Back to Journals » Stem Cells and Cloning: Advances and Applications » Volume 16
Alginate Beads as a Promising Tool for Successful Production of Viable and Pluripotent Human-Induced Pluripotent Stem Cells in a 3D Culture System
Authors Alsobaie S , Alsobaie T, Alshammary AF , Abudawood M, Mantalaris A
Received 6 April 2023
Accepted for publication 13 June 2023
Published 28 September 2023 Volume 2023:16 Pages 61—73
DOI https://doi.org/10.2147/SCCAA.S409139
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Bernard Binetruy
Sarah Alsobaie,1 Tamador Alsobaie,2 Amal F Alshammary,1 Manal Abudawood,1 Athanasios Mantalaris3
1Department of Clinical Laboratory Science, King Saud University, Riyadh, Saudi Arabia; 2Biological Systems Engineering Laboratory, Department of Chemical Engineering, Imperial College London, London, UK; 3Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology, Atlanta, GA, USA
Correspondence: Sarah Alsobaie, Department of Clinical Laboratory Science, King Saud University, Prince Turki Alawal Street, Riyadh, Saudi Arabia, Tel +966507191011, Fax +966114567888, Email [email protected]
Purpose: Two-dimensional (2D)-based cell culture systems, limited by their inherent heterogeneity and scalability, are a bottleneck in the production of high-quality cells for downstream biomedical applications. Finding the optimal conditions for large-scale stem cell culture while maintaining good cellular status is challenging. The aim of this study was to assess the effects of three-dimensional (3D) culture on the viability, proliferation, self-renewal, and differentiation of human induced pluripotent stem cells (IPSCs).
Patients and Methods: Various culture conditions were evaluated to determine the optimal conditions to maintain the viability and proliferation of human IPSCs in a 3D environment: static versus dynamic culture, type of adhesion protein added to alginate (Matrigel™ versus gelatin), and the addition of Y-27632t on long-term 3D culture. The proliferation ability of the cells was evaluated via the MTS proliferation assay; the expression levels of the pluripotency markers Nanog and Oct3/4, PAX6 as an ectoderm marker, and laminin-5 and fibronectin as markers of extracellular matrix synthesis were assessed; and HIF1α and HIF2α levels were measured using quantitative reverse transcription polymerase chain reaction.
Results: Using a high-aspect-ratio vessel bioreactor with a gentle, low-sheer, and low-turbulence environment with sufficient oxygenation and effective mass transfer of nutrients and waste, we verified its ability to promote cell proliferation and self-renewal. The findings showed that human IPSCs have the ability to maintain pluripotency in a feeder-free system and by inhibiting ROCK signaling and using hypoxia to improve single-cell viability in 3D culture. Furthermore, these cells demonstrated increased self-renewal and proliferation when inoculated as single cells in 3D alginate beads by adding RI during the culture period.
Conclusion: Dynamic 3D culture is desirable for the large-scale expansion of undifferentiated human IPSCs.
Keywords: stem cell culture, large-scale expansion, HARV bioreactor, self-renewal
A Letter to the Editor has been published for this article.
Introduction
There are several promising applications for human-induced pluripotent stem cells (IPSCs), including cell replacement therapies, tissue engineering, and high-throughput toxicological and pharmacological screening.1 The scalable expansion and differentiation of IPSCs are demanding and inefficient; in particular, they commonly require the use of two-dimensional (2D) cultures.2 Data on stem cell research have largely been acquired from studies using 2D monolayer culture.
However, even in this controlled environment, stem cells can exhibit significant heterogeneity in their behavior and characteristics. One factor that contributes to this heterogeneity is the variability among individual stem cells in terms of their gene expression profiles and epigenetic modifications, which can affect their differentiation potential and response to different stimuli. Additionally, stem cells can also exhibit micro-environmental heterogeneity, where the chemical and physical properties of the culture substrate and the culture medium can affect their behavior and differentiation.
To overcome these limitations, researchers have developed various techniques for 3D culture of stem cells, which can more closely mimic the complex micro-environment found in the body. 3D culture can promote the formation of cell clusters and tissue-like structures, which can provide more physiological insights into stem cell function and behavior.3–5
However, challenges such as mass transport limitation, the use of undefined components, and increased apoptosis and differentiation have been identified in 3D human PSC culture.6–9 Therefore, in this study, we assessed the effects of 3D culture on the viability, proliferation, self-renewal, and differentiation of human IPSCs.
Cell encapsulation involves entrapping living cells within semi-permeable membrane structures, forming a 3D structure. These membranes permit the exchange of nutrients, oxygen, and stimuli between the culture environment and the cells, excluding host antibodies that are larger than the capsule pore size, striving to create products with low immunogenic responses.10 Encapsulation11 of mouse and human embryonic stem cells (ESCs) prevents teratoma formation after transplantation into immunodeficient SCID mice, representing an important breakthrough in the field of PSCs. The 3D culture conditions enhance the proliferation, differentiation, and secretion of various cell types, particularly mesenchymal stem cells,12 mouse ESCs,11,13 human ESCs,14 and neural stem cells.15
Despite the numerous advantages of this approach, studies have highlighted significant challenges for 3D human PSC culture,16 including the use of undefined human- or animal-derived components (eg Matrigel™, serum, and/or albumin), which limit reproducibility and/or scalability, and impair the implementation of good manufacturing practice.17 Growing stem cells in 3D scaffolds, either as clumps, dissociated with single-cell inoculations, or inoculated cell aggregates, was reported to result in increased apoptosis and spontaneous differentiation along the human PSC clumps. Single-cell inoculation with Rho-kinase (ROCK) inhibitors (RI) can minimize heterogeneity, apoptosis, and variability among cell populations.18 Another obstacle to 3D culture is mass transport as a limiting factor under static conditions, as it is difficult for nutrients and gases to reach the center of the construct. This limitation is reduced when encapsulated cells are cultured in a “well-mixed” dynamic bioreactor.8,9 For example, a rotary wall vessel (RWV) bioreactor can improve mass transfer in alginate hydrogel beads by providing a constant fall motion mimicking a microgravity environment. This helps to reduce sheer stress and enhances passive diffusion transport 100-fold when using a high-aspect-ratio vessel (HARV) compared with that when using a RWV, which increases cell densities to approximately 3,000,000 cells/mL.9
The objective of this research was to examine how 3D culture would affect human IPSCs in terms of viability, proliferation, ECM production, self-renewal, and differentiation. Firstly, we observed the impact of cell adhesion protein added to alginate (Matrigel™ versus gelatin) on cell proliferation. Secondly, we analyzed the effects of long-term treatment with Y-27632 as well as the outcome of 3D static/dynamic conditions on the growth kinetics, pluripotency, ECM production, and oxygenation levels of IPSCs.
Materials and Methods
Summary of the Methods
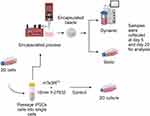
To optimize the growth of the cells in a 3D environment, the following conditions were tested: static versus dynamic culture, the addition of different adhesion proteins, and the addition of Y-27632. The proliferation of cells was measured using the MTS assay at different time points. In addition, the expression of the pluripotency markers was evaluated (Table 1) to confirm that a hypoxic niche was formed within the core of the beads due to the production of the ECM (Figure 1). For more details regarding our experimental methods, please refer to our previously published papers.19,20
 |
Table 1 Primer Sequences for Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR) |
Cell Culture
The cell line used in this study was the IMR90-1 IPSC line. The cells were purchased from the WiCell Research Institute Inc. (Madison, WI, USA).
2D Cell Cultures
Cells were cultured in Matrigel™-coated 6-well plates at approximately 200,0000 cells per well with 2 mL complete mTeSR™1 medium (STEMCELL Technologies, Vancouver, Canada) and 10 µM Y-27632 per well kept at 37 °C and 5% CO2 in a humidified incubator (Nuaire 5500E, NuAire, Caerphilly, Wales, UK). The cells were then subjected to a hypoxic environment containing 5% O2. The cells were observed using an inverted microscope (X70, Olympus-Life Science, Shinjuku-ku, Tokyo). The culture medium was replaced daily for 4 to 5 days. After this period, the colonies were dissociated using dispase in DMEM/F-12 solution (STEMCELL Technologies, Vancouver, Canada) and transferred onto new culture plates.
3D Cultures
3D alginate encapsulated cells were cultured either statically in T-bottles or dynamically on the HARV bioreactor.
Encapsulation Technique
Prior to encapsulation, cells were treated with 10 µM Y-27632 (Tocris BioScience, Bristol, UK) for two hours. To detach the cells, they were incubated with Accutase for 10 minutes at 37 °C (Innovative Cell Technologies, San Diego, CA, USA). Single-cell counts were performed using the trypan blue exclusion method according to the manufacturer’s protocol for the reagent purchased from Sigma-Aldrich (product number T 8154, Munich, Germany). A mixture of cells and alginate solution (prepared by mixing a PBS solution, low viscosity alginic acid (1.1% w/v), and bovine gelatin (0.1% v/v), obtained from Sigma-Aldrich, at pH 7.4) was dropped into a sterile 100 mM calcium chloride and 10 mM HEPES solution at pH 7 using a peristaltic pump (model P-1; Amersham Biosciences, Amersham, UK) to obtain beads of a uniform size (2.3–2.5 mm in diameter after swelling).
HARV Bioreactor
The HARV bioreactor (Synthecon Inc., Houston, TX, USA), also known as a rotating wall bioreactor, was specifically designed to study tissue and cell cultures in a low-sheer, non-turbulent environment. It has a large radius-to-depth ratio (40 × 10 mm) and provides a large surface area at the back for gas exchange through a gas-permeable membrane. The average volume of the bioreactor is 50 mL.
Alginate Bead Dissolution
To prepare the dissolving solution, a sterile solution of 50 mM trisodium citrate dihydrate (Honeywell Fluka, Morris Plains, NJ, USA), 77 mM sodium chloride (BDH Laboratory supplies, Poole, UK), and 10 mM HEPES (Sigma-Aldrich, Munich, Germany) prepared in PBS was used for Ca2+ depletion and depolymerization.
Real-Time Quantitative Polymerase Chain Reaction
Real-time quantitative polymerase-chain reaction (RT-qPCR) analysis of cDNA was performed in a StepOne Real-Time PCR System (Applied Biosystems, Waltham, MA, USA) by using the SYBR green detection system (SensiFAST™ SYBR® Hi-ROX Kit; Thermo Fisher Scientific, Waltham, MA, USA).
MTS Assay
The MTS substrate (CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS) kit; Promega, Madison, WI, USA) was prepared in a cell culture medium, added to cells in culture at a final concentration of 0.2–0.5 mg/mL of culture medium, and incubated for 1 to 4 h. The MTS standard curve was calculated using a known cell number (Supplementary Figure 1).
Live/Dead Assay
Live and dead cells were visualized in situ using the LIVE /DEAD® viability/cytotoxicity assay kit (Invitrogen, Carlsbad, CA).
Statistical Analysis
The average of three independent experimental runs ± the standard error of the mean was compared, either by unpaired two-tailed Student’s t-test or using one-way analysis of variance to analyze multiple groups. GraphPad software was used to conduct statistical analysis and plot the graphs. P values lower than 0.05 were regarded as statistically significant.
Results
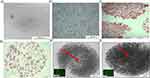
Characterization of Human IPSCs in Alginate Hydrogel Microbeads
We evaluated the morphology of undifferentiated human IPSCs encapsulated in alginate hydrogels and cultured for extended periods of time (20 days) without daily passaging or enzymatic or mechanical dissociation. Human IPSCs cultured on feeder-free Matrigel™ and mTeSR™1 monolayers were used as controls (Figure 2a). Prior to encapsulation, human IPSCs were treated with Y-27632 to produce single cells (Figure 2b). Each alginate bead contained approximately20000cells (Figure 2) following encapsulation. On day 5, aggregates were sectioned centrally; the cells were tightly packed and presented homogeneous characteristics (Figure 2c and d). Over time, the cells formed aggregates and, as shown on day 5, proliferated more in the dynamic culture group when compared to those in the static group (Figure 2e and f). The cell aggregates grew larger during the cultivation process, yet cells remained intact in the hydrogel throughout the whole culture period.
Simulated Dynamic Motion and 3D Culture Promote the Proliferation of Human IPSCs
After 24 h, the MTS and live/dead assays were performed before beads were moved into dynamic culture (Figure 3a and b). Viable single cells were observed, which permitted the normalization of time points within the first 24 h. By day 5, cellular proliferation under all conditions had increased more than two-fold compared with that on day 1. Proliferation under dynamic conditions has also increased significantly (p < 0.0001) more than that of the cells cultured in 3D static conditions (Figure 3). Moreover, the rate of proliferation under dynamic conditions was higher at all time points than that observed at day 1 of the culture and remained high until day 20, reaching 40,000 cells per bead (Figure 3b).
Effect of Adhesion Substrate on Cell Proliferation Added to Alginate Solution (Matrigel™ versus Gelatin)
To identify the optimal adhesive substrate to improve cell adhesion and subsequent proliferation, we compared the results obtained by using alginate solution mixed with Matrigel™ and that of alginate solution mixed with gelatin. Cell proliferation was quantified using the MTS assay. Favorable proliferation rates were maintained over time in both culture groups, with no significant difference between the conditions, except on day 10, when the proliferation rate of cells grown in alginate solution mixed with gelatin was higher (Figure 4).
Addition of 10 µm Y-27632 to Long-Term Culture
As an inhibitor of the ROCK signaling pathway, 10 µM Y-27632 was added to the culture media 2 h before passaging cells, which were encapsulated and maintained overnight under static conditions with 10 µM Y-27632. The first MTS measurements were taken before the beads were moved dynamically and revealed viable single cells. The cells were divided into two groups: one was treated with 10 µM Y-27632 (Dynamic RI) until day 20, and the other without Y-27632 (Dynamic without RI). Y-27632 treatment increased cell proliferation on days 5 and 20 in dynamic 3D culture compared with that in the non-treated group (Figure 5).
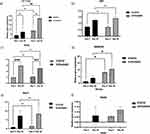
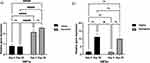
Gene Expression Profiles Associated with the Self-Renewal of Human IPSCs and Spontaneous Differentiation Markers
The gene expression of markers associated with pluripotency and spontaneous differentiation was measured. The impact of dynamic and static conditions on the expression of pluripotency markers Nanog, Utf1, Sox2, OCT3/4 and Rex1 (Figure 6a‒f) was compared at two time points: first, following short-term culture for 5 days; and second, following longer-term culture for up to 20 days, without any enzymatic treatment or mechanical stress to passage the cells.
The endogenous expression of Nanog and OCT3/4 was significantly higher on day 5 under dynamic 3D culture than under static 3D culture (Figure 6a). However, on day 20, OCT3/4 expression was more than 5-fold higher under dynamic 3D culture than under static culture; there was no significant change in Nanog expression compared with that under static culture (Figure 6b).
Both markers were significantly upregulated on day 20 compared with day 5 under dynamic culture (p < 0.01–0.001; Figure 6a‒b). Initially, under static 3D culture, there was no significant difference between days 5 and 20 for Nanog, Utf1, Rex1, and OCT3/4 expression.
PAX6 was evaluated as a marker of spontaneous differentiation.21 PAX6 mRNA was suppressed on day 5 under all 3D conditions used in this study (Figure 6c). Up to day 20, both static and dynamic conditions were able to suppress PAX6 compared with the 2D condition, which was used as a control. Thus, dynamic 3D culture can maintain human IPSCs in their pluripotent state for 20 days without passaging.
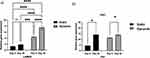
Effect of Hypoxia on Prolonged Adaption
To investigate whether the culture method in the present study formed a hypoxic environment owing to a higher rate of cellular proliferation, the relative gene expression of HIF1α and HIF2α was evaluated at the same time point as that of the pluripotency and differentiation markers. We assessed HIF1α expression between dynamic and static cultures on day 5. HIF1α regulates the response to hypoxia19 and is expressed in human IPSCs cultured under both dynamic and static conditions. HIF1α was significantly upregulated under dynamic 3D conditions (Figure 7a). Additionally, the effect of long-term 3D culture on HIF1α expression under both conditions was examined. The expression of HIF1α on day 20 remained significantly higher under the 3D dynamic condition than under the static condition (Figure 7a). Nonetheless, comparing HIF1Αα expression on days 5 and 20 under each condition revealed no significant upregulation compared with that of the control conditions (Figure 7a).
HIF2α was evaluated under all conditions as a second mediator of cellular response to hypoxia, as follows: dynamic 3D versus static 3D culture on day 5; dynamic 3D versus static 3D culture on day 20; dynamic 3D culture on day 5 versus day 20; and static 3D culture on day 5 versus day 20 (Figure 7b). HIF2α expression was identified in all culture groups, with no significant impact on the motion and elapsed time. HIF2α was significantly upregulated under static 3D culture conditions on day 5 compared with that on day 20. This may be associated with the development of hypoxia over time in static 3D culture, as there were a large number of cells under this condition, which may have prevented oxygen from reaching the center of the beads, thereby generating a hypoxic core.
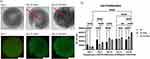
ECM Secretion by Human IPSCs Cultured in a 3D Environment
The main fibrillary components of the ECM differ and can be divided into two groups: collagens and cell-adhesive glycoproteins (eg laminin and fibronectin).22 The selection of two secreted ECM proteins, LAMA5 and FN1, was based on our previous findings that showed higher expression at the protein level on alginate encapsulated cells and also the importance of these proteins for hESCs self-renewal and viability.23 We hypothesized that higher expression was enhanced in 3D culture owing to the absence of adhesive alginate beads. Cells grown under dynamic 3D culture presented a higher expression of LAMA5 on days 5 and 20 than those grown under 2D or static conditions (Figure 8a). Moreover, we investigated whether cells could synthesize ECM throughout long-term culture. LAMA5 expression was significantly higher on day 20 than on day 5 in dynamic conditions (Figure 8b).
Next, the expression of fibronectin was evaluated in both 3D culture groups on days 5 and 20; FN1 expression was significantly increased on day 5 under dynamic conditions; however, on day 20, there was no significant difference between static and dynamic conditions. However, comparing the two conditions on different days, fibronectin gene expression was significantly increased on day 5 compared with that on day 20 in both conditions.
Discussion
In the present study, we were able to maintain high proliferation and pluripotency of IPSCs without cell passaging or use of feeder cells for prolonged culture time. Stem cells encapsulated in alginate beads maintain self-renewal for over 250 days without passaging.24 3D culture appears to maintain human ESCs in an undifferentiated state for several weeks.25 Notably, Gerecht et al encapsulated human ESCs in hyaluronic acid hydrogels, wherein the cells remained undifferentiated, presented a normal karyotype, and were capable of forming embryoid bodies.26
There was a noticeable increase in cell survival and proliferation when cultured in the HARV bioreactor. Dynamic or microgravity culture conditions can modulate cellular proliferation and survival.27,28 For example, simulated microgravity increases the proliferation of bone marrow-derived human mesenchymal stem cells.29 Additionally, it can modulate self-renewal. Simulated microgravity is sufficient to sustain pluripotency in mouse ESCs without the support of leukemia inhibitory factor.29 Additionally, 3D dynamic encapsulation alone enables fibroblast reprogramming without interference from transcription factors.30
Using a ROCK inhibitor throughout the culture time reduced apoptosis, as reported previously.31,32 The addition of 10 µM Y-27632 to the culture medium exerts a positive impact on human ESC culture, protecting cells from apoptosis after dissociation from the colony, even under serum-free suspension culture, allowing the formation of floating aggregates.18 In addition, another study tested the ability of 10 µM Y-27632 to enhance extracellular matrix production in HCS-2/8 cells, correlating with the RT-qPCR results shown in Figure 8, depicting the enhancement of ECM production in both static and dynamic 3D conditions under ROCK inhibitor treatment.33 In this study, the RT-qPCR results on day 5 revealed that the expression of FN1 and LAMA5 was significantly upregulated compared with that under the static condition. FN1 has been linked to a number of signaling pathways; for example, it phosphorylates Akt and activates PI3K/Akt signaling and genes associated with proliferation, such as C-Myc34 and caveolin-1 signaling, and their interaction in the ECM network enhances cell adhesion.35 ECM proteins, such as laminin 511 and 521, maintain human ESC pluripotency and self-renewal.36,37 Therefore, the higher expression of LAMA5 in dynamic conditions is correlated with the high expression of pluripotency markers, as previously shown (Figure 8). Proteins such as laminin and fibronectin have been used to maintain and prolong the self-renewal and proliferation of human ESCs.38–40 A mixture of LAMA5 and E-cadherin can prolong the self-renewal of human ESCs without the need for feeder cells.41 Moreover, the combination of collagen IV, fibronectin, laminin, and vitronectin has been used to replace Matrigel™ to derive and expand human PSCs under defined culture conditions.42 The ECM can also help the development of certain lineages, such as fibronectin and laminin, which stimulate neural cell lineage, and collagen, which promotes osteogenic maturation.43 Furthermore, fibronectin can interact with α8β1 to increase cell survival through the PI3K pathway via α8-mediated cell survival.44
The upregulation of hypoxia-inducible factor in the dynamic culture could be due to the enhancement in cell density and/or increment of ECM deposition. This is combined with high self-renewal. A previous study has suggested that a hypoxic environment maintains the renewal potential and proliferation of ESCs.45 Similar results were found in the present study as high hypoxic gene expression under dynamic culture corresponds with a higher cell volume and expression of pluripotency markers. Several studies have emphasized the benefits of low oxygen tension in stem cell cultures.45–47 Stem cells reside in a niche away from oxygen to prevent DNA damage and reactive oxygen species production and maintain self-renewal ability. Finding a balanced microenvironment to maintain stem cells ex vivo with a proper mixture of extrinsic and intrinsic factors such as ECM, oxygen tension, nutrients, and mechanical cues is essential in maintaining their unique properties of self-renewal and proliferation.
Alginate coated with gelatin is a non-toxic, non‑immunogenic, inexpensive, biodegradable, biocompatible material.48 The 3D culture systems are becoming increasingly popular in stem cell culture and are rapidly becoming an important tool for the industrialization of stem cell bioprocessing. This is because 3D cultures provide a more physiological microenvironment for stem cells, allowing them to interact with each other and the extracellular matrix in a way that is more representative of the in vivo environment. This can enhance stem cell proliferation, differentiation, and functionality, leading to improved stem cell production. The use of 3D culture systems also enables large-scale production of stem cells, as the culture systems can be scaled up. These systems can also be automated, which can increase efficiency and reduce labor costs.49,50
Overall, 3D culture has the potential to revolutionize the industrialization of stem cell culture and contribute to the development of advanced therapeutics and regenerative medicine applications.
Conclusion
Human IPSCs under dynamic 3D culture were able to secrete the necessary ECM components to form a suitable niche, which might help to maintain the pluripotent state. Although enhanced cellular proliferation resulted in acute hypoxia under dynamic 3D culture, low oxygen tension impacted the culture positively through the maintenance of pluripotency, proliferation, and viability. Finally, the addition of 10 µM Y-27632 to the culture improved the proliferation rate, particularly under dynamic 3D culture. Overall, these results suggest that dynamic 3D culture is desirable for the large-scale expansion of undifferentiated human IPSCs.
Abbreviations
2D, two-dimensional; 3D, three-dimensional; IPSC, induced pluripotent stem cell; ECM, extracellular matrix; ESC, embryonic stem cell; ROCK, Rho-kinase; RI, ROCK inhibitor; RWV, rotary wall vessel; HARV, high-aspect-ratio vessel; RT-qPCR, real-time quantitative polymerase-chain reaction.
Data Sharing Statement
The authors confirm that the data supporting the findings of this study are available within the article. Raw data are available from the corresponding author upon reasonable request.
Acknowledgments
The authors extend their appreciation to the deputyship for research and innovation, Ministry of Education in Saudi Arabia, for funding this research work through the project number IFKSURG-2-1743.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was funded by the deputyship for research and innovation, Ministry of Education in Saudi Arabia, under the project number IFKSURG-2-1743.
Disclosure
Part of this manuscript is based on Tamador Alsobaie’s thesis work entitled “Generation of functional mature type II pneumocytes from human induced pluripotent stem cells in 3D dynamic culture”, which was published (DOI: 10.25560/87943) by Imperial College London in March 2019. The authors report no conflicts of interest in this work.
References
1. Lewitzky M, Yamanaka S. Reprogramming somatic cells towards pluripotency by defined factors. Curr Opin Biotechnol. 2007;18:467–473. doi:10.1016/j.copbio.2007.09.007
2. Lei Y, Schaffer DV. A fully defined and scalable 3D culture system for human pluripotent stem cell expansion and differentiation. Proc Natl Acad Sci USA. 2013;110:E5039–E5048. doi:10.1073/pnas.1309408110
3. Wu X, Su J, Wei J, Jiang N, Ge X. Recent advances in three-dimensional stem cell culture systems and applications. Stem Cells Int. 2021;2021:1–13. doi:10.1155/2021/9477332
4. Chaicharoenaudomrung N, Kunhorm P, Noisa P. Three-dimensional cell culture systems as an in vitro platform for cancer and stem cell modeling. World J Stem Cells. 2019;11(12):1065–1083. doi:10.4252/wjsc.v11.i12.1065
5. Bluhmki T, Traub S, Müller A-K, et al. Functional human iPSC-derived alveolar-like cells cultured in a miniaturized 96-Transwell air–liquid interface model. Sci Rep. 2021;11(1). doi:10.1038/s41598-021-96565-4
6. Kinney MA, Sargent CY, McDevitt TC. The multiparametric effects of hydrodynamic environments on stem cell culture. Tissue Eng Part B Rev. 2011;17:249–262. doi:10.1089/ten.teb.2011.0040
7. Chowdhury F, Li Y, Poh YC, Yokohama-Tamaki T, Wang N, Tanaka TS. Soft substrates promote homogeneous self-renewal of embryonic stem cells via downregulating cell-matrix tractions. PLoS One. 2010;5(12):e15655. doi:10.1371/journal.pone.0015655
8. Hwang YS, Cho J, Tay F, et al. The use of murine embryonic stem cells, alginate encapsulation, and rotary microgravity bioreactor in bone tissue engineering. Biomaterials. 2009;30:499–507. doi:10.1016/j.biomaterials.2008.07.028
9. Randle WL, Cha JM, Hwang YS, et al. Integrated 3-dimensional expansion and osteogenic differentiation of murine embryonic stem cells. Tissue Eng. 2007;13:2957–2970. doi:10.1089/ten.2007.0072
10. Orive G, Hernández RM, Gascón AR, et al. Cell encapsulation: promise and progress. Nat Med. 2003;9:104–107. doi:10.1038/nm0103-104
11. Dean SK, Yulyana Y, Williams G, Sidhu KS, Tuch BE. Differentiation of encapsulated embryonic stem cells after transplantation. Transplantation. 2006;82:1175–1184. doi:10.1097/01.tp.0000239518.23354.64
12. Batorsky A, Liao J, Lund AW, Plopper GE, Stegemann JP. Encapsulation of adult human mesenchymal stem cells within collagen-agarose microenvironments. Biotechnol Bioeng. 2005;92:492–500. doi:10.1002/bit.20614
13. Wang X, Wang W, Ma J, Guo X, Yu X, Ma X. Proliferation and differentiation of mouse embryonic stem cells in APA microcapsule: a model for studying the interaction between stem cells and their niche. Biotechnol Prog. 2006;22(3):791–800. doi:10.1021/bp050386n
14. Dang SM, Gerecht-Nir S, Chen J, Itskovitz-Eldor J, Zandstra PW. Controlled, scalable embryonic stem cell differentiation culture. Stem Cells. 2004;22:275–282. doi:10.1634/stemcells.22-3-275
15. Liu TL, Song K, Song K, et al. Culture of neural stem cells in calcium alginate beads. Biotechnol Prog. 2006;22:1683–1689. doi:10.1002/bp060185z
16. Serra M, Brito C, Correia C, Alves PM. Process engineering of human pluripotent stem cells for clinical application. Trends Biotechnol. 2012;30:350–359. doi:10.1016/j.tibtech.2012.03.003
17. Unger C, Skottman H, Blomberg P, Dilber MS, Hovatta O. Good manufacturing practice and clinical-grade human embryonic stem cell lines. Hum Mol Genet. 2008;17:
18. Watanabe K, Ueno M, Kamiya D, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi:10.1038/nbt1310
19. Alsobaie S, Alsobaie T, Mantalaris S. Rho-associated protein kinase inhibitor and hypoxia synergistically enhance the self-renewal, survival rate, and proliferation of human stem cells. Stem Cells Cloning. 2022;15(7):43–52. doi:10.2147/SCCAA.S365776
20. Alsobaie T. Generation of functional mature type II pneumocytes from human induced pluripotent stem cells in 3D dynamic culture [PhD Thesis]. London, UK: Imperial College London; 2019. doi: 10.25560/87943.
21. Hu Q, Khanna P, Wong BSE, et al. Oxidative stress promotes exit from the stem cell state and spontaneous neuronal differentiation. Oncotarget. 2018;9:4223–4238. doi:10.18632/oncotarget.23786
22. Mecham RP. Overview of extracellular matrix. Curr Protoc Cell Biol. 2001;10:1. PMID: 18228295. doi:10.1002/0471143030.cb1001s00
23. Alsobaie S Characterisation of encapsulated embryonic stem cells using silac-based proteomics [Dissertation]. London, UK: Imperial College London; 2016. doi: 10.25560/40561.
24. Siti-Ismail N, Bishop AE, Polak JM, Mantalaris A. The benefit of human embryonic stem cell encapsulation for prolonged feeder-free maintenance. Biomaterials. 2008;29:3946–3952. doi:10.1016/j.biomaterials.2008.04.027
25. Li YJ, Chung EH, Rodriguez RT, Firpo MT, Healy KE. Hydrogels as artificial matrices for human embryonic stem cell self-renewal. J Biomed Mater Res A. 2006;79:1–5. doi:10.1002/jbm.a.30732
26. Gerecht S, Burdick JA, Ferreira LS, Townsend SA, Langer R, Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci USA. 2007;104:11298–11303. doi:10.1073/pnas.0703723104
27. Jha R, Wu Q, Singh M, et al. Simulated microgravity and 3D culture enhance induction, viability, proliferation and differentiation of cardiac progenitors from human pluripotent stem cells. Sci Rep. 2016;6:30956. doi:10.1038/srep30956
28. Grimm D, Wehland M, Pietsch J, et al. Growing tissues in real and simulated microgravity: new methods for tissue engineering. Tissue Eng Part B Rev. 2014;20:555–566. doi:10.1089/ten.teb.2013.0704
29. Yuge L, Kajiume T, Tahara H, et al. Microgravity potentiates stem cell proliferation while sustaining the capability of differentiation. Stem Cells Dev. 2006;15:921–929. doi:10.1089/scd.2006.15.921
30. Han J, Chen L, Luo G, Dai B, Wang X, Dai J. Three-dimensional culture may promote cell reprogramming. Organogenesis. 2013;9:118–120. doi:10.4161/org.24708
31. Claassen DA, Desler MM, Rizzino A. ROCK inhibition enhances the recovery and growth of cryopreserved human embryonic stem cells and human induced pluripotent stem cells. Mol Reprod Dev. 2009;76:722–732. doi:10.1002/mrd.21021
32. Chapman S, McDermott DH, Shen K, Jang MK, McBride AA. The effect of Rho kinase inhibition on long-term keratinocyte proliferation is rapid and conditional. Stem Cell Res Ther. 2014;5:60. doi:10.1186/scrt449
33. Piltti J, Bygdell J, Fernández-Echevarría C, Marcellino D, Lammi MJ. Rho-kinase inhibitor Y-27632 and hypoxia synergistically enhance chondrocytic phenotype and modify S100 protein profiles in human chondrosarcoma cells. Sci Rep. 2017;7:3708. doi:10.1038/s41598-017-03958-5
34. Park JH, Ryu JM, Han HJ. Involvement of caveolin-1 in fibronectin-induced mouse embryonic stem cell proliferation: role of FAK, RhoA, PI3K/Akt, and ERK 1/2 pathways. J Cell Physiol. 2011;226:267–275. doi:10.1002/jcp.22338
35. Yue B. Biology of the extracellular matrix: an overview. J Glaucoma. 2014;23(8 Suppl 1):S20–S23. doi:10.1097/IJG.0000000000000108
36. Laperle A, Hsiao C, Lampe M, et al. α-5 Laminin synthesized by human pluripotent stem cells promotes self-renewal. Stem Cell Rep. 2015;5:195–206. doi:10.1016/j.stemcr.2015.06.009
37. Domogatskaya A, Rodin S, Tryggvason K. Functional diversity of laminins. Annu Rev Cell Dev Biol. 2012;28:523–553. doi:10.1146/annurev-cellbio-101011-155750
38. Ahmed M, Ffrench-Constant C. Extracellular matrix regulation of stem cell behavior. Curr Stem Cell Rep. 2016;2:197–206. doi:10.1007/s40778-016-0056-2
39. Sevilla CA, Dalecki D, Hocking DC. Regional fibronectin and collagen fibril co-assembly directs cell proliferation and microtissue morphology. PLoS One. 2013;8:e77316. doi:10.1371/journal.pone.0077316
40. García AJ, Vega MD, Boettiger D. Modulation of cell proliferation and differentiation through substrate-dependent changes in fibronectin conformation. Mol Biol Cell. 1999;10:785–798. doi:10.1091/mbc.10.3.785
41. Rodin S, Antonsson L, Niaudet C, et al. Clonal culturing of human embryonic stem cells on laminin-521/E-cadherin matrix in defined and xeno-free environment. Nat Commun. 2014;5:3195. doi:10.1038/ncomms4195
42. Ludwig TE, Levenstein ME, Jones JM, et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006;24:185–187. doi:10.1038/nbt1177
43. Hosseinkhani H, Hosseinkhani M, Gabrielson NP, Pack DW, Khademhosseini A, Kobayashi H. DNA nanoparticles encapsulated in 3D tissue-engineered scaffolds enhance osteogenic differentiation of mesenchymal stem cells. J Biomed Mater Res A. 2008;85:47–60. doi:10.1002/jbm.a.31327
44. Farias E, Lu M, Li X, Schnapp LM. Integrin alpha8beta1-fibronectin interactions promote cell survival via PI3 kinase pathway. Biochem Biophys Res Commun. 2005;329:305–311. doi:10.1016/j.bbrc.2005.01.125
45. Forristal CE, Wright KL, Hanley NA, Oreffo ROC, Houghton FD. Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions. Reproduction. 2010;139:85–97. doi:10.1530/REP-09-0300
46. Pimton P, Lecht S, Stabler CT, Johannes G, Schulman ES, Lelkes SI. Hypoxia enhances differentiation of mouse embryonic stem cells into definitive endoderm and distal lung cells. Stem Cells Dev. 2015;24:663–676. doi:10.1089/scd.2014.0343
47. Hawkins KE, Sharp TV, McKay TR. The role of hypoxia in stem cell potency and differentiation. Regen Med. 2013;8:771–782. doi:10.2217/rme.13.71
48. Yasmin R, Shah M, Khan SA, Ali R. Gelatin nanoparticles: a potential candidate for medical applications. Nanotechnol Rev. 2017;6(2). doi:10.1515/ntrev-2016-0009
49. Rawat S, Sharma Y, Majood M, Mohanty S. 3D Culturing of Stem Cells: An Emerging Technique for Advancing Fundamental Research in Regenerative Medicine. IntechOpen; 2023. doi:10.5772/intechopen.109671
50. Jensen C, Teng Y. Is it time to start transitioning from 2D to 3D cell culture? Front Molec Bio. 2020;7. doi:10.3389/fmolb.2020.00033
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.