Back to Journals » Veterinary Medicine: Research and Reports » Volume 14
A Randomized, Self-Controlled Case Series Evaluating Core Osteostixis of Osseous Cyst-Like Lesions of the Navicular Bone to Improve Lameness in Horses with Podotrochlear Syndrome
Authors Brock BA, Greer HR, Honnas CM, Gilleland BE, Barrett MF, Moore JN, Cohen ND
Received 1 December 2022
Accepted for publication 27 February 2023
Published 15 March 2023 Volume 2023:14 Pages 35—46
DOI https://doi.org/10.2147/VMRR.S399835
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Young Lyoo
Supplementary video of "Osseous cyst-like lesions of the navicular bone" [ID 399835].
Views: 410
Bo A Brock,1 Hunter R Greer,1 Clifford M Honnas,2 Brad E Gilleland,3 Myra F Barrett,4 James N Moore,3 Noah D Cohen5
1Brock Veterinary Clinic, Lamesa, TX, USA; 2Texas Equine Hospital, Bryan, TX, USA; 3Department of Large Animal Medicine, College of Veterinary Medicine, Athens, GA, USA; 4Department of Environmental and Radiological Health Sciences, Colorado State University, Fort Collins, CO, USA; 5Department of Large Animal Clinical Sciences, School of Veterinary Medicine & Biomedical Sciences, Texas A&M University, College Station, TX, USA
Correspondence: Noah D Cohen, Department of Large Animal Clinical Sciences, College of Veterinary Medicine & Biomedical Sciences, Texas A&M University, College Station, TX, 77843-4475, USA, Tel +1 979 845 0741, Fax +1 979-847-8863, Email [email protected] Bo A Brock, Brock Veterinary Clinic, 1204 S Dallas Avenue, Lamesa, TX, 79331, USA, Tel +1 806 872 3183, Email [email protected]
Introduction: Podotrochlear syndrome is a common cause of lameness in Quarter Horses involving both soft tissue and bony structures within the heel region. Current surgical treatment of podotrochlear syndrome addresses pathological changes affecting the soft tissue structures of the navicular region but does not address either edema or cyst-like lesions of the navicular bone.
Objective: The objective of this randomized, self-controlled case series was to determine whether core osteostixis improved lameness in Quarter Horses with podotrochlear syndrome characterized by bilateral magnetic resonance imaging (MRI) findings of osseous cyst-like lesions of the navicular bone.
Methods: Seven Quarter Horses that had not responded to standard medical management were included. Each horse had an affected forefoot randomly assigned to surgical treatment with navicular bursoscopy and core osteostixis; the contralateral limb was assigned to navicular bursoscopy only. Video recordings were used to assign lameness scores and make comparisons of each limb at baseline and 24 weeks post-operatively by an observer blinded to the surgical treatment. A second MRI was performed 24 weeks after surgery to reevaluate navicular bone edema, osseous cyst-like lesions of the navicular bone, and tears of the deep digital flexor tendon (DDFT).
Results: Reduction of lameness score from baseline was significantly (P = 0.0254) greater for the limbs treated with core osteostixis than limbs treated with bursoscopy. New DDFT tears were noted in 3 of 7 limbs treated with core osteostixis and in 1 of 7 bursoscopy limbs.
Conclusion: Results of this study suggest that core osteostixis of the navicular bone combined with navicular bursoscopy can improve lameness in horses with osseous cyst-like lesions. Further evaluation of this technique is warranted.
Keywords: navicular disease, lameness, osteostixis, horse, surgery
Introduction
Podotrochlear syndrome, also termed palmar foot pain or navicular syndrome, refers to either unilateral or bilateral forelimb lameness originating from the podotrochlear apparatus.1,2 The condition is of great clinical importance because it is typically chronic, progressive and a highly prevalent cause of lameness, particularly in Quarter Horses.1,3 Diagnosis is generally based on a combination of the findings obtained from physical and lameness examinations, regional anesthesia, radiography, and, preferably, magnetic resonance imaging (MRI).1 Common radiographic findings indicative of navicular bone degeneration include sclerosis and radiolucent cysts within the medullary cavity, enlarged synovial invaginations and osseous fragments of the distal border, flexor cortical erosions, and alterations in the bone’s normal shape.1,3,4 The use of MRI has improved the identification of pathological findings of the navicular bone and associated soft tissue structures.5,6 MRI also allows the identification of “bone edema-like” changes within the navicular bone, which are characterized by increased bone signal on fluid-sensitive sequences such as short tau inversion recovery (STIR).5,6 The bone fluid signal likely represents changes in the medullary fluid content secondary to necrosis, fibrosis, and inflammation,6,7 which may result in alterations of intraosseous pressure and the formation of osseous cyst-like lesions of the navicular bone.3,4,7 It is speculated that these findings contribute to the pain associated with podotrochlear syndrome.3,5,8–11
Treatment of podotrochlear syndrome is challenging and relies primarily on podiatry and medical management. However, horses with medullary sclerosis and osseous cyst-like lesions of the navicular bone4 often fail to respond to this approach.12 It has been proposed that cyst-like lesions of the navicular bone occurs in 2 types: 1) cystic lesions at the proximal extent of synovial invaginations at the distal border of the navicular bone that communicate with these invaginations and 2) cystic lesions that arising from erosions at the base of the synovial invaginations.8 Although there is some evidence to support classifying cysts into these 2 categories,13 histological characterization of these cyst-like lesions varies,14 and to the authors’ knowledge, evidence is lacking that the clinical signs differ between horses with either type of cyst-like lesion (also referred to as pseudocysts).13,14 These horses require surgical management that most frequently includes the palliative procedure of palmar digital neurectomy and navicular bursoscopy for management of soft tissue injury.6,12 Bursoscopy has principally been recommended for the treatment of tears of the deep digital flexor tendon that occur either in isolation as discrete injuries which are not included in podotrochlear syndrome or in combination with other pathology of the navicular apparatus.15,16 Neither surgical approach addresses a large number of navicular bone lesions identified with MRI.
Core osteostixis via osteostixis has been used to treat human patients with osteonecrosis, a condition that shares many features with degenerative navicular bone disease.10,11 Core osteostixis of the equine navicular bone in healthy horses has been demonstrated to transiently reduce intraosseous pressure (which returned to levels higher than preoperative values 6 weeks after surgery) and result in remodeling (including mineralization of the drilled channels) and neovascularization of the bone; the drill tracts were approximately 90% filled with woven bone by 12 weeks postoperatively with signs of sclerosis in and around the drill tracts.11 Moreover, no adverse effects of osteostixis of the navicular bone were identified microscopically.11 However, the effects of core osteostixis on lameness and MRI findings in horses with clinical disease attributed to degenerative navicular bone disease are unknown. Thus, the aim of this randomized, blinded, self-controlled surgical trial was to provide an initial comparison of the effects on lameness scores of bursoscopy and core osteostixis of osseous navicular lesions (hereafter, core osteostixis) against bursoscopy only in horses with osseous cyst-like lesions and/or bone edema identified via low field, standing MRI. The hypothesis was that bursoscopy plus core osteostixis would improve lameness (ie, reduce the lameness score) relative to limbs in which only bursoscopy was performed.
Materials and Methods
Study Design and Study Population
A randomized, blinded, self-controlled trial was designed and conducted at the practice of the lead author. This practice does not have an institutional animal care and use committee, but the study design was reviewed and unanimously approved by the owners of the practice. All horses were handled within guidelines of best practices of veterinary care and in accordance with the veterinarians’ oath of the American Veterinary Medical Association. All horse-owners provided signed informed consent to the lead author to participate in the study. We estimated that it would be necessary to include 6 horses to demonstrate a significant difference if the lameness score for the core osteostixis limb improved more than the score for the bursoscopy limb based on a sign test, and a null hypothesis of equal likelihood (50%) of greater improvement in the core osteostixis limb due to chance alone. The following criteria were used for including horses in the study: 1) bilateral lameness of the forelimbs identified by visual examination that was attributed to the palmar foot area based on findings of positive response to palmar digital nerve blocks and sensitivity to hoof-testers in the heel region; 2) lameness score in each limb ≥2/5 using a lameness scale used in the practices of the lead author and the video examiner (Table 1) that is based on the American Association of Equine Practitioners lameness scale17; 3) age >5 years; 4) intended use for Western athletic performance, such as roping, cutting, or barrel racing; 5) lack of response to medical management (Table 2); 6) duration of lameness >4 months; 7) findings of bilateral bone edema, osseous cyst-like lesions or both on images of STIR sequence using a 0.27T standing MRI (Hallmarq Veterinary Imaging, Guildford, Surrey, UK); and 8) owner consent to enroll the horse in the study. Horses were excluded from the study if any of the following were present: 1) unilateral forelimb lameness determined by visual examination before and after palmar digital nerve blocks or concurrent hindlimb lameness; 2) radiographic or MRI evidence of another source of lameness other than the podotrochlear apparatus (eg, fracture of the distal phalanx, severe osteoarthritis, or chronic laminitis); 3) MRI evidence of osseous fragments in the impar ligament; 4) any prior surgical management of podotrochlear syndrome such as palmar digital neurectomy, desmotomy of the navicular bone suspensory ligament, or navicular bursoscopy; 5) medical treatment for podotrochlear syndrome within the 30-day-period prior to examination; and, 6) the owner declined to permit random assignment of the horse’s limbs for treatment.
 |
Table 1 Lameness Scale Used in This Study |
 |
Table 2 Prior Treatments Administered to Horses Before Inclusion in the Study |
Eligible horses were videotaped on a single day prior to inclusion in the study (baseline) and at and on a single day 24 weeks post-operatively. The videotaped examinations were all performed from the same vantage point with the horse trotted in a consistent pattern on a hard surface and on soft ground. Aside from one videotaped examination at 24 weeks post-operatively, each video recording took place at the same location and on the same surface. The horses were trotted in a straight line (towards and away from the videographer) and in a circle in both directions. The videotapes were reviewed by one of the investigators (CMH) who was not affiliated with the practice that conducted the surgery. This investigator was blinded to the procedure performed on each limb of each horse, but not to the time of the examination (baseline versus week 24 post-operatively). The investigator graded each lameness according to a 5-point scale (based on the AAEP guidelines,17 with a modification of adding a half-grade for horses that were deemed to be more severe than a given grade but did not meet the criteria for the grade above it); this grading system was developed by the authors independent of this project on the basis of need to be able to distinguish severity of lameness between limbs within a given grade. All grading was performed prior to data analysis, and the lameness scores were neither reviewed nor modified by the surgeon (BAB) or other investigators. Comparisons of the assigned lameness scores were made at baseline and 24 weeks post-operatively; lameness was assessed at 3 and 12 weeks solely to monitor recovery and potential adverse events related to the procedure, but these scores were not used for analysis. The same primary and assistant surgeons performed all procedures. Data collection and management was performed by a single investigator (NDC).
Each eligible horse had an affected forelimb randomly assigned to navicular bursoscopy and core osteostixis, with the contralateral limb assigned to navicular bursoscopy only. These assignments were made by an investigator remote from the practice (NDC) who was blinded to the clinical case findings. The assignments were made using a simulated coin flip with R statistical software (R Foundation for Statistical Computing, Vienna, Austria) using the sample command, an expected probability of 0.5 (unbiased coin), and a sampling frame of 0 (heads = LF) or 1 (tails = RF), for a single trial.
Surgical Procedure
Navicular bursoscopy was performed on both forelimbs of each horse as described by Smith et al.15 Briefly, the horse was placed in dorsal recumbency with the distal limb slightly flexed. The digital tendon sheath was distended with 15 mL of sterile saline before making 8-mm skin incisions on the lateral and medial borders of the DDFT approximately 8-mm proximal to the coronary band. These incisions created the medial and lateral portals for insertion of the arthroscope and surgical instruments. Using one portal, a 4-mm, forward-facing, 30° arthroscope with a blunt tip obturator within a cannula was inserted into the digital tendon sheath, replaced by the arthroscope, and passed distally over the dorsal side of the DDFT until the proximal side of the T ligament/collateral sesamoidean ligament was encountered. On the dorsal side of the DDFT, an incision was created through the proximal border of the T ligament/collateral sesamoidean ligament and was extended to the lateral and medial boundaries of the navicular bursa until the bursa communicated with the digital tendon sheath. At that time, the navicular bursa was evaluated for evidence of soft-tissue lesions. Tears involving the DDFT were resected, and adhesions were transected.
For the limb randomly assigned to receive core osteostixis, the drill-guide and drill were inserted through the other instrument portal. Radiographic and arthroscopic evaluations were used to position the drill for osteostixis. Osteostixis was performed by drilling a single hole into the medullary cavity of the proximal portion of the navicular bone with a 2.5-mm drill-bit (Figure 1; Supplementary Item 1); Supplementary Video. Intraoperative radiographic guidance determined the placement of the drill-bit and drill-guide on the navicular bone. If an osseous cyst-like lesion was identified (Figure 2A), the depth and location of the cyst dictated placement of the drill bit and the depth of drilling (Figure 2B). If navicular bone edema was identified on MRI in the absence of a cyst, a single hole was made in the middle of the proximal cortex of the navicular bone into the medullary cavity to an approximate depth of 75% of the distance between the proximal and distal margins of the bone, taking precautions not to penetrate the distal cortex. The area was lavaged with sterile saline solution (0.9% sodium chloride) during and after drilling.
All navicular bursae were lavaged with approximately 1500 mL sterile lactated Ringer’s solution throughout the procedure. The skin portals were closed using a single, simple-interrupted suture layer with 2–0 polypropylene suture. The limbs were dressed with a light sterile bandage consisting of kerlix gauze, cast padding, and Elastikon® (Johnson and Johnson, Inc., New Brunswick, New Jersey, USA).
Post-Operative Care and Rehabilitation
All horses received penicillin G procaine (20,000 IU/kg; IM; q 24 hr) and gentamicin (6.6 mg/kg; IV; q 24 hr) for 3 days after surgery, followed by 7 days of doxycycline (10 mg/kg; PO; q 12 hr). For post-operative pain, all horses received phenylbutazone (4.4 mg/kg; PO; q 24 hr) for 3 days and were maintained on firocoxib (0.22 mg/kg; PO; q 24 hr) for at least 7 days, but further duration varied on an individual basis. The limbs were rebandaged every 3 days using the materials listed above and remained bandaged until suture removal at 2 weeks post-operatively. At 3 and 12 weeks post-operatively, 6 mg of triamcinolone was infused via the basilar sesamoid approach into the tendon sheath of both limbs to reduce the likelihood of adhesion formation.
Each horse was housed in a 12- × 12-ft stall for 2 weeks after surgery and in a larger stall or pen (12- × 40-ft or 20- × 40-ft) for the remainder of the 4-month rehabilitation period. Hand-walking was initiated 2 days after surgery, and the duration was gradually increased over 2 months. During the 3rd and 4th months, light work under the saddle was performed approximately 5 days per week with a gradual increase in the level of intensity each week. Horses returned to intended use if sound at the end of the 4-month rehabilitation period.
MRI Procedure
Both limbs underwent 0.27T standing MRI (Hallmarq Veterinary Imaging, Guildford, Surrey, UK) imaging before surgery and 24 weeks post-operatively. A standard imaging protocol included the following sequences: sagittal T1GRE, T2*STIR, transverse T1 GRE, STIR, PD and T2 fast spin echo and dorsal T1GRE. The limbs were evaluated for the following outcomes: 1) navicular bone fluid signal; 2) osseous cyst-like lesions of the navicular bone, and 3) tearing of the DDFT. These outcomes were based on MRI reports provided by radiologists certified by the American College of Veterinary Radiology (ACVR). Radiologists noted whether there was an improvement in the extent of bone edema between exams but were not asked to grade the severity of bone edema; however, some radiologists occasionally described some of the navicular bone fluid signal intensity using subjective terms of mild, moderate, or severe.
Data Analysis
The primary outcome of this study was defined as a decrease in lameness score 24 weeks post-surgery from that observed at baseline. The null hypothesis tested was that the limbs in which the navicular bone underwent core osteostixis (hereafter termed core-decompressed limbs) were not significantly more likely than the limbs that underwent only bursoscopy (hereafter termed bursoscopy only limbs) to have decreased lameness scores (ie, mean paired differences between before and after surgery were not more likely to be > 0). We also compared the paired differences in lameness scores between the core decompressed versus the bursoscopy-only limbs and the right and left limbs, both at baseline and at 24 weeks. The data were analyzed using Wilcoxon sign-rank tests with R statistical software and the wilcox.test procedure, with a significance set at P < 0.05. A McNemars test was performed to compare the proportion of discordant limbs (core decompressed limb sound and bursoscopy limb unsound versus core decompressed no sound and bursoscopy limb sound) using R statistical software and the mcnemar.test procedure with significance set at P < 0.05. Only descriptive statistics were used to report MRI findings.
Results
Study Population
Seven Quarter Horses fulfilled the criteria for inclusion in this study, which was conducted from July 8, 2019, to August 22, 2019 (Table 2 and Table 3). All horses were geldings used for Western events, predominately calf roping or team roping. There was no significant difference in the severity of lameness between the RF and LF limbs at baseline (P = 0.1694), or between the core osteostixis and bursoscopy alone limbs (P = 0.4593). The limb randomly assigned to core osteostixis had a higher pre-surgical lameness score in 3 horses, a lower pre-surgical lameness score in 3 horses, and were equal before and after surgery in 1 horse (Table 3). Results of radiography, MRI and/or surgery indicated that 5 horses had bilateral cyst-like lesions in the navicular bone, all horses had tearing of the DDFT in at least 1 limb, and all but 1 horse had adhesions involving the DDFT in at least 1 limb (Table 4). Horse 3 did not have any identified tears on MRI, but a tear was identified intra-operatively in a limb of that horse.
 |
Table 3 Characteristics of the 7 Horses in the Study at Baseline |
 |
Table 4 Diagnostic Imaging and Surgical Findings for the 7 Horses in the Study |
Outcome
Clinical Findings
The primary study outcome was to test the hypothesis that the core osteostixis limbs would have a significantly greater reduction in lameness than the contralateral, bursoscopy only limbs (ie, the difference in magnitude of change in lameness score when compared to baseline would be significantly less than 0). The difference in improvement from baseline between core osteostixis limbs and bursoscopy only limbs was significantly less than 0 (P = 0.0254), indicating that the osteostixis procedure significantly improved the outcome relative to the limb not undergoing osteostixis (Table 5). Although the lameness scores at a single time-point 24 weeks after surgery (median, 0; range, 0 to 2) were lower for the core osteostixis limbs than those for the bursoscopy-only limbs (median, 1; range 0 to 2.5), this difference was not significant (P = 0.0848). Five of the 7 limbs in the core osteostixis group (71%) were sound at 24 weeks compared with only 1 (14%) of the 7 bursoscopy only limbs at 24 weeks: there were 4 horses in which the core osteostixis limb was sound and the bursoscopy only limb was unsound, 0 horses in which the bursoscopy only limb was sound and the core osteostixis limb was unsound, 2 horses in which both limbs were unsound, and 1 horse in which both limbs were sound. This difference in soundness between the paired core decompressed and bursoscopy-only limbs was not significant (P = 0.1250). Skin sensation in the heel area was evaluated at each post-operative examination in all horses, and evidence of painful neuroma formation was not detected in any horse at any examination. All horses were returned to their intended use by their owners 6 months post-operatively, despite residual lameness detected in some limbs of some horses when evaluated 24 weeks after surgery; however, continued performance past this timeframe is unknown. The decision to return to use those horses with residual lameness was made by the owners independently of recommendations of the authors.
 |
Table 5 Lameness Scores at Baseline, 24-Week Follow-Up, and the Difference Between Core Osteostixis (CO) and Bursoscopy Only (BA) Limbs |
MRI Findings
As secondary outcomes, we examined changes in 3 specific MRI findings between baseline and 24 weeks post-operatively, as reported by radiologists certified by the ACVR: 1) navicular bone fluid signal; 2) osseous cyst-like lesions in the navicular bone; and 3) tearing of the DDFT. All 7 horses had moderate to marked navicular bone fluid signal in both limbs prior to surgery. The fluid signal decreased in severity in 5 of the 7 limbs in the core osteostixis group. In contrast, the navicular bone fluid signal decreased in 3 of the 7 limbs in the bursoscopy-only group. Five of the 7 horses had cyst-like lesions bilaterally prior to surgery, and the sizes of the cysts remained unchanged after surgery in both groups. Tears of the DDFT were identified in 6 of the 7 horses pre-operatively. Post-operatively, new DDFT tears were identified in 3 of the limbs that had undergone core osteostixis, while 1 of the limbs that had undergone bursoscopy only had a newly identified tear.
Discussion
This study documents the use of core osteostixis for the treatment of degenerative navicular bone disease in a population of Western performance Quarter Horses with evidence of bone edema and osseous cyst-like lesions in the navicular bone. Results of the present study indicate that core osteostixis of the navicular bone via osteostixis reduced the severity of lameness without resulting in subsequent evidence of navicular bone damage (other than the defect from the osteostixis tract) as assessed using MRI in Western performance Quarter Horses that were refractory to standard medical treatments and shoeing. These findings are similar to studies involving human patients with bone marrow edema syndrome or in the acute phase of osteonecrosis, in which core osteostixis of the affected bone relieved pain associated with increased intraosseous pressure or bone marrow edema.18,19 Whether this procedure can benefit horses of other breeds and activities such as Sport Horses diagnosed with podotrochlear syndrome16 resulting from degenerative navicular bone disease with osseous cyst-like lesions similar to those of the horses included in our study merits investigation.
A common finding in the 7 horses in the current study was an increased navicular bone fluid signal. Such findings are consistent with MRI or histologic evidence of a bone edema-like syndrome3,7,8,11 and increased intraosseous pressure within the navicular bone in horses with degenerative navicular bone disease.20,21 In keeping with the latter finding, core osteostixis of the navicular bone transiently reduced intraosseous pressure in healthy horses and did so with few adverse effects, although it should be noted that maximal intraosseous pressure exceeded pre-osteostixis levels at 6 weeks after drilling, most likely because the drill tracts were filling with woven bone accompanied by sclerosis and fibrosis.11 However, the effectiveness of this procedure in horses with degenerative navicular bone disease had not been evaluated before the current study.
Horses with degenerative navicular bone disease frequently have accompanying soft tissue lesions of different severities, as well as the aforementioned bony changes. Thus, it is unclear whether the osteostixis alone, the repair of soft tissue lesions, or both is the reason lameness was improved in the horses in this study; however, the finding that the decompressed limbs improved significantly more than the bursoscopy only limbs suggests osteostixis was an important contributing factor to improvement in the lameness. Although navicular disease is often a bilateral disease, it is not necessarily symmetrical; as a result, it is possible that the more severely affected foot was assigned more often to one group or the other. Although randomization of the surgical treatment greatly diminishes this possibility, it does not preclude it from happening. It should be noted that preoperative (baseline) lameness scores did not differ significantly between those assigned to core osteostixis and those that were assigned to bursoscopy only. Using the contralateral limb as a control has the benefit of limiting confounding effects of factors such as nutrition, shoeing, genetics, and environment.
This project had limitations, one of which was the small sample size. Our intention with this work was, within the constraints of busy private practice and limited resources, to provide preliminary unbiased data regarding the feasibility and efficacy of the procedure. The encouraging nature of the results obtained indicates that larger-scale prospective observational or experimental (ie, clinical trials) studies should be conducted to further evaluate the therapeutic benefits of core osteostixis of the navicular bone in horses having similar clinical, radiographic and MRI findings to those reported here. Another limitation of the study was that the videos were reviewed by a single observer. Having multiple observers would have added to the validity of the reported lameness scores at 24 weeks post-surgery. A drawback associated with the latter approach is how best to amalgamate the scores from multiple observers. Regarding lameness evaluation, evidence exists that agreement is limited between observers in numerical gait-scoring.22,23 Consequently, we considered the reliability within the observer to be of primary importance and elected to use a single observer blinded to the surgical procedure used on each limb. Because the video observer (CMH) was not blinded to the timing of the examinations, we cannot exclude the possibility that the video observer was subconsciously biased to report lower lameness scores post-operatively. However, because the video observer was blinded to which limb underwent core osteostixis, biased interpretation could not explain the significantly lower lameness scores at follow-up for the core decompressed limbs than for the bursoscopy only limbs. As noted, it has been proposed that there are 2 types of osseous cyst-like lesions,8,13 and we did not attempt to distinguish between these 2 types of lesions. It is difficult to know the magnitude of this limitation in our results. We are not aware of evidence that clinical signs or pathogenesis are distinct for each of these 2 proposed types of lesions. Moreover, this was a self-controlled study, and it is highly improbable that the type of cyst-like lesion differed between limbs within horse. While other investigators have described the cyst-like lesions of navicular bones to involve full-thickness defects in the bone and cartilage, none of the horses in this study had visible evidence of fibrocartilage defects in the area of the cyst-like lesion (ie, none had arthroscopic evidence of full-thickness defects).
Using a device that quantifies gait analysis such as the Lameness Locator® (Equinosis Q, Columbia, MO, USA) would have obviated the need for an external evaluator to score lameness and would have improved the objectivity of our outcome measurements. It should be noted that some question the use of these devices for assessing lameness,24–27 although users tend to have more favorable impressions of the utility of such devices than non-users.27 Nevertheless, in retrospect, our study would have been improved by using a quantitative gait analysis device and including this as a primary outcome along with subjective lameness scoring.
Although localization of bilateral forelimb lameness using anesthesia of the posterior digital (PD) nerves was a criterion for inclusion in the study, scoring lameness was not repeated after PD nerve blocks. In horses with bilateral forelimb lameness from podotrochlear syndrome, it is common that the severity of the lameness in the limb deemed to be less severe becomes more noticeable after PD nerve block of the limb deemed to be more severe. Thus, we cannot exclude the possibility that our results might have differed had we scored horses before and after PD nerve blocks of each limb, with washout periods between blocks. We decided a priori not to evaluate horses after nerve blocks and re-evaluate after a wash-out period for several reasons. First, it provided an unbiased assessment of the horse’s lameness without having to account for variations resulting from evaluating the horse at different time-points on the same day and with effects of regional anesthesia. Second, it was less onerous for the lead author to conduct a single videorecording without having to wait for washout periods to repeat videorecording each horse. Third, it was less onerous for the evaluator because only a single examination needed to be evaluated for each horse at each study time-point. Fourth, we would have generated multiple scores from each examination from each phase of blocking which would have complicated analysis and interpretation of study findings. For example, it would have been possible that we might have observed differences in the score of a given limb without PD blocking 12 weeks after surgery than after the contralateral limb was blocked or after the nerve block wore off in the contralateral limb. Our goal was to determine whether the lameness had improved after surgery, and we selected the presentation of the horse without regional anesthesia to be an effective measure.
Another limitation of the study was that radiologists were not blinded to the assigned limbs. Consequently, the interpretation of the MRI results is subject to bias. Although the presence of bone edema was noted by the radiologists who reviewed MRI images, the radiologists were not requested to objectively quantify or subjectively classify the extent of bone edema; however, they did comment on whether bone edema had changed between the baseline and post-operative MRI examination. Although characterizing the amount of edema would have been an interesting addition to our report, the primary study outcome was a reduction in lameness score. Radiologists were not asked to comment on the character of the drill-tracts at the time of follow-up MRI. It has been reported in healthy horses that the drill-tracts are 90% filled with woven bone 12 weeks after osteostixis.11 All horses had cyst-like lesions of the navicular bone and various lesions affecting the navicular bursa or its adjacent tissues. Thus, while we can be confident that osteostixis improved lameness scores in the horses included in this study, we cannot be certain that this improvement was exclusively attributable to the bony lesions. However, it seems probable that osteostixis ameliorated lameness due to osseous lesions because navicular bursoscopy was reported to have limited success in improving horses with osseous lesions.15 It is important to note that horses with chronic podotrochlear syndrome with only osseous lesions of the navicular bone are not represented in the patient population of the lead author’s practice. The current study was also limited to Quarter Horses performing Western events. This occurred for 2 reasons. First, these horses reflect the base population of the practice in which the surgery was performed. Second, podotrochlear syndrome is highly prevalent in Quarter Horses.1,3 It is our hope that future larger scale studies will involve evaluation of core osteostixis of the navicular bone in horses of other breeds and used for other purposes.
It is important to note that there are potential complications associated with the core osteostixis procedure. For example, new tears in the DDFT were identified in 3 of the limbs that had undergone core osteostixis compared to 1 limb of the bursoscopy-only limbs (a difference that was not statistically significant). The finding that tears were more common in the limbs that underwent osteostixis suggests that some of these tears were iatrogenic; however, newly identified tears in a limb that underwent only bursoscopy raises the possibility that the process occurs naturally and that this apparent difference is simply due to chance. The clinical relevance of these tears remains to be determined, because their presence was not associated with worsening of the lameness score 24 weeks after surgery, although some experts consider all DDFT injuries to be clinically significant.
Some difficulties associated with the core osteostixis procedure may exist related to anatomical variations in the proximal margin of the navicular bone encountered during surgery. A case in point is Horse 2, which was the only participant in the study that had a higher lameness score at 24 weeks after surgery in the limb that had undergone core osteostixis as compared to the bursoscopy-only limb (Table 5). This horse was the first encountered in this study having a convex shape to the proximal border of its navicular bone, which made it more difficult for the surgeon to direct the drill bit to enter the medullary cavity of the bone as occurred in the other horses (Figure 3). In Horse 2, multiple attempts were made to enter the medullary cavity, and the drill bit inadvertently entered the navicular bursa and distal interphalangeal joint. Given these difficulties, we believe that it is imperative that the drill guide be seated directly over the proximal osseous part of the navicular bone with no soft tissue separating the drill guide from the bone. In other words, all soft-tissue structures associated closely with the proximal border of the navicular bone (eg, hypertrophied T-ligament, fibrotic scar tissue) must be removed to properly position the drill guide. Nonetheless, the lameness score for the core decompressed limb decreased more from its baseline value than the bursoscopy only limb in Horse 2.
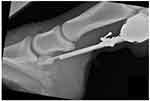 |
Figure 3 Ideal positioning of drill-bit over the proximal osseous portion of the navicular bone. |
Conclusion
Results of this case series suggest that core osteostixis combined with navicular bursoscopy as described by Smith et al16 can reduce the lameness scores of horses with podotrochlear syndrome that have failed to respond to standard medical management and shoeing. The promising results obtained in this study involving a small number of horses of a single breed justify examining the efficacy of core osteostixis in larger-scale studies including other breeds of horses and other types of use/athletic performance. Long-term follow-up of horses undergoing core osteostixis is warranted to determine the duration of improvement in lameness score and use of the horse. Although this study focused on a population with chronic disease, horses with early MRI evidence of podotrochlear syndrome also might benefit from this procedure because the edema-like signal evident early in the disease process may precede radiographic changes.7 Finally, surgeons wishing to use the core osteostixis procedure are strongly advised to remove all soft tissue from the proximal border of the navicular bone in order to appropriately seat the drill guide and direct the drill bit into the medullary cavity of the bone.
Acknowledgments
The Brock Veterinary Hospital provided support for imaging costs for this paper. Dr. Noah Cohen is supported by the Patsy Link Chair in Equine Research at Texas A&M University.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Waguespack RW, Hanson RR. Navicular syndrome in equine patients: anatomy, causes, and diagnosis. Compend Contin Educ Pract Vet. 2010;32:E1–E12.
2. Hoaglund EL, Barrett MF. Magnetic resonance imaging changes of the navicular bursa following navicular bursoscopy in seven horses. Equine Vet Educ. 2021;33:531–538. doi:10.1111/eve.13357
3. Dyson SJ. Navicular disease and other soft tissue causes of palmar foot pain. In: Ross MW, Dyson SJ, editors. Diagnosis and Management of Lameness in the Horse. Elsevier, St. Louis; 2003:286–299.
4. Dyson SJ. Radiological interpretation of the navicular bone. Equine Vet Educ. 2008;20:268–280. doi:10.2746/095777308X294306
5. Murray RC, Schramme MC, Dyson SJ, et al. Magnetic resonance imaging characteristics of the foot in horses with palmar foot pain and control horses. Veterinary Radiology. 2006;47:1–16. doi:10.1111/j.1740-8261.2005.00100.x
6. Barrett MF, Frisbie DD, King MR, et al. A review of how magnetic resonance imaging can aid in case management of common pathological conditions of the equine foot. Equine Vet Educ. 2017;29:683–693. doi:10.1111/eve.12542
7. Busoni V, Heimann M, Trenteseaux J, et al. Magnetic resonance imaging findings in the equine deep digital flexor tendon and distal sesamoid bone in advanced navicular disease--an ex vivo study. veterinary Radiology. 2005;46:279–286. doi:10.1111/j.1740-8261.2005.00051.x
8. Pool RR, Meagher DM, Stover SM. Pathophysiology of navicular syndrome. Vet Clin North Am Equine Pract. 1989;5:109–129. doi:10.1016/S0749-0739(17)30606-5
9. Rijkenhuizen ABM. Navicular disease: a review of what’s new. Equine Vet J. 2006;38:82–88. doi:10.2746/042516406775374216
10. Jenner F, Kirker‐Head C. Core decompression of the equine navicular bone: an in vitro biomechanical study. Vet Surg. 2011;40:163–170. doi:10.1111/j.1532-950X.2010.00766.x
11. Jenner F, Kirker-Head C. Core decompression of the equine navicular bone: an in vivo study in healthy horses. Vet Surg. 2011;40:151–162. doi:10.1111/j.1532-950X.2010.00765.x
12. Madison JB, Dyson SJ. Treatment and prognosis of horses with navicular disease. In: Ross MW, Dyson SJ, editors. Diagnosis and Management of Lameness in the Horse. Elsevier, St. Louis; 2003:299–304.
13. Wright IM, Kidd L, Thorp BH. Gross, histological and histomorphometric features of the navicular bone and related structures of the horse. Equine Vet J. 1998;30:220–234. doi:10.1111/j.2042-3306.1998.tb04491.x
14. Blunden A, Dyson S, Murray R, Schramme M. Histopathology in horses with chronic palmar foot pain and age-matched controls. Part 1: navicular bone and related structures. Equine Vet J. 2006;38:15–22. doi:10.2746/042516406775374298
15. Smith MR, Wright IM, Smith RK. Endoscopic assessment and treatment of lesions of the deep digital flexor tendon in the navicular bursae of 20 lame horses. Equine Vet J. 2007;39:18–24. doi:10.2746/042516407X151095
16. Smith MR, Wright IM. Endoscopic evaluation of the navicular bursa: observations, treatment and outcome in 92 cases with identified pathology. Equine Vet J. 2011;44:339–345. doi:10.1111/j.2042-3306.2011.00443.x
17. American Assossiation of Equine Practitioners. LAMENESS EXAMS: Evaluating the Lame Horse . Available from: https://aaep.org/horsehealth/lameness-exams-evaluating-lame-horseAccessed:
18. Calvo E, Fernandez-Yruegas D, Alvarez L. Core decompression shortens the duration of pain in bone marrow oedema syndrome. Int Orthop. 2000;24:88–91. doi:10.1007/s002640000120
19. Dolce M, Osher L, McEneaney P, Prins D. The use of surgical core decompression as treatment for avascular necrosis of the second and third metatarsal heads. Foot. 2007;17:162–166. doi:10.1016/j.foot.2007.04.001
20. Svalastoga E, Smith M. Navicular disease in the horse. The subchondral bone pressure. Nord Vet Med. 1983;35:31–37.
21. Pleasant RS, Baker GJ, Foreman JH, et al. Intraosseous pressure and pathologic changes in horses with navicular disease. Am J Vet Res. 1993;54:7–12.
22. Hewetson M, Christley RM, Hunt ID, Voute LC. Investigations of the reliability of observational gait analysis for the assessment of lameness in horses. Vet Rec. 2006;158:852–858. doi:10.1136/vr.158.25.852
23. Keegan KG, Dent EV, Janicek J, et al. Repeatability of subjective evaluation of lameness in horses. Equine Vet J. 2010;42:92–97. doi:10.2746/042516409X479568
24. Dyson S. Recognition of lameness: man versus machine. Vet J. 2014;201:245–248. doi:10.1016/j.tvjl.2014.05.018
25. Bathe AP, Judy CE, Dyson S. Do we have to redefine lameness in the era of quantitative gait analysis? Equine Vet J. 2018;50:273. doi:10.1111/evj.12791
26. Dyson S. Continued debate about what constitutes lameness. Equine Vet J. 2019;51:556. doi:10.1111/evj.13118
27. Hardeman AM, Van Weeren PR, Serra Bragança FM, et al. A first exploration of perceived pros and cons of quantitative gait analysis in equine practice. Equine Vet Educ. 2022;34:e438–e444. doi:10.1111/eve.13505
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


