Back to Journals » International Journal of Nanomedicine » Volume 19
A Promising Application of Injectable Hydrogels in Nerve Repair and Regeneration for Ischemic Stroke
Authors Gao Y, Zhang TL, Zhang HJ, Gao J, Yang PF
Received 1 November 2023
Accepted for publication 13 December 2023
Published 12 January 2024 Volume 2024:19 Pages 327—345
DOI https://doi.org/10.2147/IJN.S442304
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yan Shen
Yuan Gao,1,2,* Ting-Lin Zhang,3,* Hong-Jian Zhang,1,2,* Jie Gao,3 Peng-Fei Yang1,2,4
1Oriental Pan-Vascular Devices Innovation College, University of Shanghai for Science and Technology, Shanghai, People’s Republic of China; 2School of Health Science and Engineering, University of Shanghai for Science and Technology, Shanghai, People’s Republic of China; 3Changhai Clinical Research Unit, Shanghai Changhai Hospital, Naval Medical University, Shanghai, People’s Republic of China; 4Neurovascular Center, Changhai Hospital, Naval Medical University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Peng-Fei Yang, School of Health Science and Engineering, University of Shanghai for Science and Technology, Shanghai, People’s Republic of China, Tel +86-15921196312, Fax +86-2131161784, Email [email protected] Jie Gao, Changhai Clinical Research Unit, Shanghai Changhai Hospital, Naval Medical University, Shanghai, People’s Republic of China, Tel/Fax +86-21-31162092, Email [email protected]
Abstract: Ischemic stroke, a condition that often leads to severe nerve damage, induces complex pathological and physiological changes in nerve tissue. The mature central nervous system (CNS) lacks intrinsic regenerative capacity, resulting in a poor prognosis and long-term neurological impairments. There is no available therapy that can fully restore CNS functionality. However, the utilization of injectable hydrogels has emerged as a promising strategy for nerve repair and regeneration. Injectable hydrogels possess exceptional properties, such as biocompatibility, tunable mechanical properties, and the ability to provide a supportive environment for cell growth and tissue regeneration. Recently, various hydrogel-based tissue engineering approaches, including cell encapsulation, controlled release of therapeutic factors, and incorporation of bioactive molecules, have demonstrated great potential in the treatment of CNS injuries caused by ischemic stroke. This article aims to provide a comprehensive review of the application and development of injectable hydrogels for the treatment of ischemic stroke-induced CNS injuries, shedding light on their therapeutic prospects, challenges, recent advancements, and future directions. Additionally, it will discuss the underlying mechanisms involved in hydrogel-mediated nerve repair and regeneration, as well as the need for further preclinical and clinical studies to validate their efficacy and safety.
Keywords: ischemic stroke, central nervous system, hydrogels, neural restoration, nerve regeneration
Introduction
In the global context, ischemic stroke is one of the most prevalent and primary causes of disability and cognitive impairment.1 This condition is characterized by obstructions of blood vessels, leading to inadequate oxygenation and nutrition to the brain. This hypoxia/ischemia state triggers a series of neuropathological processes in tissues, causing substantial loss of brain parenchyma, diminished brain function, and profound neurological impairment.2 Ischemic stroke causes neuronal injury and death through three primary mechanisms. Firstly, it induces neurotoxic effects in glutamate, the primary neurotransmitter of the central nervous system (CNS), leading to severe neuronal death or damage.3 Secondly, neuronal damage is exacerbated by reactive oxygen species (ROS) generated due to mitochondrial dysfunction.4 Lastly, the inflammatory response triggered by ischemic stroke can also worsen nerve damage.5
As a result of ischemic stroke, neuron damage or death is particularly devastating, contributing to limited capacity for the CNS regeneration. It was historically believed that after a stroke, brain and CNS cells could not regenerate. However, evidence of neurogenesis in the adult human brain has emerged, demonstrating its occurrence in the hippocampal dentate nucleus and the subventricular region.6 Cells expressing markers associated with newborn neurons are found in the ischemic penumbra surrounding cerebral cortex infarction and tend to be predominantly situated near blood vessels.7
The addition of exogenous stem cells or therapeutic drugs to the affected region enhances their delivery and creates a favorable environment for the regeneration of damaged tissue.8 However, transplantation of stem cells and drugs can face challenges such as low survival rates and inadequate migration to the affected site. The mechanism through which stem cells contribute to nerve repair is more likely related to their ability to secrete various growth factors through paracrine action. These growth factors help to promote the endogenous brain nerve repair process, rather than solely relying on direct cell replacement. This paracrine signaling enables the stimulation of nearby cells to aid in the regeneration and recovery of damaged neural tissue. Delivering growth factors can indeed promote endogenous brain recovery,9 but one major obstacle is the limited diffusion of these molecules across the blood-brain barrier (BBB). To overcome this limitation, researchers have been exploring various strategies, such as using nanotechnology-based drug delivery systems, focused ultrasound to temporarily open the BBB, or even direct injection of growth factors into the brain. Hydrogels have garnered significant attention as an optimal framework for promoting cell proliferation and survival.
Hydrogels are described as a network of cross-linked polymeric units that form a 3D structure like the extracellular matrix (ECM) of native tissues. The ECM is a dynamic three-dimensional network of macromolecules that offer structural support for cells and tissues. Due to its inducible properties, the ECM is used as a source of injectable hydrogels for preparation in regenerative therapies.10 Hydrogels have the remarkable ability to hold a substantial amount of water within their structure, facilitating the transport of cells, drugs, and proteins, thus aiding in the repair of injured nerves. The injectable nature of hydrogels allows for precise targeting of therapeutic stem cells or small molecules to specific brain regions while minimizing invasiveness during the treatment process.11 The continuous expansion of tissue engineering, based on hydrogel, offers more possibilities for CNS regenerative therapy after ischemic stroke.
In the emerging field of hydrogel therapy for ischemic stroke, while some reviews have explored the advancements in the application of hydrogel biomaterials for the treatment of ischemic stroke, to the best of our knowledge, there is currently no comprehensive and systematic review that encompasses the various types of injectable hydrogels, design considerations for neural repair following stroke, and their underlying mechanisms of action. Therefore, there is a pressing need for a comprehensive summary and synthesis of the research progress concerning injectable hydrogels in the context of neural repair and regeneration for ischemic stroke, along with an examination of their potential applications, as well as the challenges they present. Such an effort will greatly facilitate our understanding of advanced therapies involving the design, engineering, and application of injectable hydrogels. This review introduces the classification and potential applications of hydrogels in nerve repair and regeneration after ischemic stroke, offering insights into the future possibilities for the clinical use and transformation of biomaterials (as shown in Figure 1).
Pathophysiological Mechanism of Nerve Injury in Ischemic Stroke
During cerebral artery embolization, blood flow to the corresponding brain region is impeded, leading to a significant reduction or blockage of blood supply. As a result, there is a lack of timely delivery of energy metabolites and oxygen to the affected area.12 After ischemic stroke, neurons experience disruptions in ATP synthesis, leading to energy deficiency. This deficiency causes an imbalance in ion gradients and results in the excessive release of excitatory amino acids, such as glutamic acid. Consequently, there is an influx of intracellular calcium (Ca2+), which triggers the activation of apoptosis and necrosis pathways, contributing to neuronal cell death.13 Ca2+ influx can stimulate the production of nitric oxide (NO), which, in turn, reacts with superoxide to form peroxynitrite. This peroxynitrite can lead to neurotoxicity, causing damage to neurons.14 Additionally, Ca2+ influx also contributes to an increase in the production of reactive oxygen species (ROS), primarily through oxidative phosphorylation in the mitochondria. The excessive ROS generation further exacerbates oxidative stress and cellular damage in the affected brain region.15 On one hand, ROS can have various effects on blood vessels and the blood-brain barrier, leading to nerve injury.16 ROS-induced oxidative stress can increase the permeability of the blood-brain barrier, making it more vulnerable to damage and potentially allowing harmful substances to enter the brain tissue.17 On the other hand, ROS can promote platelet aggregation, leading to the formation of blood clots, which can further impede blood flow and exacerbate ischemic damage. In addition, multiple signaling pathways participate in neuroinflammation in ischemic stroke.15 After ischemic stroke, the release of Damage-Associated Molecular Patterns (DAMPs) by injured or dead cells triggers neuroinflammatory responses. The neuroinflammatory response and the prolonged ischemia contribute to increased neuronal death and worsen the overall outcome after ischemic stroke.
After ischemia-reperfusion (I/R), blood flow recovery and reoxygenation may further aggravate brain tissue injury.18 After reperfusion, neutrophils, and other inflammatory cells in the blood infiltrate the ischemic area and release numerous inflammatory mediators, such as interleukin and nuclear factor-κB. These mediators facilitate the adhesion of inflammatory cells to endothelial cells, leading to the rupture and necrosis of endothelial cells. As a result, the blood-brain barrier is compromised, exacerbating the injury in the ischemia-reperfusion area.19 Moderate cerebral I/R injury can trigger autophagy and activate the cellular recovery system, allowing cells to survive and cope with the damage. In contrast, severe cerebral I/R injury primarily activates apoptosis and necrosis pathways, which can lead to significant cell death and exacerbate the overall injury.20
In regions surrounding an ischemic core area, cerebral blood flow (CBF) levels may be below functional thresholds required for normal cellular function. Despite this, they may still be temporarily above the threshold for cell death. This region is referred to as the penumbra area. The penumbra area is considered a critical zone where brain tissue has the potential to recover if blood flow is restored promptly and effectively. It is a target for therapeutic interventions aimed at salvaging brain tissue and reducing the extent of damage after ischemic stroke.21 Neuroprotective therapy is therefore primarily targeted at the penumbra, which represents potentially salvageable tissue.22 After I/R, the penumbra can be saved by regaining oxygen and nutrients, but it is undeniable that the nerve damage caused by I/R may also be difficult to recover.Traditional neuroprotection in stroke patients has focused primarily on mitigating the ischemic cascade reaction inside the affected brain area.23 In a new perspective, protection must focus on the entire neurovascular unit. The neurovascular unit comprises various cell types, including neurons, astrocytes, microglia, pericytes, and endothelial cells, all of which play crucial roles in maintaining the integrity and functionality of the brain’s blood vessels and neuronal network. By protecting the entire neurovascular unit, therapeutic strategies can address the complex interactions and interdependencies among these cell types, which collectively contribute to brain function and tissue repair. In the hours or days following a stroke, there is intercellular signaling between various components of the neurovascular unit, which can either amplify damage or promote protection of neurons. The interactions within the neurovascular unit are dynamic and can switch between these states (as shown in Figure 2).24 In the ischemic cascade, astrocytes and microglia respond to the “help me” signal from damaged neurons by releasing trophic factors, extracellular vesicles, and transferring mitochondria to support the neurons. Instead of solely targeting one specific component of the ischemic cascade, a promising approach is to employ cellular protection methods that can impact multiple aspects of the cascade. By considering the intricate interactions within the neurovascular unit, therapies can be designed to enhance the overall resilience of brain tissue to ischemic injury, leading to more comprehensive and effective neuroprotection.
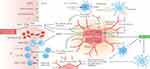 |
Figure 2 Ischemic cascade elements and mechanisms of neurovascular units in acute ischemic stroke. Notes: Printed with permission from Nature Publishing Group: Nature Reviews Neurology, Pharmacological brain cytoprotection in acute ischemic stroke — renewed hope in the reperfusion era, Marc Fisher, Sean I. Savitz. Copyright © Springer Nature Limited 2022.24 |
Hydrogels as carriers can effectively protect and maintain the bioactivity of stem cells and vesicles, creating a stable microenvironment at the injury site. This approach facilitates directed migration of stem cells and reconstruction of neurovascular units, thereby promoting effective nerve repair and regeneration. The integration of hydrogels with stem cells and vesicles offers a promising therapeutic strategy for targeted treatments of neurovascular units, leading to improved outcomes and rehabilitation for ischemic stroke patients.
Material Source-Based Classification of Injectable Hydrogels
Natural Biomaterials
Natural biological materials are substances produced under natural conditions, known for their excellent biocompatibility. In the physiological environment, these materials can gradually degrade into small molecules that already exist in the body. Eventually, they are completely absorbed or excreted through metabolism without causing toxic side effects on the body itself. Due to these characteristics, natural biological materials have been extensively utilized as hydrogels for nerve repair after ischemic stroke. By providing a favorable environment for cellular growth and facilitating therapeutic delivery, natural biological hydrogels show great potential in advancing regenerative therapies for neurological disorders, including ischemic stroke.25 The classification of hydrogels commonly used for nerve repair is shown in Figure 3 and Table 1.
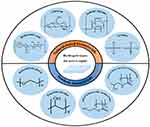 |
Figure 3 Source-based classification of the hydrogel carriers for nerve repair. Adapted with permission from Ma XY, Wang MJ, Ran YY, et al. Polymers, Design and Fabrication of Polymeric Hydrogel Carrier for Nerve Repair. Polymers (Basel). 2022 Apr; 14(8):1549.26 |
 |
Table 1 Material Source-Based Classification of Injectable Hydrogels in Nerve Repair and Regeneration |
Hyaluronic acid (HA) is a natural polysaccharide known for its exceptional gelling properties and the ability to bind water rapidly. One of the remarkable features of HA is its versatility in cross-linking or binding with various biomacromolecules. This characteristic makes it suitable for creating hydrogels with tailored properties for specific applications. HA-based hydrogels can be engineered to have desired mechanical strength, degradation rates, and drug release profiles. Moreover, HA hydrogels possess the capability to effectively encapsulate a wide range of drugs, including those in nanoscale formulations.42 In vitro experiments proved that HA hydrogel effectively promoted neurite growth,27 proliferation and differentiation of neural precursor cells and NSCs.28,29 These findings suggest that HA hydrogel has significant potential in supporting neural tissue regeneration and repair. In vivo, HA hydrogels undergo rapid degradation, forming a hydrated open web at the site of injury. This open web structure helps inhibit the formation of glial scars, which are known to secrete various axon regeneration inhibitors. By preventing the formation of glial scars, HA hydrogels create a more favorable environment for axon regeneration and functional recovery of damaged nerves after ischemic stroke. The inhibition of glial scars facilitates better neural tissue remodeling and improves the chances of successful nerve repair.26 Low molecular weight HA promoted the production of proinflammatory mediators, while high molecular weight HA inhibited the production of proinflammatory mediators.30,43 This differential effect is likely due to the varying interactions of HA with different cell receptors. Low molecular weight HA can bind to specific receptors on immune cells and activate proinflammatory pathways, leading to the production of inflammatory mediators. In contrast, high molecular weight HA interacts with different receptors, which can have anti-inflammatory effects and inhibit the release of proinflammatory mediators. The accumulation and production of high molecular weight HA by CD44-positive astrocytes inhibited myelin re-formation in the spinal cord sections of tested mice.44 HA deposits around medullated fibers and produces a lattice-like ECM substructure called the neural tract net, which surrounds neuronal cells and helps develop neuroplasticity and brain maturation.45 The modulation of HA levels and interactions may present potential therapeutic targets for promoting nerve repair and recovery in neurological disorders, including those resulting from ischemic stroke.
Collagen is the skeletal component of extracellular matrix and the most abundant protein in the human body. Collagen provides a supportive environment for cell attachment, proliferation, and differentiation.31 Gelatin is derived from the thermal denaturation of collagen, and it shares some properties with collagen but in a more processed and easily usable form. Gelatin has been utilized in various biomedical applications inducing nerve axons to regenerate due to its biocompatibility and ability to promote cell adhesion and proliferation.32 However, gelatin has poor mechanical strength and degrades rapidly. As a result, it is commonly mixed with other biological materials to enhance the performance of hydrogels.
Chitosan is derived from the deacetylation of chitin, a compound widely found in nature. Chitosan preserves the biological activity of host cells and facilitates the differentiation of neural progenitor cells.33 As a multi-purpose biocompatible polysaccharide, Chitosan have demonstrated neuroprotective effects. They provide a 3D feeder layer free culture system that promotes cell proliferation,46 which makes them promising candidates for neuroprotective therapies and nerve regeneration after ischemic stroke.34
Sodium alginate is a by-product of the extraction of iodine and mannitol from brown algae. It has antioxidant properties that protect neurons from oxidative stress.35 In addition, sodium alginate has been shown to trigger the regeneration of axon fibers and promote the formation of synapses, further supporting its potential in nerve repair and regeneration after ischemic stroke.36,47 However, one of the drawbacks of alginate-based hydrogels is that they can take months to fully degrade in the body. Additionally, these gels are considered bioinert, which means they lack the ability to actively interact with cells, limiting their ability to support cell survival and tissue integration.48
Synthetic Biomaterials
Compared with natural biomaterials, synthetic biomaterials can be produced in large quantities, have low cost and stable performance, and are also widely used in nerve repair. Due to these advantages, synthetic biomaterials have found widespread use in nerve repair and regenerative therapies.
Polyethylene glycol (PEG) is an oligomer or polymer of ethylene oxide. It is stable, non-toxic, and biocompatible. PEG exhibits various polymerization mechanisms and can be utilized to synthesize hydrogels or modify existing ones.49 By incorporating biological components, such as nutrient factors, into synthetic hydrogels, the functionality of PEG hydrogels in sustaining nerve cell activity can be significantly enhanced.50 PEG was implanted into the cavity after scar removal, and the results demonstrated that PEG facilitated long-distance axonal regeneration and led to improved nerve function in rats.37
Polyvinyl alcohol (PVA) is a crystalline polymer derived from polyvinyl acetate. PVA/HA hydrogels exhibit a broad range of stiffness and serve as valuable carriers for stem cell differentiation, cell migration, and tissue regeneration.38 However, a significant drawback of PVA is its inability to be completely degraded within the body. This limitation hinders its application in the biomedical field, as long-term persistence of PVA may impede natural tissue regeneration and clearance of the material from the body. As a result, researchers are actively exploring ways to improve the biodegradability of PVA-based hydrogels to enhance their compatibility with tissue regeneration applications.
Hydrogels based on poly (2-hydroxyethyl methacrylate) (PHEMA) is one of the most important hydrogels in the field of biomaterials. PHEMA hydrogels have garnered attention due to their biocompatibility, tunable properties, and ability to absorb and retain water, making them ideal for tissue engineering and regenerative medicine.51 The mechanical compatibility of PHEMA hydrogel allows it to restore anatomical continuity to damaged neural structures. However, PHEMA lacks inherent adhesion or attraction to neurons, which necessitates modification by incorporating substances capable of recognizing specific biological sites. Moreover, PHEMA is not readily degradable, making it susceptible to hydrogel calcification and eliciting prolonged inflammatory responses. These limitations may pose challenges in supporting long-term axonal regeneration. To address these issues, researchers are actively exploring strategies to improve PHEMA hydrogels’ degradation profiles, reduce inflammatory reactions, and enhance their overall biocompatibility for more effective nerve tissue regeneration and functional recovery.39 Lysine-coupled PHEMA hydrogel releases neurotrophic factors that significantly enhance neuronal survival and nerve growth.40 In comparison to PHEMA, poly [N-(2-hydroxypropyl)-methacrylamide] (PHPMA) demonstrates superior biocompatibility but is also less susceptible to degradation. By incorporating peptide or amino sugar sequences, PHPMA hydrogel can enhance the adhesion of nerve tissue and promote axon growth.41,52
Responsiveness-Based Classification of Injectable Hydrogels
Hydrogels are designed to mimic ECM-related properties of the brain, thereby promoting stem cell adhesion, proliferation, and differentiation to repair nerves. The application design of injectable hydrogels requires consideration of certain properties to facilitate repair.
Injectable hydrogels for nerve repair in the brain need to be thixotropic and have some stiffness and elasticity in the body.53 The elasticity of the matrix can regulate the proliferation and differentiation of neural stem cells and play an important role in the construction of scaffolding of central nervous system tissues.54 The design of injectable hydrogels must enable rapid gelation upon contact with tissue to fill the diseased defect and maintain their position. Additionally, hydrogels are expected to exhibit good biocompatibility and low toxicity. Biocompatibility of hydrogels is demonstrated in two aspects: cytocompatibility with encapsulated cells and histocompatibility with host tissues. Injectable hydrogels should integrate with host cells, degrade gradually, and avoid inducing tissue scarring and glial cell formation.55 Furthermore, hydrogels possess an optimal pore size and interconnection, which can effectively enhance the vitality, proliferation, and migration of NSCs.56 Previous studies have suggested that the optimal pore size and scaffold porosity for nerve cell growth are 10–100μm and more than 85%, respectively.57 Finally, for hydrogels to mimic stem cell ecosystems, they need to be able to sense and respond to external stimuli.58 These external stimulus responses include light, thermal, PH, magnetic, and electrical responses, which will be introduced in more detail below (as shown in Table 2).
 |
Table 2 Responsiveness-Based Classification of Injectable Hydrogels in Nerve Repair and Regeneration |
Light-Responsive Injectable Hydrogels
Light-responsive hydrogels are created by incorporating photosensitive components into their polymer structure. The polymer network of these hydrogels includes a light-receiving part that enables reversible crosslinking reactions and photothermal excitation. Upon receiving light, the photosensitive groups within the hydrogels undergo partial or complete cross-linking, degradation, expansion, or contraction.59,69 Light-responsive hydrogels hold significant potential for developing precise and controllable drug delivery systems. Photocleavage is a reaction that involves incorporating photocleavable linkers into the hydrogel’s structure to generate photocleavable nanoparticles.70 Drugs in this design can essentially maintain their efficacy in controlled release. A study60 showed that PEG-based hydrogels more accurately control the release rate of embedded model drugs via Azobenzene/Cyclodextrin Complex Tethers. In another study,71 the authors established an innovative synthetic procedure that uses a photosensitive protein as a reversible binding site for photoactivation in PEG hydrogels to control recombinant protein release. However, the clinical transformation of light-responsive hydrogels still faces some problems. One major concern is the potential for non-specific light response in normal tissues, which may lead to unintended effects or side effects. Another issue is maintaining the stability of loaded drugs within the hydrogel matrix.
Thermal-Responsive Injectable Hydrogels
Certain polymers may undergo precipitation or micellization when thermo-responsive hydrogels sense an ambient temperature above a specific threshold. Thermo-responsive hydrogels have demonstrated the capability to deliver various types of drugs, including proteins, DNA, and antibiotics.70,72 The temperature sensitivity of thermo-responsive hydrogels allows for controlled drug release in response to temperature changes, offering a promising platform for precise and on-demand drug delivery. This feature makes them particularly suitable for localized drug delivery to injured nerve tissues, where the therapeutic agents can be released in a targeted manner to facilitate nerve repair and promote functional recovery.61 Thermo-responsive hydrogel scaffolds based on xyloglucan have been reported to enhance neuronal adhesion and support neurite growth.62 Johnson et al73 combined nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF), allowing it to self-assemble into nanoparticles at their respective transition temperatures. These nanoparticles retain the bioactivity of neurotrophic proteins and serve as robust drug carriers that can be utilized for nerve regeneration. Thermal-responsive hydrogels are among the most used in situ hydrogels, offering the ability to release drugs accurately and effectively at physiological temperatures.
PH-Responsive Injectable Hydrogels
PH-responsive hydrogels undergo swelling or contraction of the polymer network in response to a specific pH environment. In the context of cerebral ischemia, CO2 accumulates in the intercellular space, leading to a decrease in the pH of the injured area.74 In addition, insufficient oxygen and glucose supply increase the consumption of glycogen and phosphocreatine, leading to the production of H+ ions and rendering the intracellular environment acidic.75 This acidic environment can further influence the behavior of pH-responsive hydrogels, making them responsive to the specific conditions in the ischemic brain tissue. In a particular study, pH-sensitive copolymers were employed to target the delivery of SDF-1α, a chemokine, with the aim of promoting neurogenesis and angiogenesis at the site of ischemia.63 By responding to the acidic conditions in the ischemic tissue, the hydrogel can release therapeutic agents or growth factors at the precise location and time, stimulating the natural regenerative processes of nerve tissue and supporting functional recovery.64 This targeted approach holds great potential for enhancing nerve repair and promoting the restoration of neural function after ischemic events.
Magnetic-Responsive Injectable Hydrogels
Hydrogels typically exhibit an inherently isotropic and disordered internal structure. However, magnetic-responsive hydrogels can be created by incorporating exogenous paramagnetic or ferromagnetic additives into the polymer matrix.76 An intriguing study77 reported a magnetic-responsive hydrogel with the ability to induce unidirectional growth of functional nerve cells. Before the matrix fully gels, the hydrogel is strategically positioned in situ, aligned with a low external magnetic field. Once the magnetic field is withdrawn, the aligned fibers become fixed, preserving the anisotropic structure of the gel. This magnetically sensitive hydrogel effectively promotes the unidirectional growth and extension of fibroblasts and nerve cells, presenting a promising approach for guiding and supporting tissue regeneration with enhanced cellular alignment and functionality.65,66 The anisotropic properties of magnetic-responsive hydrogels are more analogous to biological tissues in morphology and mechanical property, showing better biocompatibility.78 However, the potential toxicity and limited reproducibility of magnetic-responsive injectable hydrogels in in vivo studies raise concerns, and further optimization is necessary for their translation to successful clinical outcomes.79
Electrical-Responsive Injectable Hydrogels
Electrical-responsive hydrogels can facilitate endogenous brain repair after ischemic stroke injury due to the active regenerative response of nerve cells to electrical stimulation. Electrical stimulation has been shown to enhance stroke recovery by upregulating the expression of vascular endothelial growth factor (VEGF) and promoting the secretion of factors that induce cortical remodeling and endogenous angiogenesis.80 To create a conductive polymer hybrid system, heparin methacrylate (HepMA) has been incorporated into a polyvinyl alcohol (PVA) hydrogel and further electrodeposited with poly(3,4-ethylenedioxythiophene)-doped p-toluene sulfonate (PEDOT/pTS).81 The conductive polymer mixed with hydrogels can be utilized for in vitro culture and electrical stimulation of neural stem cells. Research findings indicate that this combination leads to enhanced cell proliferation and neurite growth, suggesting that the conductive properties of the polymer contribute to the promotion of neural cell development and maturation.67,68 While conductive polymers show great potential in various biomedical applications, their cytotoxicity remains a significant concern.82 Addressing cytotoxicity issues will be essential for ensuring the successful translation of conductive polymers into practical and safe biomedical applications in the future.
Different Functions of Injectable Hydrogels in Nerve Repair After Ischemic Stroke
Natural hydrogels have a similar composition to brain tissue, which makes them less likely to cause immune rejection when used in regenerative therapies. They have the potential to suppress inflammatory responses and provide a favorable environment for cell growth and tissue repair. However, the controllability and stability of natural hydrogels in vivo may not meet the requirements. The physical and chemical properties of synthetic hydrogels can be adjusted by changing the parameters in the synthesis process. Hydrogels that combine natural and synthetic properties perform better and can be gelatinized in situ, allowing local drug delivery, thereby increasing the bioavailability of therapeutic agents. At the same time, the excellent biocompatibility of the hydrogel allows the stem cells, their vesicles, and the drug to be continuously released at the site of injury without removal. The use of hydrogels has gained significant attention in the field of regenerative medicine, especially for promoting tissue repair and recovery after ischemic stroke (Figure 4).
 |
Figure 4 Application of hydrogels in central nervous system repair and regeneration. Reproduced with permission from Hasanzadeh E, Seifalian A, Mellati A, et al. Materials Today Bio, Injectable hydrogels in central nervous system: Unique and novel platforms for promoting extracellular matrix remodeling and tissue engineering. Mater Today Bio. 2023;20:100614.83 |
Physical Support and Biocompatibility
Hydrogels offer customizable properties such as elasticity and degradation behavior, making them highly versatile in various tissue engineering applications, including nerve regeneration in the brain after ischemic stroke.84 Cells cultured in a hydrogel environment exhibited lower levels of cytokine production compared to control cells cultured in a tissue culture dish. Importantly, despite the reduction in cytokine secretion, no inflammatory activity was observed in the hydrogel-cultured cells. This suggests that hydrogels have anti-inflammatory properties.85 The development of a bionic hydrogel crosslinked with the biogenic amine spermidine demonstrated biomimicry properties that closely resemble those of rabbit brain tissue. This biomimetic characteristic is advantageous for supporting the growth and development of neurons in a way that closely resembles their natural environment.86 In addition, in hydrogels were prepared by ultrasound induced gelation of regenerated fibroin protein solution and injected into mouse striatum to evaluate its biosafety. The findings from this study demonstrate that in situ fibroin hydrogels exhibit excellent biocompatibility and can seamlessly coexist with the intricate neuronal circuits responsible for controlling sensorimotor functions, learning, and memory mechanisms.87 The biocompatibility and customizable properties of hydrogels determine their ability to serve as scaffolds for nerve growth cues in the body. By customizing the pore size of the hydrogel, it is possible to influence the directed growth of nerve cells on its surface. Solid scaffolds after cutting or forming can better adapt to the irregular injury geometry, but do not fit well with the surrounding tissue. The hydrogel binding scaffolds have the advantage of being able to completely fill the irregular geometry of CNS injuries. A popular approach involves combining electrospun fibers with hydrogels to create composite scaffolds. The electrospun fibers provide the solid scaffold structure, while the hydrogel component allows for excellent cell infiltration into the injury site. This combination promotes the migration of cells to the site of injury and creates a conducive environment for nerve regeneration and tissue repair.88 Overall, hydrogels can help guide nerve repair and provide clues for nerve directed growth.
Providing a Favorable Microenvironment for Stem Cells Transplantation
In recent years, researchers have increasingly focused on the combination of biomaterial scaffolds and cell therapies for central nervous system (CNS) regeneration. Stem cells, known for their clonogenic nature and ability to self-renew, possess the potential to differentiate into various cell types.89 Many experimental studies in ischemic stroke have shown that mesenchymal stem cells (MSCs) can regulate the immune response and play a neuroprotective role by stimulating neurogenesis, astrogenesis, and angiogenesis.90 Additionally, the transplanted stem cells can exert immunomodulatory effects, helping to regulate the immune response and reduce inflammation at the site of injury.91 The integration of hydrogels with stem cell therapies presents a promising approach to enhance nerve tissue repair and regeneration, particularly in conditions like ischemic stroke or CNS injuries. These stem cells can be guided and supported by the hydrogels, which provide a conducive microenvironment for their survival, proliferation, and differentiation. Biomaterials based on hydrogels can support The stem cells to maintain normal vitality and function in the body and are critical in the transformation of cell-based therapies (as shown in Table 3).92 Wei et al93 successfully developed injectable hydrogels with an elastic modulus like brain tissue, utilizing N-carboxyethyl chitosan (CEC) and oxidized sodium alginate (OSA) as the primary structural components for the delivery of neural stem cells. Similarly, a fiber-hydrogel complex with adjustable stiffness has shown to be highly beneficial for cell attachment and proliferation. Hydrogels with a modulus of approximately 20 kPa demonstrated the most optimal performance in providing a conducive loading environment for cellular activities, further supporting nerve tissue regeneration.94 Moreover, protein-based hydrogels have been successfully employed in nerve repair and regeneration efforts following ischemic stroke. For instance, fibrin glue subdural transplantation of induced pluripotent stem cells has shown promising results in improving focal ischemic injury, leading to the restoration of neurological and behavioral functions, and providing neuroprotective effects.95 Wang et al96 prepared a genipin-cross-linked sericin hydrogel (GSH) with a porous structure and mild swelling ratio to support effective adhesion and growth of neurons in vitro (Figure 5). The combination of hydrogels with stem cells is a highly promising direction in the field of nerve repair and regeneration.97 As research continues to advance in this field, the combination of hydrogels with stem cells holds great promise for addressing the challenges posed by CNS injuries resulting from ischemic stroke.
 |
Figure 5 Diagram of sericin hydrogel used as carrier to repair neuronal cells in ischemic stroke. (A) Extraction and formation of the genipin-crosslinked sericin hydrogel (GSH). (B) Cytotoxicity of the GSH. (C) Primary neurons effectively adhere and grow in the GSH and (D) the confocal images. Adapted with permission from Wang Z, Wang J, Jin Y, et al. A neuroprotective sericin hydrogel as an effective neuronal cell carrier for the repair of ischemic stroke. ACS Appl. Mater. Interfaces. 2015;7(44):24629–24640. Copyright © 2015 American Chemical Society.96 |
 |
Table 3 Drug Type-Based Classification of Injectable Hydrogels in Nerve Repair and Regeneration |
Mediating the Release of Biologically Active Substances
Studies surface the stem cells do not integrate into resident neural networks but act indirectly through paracrine mechanisms to induce neuroprotection and promote nerve regeneration.103 Extracellular vesicles (EVs) are membrane structures containing proteins, lipids, and nucleic acids that can express properties like those of their source cells. Compared to MSCs, MSCS-derived EVs have lower immunogenicity and the ability to cross the blood-brain barrier.104 In recent studies, the effects of injecting MSCs and MSC-derived EVs on cerebral angiogenesis and neurogenesis after cerebral ischemia in mice were systematically compared. The results revealed that MSC-derived EVs showed comparable therapeutic effects to MSCs. This indicates that EVs are not inferior to MSCs in promoting the regeneration of blood vessels and nerve cells in the brain after ischemic stroke.105 Nanoscale EVs can not only evade phagocyte clearance, but also penetrate tissues more effectively. Hydrogels can serve as reservoirs, encapsulating and protecting the EVs, preventing their rapid clearance, and prolonging their retention time in the body.106 By combining nanoscale EVs with hydrogels, researchers aim to create an optimized therapeutic strategy for nerve repair after conditions like ischemic stroke. Fan and colleagues107 used EVS-loaded conductive hydrogels for spinal cord nerve repair, enhancing local neural stem cell recruitment, and promoting neuron and axon regeneration.
The hydrogels can also serve as an effective delivery system for growth factors and drugs in the context of nerve repair and regeneration after a stroke (as shown in Table 3). The therapeutic application of targeted growth factors may provide an extended therapeutic window to endogenously restore tissue damaged by stroke.98 HAMC hydrogel delivers erythropoietin (EPO), which also stimulates the migration of neural stem cells and mature neuroblasts and reduces apoptosis at the site of injury.99 Similarly, the use of gelatin hydrogel microspheres to deliver insulin-like growth factor-1 and hepatocyte growth factor, respectively, increased the number of new neurons in the subventricular region of the adult mammalian brain and the number of new neurons migrating to the damaged striatum (as shown in Figure 6).100 For injectable hydrogel slow-release growth factors and drugs, different bioactive substances require different time and speed, and the stimulation response of binding hydrogel can better achieve the slow-release effect.101,102 Hydrogels can be engineered to respond to specific internal body stimuli, allowing for precise and on-demand release of bioactive materials.108 For example, due to the ability of bioelectrical signals to regulate cell behavior, conductive polymers are incorporated into the hydrogel network to adapt to the electrophysiological properties of nerve tissue and promote the regeneration of neurons and myelin-associated axons.107
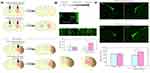 |
Figure 6 Gelatin hydrogel microspheres loaded with growth factors for preparation and augmentation of neurons. (A) Design of growth factors released by gelatin hydrogels. (B) Effects of IGF-1-containing gelatin hydrogels on SVZ neurogenesis. (C) The effect of gelatin hydrogels containing HGF on the number of new neurons migrated. Adapted with permission from Nakaguchi K, Jinnou H, Kaneko N, et al. Stem Cells International, Growth factors released from gelatin hydrogel microspheres increase new neurons in the adult mouse brain. Stem Cells Int. 2012;2012:915160.100 Abbreviations: IGF-1, insulin-like growth factor 1; SVZ, subventricular zone; HGF, hepatocyte growth factor. |
In summary, nerve repair and regeneration after ischemic stroke is a complex process involving multiple pathways and multiple signals. By providing a controlled and sustained release of growth factors and drugs, hydrogel stents serve as a significant advancement in this field. The ability of hydrogels to continuously release therapeutic substances within the body represents a crucial step towards the clinical application of ischemic stroke treatment.
Imaging of Hydrogels
Hydrogels have broad prospects in nerve repair and regeneration after ischemic stroke. However, currently, assessing their properties in vivo is challenging, and critical information such as adhesion and degradation of hydrogels at the administration site cannot be observed in real time. To date, several methods have been developed that allow the detection of hydrogels through various imaging techniques without compromising their rheological properties and biocompatibility.109–111 For example, diamagnetic chemical Exchange Saturated transfer (CEST) magnetic resonance imaging is a non-invasive analytical method that detects concentration dependent CEST signals from ECM precursors and hydrogels in vitro. In vivo, specific CEST signals detected around the periinfarction lumen correspond to endogenous ECM molecules. By subtracting the pre-implantation image from the post-implantation image, the researchers were able to observe the distribution of the hydrogel in the stroke cavity and visualize its evolution over a 7-day period.112 The imaging effect can also be achieved by synthesizing hydrogels that can image themselves, or by dynamic interactions such as ionic interactions and hydrogen bonds, or by forming covalent bonds or coordination bonds with contrast agents and encapsulating contrast agents.113 The manganese-Labeled hydrogel was able to form stable fibers when injected into the cerebrospinal fluid, and the deposition was monitored by T1-weighted magnetic resonance imaging (MRI) after injection.114 Similarly, alginate gels loaded with manganese ions enable non-invasive MR Imaging, allowing for hydrogel visualization in vivo. Manganese diffuses through the ventricular system into the brain parenchyma, and depending on the concentration of manganese, strong signals can be seen from the olfactory bulb to the brain stem.115 The controllability of hydrogel provides a possibility for its application in nerve regeneration and repair in ischemic stroke. By controlling the slow-release rate and sensitivity of hydrogels, the state of hydrogels and the release of their host cells and drugs can be monitored in vivo for a long time.
Conclusion and Outlook
Ischemic stroke is still threatened with a high recurrence rate and high disability rate after treatment. There is an increasing demand for effective central nervous system (CNS) treatment strategies to address these issues. The unique properties of hydrogels, such as their biocompatibility, tunable characteristics, and ability to mimic the extracellular matrix, make them an ideal platform to create a stable and supportive environment for nerve regeneration. By acting as scaffolds, hydrogels can guide the direction of nerve growth and provide a conducive microenvironment for cellular activities, promoting tissue repair and regeneration. There are several obstacles to overcome, such as the complexity of CNS injuries, the precise control of hydrogel properties, and the need for long-term safety and efficacy assessments. Further research and rigorous testing are required to address these challenges and ensure the safety and effectiveness of hydrogel-based therapies for ischemic stroke in human patients.
As we all know, the human brain is extremely complex, and when designing hydrogels for neural applications, we need to consider the mechanical strength of different parts of the human brain, multiple cell types, and other complexities to make them well adapted to the internal environment. Natural hydrogels have soft, viscoelastic, and tissue-like properties that reduce the likelihood of an immune response when implanted in the body. The physical and chemical properties of synthetic hydrogels are easier to adjust, but their poor mechanical properties often limit their use in soft tissues and non-load-bearing tissues, and they are easy to remove.116 Therefore, it is possible to combine natural and synthetic materials to form a composite hydrogel, combining the advantages of these two components for nerve regeneration after stroke. As an alternative drug delivery system, hydrogels can provide greater therapeutic efficiency, but safety and feasibility need to be considered. High porosity and lack of proper spatial and temporal control of the material often lead to rapid explosive release of the hydrogels, which can lead to a toxic risk of rapid release of the drug. When developing hydrogels for nerve repair, a good balance needs to be maintained before delivery efficiency and safety. The potential of using stem cells to promote neural repair is also a new way to construct cytocompatibility and effective coagulation vectors. While stem cell transplant treatments have been shown to be effective in nerve regeneration from ischemic stroke, they still have the problem of heterogeneity in the body, and stem cells grow slowly in the body, inevitably replicating aging.117 EVs has low immunogenicity and the ability to cross the blood-brain barrier. It can bind to corresponding targets through various pathways, and the therapeutic effect is no less than that of stem cell transplantation. Therefore, hydrogels coated EVs is also an effective means to target nerve repair after ischemic stroke.
Due to the complexity of the brain and the presence of BBB, the delivery method of hydrogels is generally in situ injection. More attention is being paid to nanoscale hydrogels, or hydrogel microspheres, which can be injected through blood vessels in some way to penetrate the BBB and target the treatment site. Detecting and monitoring the hydrogel using non-invasive imaging techniques provides important information on the functional status of the implant.118
In conclusion, the hydrogels discussed in this review show great potential for nerve repair and regeneration after stroke and provide a unique platform for developing new approaches to improve the efficacy of ischemic stroke treatment. Despite the existing challenges, the future of hydrogels as a biomaterial for nerve repair in ischemic stroke looks promising. As we overcome these obstacles, hydrogels have the potential to revolutionize the treatment of ischemic stroke and significantly improve patients’ quality of life.
Acknowledgments
This study was funded by the“Shuguang Plan” project of Shanghai in 2022 (approval number: 22SG37) and the National Natural Science Foundation of China (grant number: 82071278).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gorelick PB. The global burden of stroke: persistent and disabling. Lancet Neurol. 2019;18(5):417–418. doi:10.1016/S1474-4422(19)30030-4
2. Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371(9624):1612–1623. doi:10.1016/S0140-6736(08)60694-7
3. Mao R, Zong N, Hu Y, Chen Y, Xu Y. Neuronal death mechanisms and therapeutic strategy in ischemic stroke. Neurosci Bull. 2022;38(10):1229–1247. doi:10.1007/s12264-022-00859-0
4. Orellana-Urzúa S, Rojas I, Líbano L, Rodrigo R. Pathophysiology of ischemic stroke: role of oxidative stress. Curr Pharm Des. 2020;26(34):4246–4260. doi:10.2174/1381612826666200708133912
5. Maida CD, Norrito RL, Daidone M, Tuttolomondo A, Pinto A. Neuroinflammatory mechanisms in ischemic stroke: focus on cardioembolic stroke, background, and therapeutic approaches. Int J Mol Sci. 2020;21(18):6454. doi:10.3390/ijms21186454
6. Kempermann G, Song H, Gage FH. Neurogenesis in the Adult Hippocampus. Cold Spring Harb Perspect Biol. 2015;7(9):a018812. doi:10.1101/cshperspect.a018812
7. Jin K, Wang X, Xie L, et al. Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci U S A. 2006;103(35):13198–13202. doi:10.1073/pnas.0603512103
8. Chan SJ, Love C, Spector M, Cool SM, Nurcombe V, Lo EH. Endogenous regeneration: engineering growth factors for stroke. Neurochem Int. 2017;107:57–65. doi:10.1016/j.neuint.2017.03.024
9. Cooke MJ, Wang Y, Morshead CM, Shoichet MS. Controlled epi-cortical delivery of epidermal growth factor for the stimulation of endogenous neural stem cell proliferation in stroke-injured brain. Biomaterials. 2011;32(24):5688–5697. doi:10.1016/j.biomaterials.2011.04.032
10. Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15(12):786–801. doi:10.1038/nrm3904
11. Fernandez-Serra R, Gallego R, Lozano P, González-Nieto D. Hydrogels for neuroprotection and functional rewiring: a new era for brain engineering. Neural Regen Res. 2020;15(5):783–789. doi:10.4103/1673-5374.268891
12. Tuo QZ, Zhang ST, Lei P. Mechanisms of neuronal cell death in ischemic stroke and their therapeutic implications. Med Res Rev. 2022;42(1):259–305. doi:10.1002/med.21817
13. Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79(4):1431–1568. doi:10.1152/physrev.1999.79.4.1431
14. Wierońska JM, Cieślik P, Kalinowski L. Nitric oxide-dependent pathways as critical factors in the consequences and recovery after brain ischemic hypoxia. Biomolecules. 2021;11(8):1097. doi:10.3390/biom11081097
15. Qin C, Yang S, Chu YH, et al. Signaling pathways involved in ischemic stroke: molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther. 2022;7(1):215. doi:10.1038/s41392-022-01064-1
16. Andrabi SS, Parvez S, Tabassum H. Ischemic stroke and mitochondria: mechanisms and targets. Protoplasma. 2020;257(2):335–343. doi:10.1007/s00709-019-01439-2
17. He Z, Ning N, Zhou Q, Khoshnam SE, Farzaneh M. Mitochondria as a therapeutic target for ischemic stroke. Free Radic Biol Med. 2020;146:45–58. doi:10.1016/j.freeradbiomed.2019.11.005
18. Zhang Q, Jia M, Wang Y, Wang Q, Wu J. Cell death mechanisms in cerebral ischemia-reperfusion injury. Neurochem Res. 2022;47(12):3525–3542. doi:10.1007/s11064-022-03697-8
19. Datta A, Sarmah D, Mounica L, et al. Cell Death Pathways in Ischemic Stroke and Targeted Pharmacotherapy. Transl Stroke Res. 2020;11(6):1185–1202. doi:10.1007/s12975-020-00806-z
20. Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012;298:229–317. doi:10.1016/B978-0-12-394309-5.00006-7
21. Xing C, Arai K, Lo EH, Hommel M. Pathophysiologic cascades in ischemic stroke. Int J Stroke. 2012;7(5):378–385. doi:10.1111/j.1747-4949.2012.00839.x
22. Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4(5):399–415. doi:10.1038/nrn1106
23. Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67(2):181–198. doi:10.1016/j.neuron.2010.07.002
24. Fisher M, Savitz SI. Pharmacological brain cytoprotection in acute ischaemic stroke - renewed hope in the reperfusion era. Nat Rev Neurol. 2022;18(4):193–202. doi:10.1038/s41582-021-00605-6
25. Kornev VA, Grebenik EA, Solovieva AB, Dmitriev RI, Timashev PS. Hydrogel-assisted neuroregeneration approaches towards brain injury therapy: a state-of-The-art review. Comput Struct Biotechnol J. 2018;16:488–502. doi:10.1016/j.csbj.2018.10.011
26. Ma X, Wang M, Ran Y, et al. Design and fabrication of polymeric hydrogel carrier for nerve repair. Polymers. 2022;14(8):1549. doi:10.3390/polym14081549
27. Horn EM, Beaumont M, Shu XZ, et al. Influence of cross-linked hyaluronic acid hydrogels on neurite outgrowth and recovery from spinal cord injury. J Neurosurg Spine. 2007;6(2):133–140. doi:10.3171/spi.2007.6.2.133
28. Lam J, Lowry WE, Carmichael ST, Segura T. Delivery of iPS-NPCs to the stroke cavity within a hyaluronic acid matrix promotes the differentiation of transplanted cells. Adv Funct Mater. 2014;24(44):7053–7062. doi:10.1002/adfm.201401483
29. Pan L, Ren Y, Cui F, Xu Q. Viability and differentiation of neural precursors on hyaluronic acid hydrogel scaffold. J Neurosci Res. 2009;87(14):3207–3220. doi:10.1002/jnr.22142
30. Khaing ZZ, Milman BD, Vanscoy JE, Seidlits SK, Grill RJ, Schmidt CE. High molecular weight hyaluronic acid limits astrocyte activation and scar formation after spinal cord injury. J Neural Eng. 2011;8(4):046033. doi:10.1088/1741-2560/8/4/046033
31. Deng WS, Ma K, Liang B, et al. Collagen scaffold combined with human umbilical cord-mesenchymal stem cells transplantation for acute complete spinal cord injury. Neural Regen Res. 2020;15(9):1686–1700. doi:10.4103/1673-5374.276340
32. Matsumine H, Sasaki R, Tabata Y, et al. Facial nerve regeneration using basic fibroblast growth factor-impregnated gelatin microspheres in a rat model. J Tissue Eng Regen Med. 2016;10(10):E559–E567. doi:10.1002/term.1884
33. Feng X, Lu X, Huang D, et al. 3D porous chitosan scaffolds suit survival and neural differentiation of dental pulp stem cells. Cell Mol Neurobiol. 2014;34(6):859–870. doi:10.1007/s10571-014-0063-8
34. Huang HC, Hong L, Chang P, et al. Chitooligosaccharides attenuate Cu2+-induced cellular oxidative damage and cell apoptosis involving Nrf2 activation. Neurotox Res. 2015;27(4):411–420. doi:10.1007/s12640-014-9512-x
35. Matyash M, Despang F, Mandal R, Fiore D, Gelinsky M, Ikonomidou C. Novel soft alginate hydrogel strongly supports neurite growth and protects neurons against oxidative stress. Tissue Eng Part A. 2012;18(1–2):55–66. doi:10.1089/ten.tea.2011.0097
36. Blaško J, Szekiova E, Slovinska L, Kafka J, Cizkova D. Axonal outgrowth stimulation after alginate/mesenchymal stem cell therapy in injured rat spinal cord. Acta Neurobiol Exp. 2017;77(4):337–350. doi:10.21307/ane-2017-066
37. Estrada V, Brazda N, Schmitz C, et al. Long-lasting significant functional improvement in chronic severe spinal cord injury following scar resection and polyethylene glycol implantation. Neurobiol Dis. 2014;67:165–179. doi:10.1016/j.nbd.2014.03.018
38. Oh SH, An DB, Kim TH, Lee JH. Wide-range stiffness gradient PVA/HA hydrogel to investigate stem cell differentiation behavior. Acta Biomater. 2016;35:23–31. doi:10.1016/j.actbio.2016.02.016
39. Kubinová S, Horák D, Kozubenko N, et al. The use of superporous Ac-CGGASIKVAVS-OH-modified PHEMA scaffolds to promote cell adhesion and the differentiation of human fetal neural precursors. Biomaterials. 2010;31(23):5966–5975. doi:10.1016/j.biomaterials.2010.04.040
40. Jhaveri SJ, Hynd MR, Dowell-Mesfin N, Turner JN, Shain W, Ober CK. Release of nerve growth factor from HEMA hydrogel-coated substrates and its effect on the differentiation of neural cells. Biomacromolecules. 2009;10(1):174–183. doi:10.1021/bm801101e
41. Hejčl A, Růžička J, Kekulová K, et al. Modified methacrylate hydrogels improve tissue repair after spinal cord injury. Int J Mol Sci. 2018;19(9):2481. doi:10.3390/ijms19092481
42. Bayer IS. Hyaluronic Acid and Controlled Release: a Review. Molecules. 2020;25(11):2649. doi:10.3390/molecules25112649
43. Moshayedi P, Carmichael ST. Hyaluronan, neural stem cells and tissue reconstruction after acute ischemic stroke. Biomatter. 2013;3(1). doi:10.4161/biom.23863
44. Back SA, Tuohy TM, Chen H, et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005;11(9):966–972. doi:10.1038/nm1279
45. Jensen G, Holloway JL, Stabenfeldt SE. Hyaluronic acid biomaterials for central nervous system regenerative medicine. Cells. 2020;9(9):2113. doi:10.3390/cells9092113
46. Chang PH, Chao HM, Chern E, Hsu SH. Chitosan 3D cell culture system promotes naïve-like features of human induced pluripotent stem cells: a novel tool to sustain pluripotency and facilitate differentiation. Biomaterials. 2021;268:120575. doi:10.1016/j.biomaterials.2020.120575
47. Suzuki Y, Kitaura M, Wu S, et al. Electrophysiological and horseradish peroxidase-tracing studies of nerve regeneration through alginate-filled gap in adult rat spinal cord. Neurosci Lett. 2002;318(3):121–124. doi:10.1016/S0304-3940(01)02359-X
48. Novikova LN, Mosahebi A, Wiberg M, Terenghi G, Kellerth JO, Novikov LN. Alginate hydrogel and matrigel as potential cell carriers for neurotransplantation. J Biomed Mater Res A. 2006;77(2):242–252. doi:10.1002/jbm.a.30603
49. Lin CC, Anseth KS. PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm Res. 2009;26(3):631–643. doi:10.1007/s11095-008-9801-2
50. Mahoney MJ, Anseth KS. Contrasting effects of collagen and bFGF-2 on neural cell function in degradable synthetic PEG hydrogels. J Biomed Mater Res A. 2007;81(2):269–278. doi:10.1002/jbm.a.30970
51. Nazemroaya F, Soheili ZS, Samiei S, et al. Induced retro-differentiation of human retinal pigment epithelial cells on PolyHEMA. J Cell Biochem. 2017;118(10):3080–3089. doi:10.1002/jcb.26014
52. Plant GW, Woerly S, Harvey AR. Hydrogels containing peptide or aminosugar sequences implanted into the rat brain: influence on cellular migration and axonal growth. Exp Neurol. 1997;143(2):287–299. doi:10.1006/exnr.1997.6407
53. Grimaudo MA, Krishnakumar GS, Giusto E, et al. Bioactive injectable hydrogels for on demand molecule/cell delivery and for tissue regeneration in the central nervous system. Acta Biomater. 2022;140:88–101. doi:10.1016/j.actbio.2021.11.038
54. Leipzig ND, Shoichet MS. The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials. 2009;30(36):6867–6878.
55. Aurand ER, Lampe KJ, Bjugstad KB. Defining and designing polymers and hydrogels for neural tissue engineering. Neurosci Res. 2012;72(3):199–213. doi:10.1016/j.neures.2011.12.005
56. Alessandri M, Lizzo G, Gualandi C, et al. Influence of biological matrix and artificial electrospun scaffolds on proliferation, differentiation and trophic factor synthesis of rat embryonic stem cells. Matrix Biol. 2014;33:68–76. doi:10.1016/j.matbio.2013.08.001
57. Yang F, Murugan R, Ramakrishna S, Wang X, Ma YX, Wang S. Fabrication of nano-structured porous PLLA scaffold intended for nerve tissue engineering. Biomaterials. 2004;25(10):1891–1900. doi:10.1016/j.biomaterials.2003.08.062
58. Soppimath KS, Aminabhavi TM, Dave AM, Kumbar SG, Rudzinski WE. Stimulus-responsive ”smart” hydrogels as novel drug delivery systems. Drug Dev Ind Pharm. 2002;28(8):957–974. doi:10.1081/DDC-120006428
59. Goubko CA, Majumdar S, Basak A, Cao X. Hydrogel cell patterning incorporating photocaged RGDS peptides. Biomed Microdevices. 2010;12(3):555–568. doi:10.1007/s10544-010-9412-7
60. Nehls EM, Rosales AM, Anseth KS. Enhanced user-control of small molecule drug release from a poly(ethylene glycol) hydrogel via azobenzene/cyclodextrin complex tethers. J Mater Chem B. 2016;4(6):1035–1039. doi:10.1039/C5TB02004B
61. Comolli N, Neuhuber B, Fischer I, Lowman A. In vitro analysis of PNIPAAm-PEG, a novel, injectable scaffold for spinal cord repair. Acta Biomater. 2009;5(4):1046–1055. doi:10.1016/j.actbio.2008.10.008
62. Nisbet DR, Moses D, Gengenbach TR, Forsythe JS, Finkelstein DI, Horne MK. Enhancing neurite outgrowth from primary neurones and neural stem cells using thermoresponsive hydrogel scaffolds for the repair of spinal cord injury. J Biomed Mater Res A. 2009;89(1):24–35. doi:10.1002/jbm.a.31962
63. Kim DH, Seo YK, Thambi T, et al. Enhancing neurogenesis and angiogenesis with target delivery of stromal cell derived factor-1α using a dual ionic pH-sensitive copolymer. Biomaterials. 2015;61:115–125. doi:10.1016/j.biomaterials.2015.05.025
64. Gao GH, Park MJ, Li Y, et al. The use of pH-sensitive positively charged polymeric micelles for protein delivery. Biomaterials. 2012;33(35):9157–9164. doi:10.1016/j.biomaterials.2012.09.016
65. Adak A, Das G, Khan J, et al. Extracellular Matrix (ECM)-mimicking neuroprotective injectable sulfo-functionalized peptide hydrogel for repairing brain injury. ACS Biomater Sci Eng. 2020;6(4):2287–2296. doi:10.1021/acsbiomaterials.9b01829
66. Guilfoyle MR, Carpenter KL, Helmy A, Pickard JD, Menon DK, Hutchinson PJ. Matrix metalloproteinase expression in contusional traumatic brain injury: a paired microdialysis study. J Neurotrauma. 2015;32(20):1553–1559. doi:10.1089/neu.2014.3764
67. Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani MH, Ramakrishna S. Electrical stimulation of nerve cells using conductive nanofibrous scaffolds for nerve tissue engineering. Tissue Eng Part A. 2009;15(11):3605–3619. doi:10.1089/ten.tea.2008.0689
68. Pires F, Ferreira Q, Rodrigues CA, Morgado J, Ferreira FC. Neural stem cell differentiation by electrical stimulation using a cross-linked PEDOT substrate: expanding the use of biocompatible conjugated conductive polymers for neural tissue engineering. Biochim Biophys Acta. 2015;1850(6):1158–1168. doi:10.1016/j.bbagen.2015.01.020
69. Xing Y, Zeng B, Yang W. Light responsive hydrogels for controlled drug delivery. Front Bioeng Biotechnol. 2022;10:1075670. doi:10.3389/fbioe.2022.1075670
70. Jgamadze D, Liu L, Vogler S, Chu LY, Pautot S. Thermoswitching microgel carriers improve neuronal cell growth and cell release for cell transplantation. Tissue Eng Part C Methods. 2015;21(1):65–76. doi:10.1089/ten.tec.2013.0752
71. LeValley PJ, Sutherland BP, Jaje J, et al. On-demand and tunable dual wavelength release of antibody using light-responsive hydrogels. ACS Appl Bio Mater. 2020;3(10):6944–6958. doi:10.1021/acsabm.0c00823
72. Crompton KE, Goud JD, Bellamkonda RV, et al. Polylysine-functionalised thermoresponsive chitosan hydrogel for neural tissue engineering. Biomaterials. 2007;28(3):441–449. doi:10.1016/j.biomaterials.2006.08.044
73. Johnson T, Koria P. Expression and purification of neurotrophin-elastin-like peptide fusion proteins for neural regeneration. BioDrugs. 2016;30(2):117–127. doi:10.1007/s40259-016-0159-4
74. Wang C, Javadi A, Ghaffari M, Gong S. A pH-sensitive molecularly imprinted nanospheres/hydrogel composite as a coating for implantable biosensors. Biomaterials. 2010;31(18):4944–4951. doi:10.1016/j.biomaterials.2010.02.073
75. Tian WM, Zhang CL, Hou SP, et al. Hyaluronic acid hydrogel as Nogo-66 receptor antibody delivery system for the repairing of injured rat brain: in vitro. J Control Release. 2005;102(1):13–22. doi:10.1016/j.jconrel.2004.09.025
76. Guo C, Kaufman LJ. Flow and magnetic field induced collagen alignment. Biomaterials. 2007;28(6):1105–1114. doi:10.1016/j.biomaterials.2006.10.010
77. Omidinia-Anarkoli A, Boesveld S, Tuvshindorj U, Rose JC, Haraszti T, De Laporte L. An injectable hybrid hydrogel with oriented short fibers induces unidirectional growth of functional nerve cells. Small. 2017;13(36). doi:10.1002/smll.201702207
78. Xue L, Sun J. Magnetic hydrogels with ordered structure for biomedical applications. Front Chem. 2022;10:1040492. doi:10.3389/fchem.2022.1040492
79. Vangijzegem T, Stanicki D, Laurent S. Magnetic iron oxide nanoparticles for drug delivery: applications and characteristics. Expert Opin Drug Deliv. 2019;16(1):69–78. doi:10.1080/17425247.2019.1554647
80. Gopalakrishnan A, Shankarappa SA, Rajanikant GK. Hydrogel scaffolds: towards restitution of ischemic stroke-injured brain. Transl Stroke Res. 2019;10(1):1–18. doi:10.1007/s12975-018-0655-6
81. Green RA, Hassarati RT, Goding JA, et al. Conductive hydrogels: mechanically robust hybrids for use as biomaterials. Macromol Biosci. 2012;12(4):494–501. doi:10.1002/mabi.201100490
82. Green RA, Lovell NH, Wallace GG, Poole-Warren LA. Conducting polymers for neural interfaces: challenges in developing an effective long-term implant. Biomaterials. 2008;29(24–25):3393–3399. doi:10.1016/j.biomaterials.2008.04.047
83. Hasanzadeh E, Seifalian A, Mellati A, et al. Injectable hydrogels in central nervous system: unique and novel platforms for promoting extracellular matrix remodeling and tissue engineering. Mater Today Bio. 2023;20:100614. doi:10.1016/j.mtbio.2023.100614
84. Ullm S, Krüger A, Tondera C, et al. Biocompatibility and inflammatory response in vitro and in vivo to gelatin-based biomaterials with tailorable elastic properties. Biomaterials. 2014;35(37):9755–9766. doi:10.1016/j.biomaterials.2014.08.023
85. Sirova M, Van Vlierberghe S, Matyasova V, et al. Immunocompatibility evaluation of hydrogel-coated polyimide implants for applications in regenerative medicine. J Biomed Mater Res A. 2014;102(6):1982–1990. doi:10.1002/jbm.a.34873
86. Koivisto JT, Joki T, Parraga JE, et al. Bioamine-crosslinked gellan gum hydrogel for neural tissue engineering. Biomed Mater. 2017;12(2):025014. doi:10.1088/1748-605X/aa62b0
87. Fernández-García L, Marí-Buyé N, Barios JA, et al. Safety and tolerability of silk fibroin hydrogels implanted into the mouse brain. Acta Biomater. 2016;45:262–275. doi:10.1016/j.actbio.2016.09.003
88. Rivet CJ, Zhou K, Gilbert RJ, Finkelstein DI, Forsythe JS. Cell infiltration into a 3D electrospun fiber and hydrogel hybrid scaffold implanted in the brain. Biomatter. 2015;5(1):e1005527. doi:10.1080/21592535.2015.1005527
89. Weissman IL, Anderson DJ, Gage F. Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu Rev Cell Dev Biol. 2001;17:387–403. doi:10.1146/annurev.cellbio.17.1.387
90. Dabrowska S, Andrzejewska A, Lukomska B, Janowski M. Neuroinflammation as a target for treatment of stroke using mesenchymal stem cells and extracellular vesicles. J Neuroinflammation. 2019;16(1):178. doi:10.1186/s12974-019-1571-8
91. Bernstock JD, Peruzzotti-Jametti L, Ye D, et al. Neural stem cell transplantation in ischemic stroke: a role for preconditioning and cellular engineering. J Cereb Blood Flow Metab. 2017;37(7):2314–2319. doi:10.1177/0271678X17700432
92. Zhong J, Chan A, Morad L, Kornblum HI, Fan G, Carmichael ST. Hydrogel matrix to support stem cell survival after brain transplantation in stroke. Neurorehabil Neural Repair. 2010;24(7):636–644. doi:10.1177/1545968310361958
93. Wei Z, Zhao J, Chen YM, Zhang P, Zhang Q. Self-healing polysaccharide-based hydrogels as injectable carriers for neural stem cells. Sci Rep. 2016;6:37841. doi:10.1038/srep37841
94. Mungenast L, Züger F, Selvi J, et al. Directional submicrofiber hydrogel composite scaffolds supporting neuron differentiation and enabling neurite alignment. Int J Mol Sci. 2022;23(19):11525. doi:10.3390/ijms231911525
95. Chen SJ, Chang CM, Tsai SK, et al. Functional improvement of focal cerebral ischemia injury by subdural transplantation of induced pluripotent stem cells with fibrin glue. Stem Cells Dev. 2010;19(11):1757–1767. doi:10.1089/scd.2009.0452
96. Wang Z, Wang J, Jin Y, et al. A neuroprotective sericin hydrogel as an effective neuronal cell carrier for the repair of ischemic stroke. ACS Appl Mater Interfaces. 2015;7(44):24629–24640. doi:10.1021/acsami.5b06804
97. Royce Hynes S, McGregor LM, Ford Rauch M, et al. Photopolymerized poly(ethylene glycol)/poly(L-lysine) hydrogels for the delivery of neural progenitor cells. J Biomater Sci Polym Ed. 2007;18(8):1017–1030. doi:10.1163/156856207781494368
98. Cook DJ, Nguyen C, Chun HN, et al. Hydrogel-delivered brain-derived neurotrophic factor promotes tissue repair and recovery after stroke. J Cereb Blood Flow Metab. 2017;37(3):1030–1045. doi:10.1177/0271678X16649964
99. Wang Y, Cooke MJ, Morshead CM, Shoichet MS. Hydrogel delivery of erythropoietin to the brain for endogenous stem cell stimulation after stroke injury. Biomaterials. 2012;33(9):2681–2692. doi:10.1016/j.biomaterials.2011.12.031
100. Nakaguchi K, Jinnou H, Kaneko N, et al. Growth factors released from gelatin hydrogel microspheres increase new neurons in the adult mouse brain. Stem Cells Int. 2012;2012:915160. doi:10.1155/2012/915160
101. Caicco MJ, Cooke MJ, Wang Y, Tuladhar A, Morshead CM, Shoichet MS. A hydrogel composite system for sustained epi-cortical delivery of Cyclosporin A to the brain for treatment of stroke. J Control Release. 2013;166(3):197–202. doi:10.1016/j.jconrel.2013.01.002
102. Tuladhar A, Morshead CM, Shoichet MS. Circumventing the blood-brain barrier: local delivery of cyclosporin A stimulates stem cells in stroke-injured rat brain. J Control Release. 2015;215:1–11. doi:10.1016/j.jconrel.2015.07.023
103. Dabrowska S, Andrzejewska A, Strzemecki D, Muraca M, Janowski M, Lukomska B. Human bone marrow mesenchymal stem cell-derived extracellular vesicles attenuate neuroinflammation evoked by focal brain injury in rats. J Neuroinflammation. 2019;16(1):216. doi:10.1186/s12974-019-1602-5
104. Doeppner TR, Bähr M, Hermann DM, Giebel B. Concise review: extracellular vesicles overcoming limitations of cell therapies in ischemic stroke. Stem Cells Transl Med. 2017;6(11):2044–2052. doi:10.1002/sctm.17-0081
105. Doeppner TR, Herz J, Görgens A, et al. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl Med. 2015;4(10):1131–1143. doi:10.5966/sctm.2015-0078
106. Tsintou M, Dalamagkas K, Moore TL, et al. The use of hydrogel-delivered extracellular vesicles in recovery of motor function in stroke: a testable experimental hypothesis for clinical translation including behavioral and neuroimaging assessment approaches. Neural Regen Res. 2021;16(4):605–613. doi:10.4103/1673-5374.295269
107. Fan L, Liu C, Chen X, et al. Exosomes-loaded electroconductive hydrogel synergistically promotes tissue repair after spinal cord injury via immunoregulation and enhancement of myelinated axon growth. Adv Sci. 2022;9:13.
108. Ju Y, Hu Y, Yang P, Xie X, Fang B. Extracellular vesicle-loaded hydrogels for tissue repair and regeneration. Mater Today Bio. 2023;18:100522. doi:10.1016/j.mtbio.2022.100522
109. Liu J, Wang K, Luan J, et al. Visualization of in situ hydrogels by MRI in vivo. J Mater Chem B. 2016;4(7):1343–1353. doi:10.1039/C5TB02459E
110. Park GK, Kim SH, Kim K, et al. Dual-Channel fluorescence imaging of hydrogel degradation and tissue regeneration in the brain. Theranostics. 2019;9(15):4255–4264. doi:10.7150/thno.35606
111. Jin R, Yang X, Zhao D, et al. An injectable hybrid hydrogel based on a genetically engineered polypeptide for second near-infrared fluorescence/photoacoustic imaging-monitored sustained chemo-photothermal therapy. Nanoscale. 2019;11(34):16080–16091. doi:10.1039/C9NR04630E
112. Jin T, Nicholls FJ, Crum WR, Ghuman H, Badylak SF, Modo M. Diamagnetic chemical exchange saturation transfer (diaCEST) affords magnetic resonance imaging of extracellular matrix hydrogel implantation in a rat model of stroke. Biomaterials. 2017;113:176–190. doi:10.1016/j.biomaterials.2016.10.043
113. Dong YC, Bouché M, Uman S, Burdick JA, Cormode DP. Detecting and monitoring hydrogels with medical imaging. ACS Biomater Sci Eng. 2021;7(9):4027–4047. doi:10.1021/acsbiomaterials.0c01547
114. Vieira S, Strymecka P, Stanaszek L, et al. Methacrylated gellan gum and hyaluronic acid hydrogel blends for image-guided neurointerventions. J Mater Chem B. 2020;8(27):5928–5937. doi:10.1039/D0TB00877J
115. Araszkiewicz AM, Oliveira EP, Svendsen T, et al. Manganese-labeled alginate hydrogels for image-guided cell transplantation. Int J Mol Sci. 2022;23(5):2465. doi:10.3390/ijms23052465
116. El-Sherbiny IM, Yacoub MH. Hydrogel scaffolds for tissue engineering: progress and challenges. Glob Cardiol Sci Pract. 2013;2013(3):316–342. doi:10.5339/gcsp.2013.38
117. Namestnikova DD, Gubskiy IL, Cherkashova EA, et al. Therapeutic efficacy and migration of mesenchymal stem cells after intracerebral transplantation in rats with experimental ischemic stroke. Bull Exp Biol Med. 2023;175(1):116–125. doi:10.1007/s10517-023-05822-1
118. Sontyana AG, Mathew AP, Cho K-H, Uthaman S, Park I-K. Biopolymeric in situ hydrogels for tissue engineering and bioimaging applications. Tissue Eng Regen Med. 2018;15(5):575–590. doi:10.1007/s13770-018-0159-1
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

