339 and 8217040546), Precision Medicine Research and Industrial Innovation Development Fund-Individualized Medical Incubation Project 2022 (KH012023367), the Science and Technology Program of Guangzhou (20220610092 and 202103000045), the Outstanding Young Talent Trainee Program of Guangdong Provincial People’s Hospital (KJ012019087), the Talent Introduction Fund of Guangdong Provincial People’s Hospital (Y012018145), the GDPH Scientific Research Funds for Leading Medical Talents and Distinguished Young Scholars in Guangd ong Province (KJ012019457) and the GDPH Supporting Fund for Talent Program (KY0120220263). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Malhi GS, Mann JJ. Depression. Lancet. 2018;392(10161):2299–2312. doi:10.1016/S0140-6736(18)31948-2
2. Lu J, Xu X, Huang Y, et al. Prevalence of depressive disorders and treatment in China: a cross-sectional epidemiological study. Lancet Psychiatry. 2021;8(11):981–990. doi:10.1016/S2215-0366(21)00251-0
3. McCarron RM, Shapiro B, Rawles J, Luo J. Depression. Ann Intern Med. 2021;174(5):ITC65–ITC80. doi:10.7326/AITC202105180
4. Zhang FF, Peng W, Sweeney JA, Jia ZY, Gong QY. Brain structure alterations in depression: psychoradiological evidence. CNS Neurosci Ther. 2018;24(11):994–1003. doi:10.1111/cns.12835
5. London A, Benhar I, Schwartz M. The retina as a window to the brain-from eye research to CNS disorders. Nat Rev Neurol. 2013;9(1):44–53. doi:10.1038/nrneurol.2012.227
6. Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G. Optical coherence tomography angiography. Prog Retin Eye Res. 2018;64:1–55. doi:10.1016/j.preteyeres.2017.11.003
7. Kalenderoglu A, Çelik M, Sevgi-Karadag A, Egilmez OB. Optic coherence tomography shows inflammation and degeneration in major depressive disorder patients correlated with disease severity. J Affect Disord. 2016;204:159–165. doi:10.1016/j.jad.2016.06.039
8. Liu Y, Chen J, Huang L, Yan S, Gao D, Yang F. Association between changes in the retina with major depressive disorder and sleep quality. J Affect Disord. 2022;311:548–553. doi:10.1016/j.jad.2022.05.074
9. LeGates TA, Altimus CM, Wang H, et al. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature. 2012;491:594–598. doi:10.1038/nature11673
10. LeGates TA, Fernandez DC, Hattar S. Light as a central modulator of circadian rhythms, sleep and affect. Nat Rev Neurosci. 2014;15(7):443–454. doi:10.1038/nrn3743
11. Fernandez DC, Fogerson PM, Lazzerini Ospri L, et al. Light affects mood and learning through distinct retina-brain pathways. Cell. 2018;175(1):71–84.e18. doi:10.1016/j.cell.2018.08.004
12. Huang L, Xi Y, Peng Y, et al. A visual circuit related to habenula underlies the antidepressive effects of light therapy. Neuron. 2019;102(1):128–142.e8. doi:10.1016/j.neuron.2019.01.037
13. Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6(4):278–296. doi:10.1111/j.2044-8260.1967.tb00530.x
14. Zimmerman M, Thompson JS, Diehl JM, Balling C, Kiefer R. Is the DSM-5 anxious distress specifier interview a valid measure of anxiety in patients with generalized anxiety disorder: a comparison to the Hamilton anxiety scale. Psychiatry Res. 2020;286:112859. doi:10.1016/j.psychres.2020.112859
15. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi:10.1111/j.1532-5415.2005.53221.x
16. Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi:10.1016/s1389-9457(00)00065-4
17. Jia Y, Tan O, Tokayer J, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express. 2012;20(4):4710–4725. doi:10.1364/OE.20.004710
18. Liu X, Lai S, Ma S, et al. Development of a novel retina−based diagnostic score for early detection of major depressive disorder: an interdisciplinary view. Front Psychiatry. 2022;13:897759. doi:10.3389/fpsyt.2022.897759
19. Schönfeldt-Lecuona C, Schmidt A, Kregel T, et al. Retinal changes in patients with major depressive disorder - A controlled optical coherence tomography study. J Affect Disord. 2018;227:665–671. doi:10.1016/j.jad.2017.11.077
20. Greaney JL, Saunders EFH, Santhanam L, Alexander LM. Oxidative stress contributes to microvascular endothelial dysfunction in men and women with major depressive disorder. Circ Res. 2019;124(4):564–574. doi:10.1161/CIRCRESAHA.118.313764
21. Smith PJ, Blumenthal JA, Hinderliter AL, Watkins LL, Hoffman BM, Sherwood A. Microvascular endothelial function and neurocognition among adults with major depressive disorder. Am J Geriatr Psychiatry. 2018;26(10):1061–1069. doi:10.1016/j.jagp.2018.06.011
22. Nishimura Y, Hara H, Kondo M, Hong S, Matsugi T. Oxidative stress in retinal diseases. Oxid Med Cell Longev. 2017;2017:4076518. doi:10.1155/2017/4076518
23. Masuda T, Shimazawa M, Hara H. retinal diseases associated with oxidative stress and the effects of a free radical scavenger (Edaravone). Oxid Med Cell Longev. 2017;2017:9208489. doi:10.1155/2017/9208489
24. Castelli V, Paladini A, d’Angelo M, et al. Taurine and oxidative stress in retinal health and disease. CNS Neurosci Ther. 2021;27(4):403–412. doi:10.1111/cns.13610
25. Wu H, Lu B, Zhang Y, Li T. Differences in prefrontal cortex activation in Chinese college students with different severities of depressive symptoms: a large sample of functional near-infrared spectroscopy (fNIRS) findings. J Affect Disord. 2024;350:521–530. doi:10.1016/j.jad.2024.01.044
26. Rensma SP, van Sloten TT, Launer LJ, Stehouwer CDA. Cerebral small vessel disease and risk of incident stroke, dementia and depression, and all-cause mortality: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2018;90:164–173. doi:10.1016/j.neubiorev.2018.04.003
27. Geerling CF, Terheyden JH, Langner SM, et al. Changes of the retinal and choroidal vasculature in cerebral small vessel disease. Sci Rep. 2022;12(1):3660. doi:10.1038/s41598-022-07638-x
28. Cabrera DeBuc D, Somfai GM, Koller A. Retinal microvascular network alterations: potential biomarkers of cerebrovascular and neural diseases. Am J Physiol Heart Circ Physiol. 2017;312(2):H201–H212. doi:10.1152/ajpheart.00201.2016
29. Han LKM, Dinga R, Hahn T, et al. Brain aging in major depressive disorder: results from the ENIGMA major depressive disorder working group. Mol Psychiatry. 2021;26(9):5124–5139. doi:10.1038/s41380-020-0754-0
30. Labaka A, Goñi-Balentziaga O, Lebeña A, Pérez-Tejada J. Biological sex differences in depression: a systematic review. Biol Res Nurs. 2018;20(4):383–392. doi:10.1177/1099800418776082
31. Birur B, Amrock EM, Shelton RC, Li L. Sex differences in the peripheral immune system in patients with depression. Front Psychiatry. 2017;8:108. doi:10.3389/fpsyt.2017.00108
32. Myoraku A, Lang A, Taylor CT, et al. Age-dependent brain morphometry in major depressive disorder. Neuroimage Clin. 2022;33:102924. doi:10.1016/j.nicl.2021.102924
33. van der Heide FCT, Steens ILM, Geraets AFJ, et al. Association of retinal nerve fiber layer thickness, an index of neurodegeneration, with depressive symptoms over time. JAMA Network Open. 2021;4(11):e2134753. doi:10.1001/jamanetworkopen.2021.34753
34. Zhang X, Olson DJ, Le P, Lin FC, Fleischman D, Davis RM. The association between glaucoma, anxiety, and depression in a large population. Am J Ophthalmol. 2017;183:37–41. doi:10.1016/j.ajo.2017.07.021
35. Berchuck S, Jammal A, Mukherjee S, Somers T, Medeiros FA. Impact of anxiety and depression on progression to glaucoma among glaucoma suspects. Br J Ophthalmol. 2021;105(9):1244–1249. doi:10.1136/bjophthalmol-2020-316617
36. Jain NS, Ruan CW, Dhanji SR, Symes RJ. Psychotropic drug-induced glaucoma: a practical guide to diagnosis and management. CNS Drugs. 2021;35(3):283–289. doi:10.1007/s40263-020-00790-w
37. Palagini L, Cipollone G, Masci I, et al. Insomnia symptoms predict emotional dysregulation, impulsivity and suicidality in depressive bipolar II patients with mixed features. Compr Psychiatry. 2019;89:46–51. doi:10.1016/j.comppsych.2018.12.009
38. Yildiz M, Alim S, Batmaz S, et al. Duration of the depressive episode is correlated with ganglion cell inner plexifrom layer and nasal retinal fiber layer thicknesses: optical coherence tomography findings in major depression. Psychiatry Res Neuroim. 2016;251:60–66. doi:10.1016/j.pscychresns.2016.04.011
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.