Back to Journals » Nature and Science of Sleep » Volume 15
Association of Depression with Long-Term Cardiovascular Risks in Older Patients with Obstructive Sleep Apnea
Authors Zhao Z , Gao Y, Lin J, Xu R, He Z, Zhao L, Fang F, Cai W, Chen K, Fan L, Liu L
Received 24 June 2023
Accepted for publication 24 October 2023
Published 5 December 2023 Volume 2023:15 Pages 1033—1043
DOI https://doi.org/10.2147/NSS.S423550
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Ahmed BaHammam
Zhe Zhao,1,* Yinghui Gao,2,* Junling Lin,3,* Ruyi Xu,4 Zijun He,5 Libo Zhao,1 Fengfeng Fang,5 Weimeng Cai,6 Kaibin Chen,7 Li Fan,1 Lin Liu6
1Cardiology Department of the Second Medical Center & National Clinical Research Center for Geriatric Diseases, Chinese PLA General Hospital, Beijing, People’s Republic of China; 2PKU-Upenn Sleep Center, Peking University International Hospital, Beijing, People’s Republic of China; 3Department of Pulmonary and Critical Care Medicine, Beijing Chaoyang Hospital Affiliated to Capital Medical University, Beijing, People’s Republic of China; 4Sixth Medical Center, Chinese PLA General Hospital, Beijing, People’s Republic of China; 5Medical College, Yan’an University, Yan’an, People’s Republic of China; 6Department of Respiratory and Critical Care Medicine of the Second Medical Center & National Clinical Research Center for Geriatric Diseases, Chinese PLA General Hospital, Beijing, People’s Republic of China; 7Sleep Center, The Affiliated Hospital of Gansu University of Chinese Medicine, Lanzhou City, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Li Fan, Cardiology Department of the Second Medical Center & National Clinical Research Center for Geriatric Diseases, Chinese PLA General Hospital, 28 Fuxing Road, Beijing, 100853, People’s Republic of China, Tel +86 13301100573, Email [email protected] Lin Liu, Department of Respiratory and Critical Care Medicine of the Second Medical Center & National Clinical Research Center for Geriatric Diseases, Chinese PLA General Hospital, 28 Fuxing Road, Beijing, 100853, People’s Republic of China, Tel +86 13263189578, Email [email protected]
Introduction: Obstructive sleep apnea (OSA) is associated with an increased risk of cardiovascular disease (CVD). Depression is a crucial factor among the various factors that are associated with OSA and CVD.
Purpose: This study was conducted with an aim to assess the prognostic significance of depression on the MACE in older patients with OSA.
Patients and Methods: 1106 older patients with OSA, without myocardial infarction (MI), history of hospitalization for unstable angina, or heart failure at baseline were enrolled and followed up prospectively. Incidence rates were expressed as cumulative incidence. Cox proportional hazards analysis was used to estimate the risk of all events. The primary outcomes were major adverse cardiovascular events (MACE). Each patient underwent polysomnography (PSG) and GDS-12 scale assessment. Those with an apnea-hypopnea index (AHI) greater than 5 were diagnosed with OSA, while those with a scale score greater than 3 were diagnosed with depression.
Results: Among the 1106 older patients with OSA, depression was found in 133(12.0%) patients, 96(8.7%) patients experienced MACE during the follow-up. Depression was associated with a higher cumulative incidence of MACE in older patients with OSA. Multivariate analysis revealed that depression independently increased the risk of MACE (adjusted hazard ratio [aHR] = 2.29; 95% confidence interval [CI]: 1.34– 3.90; P = 0.002). Subgroup analyses showed that male patients (aHR = 2.96; 95% CI: 1.52– 5.77; P = 0.001), overweight-obese individuals (aHR = 2.98; 95% CI: 1.49– 6.00; P = 0.002), and those with moderate-severe OSA (aHR = 2.82; 95% CI: 1.55– 5.14; P = 0.001) and concurrent depression were at a higher risk for MACE.
Conclusion: Depression is common in older patients with OSA in the absence of MI, hospitalization for unstable angina, or heart failure, and confers an independent, increased risk of MACE.
Keywords: obstructive sleep apnea, OSA, depression, major adverse cardiovascular events, MACE, older adults
Introduction
Obstructive sleep apnea (OSA), which is estimated to affect 34% and 17% of males and females, respectively, may have an even higher actual prevalence.1 The prevalence of OSA is on the rise worldwide, affecting approximately 22% of the population, with a particularly high incidence among the older patients.2 OSA is one of the most common sleep disorders, and is associated with an increased risk of hypertension and cardiovascular disease (CVD).3 In particular, OSA occurs in 40–80% of patients with hypertension, heart failure (HF), coronary artery disease, pulmonary hypertension (PH), atrial fibrillation (AF), or stroke.4 Epidemiological investigation5 revealed that OSA is associated with an increased incidence and progression of coronary heart disease, heart failure, stroke, and AF. Undiagnosed OSA is associated with increased cardiovascular risk in the general population,6 therefore, it is important to understand the relationship between OSA and CVD to enable the development of clinical preventive and therapeutic strategies.
Depression is a crucial factor among the various factors that are associated with OSA and CVD. Worldwide, the most common psychiatric disorder, depression, remains a major public health problem.7 The effect of depression and anxiety on CVD is evident, not only in the increased risk for and accelerated pathogenesis of CVD but also in the frequent poorer prognosis.8 Earlier identification and prevention of depression can markedly enhance the quality of patient survival. Several evidences9–11 indicate a higher incidence of depression among individuals with OSA. Both OSA and depression are risk factors for cardiovascular disease, and their coexistence may lead to adverse cardiovascular outcomes. In 2021, Bouloukaki reported12 that depression is an important characteristic of OSA patients with a high risk for CVD. However, their study did not elucidate whether depression increases the probability of CVD risk in OSA patients. The incidence and long-term risk of cardiovascular disease related to depression in older patients with OSA remains uncharacterized.
Therefore, this study was conducted with an aim to assess the prognostic significance of depression on the major adverse cardiac events (MACE) complex (cardiovascular death, MI, hospitalization for unstable angina, or heart failure) in a cohort of OSA patients without previous MI, history of hospitalization for unstable angina, or heart failure at baseline.
Materials and Methods
Study Population
This multicenter, observational cohort study that recruited older OSA patients (age ≥60 years) without previous MI, history of hospitalization for unstable angina, or heart failure at baseline. OSA was diagnosed between January 2015 and October 2017 in departments or sleep medicine centers at 6 hospitals, including the General Hospital of the People’s Liberation Army, Peking University International Hospital, Peking University People’s Hospital, Beijing Chaoyang Hospital, the 960th Hospital of the People’s Liberation Army, and the Affiliated Hospital of Gansu University of Traditional Chinese Medicine. OSA was clinically diagnosed based on an apnea–hypopnea index (AHI) ≥5 breaths per hour, which was calculated as the number of apnea and hypoventilation events per hour of sleep. In summary, we recruited 1290 consecutive patients with an incident diagnosis of OSA after undergoing an overnight sleep study and were clinically stable during their stay (within 1 week of admission) in the sleep centers at the six study centers. Study flow is shown in Figure 1.The study enrolled a total of 1106 elderly patients with obstructive sleep apnea (OSA), whose inclusion criteria comprised: 1) age ≥60 years, and 2) a confirmed diagnosis of OSA. Exclusion criteria encompassed: 1) a prior diagnosis of MI, history of hospitalization for unstable angina, or heart failure; 2) a history of malignancy; 3) psychiatric disorders; 4) systemic disease; and 5) previously received continuous positive airway pressure (CPAP) therapy. Furthermore, patients who were diagnosed with sleep-disordered breathing other than OSA and those who were lost to follow-up were also excluded from this study. Ultimately, the final study population consisted of 1106 older patients with OSA who satisfied all of the aforementioned criteria.
 |
Figure 1 Study flowchart. CPAP indicates continuous positive airway pressure; MI, myocardial infarction. |
This study complies with the Guidelines for Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) and was conducted in accordance with the tenets underlying the Declaration of Helsinki. This study was approved by the Ethics Committee of the General Hospital of the Chinese People’s Liberation Army (S2019-352-01), and all participants provided written informed consent prior to their study enrolment.
Overnight Sleep Study
OSA was diagnosed and sleep tests are scored in accordance with the American Academy of Sleep Medicine guidelines (2017). All participants underwent an overnight sleep study (from 21:00 to 07:00 on the next day) in the sleep center within 1 week of admission. Sleep studies were performed using a portable laboratory polysomnography (Compumedics, Melbourne, Australia), and data were subjected automatically to computerized analysis and then manually corrected by two sleep technologists and a senior physician. As OSA was defined as an AHI ≥5 events/h, the AHI was calculated as the total number of apnea and hypoventilation events divided by the duration of sleep (hours). Accordingly, OSA was classified as mild (AHI 5.0–14.9), moderate (AHI 15.0–30.0), or severe (AHI >30.0).13
Covariates
Covariates included baseline clinical and demographic variables. Baseline data with statistically significant differences between the two patient groups or indicators that are significantly associated with MACE were included as covariates. These included age, smoking and alcohol use, comorbidities [hypertension, atrial fibrillation (AF), chronic obstructive pulmonary disease (COPD), renal dysfunction, cerebrovascular disease, carotid atherosclerosis, coronary heart disease (CHD)]; laboratory data [creatinine, uric acid] and sleep parameters [AHI, oxygen desaturation index (ODI), mean pulse oxygen saturation (MSpO2), lowest pulse oxygen saturation (LSpO2), and Maximum apnea time].
Depression Assessment
The participants were assessed according to the 12-item Geriatric Depression Scale (GDS), Depression was defined as meeting at least 4 of the 12 symptoms.14
Procedures, Follow-Up, and Outcomes
Within 7 days after admission, all patients underwent PSG and a comprehensive suite of laboratory tests. Subsequently, patients were prospectively followed up for a duration of approximately 4 years following their PSG assessment and diagnosis of OSA, with all follow-ups being concluded by December 2020. Follow-ups were terminated upon the occurrence of the first MACE. Two investigators who were blinded to the patients’ PSG results contacted the participants at 1 month, 3 months, 6 months, 1 year, and every 6 months thereafter (at minimum intervals of 3 months and up to 1 year). Patient follow-up outcomes were additionally verified via clinical visits and medical record reviews, which remained in effect until the study’s conclusion. The study’s primary endpoint consisted of MACE, which was defined as MI, cardiovascular death, or hospitalization due to unstable angina or heart failure.
Statistical Analysis
Continuous variables are presented as means (standard deviations) for normally distributed data or as medians (interquartile ranges) for skewed data. Categorical variables are displayed as counts and proportions (%). As the quantitative variables were not normally distributed, a Mann Whitney U-test was utilized to compare two groups. The relationship between depression and time-to-event endpoints was summarized using the Kaplan-Meier method, and the curves were compared using the Log rank test. Cox proportional hazards regression models were utilized to compute crude and adjusted hazard ratios (aHR) and their corresponding 95% confidence intervals (CI) to investigate the association between depression and the risk of cardiovascular events. Model 1 was unadjusted, while Model 2 was adjusted for potential risk factors, including depression, age, AHI, ODI, Maximum apnea time, MSaO2, LSaO2, creatinine, uric acid, hypertension, CHD, Cerebrovascular disease, carotid atherosclerosis, AF, COPD, renal dysfunction, smoking, and alcohol use. Statistical significance was determined at bilateral P <0.05. All analyses were conducted in SPSS version 25.0 (SPSS Inc, Chicago, IL, USA).
Results
Baseline Characteristics
After excluding patients with myocardial infarction, history of hospitalization for unstable angina, or heart failure at screening, 1106[male (n=686) and female (n=420)] eligible patients were identified. Among these, 71 patients had been treated with CPAP, 17 patients could not be followed up. Thus, a total of 1106 patients were included in the analysis (Table 1). Baseline participant characteristics (median age, 70.0 years, 61.6% male) are shown in Table 1. Of the 1106 patients, 973 (87.7%) were not diagnosed with depression (median age, 65.0 years, 61.8% male) and 133 (12.0%) were diagnoses with depression (median age, 70.0 years, 63.9% male), with AHI values of 30.9 and 25.4 (P = 0.006).
 |
Table 1 General Characteristics of Study Subjects According to Depression |
Participants with depression in the group had higher median age (70 years vs 65 years), ODI (24.1 vs 19.4), creatinine (76.1 vs 72.0), uric acid (363.0 vs 340.0), SBP (140 mmHg vs 130 mmHg), Maximum apnea time (61.8 s vs 48.0 s), longest pause time (61.4 s vs 50.5 s), drinking status (18.5% vs 11.2%), and smoking status (32.3% vs 21.2%) than those in the control group (P < 0.05). MSaO2 (92.2% vs 94.0%) and LSaO2 (78.0% vs 81.0%) were significantly higher in patients without depression compared with those patients with depression. Furthermore, the proportion of comorbidities was significantly higher (P < 0.05) in patients with depression. For instance, AF (12.0% vs 8.2%), CHD (31.6% vs 21.9%), COPD (13.5% vs 6.0%), hypertension (73.7% vs 61.8%), carotid atherosclerosis (34.6% vs 24.5%), renal dysfunction (9.8% vs 2.7%), and cerebrovascular disease (29.3% vs 15.5%). In contrast, the proportions of participants with and without depression did not differ significantly (all P > 0.05) according to the sex, BMI, mean heart rate, hyperlipidemia, and other sleep parameters.
Mace
A total of 1106 participants were prospectively followed for 42 months or until the occurrence of MACE; during the median follow-up of 42 months (range 1–72 months), 96 patients (8.7%) experienced MACE, among whom 25 patients (26.0%) had comorbid depression. The incidence of MACE between these two groups was significantly different (P < 0.001; Table 2). Kaplan-Meier curves plotting the relationship between depressive status and the cumulative incidence of MACE indicated that patients with depression had a significantly higher cumulative event rate of MACE than those without depression (Log rank test, P < 0.001; Figure 2). In the unadjusted Cox proportional hazard model, depression was associated with a hazard ratio (HR) of 2.63 (95% CI, 1.66–4.14; P < 0.001; Table 3) for MACE. This association persisted even after multivariable adjustment for the depression and potential risk factors including age, AHI, ODI, maximum apnea time, mean and lowest oxygen saturation, creatinine, uric acid, hypertension, CHD, cerebrovascular disease, carotid atherosclerosis, AF, COPD, renal dysfunction, smoking, and alcohol use (aHR, 2.29; 95% CI, 1.34–3.90; P = 0.002; Table 3).
 |
Table 2 Crude Number of Adverse Events During Follow-Up |
 |
Table 3 Association Between Depression and Incidence of All Events |
 |
Figure 2 Kaplan-Meier curve of depression group and non- depression group on the risk of MACE. 0: non-depression group, 1: depression group, Log Rank P< 0.000. |
The Interaction Between AHI and Depression on the Risk of MACE
After adjusting for AHI, depression, and their interaction term in COX regression analysis, we observed a significant multiplicative interaction between AHI and depression on MACE in patients with hypertension (P < 0.05; Table 4). However, due to the inherent relationship between hypertension itself and cardiovascular disease, the clinical implications of this finding are constrained. While, no significant interaction was evident between AHI and depression concerning the incidence of MACE in the overall population or other subpopulations (P > 0.05; Table 4).
 |
Table 4 The Interaction Between AHI and Depression on the Risk of MACE |
Subgroup
Upon conducting a subgroup analysis, it was discovered that the aHR for MACE associated with depression was notably higher in individuals classified as overweight-obese (aHR, 2.98; 95% CI, 1.49–6.00, P = 0.002; Table 5), male (aHR, 2.96; 95% CI, 1.52–5.77, P = 0.001; Table 5), and those diagnosed with moderate-severe obstructive sleep apnea (OSA) (aHR, 2.82; 95% CI, 1.55–5.14, P = 0.001; Table 5). However, no statistically significant interaction was observed between depression and the variables in each group. Therefore, clinical trials are required to validate whether depression increases cardiovascular burden in these specific populations.
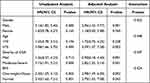 |
Table 5 Subgroup Analysis of the Associations Between Depression and MACE |
Discussion
In this multicenter, prospective, observational study of 1106 older participants with OSA without MI, heart failure, or hospitalization for unstable angina at baseline, we found that more than one-tenth of patients in our study population suffered from depression, as ascertained according to the GDS-12 criteria, depression was associated with the incidence of MACE. Furthermore, at a median follow-up of 42 months, patients with depression were at higher risk of MACE than were patients without depression. Depressive symptoms can impose cardiovascular burden on the elderly population with OSA. Therefore, this study has important implications for addressing the public health burden of OSA-related complications. To our knowledge, this is the first study to assess the relationship between depression and MACE risk in the older OSA population in mainland China.
Approximately 17% of the total population is affected by OSA, and OSA prevalence varies widely according to the age, ethnicity, and sex of the population studied.15 The clinical symptoms of the depression overlap with OSA, and untreated OSA can lead to affective disorders.16 Decreased sleep quality and reduced sleep duration due to sleep apnea may increase the risk of depression in individuals with OSA.17 Epidemiological studies18 estimate an up to 28.1% prevalence of depression in OSA populations. Depression, as a common psychiatric disorder, has been the subject of many studies that have evaluated the factors of CVD.19 However, Researchers have so far done little to investigate the expression of the combined or synergistic effect of both OSA and depression on the CVD risk in the older population. In our study, patients with depression showed a greater burden of comorbidities, such as hypertension and carotid atherosclerosis, all of which were associated with the long-term prognosis of patients, and these very intuitively reflected in our study, wherein the incidence of MACE was three times higher in patients with depression than in patients without depression, and there were intergroup differences in age, medical history, oxygen reduction index, and various sleep breathing parameters. Furthermore, the differential indicators were used as adjustment factors to determine their influence on the incidence of MACE, depression was found to be correlated with the risk of MACE.
Depressive symptoms have been correlated with CVD prevalence in the population, and some studies20 have reported that patients with depression are more likely to develop endothelial dysfunction compared to the normal population, this may constitute a possible mechanism for the association of depression with CVD. Furthermore, animal experiments have shown that depressive behavior in mice correlates with left ventricular wall thickness and elevated levels of acetyl heparin (HS) and chondroitin sulfate (CS) in the heart,21 which suggest that depression may alter cardiovascular physiology and lead to adverse cardiovascular events.
In this study, we did not identify any interaction between AHI and depression with respect to MACE risk in the overall population. Nonetheless, our findings suggest that such an interaction may be linked to unfavorable outcomes in patients with hypertension. It can be said that when patients with hypertension suffered from both of symptom of high AHI and depression, there was a higher risk of MACE. Among the chronic diseases found in older people, hypertension is one of the most common, and a high percentage of OSA patients are tend to be diagnosed with hypertension. Because of various complications and long-term effects of medication, patients with hypertension are often accompanied by depression.22,23 Given the potential impact of long-term complications, reduced quality of life, and impaired self-management of hypertension among individuals with OSA, comorbid hypertension, and depression, it is crucial to emphasize the importance of prognostic evaluation for this population.Additionally, a high AHI has been prevalent in hypertensive patients,24 which may contribute to a poor prognosis in this population.
Our study revealed that the concomitant presence of elevated AHI and depression may heighten the risk of MACE in elderly individuals with obstructive sleep apnea (OSA) and hypertension. These findings underscore the significance of closely monitoring sleep apnea treatments and depressive symptoms among hypertensive patients, promoting adaptive coping strategies such as CPAP and regular physical activity to mitigate the risk of MACE. While our results support the presence of an interaction between high AHI and depression on MACE risk in this population, further prospective investigations are warranted to corroborate our findings and elucidate underlying mechanisms.
Subgroup analysis showed that older male OSA patients with depression had a higher risk of MACE. Previous findings have shown that depression has predictive value for CVD in males with OSA.12 Compared to females, males are more socially isolated and less socially supported, and both isolation and social support are inherently associated with CVD.25 Eriksson26 found that the mechanism linking depression to CVD in male patients may be related to arterial stiffness. Decreased testosterone levels due to reduced sleep duration and sleep fragmentation in male patients were associated with an increased risk for depressive symptoms,27 which may explain the high risk of depression in the older male OSA population.
The risk of depression correlates with OSA severity, and patients with severe OSA are more likely to be depressed. The association between OSA-induced sleep disturbance and depression is bidirectional.28 Animal experiments29 have shown that OSA-related intermittent hypoxia causes rapid eye movement (REM) sleep abnormalities. Comparisons of patients with different sleep polysomnography findings show that patients with REM-OSA have a greater extent of airway collapse during REM sleep,30 and REM-OSA in males are associated with depression.31 Sleep disturbances are both a risk factor for and a symptom of depression. The onset of depression may be associated with the alterations of REM sleep.32 The characteristic sleep EEG changes in patients with depression include disinhibition of REM sleep, changes in sleep continuity, and impaired non-REM sleep. Furthermore, REM sleep alterations are associated with dysregulation of monoamine neurotransmitters, which occur in patients of depression.28 Thus, OSA and depression are likely to interact each other, and work together to induce MACE.
Obesity is a major factor in the increased risk for CVD-related morbidity and mortality and, in this study, overweight patients with OSA and depression were at greater risk of MACE in a BMI-based subgroup analysis. Obesity can increase the risk of CVD through multiple regulatory mechanisms, including excess adipose tissue leading to insulin resistance, inflammatory responses, activation of the renin–angiotensin–aldosterone system, and structural cardiac remodeling. Moreover, obese individuals, especially those who are metabolically unhealthy, have a higher risk of depression than metabolically healthy obese individuals.33
Study Strengths and Limitations
The sample size of our study is large, and there is a fairly long period of follow-up, but the homogenous Asian study population means that there is a need for further research into whether our results would apply to other populations. Depression is a complex disorder with multiple diagnostic criteria, this could have biased the results. The processes that lead to MACE are complex, multivariable models may not incorporate certain confounders or be incompletely adjusted. However, these limitations do not affect the main conclusion of our study.
Conclusion
Our study used Asian population-based multicenter cohort, depression was a complex risk factor that independently increased the risk of MACE in older patients with OSA in the absence of MI, history of hospitalization for unstable angina, or heart failure. In the subgroup analysis, obese males with moderate-severe OSA combined with depression exhibited a higher risk of MACE. Our findings strengthen the clinical utility of screening for depression as part of CVD risk assessment in older patients with OSA. Furthermore, we suggest that depression should be considered a modifiable risk factor for CVD prevention in patients with OSA and this recommendation needs to be validated in future clinical trials.
Data Sharing Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Statement of Ethics
This study complies with the Guidelines for Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) and was conducted in accordance with the tenets underlying the Declaration of Helsinki. This study was approved by the Ethics Committee of the General Hospital of the Chinese People’s Liberation Army (S2019-352-01), and all participants provided written informed consent prior to their study enrolment.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Master reports a grant from Army experimental animal special research project (SYDW_KY[2021]04), Military Health Care Project (23BJZ27, 22BJZ52, 19BJZ38) and Military Equipment Construction Application Research Project (LB20211A010013).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi:10.1093/aje/kws342
2. Liu YY, Wang Y, Walsh TR, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16(2):161–168. doi:10.1016/S1473-3099(15)00424-7
3. Salman LA, Shulman R, Cohen JB. Obstructive sleep apnea, hypertension, and cardiovascular risk: epidemiology, pathophysiology, and management. Curr Cardiol Rep. 2020;22(2):6. doi:10.1007/s11886-020-1257-y
4. Javaheri S, Barbe F, Campos-Rodriguez F, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol. 2017;69(7):841–858. doi:10.1016/j.jacc.2016.11.069
5. Drager LF, McEvoy RD, Barbe F, Lorenzi-Filho G, Redline S. Sleep apnea and cardiovascular disease: lessons from recent trials and need for team science. Circulation. 2017;136(19):1840–1850. doi:10.1161/CIRCULATIONAHA.117.029400
6. Chan MTV, Wang CY, Seet E, et al. Association of unrecognized obstructive sleep apnea with postoperative cardiovascular events in patients undergoing major noncardiac surgery. JAMA. 2019;321(18):1788–1798. doi:10.1001/jama.2019.4783
7. Liu Q, He H, Yang J, Feng X, Zhao F, Lyu J. Changes in the global burden of depression from 1990 to 2017: findings from the global burden of disease study. J Psych Res. 2020;126:134–140. doi:10.1016/j.jpsychires.2019.08.002
8. Penninx BW. Depression and cardiovascular disease: epidemiological evidence on their linking mechanisms. Neurosci Biobehav Rev. 2017;74(Pt B):277–286. doi:10.1016/j.neubiorev.2016.07.003
9. Wheaton AG, Perry GS, Chapman DP, Croft JB. Sleep disordered breathing and depression among U.S. adults: national health and nutrition examination survey, 2005–2008. Sleep. 2012;35(4):461–467. doi:10.5665/sleep.1724
10. Hayley AC, Williams LJ, Venugopal K, Kennedy GA, Berk M, Pasco JA. The relationships between insomnia, sleep apnoea and depression: findings from the American national health and nutrition examination survey, 2005–2008. Austral New Zealand J Psychiat. 2015;49(2):156–170. doi:10.1177/0004867414546700
11. Geovanini GR, Gowdak LHW, Pereira AC, et al. OSA and depression are common and independently associated with refractory angina in patients with coronary artery disease. Chest. 2014;146(1):73–80. doi:10.1378/chest.13-2885
12. Bouloukaki I, Fanaridis M, Stathakis G, et al. Characteristics of patients with obstructive sleep apnea at high risk for cardiovascular disease. Medicina. 2021;57(11). doi:10.3390/medicina57111265
13. Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. deliberations of the sleep apnea definitions task force of the American Academy of sleep medicine. J Clin Sleep Med. 2012;8(5):597–619. doi:10.5664/jcsm.2172
14. Jongenelis K, Pot AM, Eisses AM, et al. Diagnostic accuracy of the original 30-item and shortened versions of the geriatric depression scale in nursing home patients. Int J Geriatr Psychiatry. 2005;20(11):1067–1074. doi:10.1002/gps.1398
15. Framnes SN, Arble DM. The bidirectional relationship between obstructive sleep apnea and metabolic disease. Front Endocrinol. 2018;9:440. doi:10.3389/fendo.2018.00440
16. Vanek J, Prasko J, Genzor S, et al. Obstructive sleep apnea, depression and cognitive impairment. Sleep Med. 2020;72:50–58. doi:10.1016/j.sleep.2020.03.017
17. Li J, Cao D, Huang Y, et al. Sleep duration and health outcomes: an umbrella review. Sleep Breath. 2022;26(3):1479–1501. doi:10.1007/s11325-021-02458-1
18. Acker J, Richter K, Piehl A, Herold J, Ficker JH, Niklewski G. Obstructive sleep apnea (OSA) and clinical depression-prevalence in a sleep center. Sleep Breath. 2017;21(2):311–318. doi:10.1007/s11325-016-1411-3
19. Kyrou I, Kollia N, Panagiotakos D, et al. Association of depression and anxiety status with 10-year cardiovascular disease incidence among apparently healthy Greek adults: the ATTICA Study. Europ J Prevent Cardiol. 2017;24(2):145–152. doi:10.1177/2047487316670918
20. Waclawovsky AJ, de Brito E, Smith L, Vancampfort D, da Silva AMV, Schuch FB. Endothelial dysfunction in people with depressive disorders: a systematic review and meta-analysis. J Psychiat Res. 2021;141:152–159. doi:10.1016/j.jpsychires.2021.06.045
21. Luong H, Singh S, Patil M, Krishnamurthy P. Cardiac glycosaminoglycans and structural alterations during chronic stress-induced depression-like behavior in mice. Am J Physiol Heart Circul Physiol. 2021;320(5):H2044–h2057. doi:10.1152/ajpheart.00635.2020
22. Polishchuk OY, Tashchuk VK, Barchuk NI, Amelina TM, Hrechko SI, Trefanenko IV. Anxiety and depressive disorders in patients with arterial hypertension. Wiadom Lekarsk. 2021;74(3):455–459. doi:10.36740/WLek202103113
23. Liu Q, Wang H, Liu A, et al. Adherence to prescribed antihypertensive medication among patients with depression in the United States. BMC Psychiatry. 2022;22(1):764. doi:10.1186/s12888-022-04424-x
24. Brown J, Yazdi F, Jodari-Karimi M, Owen JG, Reisin E. Obstructive sleep apnea and hypertension: updates to a critical relationship. Current Hypert Rep. 2022;24(6):173–184. doi:10.1007/s11906-022-01181-w
25. Hu J, Fitzgerald SM, Owen AJ, et al. Social isolation, social support, loneliness and cardiovascular disease risk factors: a cross-sectional study among older adults. Int J Geriatr Psychiatry. 2021;36(11):1795–1809. doi:10.1002/gps.5601
26. Eriksson MD, Eriksson JG, Kautiainen H, et al. Higher carotid-radial pulse wave velocity is associated with non-melancholic depressive symptoms in men - findings from Helsinki birth cohort study. Anna Med. 2021;53(1):531–540. doi:10.1080/07853890.2021.1904277
27. Morssinkhof MWL, van Wylick DW, Priester-Vink S, et al. Associations between sex hormones, sleep problems and depression: a systematic review. Neurosci Biobehav Rev. 2020;118:669–680. doi:10.1016/j.neubiorev.2020.08.006
28. Fang H, Tu S, Sheng J, Shao A. Depression in sleep disturbance: a review on a bidirectional relationship, mechanisms and treatment. J Cell Molec Med. 2019;23(4):2324–2332. doi:10.1111/jcmm.14170
29. Polotsky VY, Rubin AE, Balbir A, et al. Intermittent hypoxia causes REM sleep deficits and decreases EEG delta power in NREM sleep in the C57BL/6J mouse. Sleep Med. 2006;7(1):7–16. doi:10.1016/j.sleep.2005.06.006
30. Joosten SA, Landry SA, Wong AM, et al. Assessing the physiologic endotypes responsible for REM- and NREM-Based OSA. Chest. 2021;159(5):1998–2007. doi:10.1016/j.chest.2020.10.080
31. Lee SA, Paek JH, Han SH. REM-related sleep-disordered breathing is associated with depressive symptoms in men but not in women. Sleep Breath. 2016;20(3):995–1002. doi:10.1007/s11325-016-1323-2
32. Wang YQ, Li R, Zhang MQ, Zhang Z, Qu WM, Huang ZL. The neurobiological mechanisms and treatments of REM sleep disturbances in depression. Curr Neuropharmacol. 2015;13(4):543–553. doi:10.2174/1570159X13666150310002540
33. Speed MS, Jefsen OH, Børglum AD, Speed D, Østergaard SD. Investigating the association between body fat and depression via Mendelian randomization. Translat Psychiat. 2019;9(1):184. doi:10.1038/s41398-019-0516-4
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
