Back to Journals » International Journal of Nanomedicine » Volume 16
3D Printing of Micro- and Nanoscale Bone Substitutes: A Review on Technical and Translational Perspectives
Authors Cheng L , Suresh K S , He H, Rajput RS, Feng Q, Ramesh S, Wang Y, Krishnan S, Ostrovidov S , Camci-Unal G, Ramalingam M
Received 16 March 2021
Accepted for publication 17 May 2021
Published 24 June 2021 Volume 2021:16 Pages 4289—4319
DOI https://doi.org/10.2147/IJN.S311001
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Yan Shen
Lijia Cheng, 1 Shoma Suresh K, 2 Hongyan He, 1 Ritu Singh Rajput, 2 Qiyang Feng, 1 Saravanan Ramesh, 2 Yuzhuang Wang, 1 Sasirekha Krishnan, 2 Serge Ostrovidov, 3 Gulden Camci-Unal, 4 Murugan Ramalingam 2
1School of Basic Medicine, Chengdu University, Chengdu, 610106, People’s Republic of China; 2Biomaterials and Organ Engineering Group, Centre for Biomaterials, Cellular, and Molecular Theranostics, School of Bio Sciences and Technology, Vellore Institute of Technology, Vellore, Tamil Nadu, 632014, India; 3Department of Radiological Sciences, University of California Los Angeles, Los Angeles, CA, 90095, USA; 4Department of Chemical Engineering, University of Massachusetts Lowell, Lowell, MA, 01854, USA
Correspondence: Lijia Cheng
School of Basic Medicine, Chengdu University, Chengdu, 610106, People’s Republic of China
Email [email protected]
Murugan Ramalingam
Biomaterials & Organ Engineering Group, Centre for Biomaterials, Cellular, and Molecular Theranostics, Vellore Institute of Technology, Vellore, Tamil Nadu, 632014, India
Tel +91-416-220-2736
Fax +91-416-2243092
Email [email protected]
Abstract: Recent developments in three-dimensional (3D) printing technology offer immense potential in fabricating scaffolds and implants for various biomedical applications, especially for bone repair and regeneration. As the availability of autologous bone sources and commercial products is limited and surgical methods do not help in complete regeneration, it is necessary to develop alternative approaches for repairing large segmental bone defects. The 3D printing technology can effectively integrate different types of living cells within a 3D construct made up of conventional micro- or nanoscale biomaterials to create an artificial bone graft capable of regenerating the damaged tissues. This article reviews the developments and applications of 3D printing in bone tissue engineering and highlights the numerous conventional biomaterials and nanomaterials that have been used in the production of 3D-printed scaffolds. A comprehensive overview of the 3D printing methods such as stereolithography (SLA), selective laser sintering (SLS), fused deposition modeling (FDM), and ink-jet 3D printing, and their technical and clinical applications in bone repair and regeneration has been provided. The review is expected to be useful for readers to gain an insight into the state-of-the-art of 3D printing of bone substitutes and their translational perspectives.
Keywords: 3D printing, artificial bone, bone tissue engineering, biomaterials, nanomaterials
Graphical Abstract:
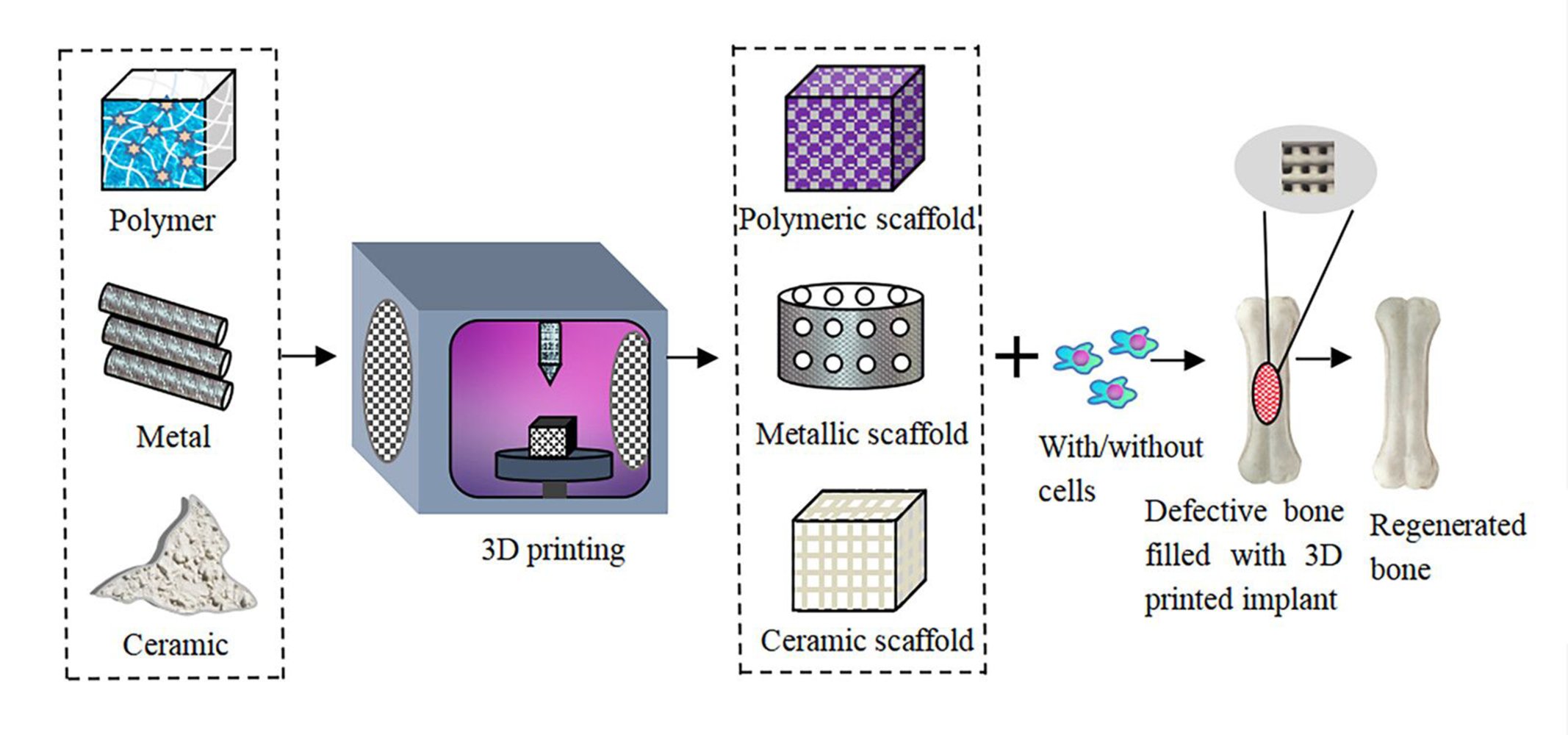
Introduction
Bone and its related disorders represent a majority of chronic diseases in patients over the age of 40 years and still exist as a critical clinical concern.1 Even though bones possess a limited regeneration capacity, their inability to repair large segmental bone defects remains an unmet clinical challenge. Bone tumor resection, traffic accidents, rarefaction of bone, and osteomyelitis are some of the main reasons that bone injuries.2,3 Annually, more than 2.5 million reconstructive surgery procedures are carried out worldwide in response to these primary causes of bone damage and defects.4,5 Autologous bone grafting has still been considered the “gold standard” in orthopedic trauma for repairing large defective bones. However, the hurdles of autologous bone grafting are the limited availability of autologous bones, the time required to harvest the graft, molding problems, the morbidity of the donor site, graft resorption, and the high risk of fracture. On the other hand, allo- and xeno-grafts face the challenge of insufficient levels of cellularity, immune rejection, and the possibility of disease transmission.6 These limitations are hampering their extensive use in clinical applications.7 Hence, it is imperative to develop alternative strategies for the efficient repair of bone.
Three-dimensional (3D) printing is an additive manufacturing technology that has recently made advances in the field of tissue engineering for the repair and regeneration of damaged tissues. The 3D printing (3DP) process integrates engineering technology and biological sciences to print scaffolds, often loaded with cells and bioactive growth factors that can be potentially used as replacements for traditional tissue grafts.8 3D printing technology can transform digital signals into physical objects, via the integrated application of computer-aided design (CAD), computer-aided manufacturing (CAM), computer numerical control (CNC), laser technology, and computed tomography (CT). Combined with these technologies, digital imaging and communications in medicine (DICOM), such as magnetic resonance imaging (MRI) and computed tomography (CT), can be converted into file types recognized by 3D printers.9 The basic principle of 3D printing is dispersion/accumulation molding, which is the creation of three-dimensional solids on layers of plastic, metal powder, and ceramic powder. First used in 1986 by Charles Hull, the technology has expanded to currently include different types of 3D printing technologies such as photopolymerization, lamination, extrusion, and ink-jet printing. A summary of 3DP technologies, their advantages, and disadvantages, and the corresponding references are shown in Table 1.
 |
 |
 |
Table 1 The Advantages and Disadvantages of 3DP Technologies |
The energy consumption in the additive manufacturing method is high during the build phase of the material and around 25% of energy spent only for post-treatment processes. When the component’s complexity increases its impact on the environment is also increased. The negative impact on the environment of 3D printing technologies such as wasting of energy for preheating the equipment, material preparation consumed a higher amount of energy, and consumption of natural resources in the form of gases. It heavily affects the aquatic ecosystem and terrestrial ecotoxicity. However, additive manufacturing techniques show lesser environmental footprints compared to other types of techniques.10
Depending on the type of material used for 3D printing, the properties of the scaffold and its application can be modified. Several studies have highlighted the use of conventional biomaterials such as metals, polymers, ceramics, and their composites for 3D bioprinting. However, the applications of these materials are limited by their microporous structure. Nanomaterials offer advantages over these conventional biomaterials because they possess high surface area to volume ratios and the nanoscale size ranges remain closer for mimicking the native bone structure to improve cell adhesion and proliferation rates. Therefore, combining 3D bioprinting techniques and nanomaterials allows for the fabrication of scaffolds with enhanced physicochemical properties, suitable for translation to the clinic.11,12
The rationale behind this review is to assess the current advances and application of 3D bioprinting for micro- and nanoscale bone substitutes, focusing on the technical aspects and translational potential into the clinic.
Impact of Bone Tissue Engineering
Bone is a substantially active and dynamic connective tissue, both structurally and functionally, which provides vital organ protection, mechanical support, locomotion, structural body framework, and flexibility to the body.13,14 In addition to these functions, it also helps in homeostasis, mineral storage, and blood pH regulation and maintenance.14 Bone tissue typically comprises compact (ie, cortical bone) or trabecular (ie, cancellous bone).15 The primary causes of bone defects include trauma, fractures, compromised blood supply, surgery, tumor resection, infection, and congenital malformation.4,16 Additionally, diseases such as osteoporosis and rheumatoid arthritis result in bone degeneration. Certain clinical conditions, such as vascular necrosis and atrophic non-unions, also lead to major defects in bone further requiring bone transplants.5,17 Globally, bone is one of the most frequently transplanted tissues, second only to blood transfusion.18
The smallest size intraosseous wounds unable to self-heal are called critical-sized bone defects and need external interventions and/or regenerative techniques to be repaired.19,20 There are not any agreed and one standard definition of a critical-size defect in humans.21,22 Although Court-Brown defined, it as a defect involving 50% of the cortical diameter with a minimum length of 1 cm, and this was used in the Study to Prospectively evaluate Intramedullary Nails in Tibial fractures (SPRINT).23 However, the general guidelines that have been suggested in the literature include defect size length greater than 1–2 cm and greater than 50% loss of the circumference of the bone.24–26
Recent advances have been made in bone tissue engineering to develop functional bone substitutes for repairing defective bone. Bone tissue engineering targets to examine the bone structure, bone dynamics, and tissue development as it seeks to develop new functional bone substitutes.16 There is a wide range of materials used to construct bone substitutes such as metals, naturally derived and synthetic polymers, composite materials, growth factor integrated materials, and cell encapsulated materials.27 Bone scaffolds should possess appropriate mechanical, physical, and biological properties such as biodegradability, swelling kinetics, high porosity, stiffness, and demonstrate increased cell adherence, proliferation, and differentiation.28 The intrinsic reparability aspect of bone substitutes provides a suitable model to mimic the natural extracellular matrix (ECM) and has increased the osteoconductivity, osteoinductivity, and osseointegration in previously reported literature examples to repair defective bone.29 Over the past decades, a wealth of research has been accomplished in bone tissue engineering, especially in obtaining reliable cell sources, cultivating biocompatible scaffolds, developing bioreactors to increase osteogenic priming in vitro, and discovering cytokines that can promote bone and vascular formation.30 Numerous pre-clinical experiments of different animal species yielded promising findings.31
Despite these advances, the limited translation of bone grafts into clinical uses indicates hurdles such as lack of cell source management and proper selection of biomaterial, inadequate in vitro preparation, and mode of delivery. In recent years, the use of 3D printing techniques has increased for developing bone substitutes. 3D printing is a flexible method to manufacture a variety of materials such as polymers, ceramics, metals, and composites with unique geometries and macro/microporous architectures.32 In bone tissue engineering, 3D printing methods have numerous advantages such as the control of fine features including interconnected porosity, there are no contamination issues related with any second material for the support structures, and it showed the ability of direct printing with both metallic and ceramic materials.33–35 Compared to traditional 3D printing methods, 3D bioprinting has different advantages such as multi-cell spatial directional control, controllability for the deposition of different cell densities, inexpensive, and high-throughput capability.8,36–38 The uniqueness of this method is the bioprinting of patient-specific organs and tissue patches which reduces the chance of rejection in the patient’s body and can eliminate the need for organ donors.39,40
Researchers have effectively utilized 3D-printed scaffolds with complex geometries for long bone reconstruction in different in vivo models, through the use of additive manufacturing technologies.41 Furthermore, mandibular and calvarial bone fabrication has been carried out using an integrated tissue-organ printer (ITOP), which could also be used for fabricating tissue constructs of any shape.42 The next section will discuss the technological advancements in 3D printing over the years and highlight the important 3D printing techniques that have been commonly used for bone tissue engineering.
History and Technical Advances of 3D Printing
Organ failure is the leading cause of death across the world. The main treatment for organ failure is the use of allografts; however, this approach is limited by donor organ shortage, lifelong immune rejection, and ethical conflicts.43 According to clinical statistics, only less than one-third of patients can receive matched organs.44 In this context, 3D printing is a favorable technology to generate 3D tissue replacements and address the shortage of donor organs. Clinical studies were first conducted in 1998, by the Wake Forest Institute for Regenerative Medicine, where the researchers successfully implanted a 3D-printed human bladder into a human.12,45,46 Currently, the most common 3D printed artificial organs include skin, blood vessels, nerve tissue, cartilage, and bone.47–49 The 3D-printed skin tissues have great potential in fields of cosmetic development, drug screening, and skin grafting. Preto et al47 prepared 3D-printed skin which mimicked the natural skin tissue better compared to the traditional artificial skin tissues. In addition, the 3D-printed skin simulated the interaction between cells that induced the formation of complete barrier function. Marone et al48 used 3D-printed aortic models to provide patients with an overview of their diseases. In another study, Liu et al49 applied 3D printing to a personalized model of a skull tumor including brain nerves for preoperative diagnosis and surgical design of skull base surgery. In medicine, 3D printing can be applied to fabricate highly complex tissues with living cells and extracellular matrices, such as bone and cartilage. Due to its scalability, 3D printing can be used to manufacture tissues for repairing defects of different sizes and geometries.50 To be specific, according to the preoperative CT data of orthopedic patients, 3D printing can produce artificial bone models consistent with defects and assist in the design of surgical plans to reduce the risk of surgery, whereas improving the accuracy of surgery. 3D printed artificial bone holds great promise in clinical orthopedics. The most common 3D printing techniques used for the fabrication of artificial bone grafts are stereolithography (SLA), selective laser sintering (SLS), fused deposition modeling (FDM), ink-jet 3D printing, direct metal laser sintering (DMLS), selective laser melting (SLM), and digital light processing (DLP).
Techniques Used in 3D Printing
The main techniques used in 3DP include SLA, SLS, FDM, and ink-jet 3DP. The next section will review the principles, raw materials, and clinical applications in these types of 3D printing technologies. The schematic for the working principles of these 3D printing technologies is displayed in Figure 1.
 |
Figure 1 The schematic diagram of stereolithography appearance (SLA), selective laser sintering (SLS), fused deposition modeling (FDM), and ink-jet 3D printing techniques. |
Stereolithography Appearance
As the earliest rapid prototyping technology, SLA is the only photocurable 3D printing technology that is currently capable of printing large-size models.51 The principle of SLA is similar to SLS; both of them are variations of laser-assisted 3D printing technology. The main difference between these techniques lies in different types of lasers and raw printing materials. The commonly used printing materials in SLA for artificial bone engineering are photosensitive resins. However, due to the presence of light residue of the initiator during the printing process, some of the photosensitive resins show cytotoxicity and low biocompatibility in vivo. Therefore, SLA printed artificial bone is often used in the preoperative simulation, and preparation of teaching mold, but less commonly used for in vivo experiments. In recent years, to improve the application of SLA, a mixture of photosensitive resins and other biological materials, such as poly (propylene fumarate) (PPF) and gelatin, has been used as raw materials. Among them, PPF showed excellent mechanical properties and was biodegradable, non-toxic with adjustable characteristics, and has been used in many preclinical applications, including repair of bone defects. Nettleton et al52 printed PPF scaffolds using SLA technology, implanted the scaffolds into the calvarial defects of critical size in rats, and evaluated the bone regeneration. A significant increase in bone growth was observed at 4 weeks post-operation, and bone continued to grow at 12 weeks without inducing a long-term inflammatory response.
To explore the feasibility of in-situ 3D printing for the treatment of diseases, such as bone and cartilage defects, Li et al53 used SLA to print alginate gel scaffolds for the defects of humerus injury and found a strong osteogenic effect. Furthermore, Le Guéhennec et al54 used SLA technology to print scaffolds to evaluate the biocompatibility and osteoinductive properties of hydroxyapatite (HA) and hydroxyapatite-tri calcium phosphate (HA-TCP) scaffolds in vivo and in vitro. The materials used for printing were mixtures of bioceramics and organic components (polyfunctional acrylic resin and photoinitiator) and the scaffolds were printed in the form of pellets. In vitro experiments showed that these materials induced biomimetic cell behavior after 3 days of culture, and the viability of cells indicated that they did not induce cytotoxicity. In the in vivo experiments, calcium phosphate pellets were subperiosteally implanted into defects in a calvarial rat model, and the result showed that all animals recovered without complications. Although pellet volumes showed an initial decrease, they were further maintained stable over the course of 3–6 months. New bone formation was detected initially at 3 months post-implantation. In another study, Danilevicius et al55 designed and made complex, geometrically controlled 3D scaffolds with SLA. The pore sizes of the scaffolds ranged from 25 to 110 μm, the porosity was 70%, 82%, 86%, and 90%, respectively. Among them, osteoblasts demonstrated strong adhesion and proliferation ability on the scaffolds with 86% porosity with the highest proliferation efficiency.
Selective Laser Sintering
SLS is a 3D model made from metal or polymer powder by a laser beam. The process of SLS printing works as the following: on a heating workbench uniformly covered with powder materials, the computer commands the laser beam to scan on the workbench collectively makes the powder caking mutually. After the worktable completes sintering in the upper layer, it moves down a certain height, and the process is repeated until the printing is finished.56,57 SLS can also print high viscosity materials. The raw materials for SLS include most of the thermoplastic polymers, bioceramics, metals, polycaprolactone (PCL), and polyetheretherketone (PEEK) powders. Compared with ink-jet printing, SLS can use a wider variety of inks. However, the side effects of laser irradiation on cells are unclear when a bioink is used. Besides, high resolution and high-intensity laser diodes are costly, and the control of laser printing systems is complex, so the application of this technology is somewhat limited.58
Among them, PCL is regarded as one of the most common polymers in orthopedic applications owing to its biocompatibility and slow degradation rate which matches the rate of bone regeneration. However, due to its lack of ability to induce bone regeneration, its low compression modulus, and mechanical strength, PCL is often used in combination with bioceramics and biological factors to improve the osteogenesis process.59 Brunello et al60 implanted a PCL scaffold printed by SLS into the femoral defect of rabbits and found that the PCL scaffold had high biocompatibility and was conducive to promoting the healing of bone defects. In another study, Williams et al61 prepared PCL scaffolds by the SLS method for bone tissue formation. The scaffold was implanted with bone morphogenetic protein-7 (BMP-7) in vivo, and the results showed that PCL scaffolds prepared by SLS had sufficient compressive strength and trabecular modulus, which could possibly support bone growth. Furthermore, as HA is the main inorganic component of natural bone and has enough mechanical strength, it is deemed as a model material for bone defect repair in the clinic.62 Du et al63 evaluated the feasibility of SLS manufacturing gradient multilayer scaffolds. Using SLS technology, multilayer scaffolds containing HA were produced and implanted into a rabbit cartilage defect model. At 6 to 12 weeks after the placement of the multilayer stent, the repair capacity of bone defects was enhanced, and smooth cartilaginous tissue was found to form in the multilayer stent. The results showed that multilayer scaffolds containing HA could effectively enhance osteochondral repair, and the newly formed tissues were articular cartilage, subchondral bone along with the reconstructed osteochondral interface. Furthermore, Shuai et al64 investigated the influence of different preheating temperatures and laser speed on the surface performance of nano HA-based artificial bone scaffolds printed by SLS. They suggested that the scaffolds that meet the requirements of the mechanical and biological properties of bone can be obtained by optimizing the sintering time. In addition, Roskies et al65 used SLS to print PEEK scaffolds and co-culture with rat BMSCs and adipose-derived stem cells (ADSCs). The results showed that ADSCs had higher osteogenic differentiation ability than BMSCs.
Fused Deposition Modeling
FDM technique can squeeze the continuous filament materials onto the previous layer of material on the platform which is melted semi-liquid material according to the computer-aided design (CAD) path. Then the materials solidify and fuse at room temperature to form a continuous 3D structure.66 The model printed by FDM is more accurate than the traditional plaster model.67 Chen et al68 fabricated FDM printed skull model with PLA and applied it to the anatomy course, the medical students received the model well based on a randomized controlled trial. Moreover, FDM can produce complex and highly uniform 3D scaffolds to customize personalized bone substitutes that are conducive to bone tissue regeneration. In another study, Chiulan et al69 proposed that FDM could be used to produce a PLA scaffold with complex geometry and high uniformity. Furthermore, Liu et al70 prepared HA-PCL porous scaffolds loaded with heparin sulfate (HS) and used them to repair femoral condyle defects in a rabbit model. The study showed that 3D printed PCL-HA-HS composite scaffold could speed up repairing bone defects, which is indicative of effective biomaterials for orthopedic applications. In another study, Kim et al71 reported an example of reconstruction after tumor resection using a 3D printing stent in a canine model. According to CT images, it was found that the defect in the left upper jaw of a 12-year-old female dog was caused by the presence of the oral mass. Then they designed the operation plan, removed the oral mass, and transplanted PCL/β-TCP scaffolds that were 3D-printed printed via FDM. After 8 months of follow-up, CT scans and oral examination were normal. Moreover, Zou, et al72 used FDM with PVA as raw material to manufacture scaffolds, which were integrated components of large-scale microfluidic channel networks and hollow complex tissues and organs characterized by a Valentine-shaped heart. Besides, Jiménez, et al73 used acrylonitrile butadiene styrene (ABS) to fabricate a skull model to treat four patients with craniosynostosis. The results showed that four models were formed, one for each case, two of them were frontal suture and the others were sagittal suture. It has been demonstrated that ABS is a suitable polymer for operable skull fusion models in terms of anatomical and mechanical properties as well as in response to surgical instruments.
Ink-Jet 3D Printing
The principle of ink-jet 3D printing is similar to a traditional ink-jet printer. During the printing process, the binder is placed on a powder bed for coagulation, creating the desired structure. Ink-jet 3D printing relies on electrically charged crystals that are mechanically deformed to deposit the printing material on the substrate. This process is repeated layer by layer in a preset way until the model structure is produced. Then, the unbound powder is washed away. Direct ink-jet printing allows for achieving features with high resolution, high control over complex geometric structures, and the introduction of microscopic or macroscopic voids into the structures.50 In theory, ink-jet 3D printing can use almost any powder material, such as biopolymers, ceramics, or metal powders. Collagen is a natural polymer and is the key organic component of bone. Collagen scaffolds feature high porosity, permeability, and biocompatibility. However, these scaffolds are fragile.74 Inzana et al printed scaffolds made of a combination of collagen and calcium phosphate materials to evaluate the parameter optimization of calcium phosphate materials.62
Hydrogels are hydrophilic polymeric 3D networks that demonstrate viscoelastic properties mimicking native tissues. They are widely used to simulate the ECM because they can provide a suitable environment for living cells during and after scaffold manufacturing.75 Sodium alginate is a non-toxic naturally derived hydrogel with high biocompatibility, degradability, and 3D printability, which is often used in cartilage tissue engineering. Alginate possesses low cell adhesivity and a slow degradation rate. Therefore, alginate can be combined with an array of growth factors, such as transforming growth factor-β (TGF-β) to enhance tissue formation. A limitation of using alginate is its high degradation rate, which can be controlled by using oxidized alginate and regulating the degradation rate in 3D bioprinting.76 Vehmeijer et al77 treated a patient with orbital floor fracture, enophthalmos, and diplopia using a molded autologous bone implant. A 3D virtual model of the fracture site was printed based on the CT image from patients. A personalized mold for the defect area was made by ink-jet printing, and a personalized autologous orbital implant was carved using this mold as a template. The implants showed excellent anastomosis in vivo. Also, the use of 3D printing improved the accuracy and efficiency of the surgical operation. The study showed that this method provides a precise and cost-efficient treatment for orbital floor fractures.
Biomaterials for 3D Printing of Bone Substitutes
Bone tissue engineering focuses on designing substitute materials for traditional grafts using biocompatible materials. These biomaterials play a key role in 3D printing of bone substitutes; hence, it is imperative to understand their properties and utilize them for suitable applications. This section focuses on metallic, polymeric, ceramic, and nano biomaterials that have been utilized for generating bone grafts and substitutes using 3D printing technologies.
Metallic Biomaterials
Titanium Alloys
Bone reconstruction and surgical fixation for conditions such as knee arthroplasty, hip replacement, lumbar fusion, and fixation have long since been aided with the use of titanium alloys.78 Typically, 6% aluminum and 4% vanadium are alloyed in titanium (Ti 90%, Al 6%, V 4%) in small amounts.79 The crystallographic transformation temperature of pure titanium is 885°C. Titanium alloys can mainly be grouped into three categories: α-type (below 885°C, pure Ti has a hexagonal-closed packed crystalline structure), β-type (above 885°C, pure Ti has a body-centered cubic crystalline structure), and (α + β)-type.78 Ti-6Al-4V and Ti-6Al-7Nb are two of the frequently used titanium alloys.80 Metallic biomaterials are often used for load-bearing orthopedic remedies since they possess good strength, low elastic modulus, low density, and alloying characteristics. These are some of the most important properties to consider in addition to biocompatibility, corrosion resistance, and adequate mechanical and physical properties for designing suitable bone implants.78,81–83 Electron beam melting (EBM) and selective laser melting (SLM) are the common 3D printing techniques applied with titanium alloys.84 Thus, Hollander et al used Ti-6Al-4V powder, and the study outcomes indicated that SLM printed material exhibits excellent biocompatibility and may well be used to substitute biological fragments during clinical application.85 In 2014, Imanishi et al assessed the compatibility of a 3D-printed titanium heel prosthesis implanted in a patient (Figure 2) previously diagnosed with calcaneus cancer. Follow-up evaluation at the end of five months post-operation revealed that the patient was clear of complications and pain and able to walk unsupported or with the help of a Controlled Ankle Movement (CAM) walker boot.86 Punyaratabandhu et al used a 3D-printed titanium prosthesis to replace phalanges on a patient who suffered from bone deterioration due to the presence of a tumor (Figure 3). This prosthesis was advantageous over autologous implants, as they offer limited flexibility and range of motion. The titanium prosthesis was surgically implanted and linked the phalanges to the nearest tendon. This study revealed that 2 years following surgery, the patient experienced no pain and although a 5 mm shortening of the thumb was observed, the patient was still able to move their thumb.87
 |
Figure 2 A photograph showing the resected specimen (Achilles tendon) and 3D printed titanium heel prosthesis used to replace the defect. Notes: Arrows represent the anchor points employed to attach the ligaments to the prosthesis. Reproduced from Imanishi J, Choong PFM. Three-dimensional printed calcaneal prosthesis following total calcanectomy. Int J Surg Case Rep. 2015.86 Copyright © 2015 The Authors. Creative Commons (https://creativecommons.org/licenses/by-nc-nd/4.0/legalcode). |
 |
Figure 3 (A and B) Photographs of a 3D printed titanium prosthesis before implantation; (C and D) intraoperative photographs displaying the implant; (E and F) radiographs of the prosthesis. Notes: Reproduced from Punyaratabandhu T, Lohwongwatana B, Puncreobutr C, Kosiyatrakul A, Veerapan P, Luenam S. A Patient-Matched Entire First Metacarpal Prosthesis in Treatment of Giant Cell Tumor of Bone. Case Rep Orthop. 2017;2017:Article ID 4101346.87 Copyright © 2017 Thipachart Punyaratabandhu et al. Creative Commons (https://creativecommons.org/licenses/by/4.0/legalcode). |
Cobalt-Chromium Alloy
Cobalt–chromium (CoCr) alloy is one of the most comprehensively analyzed metallic biomedical implant which has been extensively used in several cardiovascular devices, as well as orthopedic implants including artificial hips, knee joints, and regularly used in dental applications owing to their unique mechanical characteristics, high strength, high-temperature, wear and corrosion resistance, flexibility, in addition to excellent biocompatibility, which marks these materials as good load-bearing implants.88,89 SLM technique is most commonly used with CoCr alloys.89 Hazlehurst et al90 evaluated the stress shielding attributes of orthopedic implants that resemble the behavior of bone. Thus, SLM was used to fabricate square pore cobalt-chrome-molybdenum (CoCrMo) cellular structures, characterized by porosity extending between 25% and 95% and effective elastic moduli. The results revealed that the alloy stiffness and strength were quite comparable to those of the human femur. The CoCrMo cellular structures showed an effective elastic modulus with a volumetric porosity of 65% and above.90
Polymeric Biomaterials
Polylactic Acid
Polylactic acid (PLA) is a long linear chain, consisting of recurring monomeric units of lactic acid (LA), as shown in Figure 4.91 It is naturally present in two enantiomeric forms, that is, the L- and D-optical isomers.92 It can be obtained by fermentation of renewable forms of sugar derivatives which make it eco-friendly, thereby enabling its usage in the human body.93 The common fermentation processes mostly generate the L-isomer but sometimes an equal quantity of L- and D-type of lactic acids can be present, which is termed as meso-lactic acid. PLA made up of meso-lactic acid is called poly-DL-lactic acid (PDLLA) or meso-PLA.92,94 On the other hand, PLA that is solely comprised of L-lactic acid or D-lactic acid is called poly-L-lactide (PLLA) or poly-D-lactide (PDLA), respectively. The commercially available PLA is a copolymer of PLLA and PDLA.94 PLA, along with its copolymers finds extensive uses in orthopedic regenerative engineering applications, for instance, sutures, and bone fixation devices, including but not limited to screws, rods, pins, and plates. These polymeric materials exhibit good mechanical strength, versatility in fabrication, renewability, biocompatibility, and excellent biodegradability.92,95,96 Even though PLA has an extensive range of applications, there are certain drawbacks such as its brittle nature and poor thermal stability.93 However, several methods have been adapted to decrease PLA brittleness, mainly by blending PLA with a variety of polymers such as PCL, polyethylene oxide (PEO), and polyethylene glycol (PEG).91,97
 |
Figure 4 Structures of polymeric biomaterials (A) PCL, (B) PLA, (C) PLGA, and (D) PEEK. Notes: Reproduced from Hirsch E, Nacsa M, Ender F, Mohai M, Nagy ZK, Marosi GJ. Preparation and characterization of biocompatible electrospun nanofiber scaffolds. Period Polytech Chem Eng. 2018;62(4):510–518.260 Creative Commons. |
Poly-ε-Caprolactone
PCL is an aliphatic polyester, comprising repeating units of hexanoate (Figure 4).98,99 It is semi-crystalline at room and human body temperatures and possesses a melting point of close to 60°C and a glass transition temperature (Tg) of −60°C.100 PCL is a hydrophobic material soluble in organic solvents, and its low melting point and compatibility with other biomaterials make it readily processable.100–102 PCL is frequently used as the primary material in long-term implants utilized for cartilage, bone, tendon and ligaments, cardiovascular tissue, blood vessels, skin, and nerve tissue scaffolds for various tissue engineering applications.98,100,101,103 The versatility of PCL is due to its mechanical and physicochemical properties, which can be adapted to fit in the target application.98,104 Although biodegradable, PCL is highly stable in comparison with polylactides. This stability arises from the fact that it possesses a lesser number of ester bonds per monomer, and thereby, its degradation time is 2−5 years.100 Mainly the degradation of the material depends upon molecular weight, shape, and residual monomer content. FDM is one of the most frequently applied 3D printing techniques with PCL. Daentzer et al105 evaluated the use of a bioresorbable cage made of magnesium and polymer (PCL) implanted into a sheep ovine model (Figure 5). No complications were observed for vascular, neurologic, and wound healing even after 24 weeks post-surgery.105
 |
Figure 5 (A) Intraoperative images of the magnesium-PCL implant. (B) Bone graft. (C) Plate stabilization. Notes: Reproduced from Daentzer D, Floerkemeier T, Bartsch I, et al. Preliminary results in anterior cervical discectomy and fusion with an experimental bioabsorbable cage - clinical and radiological findings in an ovine animal model. Springerplus. 2013;2:418.105 Creative Commons (https://creativecommons.org/licenses/by/2.0). |
Poly Lactic-Co-Glycolic Acid
Poly lactic-co-glycolic acid (PLGA) is a copolymer of polylactic acid (PLA) and polyglycolic acid (PGA) where both acids are generally combined in an equal ratio.106,107 Figure 4 shows the structure of PLGA. The Tg of PLGA copolymers is usually in the range of 40–60°C.108 PLGA is a synthetic polymer that has been regularly used in orthopedic implants, surgical technique development, drug delivery systems, and tissue engineering scaffolds due to its exceptional mechanical and physical properties, biodegradability, and biocompatibility.106,109–111 PLGA is one of the best characterized biodegradable copolymers thereby accounting for its long clinical experience and use in developing sustained drug delivery systems.106,112 It has been employed as a scaffold to augment the bone healing process in several critical size bone defects with the help of an extrusion-based 3D printing technique.113,114
Moreover, Frosch et al115 evaluated the clinical use of a scaffold, Milagro, consisting of 30% β-TCP and 70% PLGA in a case of ligament graft fixation with bioabsorbable interference screws. The tibial screws and femoral screws showed volume loss after 6 months of evaluation. The results of this study revealed that all patients showed bone ingrowth. Also, the screw resorption rate showed consistent behavior with the graft healing process. In the first few months, Milagro did not prevent tunnel enlargement, but the growth of bone tissue in the screws diminished the tunnel volume following 12 months.91,115
Polyether Ether Ketone
PEEK is a colorless aromatic thermoplastic polymer, having the chemical formula (–C6H4–O–C6H4–O–C6H4–CO–)n (structure shown in Figure 4).116,117 It is widely used in spine surgery, maxillofacial skull reconstruction, femoral reconstruction, cardiac surgery, oral implant, scaffoldings, drug vessels, hip-replacement, orthopedic devices, cardiac surgery, scaffoldings, drug vessels, aerospace, and automotive due to its excellent mechanical properties, less coefficient of friction, high capability of load-bearing, biocompatibility, dimensional stability, stiffness, and thermal properties which makes it stable in the human body.117–120 PEEK is a semicrystalline material having a melting temperature of 334°C, Young’s modulus of 3.6 GPa, Tg of 145°C, and tensile strength around 90–100 MPa.121
Ceramic Biomaterials
Calcium Phosphate
Calcium phosphate (CaP) bioceramics find numerous applications in orthopedics, for use as implants, bone grafting materials, and coatings on dental and orthopedic prostheses.122–124 Among other calcium phosphates, tri-calcium phosphate (TCP) [(Ca3(PO4)2], otherwise known as tribasic calcium phosphate, has been widely used owing to its high osteoconductivity, bioresorbable nature, bioactivity, and excellent biocompatibility.122,123,125 Tarafder et al126 assembled 3D printed interconnected macroporous TCP scaffolds with precise internal architecture, with pore sizes ranging from 500 to 1000 μm. Two weeks after implantation of the scaffolds into a rat model of a femoral defect, the evaluation showed that new bone formation occurred in the central fibrous zone, histologically called the fibrous interzone (FIZ) present between the pores, and the mechanical strength was obtained by microwave sintering of the TCP scaffolds.126
Hydroxyapatite (HA), with the chemical formula [Ca10(PO4)6(OH)2], is a naturally occurring mineral form of calcium apatite that is frequently used in bone regeneration as implant materials, bone graft materials, and bone fillers.127,128 The nature of HA is quite comparable to the mineral component of bones, teeth, and mineralized cartilage.129 HA has been widely used in hard tissue repair or regeneration, dental prosthetics, hip replacements, dental implants, bone conduction implants, and bone grafts due to its bioactivity, biocompatibility, and excellent osteoconductivity.127,130 Figure 6 shows SEM images of 3D-printed hydroxyapatite scaffolds with their porous structures.131 Luo et al132 evaluated that strontium-doped hydroxyapatite (Sr-HAP) based 3D-printed scaffolds for repairing calvarial defects in rabbits. In this study, Sr-HAP was produced using collagen type I and citrate. Micro-CT imaging showed that no significant differences were present between the scaffolds fabricated with strontium and without strontium. The results revealed that 12 weeks after surgery, the Sr-HAP group presented greater bone growth than the control, as well as improved cell adhesion and proliferation, with increased alkaline phosphatase activity, and enhanced osteogenic capacity.132
 |
Figure 6 3D printed hydroxyapatite scaffold with defined macroporosity. Scale bars: 0.5 cm (A), 500 µm (B), and 5 µm (C). Notes: Reproduced from Burgio F, Rimmer N, Pieles U, Buschmann J, Beaufils-Hugot M. Characterization and in ovo vascularization of a 3D-printed hydroxyapatite scaffold with different extracellular matrix coatings under perfusion culture. Biol Open. 2018;7(12):bio034488.131 Creative Commons (https://creativecommons.org/licenses/by/4.0/). |
Nanomaterials for 3D Printing
Nanotechnology performs an imperative part in the production and development of nanophase biomaterials.133–135 These biomaterials are used for 3D printing and consist of bio-ink, which is an integral part of the bioprinting technique.11,12,136 Bioprinting technology depends upon several parameters for better efficiency such as biocompatibility of the material and remodeling by the cells.12 Nanophase biomaterials have been recently used to achieve these conditions and overcome the limitations of conventional biomaterials. Bio-ink is an important factor that maintains the 3D environment for the cells to grow efficiently.137–140 Additionally, bio-ink plays a role as a protective shield for cells during the printing process when the biological constructs are fabricated.141–143 Nanomaterial-based bio-inks provide easier processability, higher stiffness and degradation, and functional ability due to their physical properties and nanoscale features to promote cell and bone growth, reduce infection rates, and enhance tissue regeneration.12,144–146 A porous scaffold for cell growth and tissue formation is a significant constituent for tissue engineering. Numerous inks have been synthesized from different available sources including natural (eg, collagen, chitin, alginate, agarose) and synthetic (eg, PLLA, PLGA, PCL) sources.6,95 An ideal bio-ink should contain more than one biomaterial for getting better 3D printing efficiency.138 Several types of nanoparticle-based biomaterial constituents can be crafted from a number of materials, which include carbon nanomaterials,147 self-assembly nanomaterials, natural or synthetic polymers, and ceramics.148
Metallic Nanomaterials
Metallic scaffolds have vast potential for healing bones in load-bearing areas because of their mechanical properties. However, the development of biodegradable metallic implants is a complicated process due to the specific medical requirements for the patients depending on the type of bone, location of the defect, healing rate of the bone.149 Titanium (Ti) and its alloys are broadly used as implants in clinical orthopedic applications due to their stable nature and excellent biocompatibility.150,151 Nonetheless, Ti-based alloys have some limitations as implant material such as non-biodegradability because of its superior corrosion resistance and high stiffness, which may result in implant failure.152 In comparison with Ti-based metals, iron (Fe)-based metals are the most commonly used biodegradable metals and it exhibits mechanical characteristics resembling that of natural bone.153,154 However, Fe exhibits a relatively slower rate of degradation, which gives an adequate period for new bone ingrowth, while also providing the required mechanical support.155,156 The biocompatibility of Fe needs further research, thereby limiting its applications in bone tissue regeneration. To overcome this limitation, bioceramic surface coating is one of the approaches which can enhance biocompatibility.155 HA coating on metallic implants shows magnificent bone integration because it is the chief mineral component of bone.157,158 To evaluate their property, Yang et al159 applied nanostructured HA onto 3D-printed Fe scaffolds for better bone regeneration. In this study, the pure Fe scaffold was fabricated by the use of the 3D-printing method and then the surface was modified with HA. This surface modification allowed for the survival of rabbit bone marrow mesenchymal stem cells (BMSCs). These scaffolds possessed high porosities, while the compressive yield strength and Young’s modulus corresponded to those seen in natural bone. The scaffolds showed a high binding ability to bone, enhanced viability, alkaline phosphatase activity, and osteogenic differentiation of BMSCs cultured on the scaffold. Therefore, for load-bearing bones, 3D-printed Fe scaffolds coated with nano-HA may be a favorable choice for bone regeneration.159
Carbon-Based Nanomaterials
Carbon-based nanomaterials are also used for the development of 3D-printed bone tissue engineering scaffolds.160,161 There are different types of carbon-based nanomaterials with different dimensions such as carbon nanotubes, fullerenes, and graphite that have been prosperously employed in bone tissue engineering owing to their strong mechanical stability and electrical conductivity.162–164
Among these nanomaterials, carbon nanotube (CNTs) have been extensively used owing to their different properties like mechanical, electrical, thermal, non-cytotoxic effect, elasticity, fatigue resistance, and porosity which make them suitable material to incorporate into 3D printing polymers.165–172 Carbon nanotubes contain hollow cylindrical nanostructures that are made from graphene sheets.161 Carbon nanotubes can be allocated into two subtypes: single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs).147,173 MWCNTs deliver appropriate nucleation sites that permit solid interactions with polymers and also allow better cross-linking and functionalization.174,175
Thus, Cui et al176 evaluated the use of MWCNTs integrated into a polyion complex (PIC) hydrogel which was 3D printed using an extrusion-based technique to form the PIC/MWCNT biohybrid hydrogels. These hydrogels were used in a rat calvarial defect model. The scaffolds exhibited high bone volume/total volume ratios and bone mineral densities, in addition to promoting regeneration of bone tissue. Additionally, PIC/MWCNT hydrogels exhibited high biocompatibility with rat BMSCs and enhanced osteogenic differentiation. This study showed that these nanocomposite scaffolds have strong biocompatibility, mechanical strength, adequate porosity for cell ingrowth, and induced bone tissue formation.176,177
Gnanasekaran et al178 used FDM for 3D printing of CNT and graphene-based polybutylene terephthalate (PBT). The PBT composite filaments with CNT and graphene were fabricated by melt extrusion process and the electrical percolation threshold by 3D printed to monolayers was determined, which is shown in Figure 7. The 3D-printed PBT/CNT objects were found to have higher conductivity and mechanical characteristics and showed better efficiency than 3D printed PBT/graphene structures in terms of printability, electrical conductivity, and mechanical stability.178 Similarly, to overcome the 3D printing limitation of making desired microscale features and electrochemical properties, Liu et al161 evaluated the rapid and homogeneous one-step functionalization of carbon nanotube using a 3D printing technology. In this study, CNTs and single-stranded deoxyribonucleic acid (ssDNA) were blended to produce a negatively charged ssDNA@CNT nano-complex. Additionally, free amine groups were generated from 3D-printed PPF scaffolds and then applied to the 3D-printed scaffolds. The results revealed that the rapid and simple functionalization provides a uniform and non-toxic coating of CNTs onto the scaffold, thereby enhancing cell adhesion, proliferation, and differentiation. It also enhanced the cells’ ALP activity, osteocalcin (OCN), osteopontin (OPN), and other osteogenic gene marker expressions. The overall result demonstrates that 3D-printed scaffolds blended with CNTs can be considered as suitable candidates for use in orthopedics.161
 |
Figure 7 (A) Extruded PBT/CNT composite filament. (B) 3D printed monolayer of PBT/CNT composite. (C) SEM image of the PBT/CNT monolayer illustrating the ridges. (D) Extruded PBT/G composite filament. (E) 3D printed monolayer of PBT/G composite. (F) SEM image of the PBT/G monolayer illustrating the ridges. Black scale bars are 1 cm and white scale bars are 500 μm. Notes: Reproduced from Gnanasekaran K, Heijmans T, van Bennekom S, et al. 3D printing of CNT- and graphene-based conductive polymer nanocomposites by fused deposition modeling. Appl Mater Today. 2017;9:21–28.178 Creative Commons (https://creativecommons.org/licenses/by/4.0/). |
Carbon dots (CDs) are zero-dimensional nanomaterials with a size less than 10 nm, consist of sp2 carbon that is formed by 2–3 parallel graphene sheets.179–183 CDs consist of a carbogenic core with substantial quantities of oxygen, hydrogen, and carbon on their surface.180 CDs have gained many researchers’ attention because of their superior properties in comparison with other semiconductor quantum dots, which include optical properties, excellent solubility in water, low toxicity, easily functionalized surface, ultra-small size of the particle, and good biocompatibility.184–186 The main advantage of CDs is the low cost of their synthesis.180 Some of the research studies suggested that the carbon dots do not affect cell viability, proliferation, metabolism, and differentiation.187
Gogoi et al188 developed a bio-nano-macromolecular approach for bone tissue engineering, in which a tannic acid-based water-dispersible hyperbranched polyurethane is fabricated with bionanohybrids of carbon dot and four different peptides that impart target specific in vivo bone healing ability. This bio-nano-macromolecule had been blended with 10 wt% of gelatin. In vitro study shows that the developed polymeric system reveals good osteoblast adhesion, proliferation, and differentiation. The in-vivo study result shows ectopic bone formation ability and the occurrence of calcification and blood vessel formation. Thus, this study demonstrates that the carbon-dot-peptide functionalized hyperbranched polyurethane gel is useful in bone tissue engineering applications.188 In another study, Sarkar et al189 developed carbon dots conjugated carboxymethyl cellulose-hydroxyapatite nanocomposite as a material for osteogenic bone regeneration scaffolds. Here the nanocomposite had been synthesized by the simple one-pot fabrication method. The study results reveal that it has good biocompatibility, excellent ability for drug loading, and specific bone regeneration properties which were highly economical.189
Ceramic Based Nanomaterials
Nanoceramics with their crystallographic structure and strong atomic bonds have gained attention in bone repair owing to their superior characteristics such as high thermal stability, high corrosion resistance, chemical stability, biocompatibility, stability, increased hardness, cell-matrix interaction, strength, and wear resistance.190–192 In comparison with conventional ceramics, nanoceramics possessed distinctive properties such as processing, mechanical, and surface characteristics, which make them suitable materials for bone tissue engineering applications.193 Nevertheless, there are certain limitations of nanoceramic materials such as weak tensile modulus, brittle nature, and lower toughness. To overcome these drawbacks, nanoceramic materials were incorporated into polymer matrices.190 Mondal et al194 evaluated the 3D printed PLA scaffold with reinforced nano-HA bioceramics. The scaffolds were 3D printed in 0°, 45°, and 90° printing angles on the XY plane. This study revealed that the 90° orientation provided PLA scaffolds with maximum compressive stress and porosity, along with enhanced cell attachment and proliferation capability. These results showed that HA nanoparticles enhanced scaffold surface activity and mechanical strength making them a suitable material for bone tissue engineering.194
Composite Nanomaterials
3D printed composite scaffolds have previously been used for various applications because of lower production costs and their ability to assemble complex geometries.195,196 There is increasing attention to the development of printing materials with a broad range of applications and blending different materials with unique properties for generating high-performance composites.197–200 The nanocomposite scaffolds showed advanced mechanical, thermal, chemical properties, and improved cell behavior when compared against conventional composite scaffolds.201,202
Zhang et al203 evaluated the properties of multifunctional magnetic Fe3O4 nanoparticles embedded in mesoporous bioactive glass/polycaprolactone (Fe3O4/MBG/PCL) scaffolds. This study revealed that the integration of magnetic Fe3O4 nanoparticles within the MBG/PCL scaffolds enhanced their properties such as physicochemical, inducing ALP activity, proliferation rate, osteogenic activity, and extracellular matrix (ECM) mineralization when human BMSCs were cultured on the scaffolds. Additionally, the Fe3O4/MBG/PCL scaffolds presented high compressive strengths of 13–16 MPa, 400 μm pore size, and 60% porosity. These desirable characteristics are of great significance for orthopedic applications.203
The 3D printing technique has displayed potential in the fabrication of 3D-printed scaffolds for regenerative medicine.204–208 There is a restricted selection of synthetic polymers for 3D printing because bio-ink must have bioactivity, mechanical strength, printability, and biological characteristics.209–211 Synthetic polymeric biomaterials used for 3D printing are typically bioinert; however, bioactive nanomaterials can be incorporated into them for direct and improved cell functioning.212–215
Carrow et al evaluated the influence of 2D nano silicate addition to poly (ethylene oxide terephthalate) (PEOT)/poly (butylene terephthalate) (PBT) scaffolds. Human MSCs were seeded on scaffolds with or without the presence of nanosilicates, to determine the extent of differentiation on the nanocomposites surface and the influence of nanosilicates on hMSCs adhesion (Figure 8). The mechanical stability of the 3D scaffolds was ascertained after the addition of nanosilicates with PEOT/PBT, by using uniaxial and cyclic compression testing. The results revealed that the incorporation of nanosilicates with PEOT/PBT induced a decrease in scaffold degradation, improves bioactivity, enhanced mineralized matrix production, and upregulated the expression of osteo-related proteins (Figure 9). However, there was no significant rise in the mechanical stiffness of the fabricated scaffolds. Overall results demonstrate that the collaborative capability of nanosilicates and PEOT/PBT can be exploited for fabricating bioactive scaffolds for bone regeneration.216
 |
Figure 8 SEM Images of nanocomposite scaffold architecture following extrusion with 400-µm diameter nozzle. (A) Top view of single layer print; (B) side view of seven-layer print displaying inter-layer spacing and lateral spacing between fibers; (CandD) cross-sectional view of single fibers following biopsy from the macrostructure. Notes: Reproduced from Carrow JK, Di Luca A, Dolatshahi-Pirouz A, Moroni L, Gaharwar AK. 3D-printed bioactive scaffolds from nanosilicates and PEOT/PBT for bone tissue engineering. Regen Biomater. 2019;6(1):29–37.216 Copyright © 2018, Oxford University Press. Creative Commons (https://creativecommons.org/licenses/by/4.0/legalcode). |
 |
Figure 9 In vitro studies on bioactive nanocomposite scaffolds. (A) hMSCs seeded on 3D scaffolds proliferated over one week. The effect of nano silicate on hMSCs differentiation was evaluated by monitoring. (B) ALP activity and production of mineralized matrix. The presence of nanosilicates upregulated the peak ALP activity (Day 14) and production of a mineralized matrix (Day 21). ***P<0.001. Notes: Reproduced from Carrow JK, Di Luca A, Dolatshahi-Pirouz A, Moroni L, Gaharwar AK. 3D-printed bioactive scaffolds from nanosilicates and PEOT/PBT for bone tissue engineering. Regen Biomater. 2019;6(1):29–37.216 Copyright © 2018, Oxford University Press. Creative Commons (https://creativecommons.org/licenses/by/4.0/legalcode). |
Liu et al217 evaluated the use of a 3D-printed anterior cruciate ligament surgical implant in male rabbit models. The rabbits were categorized into three groups based on the implant type received including the PLA group, PLA/HA group, and the mesenchymal stem cells (MSCs) group. For this study, orthogonal and porous polylactic acid (PLA) screw-like scaffolds coated with HA were used, which considerably improved its osteoconductivity and also enhanced cellular adhesion. MSCs were loaded within the scaffold for internal fixation and cellular delivery. Assessments were conducted in vitro and in vivo using the cell-seeded scaffolds to monitor the bone-ligament healing. The results revealed that MSCs that were loaded in the PLA/HA scaffolds presented notable bone ingrowth, in addition to improved collagen fiber deposition along the new bone and tendon. The outcome of this study demonstrates that PLA-based scaffolds can improve bone growth while overcoming the limitations of slow degradation of pristine PLA during the growth process.217 In 2017, Yeon et al218 evaluated a 3D-printed bone clip that was made up of PLA, HA, and silk materials. Compared with the PLA/HA and PLA clips, the PLA/HA/silk composite bone clip exhibited good mechanical stability, higher cell growth, excellent biocompatibility, and was considerably non-invasive compared to traditional methods involving drilling in the bone.218
Different polymeric composites are used in different bone tissue engineering applications, especially due to their biocompatible nature and biodegradation rate.219,220 To augment the mechanical properties of polymer-based composites, researchers incorporated metallic materials to obtain higher mechanical stiffness. The favorable degradation characteristic of PLGA has urged researchers to concentrate on the making of PLGA porous scaffold with the assistance of 3D-printing technology. Indeed, the chemical hydrolysis reaction of ester bonds on the PLGA backbone results in the assembly of carboxylic acid groups, which decreases the pH and diminishes the mechanical properties of the scaffolds.221,222 To overcome these limitations, Rasoulianboroujeni et al223 evaluated 3D-printed PLGA scaffolds blended with nanocomposites of TiO2.224 The study showed that PLGA/TiO2 exhibited a significant increase in thermal decomposition, transition temperature, and ALP activity in comparison to PLGA scaffolds. The laser microscope imaging revealed a conformed pore size of 450–500 μm for PLGA/TiO2 scaffolds which exhibited higher compressive modulus, while osteoblasts cultured on these nanocomposite scaffolds exhibited higher calcium secretion.223
Impact of 3D Printing in Bone Regeneration Applications
Bone transplantation has become the second most common transplant after blood transfusion, since many organs in the body cannot repair themselves, such as large bone defects. Bone grafts are essential to treat the majority of bone defects caused by fractures, bone tumors, osteogenesis imperfecta congenita, and other bone diseases. The 3D bioprinted bone grafts are becoming promising implants in the clinic. Currently, 3D bioprinted bone is often applied to make prosthetic limbs and surgery planning tools. It is possible to make the shape of each implant specific to each patient and control the porosity of different regions of the implant. This aspect facilitates the osseointegration process among the implant and the natural bone tissue and promotes the growth of cells in the implant gap.225
Artificial bone made with 3D printing requires certain properties, and one of the key parameters is the materials used for printing. The materials can be split into natural biomaterials and synthetic materials according to their sources, including metal, natural biomaterials, polymer composites, nanomaterials, and bioceramics. The commonly used metallic materials are stainless steel alloy, vitallium, and titanium alloy. Among them, titanium alloy is commonly used in the repair of load-bearing bone. Naturally derived biomaterials include alginate, chitosan, chitin, and coral collagen, which are widely available, non-toxic, low-cost, and have good biocompatibility.226,227 Figure 10 shows porous gelatin scaffolds with uniform pores and complex architecture.228 Polymer composites include polylactic acid (PLA), polyglycolic acid (PGA), poly lactic-co-glycolic acid (PLGA), and polycaprolactone (PCL). Besides, researchers also focus on bioceramics due to their strong biocompatibility, osteoinductivity, and degradability, such as hydroxyapatite (HA), bioactive glass (BG), tricalcium phosphate (TCP), self-setting calcium phosphate cement (CPC), and biphasic calcium phosphate (BCP).
 |
Figure 10 Uniformly formed highly porous interconnected network via the combination of indirect 3D printing and foaming processes. (A-D) Indirect fabrication can be combined with a foaming process to produce highly and uniformly porous gelatin scaffolds with complex channel architectures. (E and F) The order of this structure can be improved further by incorporating monodispersed microspheres into the casting process. Notes: Reproduced from An J, Teoh JEM, Suntornnond R, Chua CK. Design and 3D Printing of Scaffolds and Tissues. Engineering. 2015;1(2):261–268.228 Creative Commons (https://creativecommons.org/licenses/by/4.0/). |
Pore size and porosity along with its distribution and geometry collectively referred to as pore parameters. In order to strike a perfect balance between the mechanical strength of the bone substitute and its responsiveness to its biological environment, pore parameter optimization is necessary. Larger pore size better facilitates the infiltration, mobility, and migration of osteocytes along with promoting vascularization and osteogenesis.229 It happens because the larger the pore size, the better the cell–cell signaling will be. On the other hand, the larger the pore size, the lesser is the mechanical strength of the bone substitute and hence, pore parameter optimization is necessary. It is not like that the pore size is the only important parameter, pore geometry and distribution should be relatively similar to that of in regular bone, to mimic the bone connectivity as closely as possible. Consistent porosity facilitates improved cellular mobility, adhesion, and increased rate of proliferation when compared to the substitute in which porosity percent is similar but the distribution is not. Optimized pore parameters for different biomaterials were compiled so that they may serve as a point of future reference.230–232
The growth of 3D printing in the field of science and technology has been enormous mainly due to its flexibility in design, composition and thereby providing a wide number of applications.
In the future, 3D printing has the potential to develop into a useful tool for fabricating bone substitutes and for use in the field of tissue engineering. 3D printing technology allows the usage of various input materials, which can be mixed and utilized for testing the compatibility with several cells and tissues. Technological advances can improve the design and fabrication of complex, structural details in the bone substitutes, which will allow the scaffolds to mimic the intricate details of the natural bone. Also, pre-vascularized constructs with capillary networks capable of integrating with the natural bone will vastly improve the chances of the bone substitute attaching to the surgical site. These pre-vascularized constructs can be fabricated with the use of different bio-inks and multi-head bioprinters. The constructs can be modified to tolerate mechanical stresses, especially at heavy load-bearing sites, which are common in bones. These custom-designed scaffolds will further be able to provide patient-specific bone substitutes, which would improve healing rate and physical appearance as well.32,233,234 Figure 11 shows the schematics of a 3D-printed scaffold with a complex porous architecture used for bone regeneration application in a rabbit model.235
 |
Figure 11 (A) The implantation process of a 3D printed scaffold for bone defects of rabbits. (B) Microstructure of human cortical bone. (C) Schematic diagram of the channel structure which is an ideal space for bone tissue ingrowth; a channel structure has been observed along with the black arrow. (D) Gross morphology of the 3d-printed scaffold (E) Photo of the side of the printed scaffold. (F) Photo of the top surface of the printed scaffold. (G) The channel is shown in the sketch map of the 3d-printed scaffold structure. Notes: Reproduced from Ma J, Lin L, Zuo Y, Zuo Q, Li J, Li Y. Modification of 3D printed PCL scaffolds by PVAc and HA to enhance cytocompatibility and osteogenesis. RSC Adv. 2019;9:5338–5346.235 Creative Commons (https://creativecommons.org/licenses/by-nc/3.0/). |
With the continuous and rapid development in the population and the rise in average life expectancy across the world, the demand for tissue engraftments and organ transplantations sees an upsurge. Present treatments related to injured organs and tissues are non-ideal. In addition, patients undergo painful surgeries and expose to longer recovery periods without retaining complete restoration of the tissue and organ function.135,236 These are some of the limitations of the existing treatments. Surgeons either compromise the rate of progress to avoid rapid degeneration or sacrifice the other healthy tissues.237 One way of overcoming these problems is to increase the understanding of cell and tissue interactions at a nanoscale level and thus crafting biomimetic nanostructured tissue constructs using nanomaterials to mimic biological patterns.238,239 In principle, the nanoscale of dimensions is the intermediate region that exists between the macro level and the molecular level, in which the material has at least one dimension sized from 1 to 100 nanometers, thereby constituting a nano-structure (such as a synthetically produced nanoparticle or nanomaterial).145,240,241 Nanotechnology can revolutionize and transform the fields of bone tissue engineering, implantable materials, diagnostics, and treatment of orthopedic surgical conditions.241,242 3D bioprinting using nanotechnology is emerging as a promising strategy for research and development.12,243,244
In 2013, Maleki245 proposed an efficient one-pot multicomponent reaction for diazepine synthesis. It uses silica-supported superparamagnetic iron oxide nanoparticles as a nanocatalyst. To its advancements, Maleki246 describes a green technique called ultrasonic irradiation using multiwall carbon nanotubes and a TiO2 hybrid nanostructure catalyst. This protocol has many benefits, including higher yields, shorter reaction times, mild reaction conditions, and environmental friendliness. Alcohols and olefins can be selectively converted to chemically essential aldehydes. Mani et al247 described an electrospinning technique to create an electrospun polyurethane scaffold for bone tissue engineering with nickel oxide nanoparticles and groundnut oil. Nickel oxide nanoparticles were synthesized using a microwave-assisted green synthesis process with Plectranthus amboinicus. For bone tissue engineering applications, fabricated nanocomposites act as a suitable candidate because of their effective physicochemical properties and mineral deposition.
Plant-based natural polymers are important as scaffolds in tissue engineering, according to Iravani et al,248 because they are readily available, non-toxic, and inexpensive, as well as biocompatible. The porous scaffolds of zein/polycaprolactone biocomposite were used by Wu et al249 with a porosity of about 70% and increased hydrophilicity.
In 2013, Mututuvari et al250 used a cellulose-chitosan-hydroxyapatite composite material; cellulose and chitosan are polysaccharides that can be used to build scaffolds. Similarly, silk fibroin with graphene oxide aids in enzyme resistance251 Zhou et al252 proposed a more efficient and environmentally friendly method in 2015, based on an electrospun collagen/hydroxyapatite composite with a uniform and continuous fibrous morphology. To resemble natural bone, the composite is oriented in a longitudinal direction. The collagen/HAP composites had a 40-fold higher tensile strength. The collagen/HAP fibrous composites had no cytotoxic effects and could enhance cell viability and proliferation in vitro with U2-OS cells.
Translational Aspects of 3D Printed Bone Substitutes
Bone tissue engineering typically aims to expedite the regeneration of tissues, especially critical size bone defects, through a combination of cells, bioactive factors, and scaffolds. Although in vivo studies have been performed extensively with several producing good results, very few products are present today at the clinical stage. The challenges in translation can vary from designing and manufacturing the bone substitute using current Good Manufacturing Practices (cGMP) to obtaining regulatory approval for marketing the product. There is a pressing need to focus on patient-specific implants and personalized treatment methods, to achieve effective clinical translation.
Potamianos et al253 used SLS technology to print surgical models for the treatment of right shoulder injuries that may result in a double fracture of the clavicle and scapula. By SLS model analysis, researchers observed that the clavicle of the patient was intact and partially adhered to the scapula. Therefore, the surgery was not necessary. In this case, the SLS model was used for analysis to avoid the risk of surgery for patients. In another study, Zou et al254 selected 61 types of SLA printed bones or prosthesis models from 39 patients who received 3D printing auxiliary diagnosis, preoperative planning, and surgical simulation. The models were divided into three groups: long bone, irregular bone, and prosthesis. The result of the study showed that the 3D error and relative/absolute difference between the 3D model printed by SLA and the model data obtained from CT data of bone were within an acceptable range, which proves that the use of the SLA printed model in the identification and treatment of bone diseases was effective.
Maini et al255 fabricated real-time 3D pelvic models of acetabular fracture patients for accurate preoperative planning based on SLS technology with nylon polyamide as a raw material in a case study. The results showed that postoperative hemorrhage, operation time, and postoperative reduction effect of the case group were all improved to some extent compared with the control group. In another report, Ackland et al256 adopted the technique to treat a patient with grade 5 osteoarthritis of the left temporomandibular joint. In the process, the researchers sterilized the 3D printed personalized prosthesis and implanted it into the patient, and then they measured its function 12 months after the operation. The experiment proved the 3D printed personalized prosthesis was effective.
Jalbert et al257 removed large lesions in the frontal orbital region and used PEEK-specific implants for the best primary skull reconstruction. To reduce the operation time and avoid complications, preoperative large area resection was performed virtually on 3D imaging to obtain a correct definition of the final defect and to precisely fabricate the correlated implants, thereby achieving aesthetic and functional effects after the real operation. In the treatment of a 43-year-old patient with chondrosarcoma, Kang et al258 implanted a PEEK rib prosthesis printed by FDM into the patient. Unlike traditional design methods, the prosthesis used in this study was designed according to the centroid trajectory of the replaced rib. The patient was discharged from the hospital ten days after the operation, and the results of the chest scan showed that bone reconstruction was stable and the shape of the chest was retained.
Kanno et al259 reported a clinical trial in which 3D-printed bones were prepared from CT images by ink-jet 3D printing using calcium phosphate as raw material and were applied in the treatment of 20 patients having facial bone deformities caused by congenital malformation, tumor, or trauma. During the follow-up of patients from one to seven years, no significant systemic events caused by transplantation were found in one year post-surgery. This trial proved that these artificial bones had favorable biocompatibility with the recipients.
Summary and Future Perspectives
The technological advancements in the field of 3D printing for engineering bone have been summarized in this review. This strategy has also enabled the integration of bioactive factors and cells into complex 3D tissue constructs printed through different techniques, such as SLA, SLS, FDM, and ink-jet 3D printing. Conventional biomaterials such as metals, polymers, and ceramics are used in clinical applications with moderate success rates. Therefore, the use of 3D-printed prostheses offers promising potential in regenerative medicine and can possibly change the way that orthopedic surgeries are conducted.
It is anticipated that there would be differences between natural and artificial tissues; therefore, researchers must maintain an appropriate balance while integrating the different cell types and 3D printing techniques to synthesize the scaffolds. Additionally, the types of defects that would be repaired with the help of tissue engineering, such as ligaments and osteochondral defects, require the integration of more than one cell type. Therefore, a successful 3D bioprinting process is expected to support simultaneous growth and differentiation of different cell types and tissues. The complex design restraints limit the efficiency of the currently available methods, particularly while trying to regenerate clinically relevant size injuries. Some of these limitations can be addressed by the use of nanobiomaterials. Nanomaterials conveniently offer the tunability and flexibility required for the scaffolds to maintain and adhere to the tissue microenvironment. Moreover, nanomaterial-based scaffolds can be tailored according to the patient’s needs with the help of 3D printing technology and image technologies (eg CT, CAD) which specifically scans, records, and analyzes imaging data of the injury from which the scaffold will be printed. The current developments pertaining to the 3D printing of bone substitutes using various types of biomaterials and nanomaterials can further lead to implementing patient-specific health care. A healthy and adaptive community has driven the opportunity for researchers and clinicians worldwide to implement 3D printing technologies, thereby increasing its potential for use in regenerative medicine.
Acknowledgments
This work was supported by the Health Research Project of Health Department of Sichuan Province, China (19PJ161), National Natural Science Foundation of China (No. 81803561), VIT SEED grant, and Department of Science and Technology of Government of India (Project No. CRG/2018/003965).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Baroli B. From natural bone grafts to tissue engineering therapeutics: brainstorming on pharmaceutical formulative requirements and challenges. J Pharm Sci. 2009;98(4):1317–1375. doi:10.1002/jps.21528
2. Wang H, Xu T, Shou B. Determination of material strengths by Hydraulic Bulge Test. Mater (Basel, Switzerland). 2016;10(1):23. doi:10.3390/ma10010023
3. Laurencin C, Khan Y, El-Amin SF. Bone graft substitutes. Expert Rev Med Devices. 2006;3(1):49–57. doi:10.1586/17434440.3.1.49
4. Jimi E, Hirata S, Osawa K, Terashita M, Kitamura C, Fukushima H. The current and future therapies of bone regeneration to repair bone defects. Int J Dent. 2012;2012:1–7. doi:10.1155/2012/148261
5. Kashte S, Jaiswal AK, Kadam S. Artificial bone via bone tissue engineering: current scenario and challenges. Tissue Eng Regen Med. 2017;14(1):1–14. doi:10.1007/s13770-016-0001-6
6. Meijer GJ, de Bruijn JD, Koole R, van Blitterswijk CA. Cell-based bone tissue engineering. PLoS Med. 2007;4(2):e9–e9. doi:10.1371/journal.pmed.0040009
7. Rogers GF, Greene AK. Autogenous bone graft: basic science and clinical implications. J Craniofac Surg. 2012;23(1):323–327. doi:10.1097/SCS.0b013e318241dcba
8. Gu Z, Fu J, Lin H, He Y. Development of 3D bioprinting: from printing methods to biomedical applications. Asian J Pharm Sci. 2020;15(5):529–557. doi:10.1016/j.ajps.2019.11.003
9. Chepelev L, Wake N, Ryan J, et al. Radiological Society of North America (RSNA) 3D printing Special Interest Group (SIG): guidelines for medical 3D printing and appropriateness for clinical scenarios. 3D Print Med. 2018. doi:10.1186/s41205-018-0030-y
10. Shuaib M, Haleem A, Kumar S, Javaid M. Impact of 3D Printing on the environment: a literature-based study. Sustain Oper Comput. 2021;2:57–63. doi:10.1016/j.susoc.2021.04.001
11. Rana D, Kumar TSS, Ramalingam M. Impact of nanotechnology on 3D bioprinting. J Bionanoscience. 2017;11(1):1–6. doi:10.1166/jbns.2017.1417
12. Bhatt A, Anbarasu A. Nanoscale Biomaterials for 3D printing. IOSR J Pharm Biol Sci. 2017. doi:10.9790/3008-1203068086
13. Sowjanya JA, Singh J, Mohita T, et al. Biocomposite scaffolds containing chitosan/alginate/nano-silica for bone tissue engineering. Colloids Surfaces B Biointerfaces. 2013;109:294–300. doi:10.1016/j.colsurfb.2013.04.006
14. Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng. 2012;40(5):363–408. doi:10.1615/CritRevBiomedEng.v40.i5.10
15. Rho JY, Kuhn-Spearing L, Zioupos P. Mechanical properties and the hierarchical structure of bone. Med Eng Phys. 1998;20(2):92–102. doi:10.1016/S1350-4533(98)00007-1
16. Orciani M, Fini M, Di Primio R, Mattioli-Belmonte M. Biofabrication and bone tissue regeneration: cell source, approaches, and challenges. Front Bioeng Biotechnol. 2017;5. doi:10.3389/fbioe.2017.00017
17. Ringe J, Sittinger M. Tissue engineering in the rheumatic diseases. Arthritis Res Ther. 2009;11(1):211. doi:10.1186/ar2572
18. Wang W, Yeung KWK. Bone grafts and biomaterials substitutes for bone defect repair: a review. Bioact Mater. 2017. doi:10.1016/j.bioactmat.2017.05.007
19. Hollinger JO. The critical size defect as an experimental model to test bone repair materials. J Craniofac Surg. 1990;1(1):60–68. doi:10.1097/00001665-199001000-00011
20. Gosain AK, Song L, Yu P, et al. Osteogenesis in cranial defects: reassessment of the concept of critical size and the expression of TGF-β isoforms. Plast Reconstr Surg. 2000;106(2):360–371. doi:10.1097/00006534-200008000-00018
21. Bezstarosti H, Metsemakers WJ, van Lieshout EMM, et al. Management of critical-sized bone defects in the treatment of fracture-related infection: a systematic review and pooled analysis. Arch Orthop Trauma Surg. 2020. doi:10.1007/s00402-020-03525-0
22. Schemitsch EH. Size matters: defining critical in bone defect size! J Orthop Trauma. 2017;31(5):S20–S22. doi:10.1097/BOT.0000000000000978
23. Bhandari M, Guyatt G, Walter SD, et al. Randomized trial of reamed and unreamed intramedullary nailing of tibial shaft fractures. J Bone Jt Surg Ser A. 2008;90(12):2567–2578. doi:10.2106/JBJS.G.01694
24. Nauth A, McKee MD, Einhorn TA, Watson JT, Li R, Schemitsch EH. Managing bone defects. J Orthop Trauma. 2011;25(8):462–466. doi:10.1097/BOT.0b013e318224caf0
25. Keating JF, Simpson AHRW, Robinson CM. The management of fractures with bone loss. J Bone Jt Surg Ser B. 2005;87-B(2):142–150. doi:10.1302/0301-620X.87B2.15874
26. Sanders DW, Bhandari M, Guyatt G, et al. Critical-sized defect in the Tibia: is it critical? Results from the SPRINT trial. J Orthop Trauma. 2014;28(11):632–635. doi:10.1097/BOT.0000000000000194
27. Henkel J, Woodruff MA, Epari DR, et al. Bone regeneration based on tissue engineering conceptions-A 21st Century Perspective. Bone Res. 2013;1(3):216–248. doi:10.4248/BR201303002
28. Ghassemi T, Shahroodi A, Ebrahimzadeh MH, Mousavian A, Movaffagh J, Moradi A. Current concepts in scaffolding for bone tissue engineering. Arch Bone Jt Surg. 2018;6(2):90–99.
29. Yuan B, Zhou S-Y, Chen X-S. Rapid prototyping technology and its application in bone tissue engineering. J Zhejiang Univ Sci B. 2017;18(4):303–315. doi:10.1631/jzus.B1600118
30. Salgado AJ, Coutinho OP, Reis RL. Bone tissue engineering: state of the art and future trends. Macromol Biosci. 2004;4(8):743–765. doi:10.1002/mabi.200400026
31. Cancedda R, Giannoni P, Mastrogiacomo M. A tissue engineering approach to bone repair in large animal models and in clinical practice. Biomaterials. 2007;28(29):4240–4250. doi:10.1016/j.biomaterials.2007.06.023
32. Wang C, Huang W, Zhou Y, et al. 3D printing of bone tissue engineering scaffolds. Bioact Mater. 2020;5(1):82–91. doi:10.1016/j.bioactmat.2020.01.004
33. Leukers B, Gülkan H, Irsen SH, et al. Biocompatibility of ceramic scaffolds for bone replacement made by 3D printing. Materwiss Werksttech. 2005;36(12):781–787. doi:10.1002/mawe.200500968
34. Detsch R, Schaefer S, Deisinger U, Ziegler G, Seitz H, Leukers B. In vitro-osteoclastic activity studies on surfaces of 3D printed calcium phosphate scaffolds. J Biomater Appl. 2011;26(3):359–380. doi:10.1177/0885328210373285
35. Bose S, Vahabzadeh S, Bandyopadhyay A. Bone tissue engineering using 3D printing. Mater Today. 2013;16(12):496–504. doi:10.1016/j.mattod.2013.11.017
36. Genova T, Roato I, Carossa M, Motta C, Cavagnetto D, Mussano F. Advances on bone substitutes through 3d bioprinting. Int J Mol Sci. 2020;21(19):7012. doi:10.3390/ijms21197012
37. Forrestal DP, Klein TJ, Woodruff MA. Challenges in engineering large customized bone constructs. Biotechnol Bioeng. 2017;114(6):1129–1139. doi:10.1002/bit.26222
38. Bahraminasab M. Challenges on optimization of 3D-printed bone scaffolds. Biomed Eng Online. 2020. doi:10.1186/s12938-020-00810-2
39. Kačarević ŽP, Rider PM, Alkildani S, et al. An introduction to 3D bioprinting: possibilities, challenges and future aspects. Materials (Basel). 2018;11(11):2199. doi:10.3390/ma11112199
40. Singh AV, Dad Ansari MH, Wang S, et al. The adoption of three-dimensional additive manufacturing from biomedical material design to 3D organ printing. Appl Sci. 2019. doi:10.3390/app9040811
41. Liao W, Xu L, Wangrao K, Du Y, Xiong Q, Yao Y. Three-dimensional printing with biomaterials in craniofacial and dental tissue engineering. PeerJ. 2019. doi:10.7717/peerj.7271
42. Kang HW, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol. 2016;34(3):312–319. doi:10.1038/nbt.3413
43. Wang X. Advanced polymers for three-dimensional (3D) organ bioprinting. Micromachines. 2019;10(12):814. doi:10.3390/mi10120814
44. Ozbolat IT, Yu Y. Bioprinting toward organ fabrication: challenges and future trends. IEEE Trans Biomed Eng. 2013;60(3):691–699. doi:10.1109/TBME.2013.2243912
45. Atala A. Tissue engineering of human bladder. Br Med Bull. 2011;97(1):81–104. doi:10.1093/bmb/ldr003
46. Whitaker M. The history of 3D printing in healthcare. Bull R Coll Surg Engl. 2014;96(7):228–229. doi:10.1308/147363514x13990346756481
47. Preto N, Klügel A, Himmler T, et al. 3D bioprinting of skin tissue: from pre-processing to final product evaluation. Adv Drug Deliv Rev. 2018. doi:10.1002/adhm.201700897
48. Marone EM, Rinaldi LF, Pietrabissa A, Argenteri A. Effectiveness of 3d printed models in obtaining informed consent to complex aortic surgery. J Cardiovasc Surg (Torino). 2018. doi:10.23736/S0021-9509.17.10293-4
49. Lin J, Zhou Z, Guan J, et al. Using three-dimensional printing to create individualized cranial nerve models for skull base tumor surgery. World Neurosurg. 2018;120:e142–e152. doi:10.1016/j.wneu.2018.07.236
50. Dhawan A, Kennedy PM, Rizk EB, Ozbolat IT. Three-dimensional bioprinting for bone and cartilage restoration in orthopaedic surgery. J Am Acad Orthop Surg. 2019;27(5):e215–e226. doi:10.5435/JAAOS-D-17-00632
51. Quan H, Zhang T, Xu H, Luo S, Nie J, Zhu X. Photo-curing 3D printing technique and its challenges. Bioact Mater. 2020;5(1):110–115. doi:10.1016/j.bioactmat.2019.12.003
52. Nettleton K, Luong D, Kleinfehn AP, Savariau L, Premanandan C, Becker ML. Molecular mass-dependent resorption and bone regeneration of 3D printed PPF scaffolds in a critical-sized rat Cranial Defect Model. Adv Healthc Mater. 2019;8(17):1900646. doi:10.1002/adhm.201900646
53. Li L, Yu F, Shi J, et al. In situ repair of bone and cartilage defects using 3D scanning and 3D printing. Sci Rep. 2017. doi:10.1038/s41598-017-10060-3
54. Le Guéhennec L, Van Hede D, Plougonven E, et al. In vitro and in vivo biocompatibility of calcium-phosphate scaffolds three-dimensional printed by stereolithography for bone regeneration. J Biomed Mater Res Part A. 2020;108(3):412–425. doi:10.1002/jbm.a.36823
55. Danilevicius P, Georgiadi L, Pateman CJ, Claeyssens F, Chatzinikolaidou M, Farsari M. The effect of porosity on cell ingrowth into accurately defined, laser-made, polylactide-based 3D scaffolds. Appl Surf Sci. 2015;336:2–10. doi:10.1016/j.apsusc.2014.06.012
56. Kruth JP, Mercelis P, Van Vaerenbergh J, Froyen L, Rombouts M. Binding mechanisms in selective laser sintering and selective laser melting. Rapid Prototyp J. 2005;11(1):26–36. doi:10.1108/13552540510573365
57. Mazzoli A. Selective laser sintering in biomedical engineering. Med Biol Eng Comput. 2013;51(3):245–256. doi:10.1007/s11517-012-1001-x
58. Mandrycky C, Wang Z, Kim K, Kim DH. 3D bioprinting for engineering complex tissues. Biotechnol Adv. 2016;34(4):422–434. doi:10.1016/j.biotechadv.2015.12.011
59. Gerdes S, Mostafavi A, Ramesh S, et al. Process-structure-quality relationships of three-dimensional printed Poly(Caprolactone)-Hydroxyapatite scaffolds. Tissue Eng Part A. 2020;26(5–6):279–291. doi:10.1089/ten.tea.2019.0237
60. Brunello G, Sivolella S, Meneghello R, et al. Powder-based 3D printing for bone tissue engineering. Biotechnol Adv. 2016;34(5):740–753. doi:10.1016/j.biotechadv.2016.03.009
61. Williams JM, Adewunmi A, Schek RM, et al. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials. 2005;26(23):4817–4827. doi:10.1016/j.biomaterials.2004.11.057
62. Inzana JA, Olvera D, Fuller SM, et al. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials. 2014;35(13):4026–4034. doi:10.1016/j.biomaterials.2014.01.064
63. Du Y, Liu H, Yang Q, et al. Selective laser sintering scaffold with hierarchical architecture and gradient composition for osteochondral repair in rabbits. Biomaterials. 2017;137:37–48. doi:10.1016/j.biomaterials.2017.05.021
64. Shuai C, Gao C, Nie Y, Hu A, Qu H, Peng S. Structural design and experimental analysis of a selective laser sintering system with nano-hydroxyapatite powder. J Biomed Nanotechnol. 2010;6(4):370–374. doi:10.1166/jbn.2010.1139
65. Roskies M, Jordan JO, Fang D, et al. Improving PEEK bioactivity for craniofacial reconstruction using a 3D printed scaffold embedded with mesenchymal stem cells. J Biomater Appl. 2016;31(1):132–139. doi:10.1177/0885328216638636
66. Tao O, Kort-Mascort J, Lin Y, et al. The applications of 3D printing for craniofacial tissue engineering. Micromachines. 2019;10(7):480. doi:10.3390/mi10070480
67. Rebong RE, Stewart KT, Utreja A, Ghoneima AA. Accuracy of three-dimensional dental resin models created by fused deposition modeling, stereolithography, and Polyjet prototype technologies: a comparative study. Angle Orthod. 2018;88(3):363–369. doi:10.2319/071117-460.1
68. Chen S, Pan Z, Wu Y, et al. The role of three-dimensional printed models of skull in anatomy education: a randomized controlled trail. Sci Rep. 2017. doi:10.1038/s41598-017-00647-1
69. Chiulan I, Frone AN, Brandabur C, Panaitescu DM. Recent advances in 3D printing of aliphatic polyesters. Bioengineering. 2018. doi:10.3390/bioengineering5010002
70. Liu Y, Wang R, Chen S, et al. Heparan sulfate loaded polycaprolactone-hydroxyapatite scaffolds with 3D printing for bone defect repair. Int J Biol Macromol. 2020. doi:10.1016/j.ijbiomac.2020.01.109
71. Kim SE, Shim KM, Jang K, Shim JH, Kang SS. Three-dimensional printing-based reconstruction of a maxillary bone defect in a dog following tumor removal. In Vivo (Brooklyn). 2018;32(1):63–70. doi:10.21873/invivo.11205
72. Zou Q, Grottkau BE, He Z, et al. Biofabrication of valentine-shaped heart with a composite hydrogel and sacrificial material. Mater Sci Eng C. 2020;108:110205. doi:10.1016/j.msec.2019.110205
73. Jiménez Ormabera B, Díez Valle R, Zaratiegui Fernández J, Llorente Ortega M, Unamuno Iñurritegui X, Tejada Solís S. Impresión 3 D en neurocirugía: modelo específico para pacientes con craneosinostosis. Neurocirugia. 2017;28(6):260–265. doi:10.1016/j.neucir.2017.05.001
74. Jariwala SH, Lewis GS, Bushman ZJ, Adair JH, Donahue HJ. 3D printing of personalized artificial bone scaffolds. 3D Print Addit Manuf. 2015;2(2):56–64. doi:10.1089/3dp.2015.0001
75. Pescosolido L, Schuurman W, Malda J, et al. Hyaluronic acid and dextran-based semi-IPN hydrogels as biomaterials for bioprinting. Biomacromolecules. 2011;12(5):1831–1838. doi:10.1021/bm200178w
76. Axpe E, Oyen ML. Applications of alginate-based bioinks in 3D bioprinting. Int J Mol Sci. 2016;17(12):1976. doi:10.3390/ijms17121976
77. Vehmeijer M, van Eijnatten M, Liberton N, Wolff J. A novel method of orbital floor reconstruction using virtual planning, 3-dimensional printing, and autologous bone. J Oral Maxillofac Surg. 2016;74(8):1608–1612. doi:10.1016/j.joms.2016.03.044
78. Kumar A, Misra RDK. 3D-printed titanium alloys for orthopedic applications. In: Froes FH, Qian M, editors. Titanium in Medical and Dental Applications. Woodhead Publishing; 2018:251-275. doi:10.1016/B978-0-12-812456-7.00012-3
79. Vandenbroucke B, Kruth JP. Selective laser melting of biocompatible metals for rapid manufacturing of medical parts. Rapid Prototyp J. 2007;13(4):196–203. doi:10.1108/13552540710776142
80. Qu H, Fu H, Han Z, Sun Y. Biomaterials for bone tissue engineering scaffolds: a review. RSC Adv. 2019;9(45):26252–26262. doi:10.1039/c9ra05214c
81. Shah FA, Trobos M, Thomsen P, Palmquist A. Commercially pure titanium (cp-Ti) versus titanium alloy (Ti6Al4V) materials as bone anchored implants - Is one truly better than the other? Mater Sci Eng C. 2016;62:960–966. doi:10.1016/j.msec.2016.01.032
82. Lin X, Xiao X, Wang Y, et al. Biocompatibility of Bespoke 3D-printed titanium alloy plates for treating acetabular fractures. Biomed Res Int. 2018. doi:10.1155/2018/2053486
83. Srinivasan M, Ramesh S. Synthesis and characterization of titanium alloy (Ti-6Al-4V) by high energy ball milling. Int J Appl Eng Res. 2015.
84. Sidambe AT. Biocompatibility of advanced manufactured titanium implants-A review. Materials (Basel). 2014;7(12):8168–8188. doi:10.3390/ma7128168
85. Hollander DA, Von Walter M, Wirtz T, et al. Structural, mechanical and in vitro characterization of individually structured Ti-6Al-4V produced by direct laser forming. Biomaterials. 2006;27(7):955–963. doi:10.1016/j.biomaterials.2005.07.041
86. Imanishi J, Choong PFM. Three-dimensional printed calcaneal prosthesis following total calcanectomy. Int J Surg Case Rep. 2015;10:83–87. doi:10.1016/j.ijscr.2015.02.037
87. Punyaratabandhu T, Lohwongwatana B, Puncreobutr C, Kosiyatrakul A, Veerapan P, Luenam S. A patient-matched entire first metacarpal prosthesis in treatment of giant cell tumor of bone. Case Rep Orthop. 2017;2017:1–6. doi:10.1155/2017/4101346
88. Kim SC, Jo WL, Kim YS, Kwon SY, Cho YS, Lim YW. Titanium powder coating using metal 3D printing: a novel coating technology for cobalt–chromium alloy implants. Tissue Eng Regen Med. 2019;16(1):11–18. doi:10.1007/s13770-018-0168-0
89. Lu Y, Wu S, Gan Y, et al. Investigation on the microstructure, mechanical property and corrosion behavior of the selective laser melted CoCrW alloy for dental application. Mater Sci Eng C. 2015. doi:10.1016/j.msec.2015.01.023
90. Hazlehurst K, Wang CJ, Stanford M. Evaluation of the stiffness characteristics of square pore CoCrMo cellular structures manufactured using laser melting technology for potential orthopaedic applications. Mater Des. 2013;51:949–955. doi:10.1016/j.matdes.2013.05.009
91. Chen Y, Geever LM, Killion JA, Lyons JG, Higginbotham CL, Devine DM. Review of multifarious applications of Poly (Lactic Acid). Polym Plast Technol Eng. 2016;55(10):1057–1075. doi:10.1080/03602559.2015.1132465
92. Narayanan G, Vernekar VN, Kuyinu EL, Laurencin CT. Poly (lactic acid)-based biomaterials for orthopaedic regenerative engineering. Adv Drug Deliv Rev. 2016. doi:10.1016/j.addr.2016.04.015
93. Singhvi MS, Zinjarde SS, Gokhale DV. Polylactic acid: synthesis and biomedical applications. J Appl Microbiol. 2019;127(6):1612–1626. doi:10.1111/jam.14290
94. Madhavan Nampoothiri K, Nair NR, John RP. An overview of the recent developments in polylactide (PLA) research. Bioresour Technol. 2010;101(22):8493–8501. doi:10.1016/j.biortech.2010.05.092
95. Pina S, Ferreira JMF. Bioresorbable plates and screws for clinical applications: a review. J Healthc Eng. 2012;3(2):243–260. doi:10.1260/2040-2295.3.2.243
96. Athanasiou KA, Agrawal CM, Barber FA, Burkhart SS. Orthopaedic applications for PLA-PGA biodegradable polymers. Arthroscopy. 1998;14(7):726–737. doi:10.1016/S0749-8063(98)70099-4
97. Martin O, Avérous L. Poly(lactic acid): plasticization and properties of biodegradable multiphase systems. Polymer (Guildf). 2001;42(14):6209–6219. doi:10.1016/S0032-3861(01)00086-6
98. Guarino V, Gentile G, Sorrentino L, Ambrosio L. Polycaprolactone: synthesis, properties, and applications. In: Matyjaszewski K, editor. Encyclopedia of Polymer Science and Technology. John Wiley & Sons, Ltd; 2017:1-36. doi:10.1002/0471440264.pst658
99. Bhullar SK, Rana D, Lekesiz H, et al. Design and fabrication of auxetic PCL nanofiber membranes for biomedical applications. Mater Sci Eng C. 2017;81:334–340. doi:10.1016/j.msec.2017.08.022
100. Malikmammadov E, Tanir TE, Kiziltay A, Hasirci V, Hasirci N. PCL and PCL-based materials in biomedical applications. J Biomater Sci Polym Ed. 2018;29(7–9):863–893. doi:10.1080/09205063.2017.1394711
101. Ong KL, Yun BM, White JB. New biomaterials for orthopedic implants. Orthop Res Rev. 2015. doi:10.2147/ORR.S63437
102. Singh NK, Maiti P. Polycaprolactone based Nanobiomaterials. In: Tiwari A, Ramalingam M, Kobayashi H, Turner A, editors. Biomedical Materials and Diagnostic Devices. Scrivener Publishing LLC; 2012:115-155. doi:10.1002/9781118523025.ch4
103. Prakasam M, Locs J, Salma-Ancane K, Loca D, Largeteau A, Berzina-Cimdina L. Biodegradable materials and metallic implants-A review. J Funct Biomater. 2017;8(4):44. doi:10.3390/jfb8040044
104. Boia R, Dias PAN, Martins JM, et al. Porous poly(ε-caprolactone) implants: a novel strategy for efficient intraocular drug delivery. J Control Release. 2019;316:331–348. doi:10.1016/j.jconrel.2019.09.023
105. Daentzer D, Floerkemeier T, Bartsch I, et al. Preliminary results in anterior cervical discectomy and fusion with an experimental bioabsorbable cage - clinical and radiological findings in an ovine animal model. Springerplus. 2013;2(1). doi:10.1186/2193-1801-2-418
106. Makadia HK, Siegel SJ. Poly Lactic-co-Glycolic Acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers (Basel). 2011;3(3):1377–1397. doi:10.3390/polym3031377
107. Medine C, Khan F, Pernagallo S, et al. Identification and application of polymers as biomaterials for tissue engineering and regenerative medicine. In: Ramalingam M, Ramakrishna S, Best S, editors. Biomaterials and Stem Cells in Regenerative Medicine. 2012:1-30. doi:10.1201/b12083-2
108. Prabhu B, Karau A, Wood A, Dadsetan M, Liedtke H, Dewitt T. Bioresorbable materials for orthopedic applications (Lactide and glycolide based). In: Li B, Webster T, editors. Orthopedic Biomaterials: Progress in Biology, Manufacturing, and Industry Perspectives. Springer International Publishing; 2018:287–344. doi:10.1007/978-3-319-89542-0_13
109. Gentile P, Chiono V, Carmagnola I, Hatton PV. An overview of poly(lactic-co-glycolic) Acid (PLGA)-based biomaterials for bone tissue engineering. Int J Mol Sci. 2014;15(3):3640–3659. doi:10.3390/ijms15033640
110. Park JW, Hwang JU, Back JH, et al. High strength PLGA/Hydroxyapatite composites with tunable surface structure using PLGA direct grafting method for orthopedic implants. Compos Part B Eng. 2019;178:107449. doi:10.1016/j.compositesb.2019.107449
111. Ortega-Oller I, Padial-Molina M, Galindo-Moreno P, O’Valle F, Jódar-Reyes AB, Peula-García JM. Bone regeneration from PLGA micro-nanoparticles. Biomed Res Int. 2015;2015:1–18. doi:10.1155/2015/415289
112. Rezvantalab S, Drude NI, Moraveji MK, et al. PLGA-based nanoparticles in cancer treatment. Front Pharmacol. 2018;9. doi:10.3389/fphar.2018.01260
113. Meyer F, Wardale J, Best S, Cameron R, Rushton N, Brooks R. Effects of lactic acid and glycolic acid on human osteoblasts: a way to understand PLGA involvement in PLGA/calcium phosphate composite failure. J Orthop Res. 2012;30(6):864–871. doi:10.1002/jor.22019
114. Guo T, Holzberg TR, Lim CG, et al. 3D printing PLGA: a quantitative examination of the effects of polymer composition and printing parameters on print resolution. Biofabrication. 2017;9(2):024101. doi:10.1088/1758-5090/aa6370
115. Frosch KH, Sawallich T, Schütze G, et al. Magnetic resonance imaging analysis of the bioabsorbable MilagroTM interference screw for graft fixation in anterior cruciate ligament reconstruction. Strateg Trauma Limb Reconstr. 2009;4(2):73–79. doi:10.1007/s11751-009-0063-2
116. Fan JP, Tsui CP, Tang CY, Chow CL. Influence of interphase layer on the overall elasto-plastic behaviors of HA/PEEK biocomposite. Biomaterials. 2004;25(23):5363–5373. doi:10.1016/j.biomaterials.2003.12.050
117. Haleem A, Javaid M. Polyether ether ketone (PEEK) and its 3D printed implants applications in medical field: an overview. Clin Epidemiol Glob Health. 2019;7(4):571–577. doi:10.1016/j.cegh.2019.01.003
118. Feng X, Yu H, Liu H, et al. Three-dimensionally-printed polyether-ether-ketone implant with a cross-linked structure and acid-etched microporous surface promotes integration with soft tissue. Int J Mol Sci. 2019;20(15):3811. doi:10.3390/ijms20153811
119. Honigmann P, Sharma N, Okolo B, et al. Patient-specific surgical implants made of 3D printed PEEK: material, technology, and scope of surgical application. Biomed Res Int. 2018. doi:10.1155/2018/4520636
120. Singh S, Prakash C, Ramakrishna S. 3D printing of polyether-ether-ketone for biomedical applications. Eur Polym J. 2019;114:234–248. doi:10.1016/j.eurpolymj.2019.02.035
121. Panayotov IV, Orti V, Cuisinier F, Yachouh J. Polyetheretherketone (PEEK) for medical applications. J Mater Sci Mater Med. 2016. doi:10.1007/s10856-016-5731-4
122. Khalyfa A, Vogt S, Weisser J, et al. Development of a new calcium phosphate powder-binder system for the 3D printing of patient specific implants. J Mater Sci Mater Med. 2007;18(5):909–916. doi:10.1007/s10856-006-0073-2
123. Bergmann C, Lindner M, Zhang W, et al. 3D printing of bone substitute implants using calcium phosphate and bioactive glasses. J Eur Ceram Soc. 2010;30(12):2563–2567. doi:10.1016/j.jeurceramsoc.2010.04.037
124. Victor SP, Sharma CP. Calcium phosphates and nanocrystalline apatites for medical applications. In: Ramalingam M, Tiwari A, Ramakrishna S and Kobayashi H, editors. Integrated Biomaterials for Biomedical Technology. Scrivener Publishing LLC; 2012:121-144. doi:10.1002/9781118482513.ch4
125. Trombetta R, Inzana JA, Schwarz EM, Kates SL, Awad HA. 3D printing of calcium phosphate ceramics for bone tissue engineering and drug delivery. Ann Biomed Eng. 2017;45(1):23–44. doi:10.1007/s10439-016-1678-3
126. Tarafder S, Balla VK, Davies NM, Bandyopadhyay A, Bose S. Microwave-sintered 3D printed tricalcium phosphate scaffolds for bone tissue engineering. J Tissue Eng Regen Med. 2013;7(8):631–641. doi:10.1002/term.555
127. Shao H, He J, Lin T, Zhang Z, Zhang Y, Liu S. 3D gel-printing of hydroxyapatite scaffold for bone tissue engineering. Ceram Int. 2019. doi:10.1016/j.ceramint.2018.09.300
128. Murugan R, Ramakrishna S. Crystallographic study of hydroxyapatite bioceramics derived from various sources. Cryst Growth Des. 2005;5(1):111–112. doi:10.1021/cg034227s
129. Prakasam M, Locs J, Salma-Ancane K, Loca D, Largeteau A, Berzina-Cimdina L. Fabrication, properties and applications of dense hydroxyapatite: a review. J Funct Biomater. 2015;6(4):1099–1140. doi:10.3390/jfb6041099
130. Kumar A, Kargozar S, Baino F, Han SS. Additive manufacturing methods for producing hydroxyapatite and hydroxyapatite-based composite scaffolds: a review. Front Mater. 2019;6. doi:10.3389/fmats.2019.00313
131. Burgio F, Rimmer N, Pieles U, Buschmann J, Beaufils-Hugot M. Characterization and in ovo vascularization of a 3D-printed hydroxyapatite scaffold with different extracellular matrix coatings under perfusion culture. Biol Open. 2018. doi:10.1242/bio.034488
132. Luo Y, Chen S, Shi Y, Ma J. 3D printing of strontium-doped hydroxyapatite based composite scaffolds for repairing critical-sized rabbit calvarial defects. Biomed Mater. 2018;13(6):065004. doi:10.1088/1748-605X/aad923
133. Liu W, Li Y, Liu J, Niu X, Wang Y, Li D. Application and performance of 3D printing in nanobiomaterials. J Nanomater. 2013. doi:10.1155/2013/681050
134. Elder B, Neupane R, Tokita E, Ghosh U, Hales S, Kong YL. Nanomaterial patterning in 3D printing. Adv Mater. 2020;32(17):1907142. doi:10.1002/adma.201907142
135. Murugan R, Ramakrishna S. Development of nanocomposites for bone grafting. Compos Sci Technol. 2005;65(15–16):2385–2406. doi:10.1016/j.compscitech.2005.07.022
136. Shahrubudin N, Lee TC, Ramlan R. An overview on 3D printing technology: technological, materials, and applications. Procedia Manuf. 2019;35:1286–1296. doi:10.1016/j.promfg.2019.06.089
137. Pataky K, Braschler T, Negro A, Renaud P, Lutolf MP, Brugger J. Microdrop printing of hydrogel bioinks into 3D tissue-like geometries. Adv Mater. 2012;24(3):391–396. doi:10.1002/adma.201102800
138. Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32(8):773–785. doi:10.1038/nbt.2958
139. Mironov V, Kasyanov V, Markwald RR. Organ printing: from bioprinter to organ biofabrication line. Curr Opin Biotechnol. 2011;22(5):667–673. doi:10.1016/j.copbio.2011.02.006
140. Liu S, Zhang H, Hu Q, Shen Z, Rana D, Ramalingam M. Designing vascular supportive albumen-rich composite bioink for organ 3D printing. J Mech Behav Biomed Mater. 2020. doi:10.1016/j.jmbbm.2020.103642
141. Rastin H, Zhang B, Bi J, Hassan K, Tung TT, Losic D. 3D printing of cell-laden electroconductive bioinks for tissue engineering applications. J Mater Chem B. 2020;8(27):5862–5876. doi:10.1039/d0tb00627k
142. Rutz AL, Lewis PL, Shah RN. Toward next-generation bioinks: tuning material properties pre- and post-printing to optimize cell viability. MRS Bull. 2017;42(08):563–570. doi:10.1557/mrs.2017.162
143. Ashammakhi N, Ahadian S, Xu C, et al. Bioinks and bioprinting technologies to make heterogeneous and biomimetic tissue constructs. Mater Today Biol. 2019. doi:10.1016/j.mtbio.2019.100008
144. How PDP. Nanotechnology can really improve the future of orthopedic implants and scaffolds for bone and cartilage defects. J Nanomedine Biotherapeutic Discov. 2013. doi:10.4172/2155-983x.1000114
145. Garimella R, Eltorai AEM. Nanotechnology in orthopedics. J Orthop. 2017;14(1):30–33. doi:10.1016/j.jor.2016.10.026
146. Smith IO, Liu XH, Smith LA, Ma PX. Nanostructured polymer scaffolds for tissue engineering and regenerative medicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(2):226–236. doi:10.1002/wnan.26
147. Ahadian S, Batmanghelich F, Obregón R, et al. Carbon-based nanobiomaterials. In: Wang X, Ramalingam M, Kong X and Zhao L, editors. Nanobiomaterials: Classification, Fabrication and Biomedical Applications. Wiley-VCH Verlag GmbH & Co. KGaA; 2018:85-104. doi:10.1002/9783527698646.ch4
148. Sitharaman B. Nanotechnology in tissue engineering and regenerative medicine. Tissue Eng Part B Rev. 2012;18(1):76. doi:10.1089/ten.teb.2011.1501
149. Dhivya S, Ajita J, Selvamurugan N. Metallic nanomaterials for bone tissue engineering. J Biomed Nanotechnol. 2015;11(10):1675–1700. doi:10.1166/jbn.2015.2115
150. Prodanov L, Lamers E, Domanski M, Luttge R, Jansen JA, Walboomers XF. The effect of nanometric surface texture on bone contact to titanium implants in rabbit tibia. Biomaterials. 2013;34(12):2920–2927. doi:10.1016/j.biomaterials.2013.01.027
151. Ueno T, Ikeda T, Tsukimura N, et al. Novel antioxidant capability of titanium induced by UV light treatment. Biomaterials. 2016;108:177–186. doi:10.1016/j.biomaterials.2016.08.050
152. Liang CY, Jiang XJ, Ji RL, et al. Preparation and surface modification of 3D printed Ti–6Al–4V porous implant. Rare Met. 2020:1–9. doi:10.1007/s12598-020-01413-5
153. Chou DT, Wells D, Hong D, Lee B, Kuhn H, Kumta PN. Novel processing of iron-manganese alloy-based biomaterials by inkjet 3-D printing. Acta Biomater. 2013;9(10):8593–8603. doi:10.1016/j.actbio.2013.04.016
154. Hong D, Chou DT, Velikokhatnyi OI, et al. Binder-jetting 3D printing and alloy development of new biodegradable Fe-Mn-Ca/Mg alloys. Acta Biomater. 2016;45:375–386. doi:10.1016/j.actbio.2016.08.032
155. Huang T, Zheng Y, Han Y. Accelerating degradation rate of pure iron by zinc ion implantation. Regen Biomater. 2016;3(4):205. doi:10.1093/rb/rbw020
156. Wang H, Zheng Y, Li Y, Jiang C. Improvement of in vitro corrosion and cytocompatibility of biodegradable Fe surface modified by Zn ion implantation. Appl Surf Sci. 2017;403(403):168–176. doi:10.1016/j.apsusc.2017.01.158
157. Huang CH, Chen RS, Yoshimura M. Direct bioactive ceramics coating via reactive growing integration layer method on α-Ti-alloy. Mater Sci Eng C. 2017;76:1216–1223. doi:10.1016/j.msec.2017.03.182
158. Lim HK, Byun SH, Lee JY, et al. Radiological, histological, and hematological evaluation of hydroxyapatite-coated resorbable magnesium alloy screws placed in rabbit tibia. J Biomed Mater Res Part B Appl Biomater. 2017;105(6):1636–1644. doi:10.1002/jbm.b.33703
159. Yang C, Huan Z, Wang X, Wu C, Chang J. 3D printed Fe scaffolds with HA nanocoating for bone regeneration. ACS Biomater Sci Eng. 2018;4(2):608–616. doi:10.1021/acsbiomaterials.7b00885
160. Bai RG, Muthoosamy K, Manickam S, Hilal-Alnaqbi A. Graphene-based 3D scaffolds in tissue engineering: fabrication, applications, and future scope in liver tissue engineering. Int J Nanomedicine. 2019. doi:10.2147/IJN.S192779
161. Liu X, George MN, Park S, et al. 3D-printed scaffolds with carbon nanotubes for bone tissue engineering: fast and homogeneous one-step functionalization. Acta Biomater. 2020. doi:10.1016/j.actbio.2020.04.047
162. Martinelli V, Bosi S, Penã B, et al. 3D carbon-nanotube-based composites for cardiac tissue engineering. ACS Appl Bio Mater. 2018;1(5):1530–1537. doi:10.1021/acsabm.8b00440
163. Shin SR, Li YC, Jang HL, et al. Graphene-based materials for tissue engineering. Adv Drug Deliv Rev. 2016;105:255–274. doi:10.1016/j.addr.2016.03.007
164. Eivazzadeh-Keihan R, Maleki A, de la Guardia M, et al. Carbon based nanomaterials for tissue engineering of bone: building new bone on small black scaffolds: a review. J Adv Res. 2019;18:185–201. doi:10.1016/J.JARE.2019.03.011
165. Postiglione G, Natale G, Griffini G, Levi M, Turri S. Conductive 3D microstructures by direct 3D printing of polymer/carbon nanotube nanocomposites via liquid deposition modeling. Compos Part a Appl Sci Manuf. 2015;76:110–114. doi:10.1016/j.compositesa.2015.05.014
166. Nayak TR, Jian L, Phua LC, Ho HK, Ren Y, Pastorin G. Thin films of functionalized multiwalled carbon nanotubes as suitable scaffold materials for stem cells proliferation and bone formation. ACS Nano. 2010;4(12):7717–7725. doi:10.1021/nn102738c
167. Wang W, Liao S, Liu M, Zhao Q, Zhu Y. Polymer composites reinforced by nanotubes as scaffolds for tissue engineering. Int J Polym Sci. 2014;2014:1–14. doi:10.1155/2014/805634
168. Li JL, Yang Z, Loo WTY, et al. In vitro and in vivo biocompatibility of multi-walled carbon nanotube/biodegradable polymer nanocomposite for bone defects repair. J Bioact Compat Polym. 2014;29(4):350–367. doi:10.1177/0883911514533867
169. Dervishi E, Hategekimana F, Boyer L, et al. The effect of carbon nanotubes and graphene on the mechanical properties of multi-component polymeric composites. Chem Phys Lett. 2013;590:126–130. doi:10.1016/j.cplett.2013.10.060
170. Pan L, Pei X, He R, Wan Q, Wang J. Multiwall carbon nanotubes/polycaprolactone composites for bone tissue engineering application. Colloids Surfaces B Biointerfaces. 2012;93:226–234. doi:10.1016/j.colsurfb.2012.01.011
171. Harrison BS, Atala A. Carbon nanotube applications for tissue engineering. Biomaterials. 2007;28(2):344–353. doi:10.1016/j.biomaterials.2006.07.044
172. Gupta A, Main BJ, Taylor BL, et al. In vitro evaluation of three-dimensional single-walled carbon nanotube composites for bone tissue engineering. J Biomed Mater Res A. 2014;102(11). doi:10.1002/JBM.A.35088
173. Pei B, Wang W, Dunne N, Li X. Applications of carbon nanotubes in bone tissue regeneration and engineering: superiority, concerns, current advancements, and prospects. Nanomaterials. 2019;9(10):1501. doi:10.3390/nano9101501
174. McCarthy B, Coleman JN, Curran SA, et al. Observation of site selective binding in a polymer nanotube composite. J Mater Sci Lett. 2000;19(24):2239–2241. doi:10.1023/A:1006776908183
175. Acquah SFA, Leonhardt BE, Nowotarski MS. et al. Carbon nanotubes and graphene as additives in 3D printing. In: Berber MR, Hafez IH, editors. Carbon Nanotubes - Current Progress of Their Polymer Composites. InTech; 2016:227–251. doi:10.5772/63419
176. Cui H, Yu Y, Li X, et al. Direct 3D printing of a tough hydrogel incorporated with carbon nanotubes for bone regeneration. J Mater Chem B. 2019;7(45):7207–7217. doi:10.1039/c9tb01494b
177. Eatemadi A, Daraee H, Karimkhanloo H, et al. Carbon nanotubes: properties, synthesis, purification, and medical applications. Nanoscale Res Lett. 2014;9(1):393. doi:10.1186/1556-276X-9-393
178. Gnanasekaran K, Heijmans T, van Bennekom S, et al. 3D printing of CNT- and graphene-based conductive polymer nanocomposites by fused deposition modeling. Appl Mater Today. 2017;9:21–28. doi:10.1016/j.apmt.2017.04.003
179. Kumar R, Kumar VB, Gedanken A. Sonochemical synthesis of carbon dots, mechanism, effect of parameters, and catalytic, energy, biomedical and tissue engineering applications. Ultrason Sonochem. 2020;64:105009. doi:10.1016/j.ultsonch.2020.105009
180. De Armentia SL, Del Real JC, Paz E, Dunne N. Advances in biodegradable 3D printed scaffolds with carbon-based nanomaterials for bone regeneration. Materials (Basel). 2020. doi:10.3390/ma13225083
181. Ghosal K, Ghosh A. Carbon dots: the next generation platform for biomedical applications. Mater Sci Eng C. 2019;96:887–903. doi:10.1016/j.msec.2018.11.060
182. Sun YP, Zhou B, Lin Y, et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J Am Chem Soc. 2006;128(24):7756–7757. doi:10.1021/ja062677d
183. Ray SC, Saha A, Jana NR, Sarkar R. Fluorescent carbon nanoparticles: synthesis, characterization, and bioimaging application. J Phys Chem C. 2009;113(43):18546–18551. doi:10.1021/jp905912n
184. Su Y, Zhou X, Long Y, Li W. Immobilization of horseradish peroxidase on amino-functionalized carbon dots for the sensitive detection of hydrogen peroxide. Microchim Acta. 2018;185(2). doi:10.1007/s00604-017-2629-x
185. Zhou Y, Mintz KJ, Sharma SK, Leblanc RM. Carbon dots: diverse preparation, application, and perspective in surface chemistry. Langmuir. 2019. doi:10.1021/acs.langmuir.9b00595
186. Peng Z, Zhao T, Zhou Y, Li S, Li J, Leblanc RM. Bone tissue engineering via carbon-based nanomaterials. Adv Healthc Mater. 2020;9(5):1901495. doi:10.1002/adhm.201901495
187. Shang W, Zhang X, Zhang M, et al. The uptake mechanism and biocompatibility of graphene quantum dots with human neural stem cells. Nanoscale. 2014;6(11):5799–5806. doi:10.1039/c3nr06433f
188. Gogoi S, Maji S, Mishra D, Devi KSP, Maiti TK, Karak N. Nano-bio engineered carbon dot-peptide functionalized water dispersible hyperbranched polyurethane for bone tissue regeneration. Macromol Biosci. 2017;17(3):1600271. doi:10.1002/mabi.201600271
189. Sarkar C, Chowdhuri AR, Kumar A, et al. One pot synthesis of carbon dots decorated carboxymethyl cellulose- hydroxyapatite nanocomposite for drug delivery, tissue engineering and Fe3+ ion sensing. Carbohydr Polym. 2018;181:710–718. doi:10.1016/j.carbpol.2017.11.091
190. Lakshmi Priya M, Rana D, Ramalingam M. Ceramic nanofiber composites. In: Ramalingan M, Ramakrishna S, editors. Nanofiber Composites for Biomedical Applications. Elsevier Ltd; 2017:33–54. doi:10.1016/B978-0-08-100173-8.00030-2
191. Kiani A, Rahmani M, Manickam S, Tan B. Nanoceramics: synthesis, characterization, and applications. J Nanomater. 2014;2014:1–2. doi:10.1155/2014/528348
192. Ben-Nissan B, Choi AH. Nanoceramics for medical applications. In: Geckeler KE, Nishide H, editors. Advanced Nanomaterials. WILEY-VCH Verlag GmbH & Co. KGaA; 2010:523–553. doi:10.1002/9783527628940.ch16
193. Singh Z. Nanoceramics in bone tissue engineering: the future lies ahead. Trends J Sci Res. 2018;3(3):120–123. doi:10.31586/nanomaterials.0303.03
194. Mondal S, Nguyen TP, Pham VH, et al. Hydroxyapatite nano bioceramics optimized 3D printed poly lactic acid scaffold for bone tissue engineering application. Ceram Int. 2020;46(3):3443–3455. doi:10.1016/j.ceramint.2019.10.057
195. Dimas LS, Bratzel GH, Eylon I, Buehler MJ. Tough composites inspired by mineralized natural materials: computation, 3D printing, and testing. Adv Funct Mater. 2013;23(36):4629–4638. doi:10.1002/adfm.201300215
196. Quan Z, Wu A, Keefe M, et al. Additive manufacturing of multi-directional preforms for composites: opportunities and challenges. Mater Today. 2015;18(9):503–512. doi:10.1016/j.mattod.2015.05.001
197. Wang J, Shaw LL. Fabrication of functionally graded materials via Inkjet color printing. J Am Ceram Soc. 2006;89(10):3285–3289. doi:10.1111/j.1551-2916.2006.01206.x
198. Kumar S, Kruth JP. Composites by rapid prototyping technology. Mater Des. 2010;31(2):850–856. doi:10.1016/j.matdes.2009.07.045
199. Ghosh SK, Saha P. Crack and wear behavior of SiC particulate reinforced aluminium based metal matrix composite fabricated by direct metal laser sintering process. Mater Des. 2011;32(1):139–145. doi:10.1016/j.matdes.2010.06.020
200. Winkel A, Meszaros R, Reinsch S, et al. Sintering of 3D-printed glass/HAp composites. J Am Ceram Soc. 2012;95(11):3387–3393. doi:10.1111/j.1551-2916.2012.05368.x
201. Kalsoom U, Nesterenko PN, Paull B. Recent developments in 3D printable composite materials. RSC Adv. 2016;6(65):60355–60371. doi:10.1039/c6ra11334f
202. Eslami H, Solati-Hashjin M, Tahriri M. The comparison of powder characteristics and physicochemical, mechanical and biological properties between nanostructure ceramics of hydroxyapatite and fluoridated hydroxyapatite. Mater Sci Eng C. 2009;29(4):1387–1398. doi:10.1016/j.msec.2008.10.033
203. Zhang J, Zhao S, Zhu M, et al. 3D-printed magnetic Fe3O4/MBG/PCL composite scaffolds with multifunctionality of bone regeneration, local anticancer drug delivery and hyperthermia. J Mater Chem B. 2014. doi:10.1039/c4tb01063a
204. Jakus AE, Rutz AL, Shah RN. Advancing the field of 3D biomaterial printing. Biomed Mater. 2016;11(1):014102. doi:10.1088/1748-6041/11/1/014102
205. Melchels FPW, Domingos MAN, Klein TJ, Malda J, Bartolo PJ, Hutmacher DW. Additive manufacturing of tissues and organs. Prog Polym Sci. 2012;37(8):1079–1104. doi:10.1016/j.progpolymsci.2011.11.007
206. Mota C, Puppi D, Chiellini F, Chiellini E. Additive manufacturing techniques for the production of tissue engineering constructs. J Tissue Eng Regen Med. 2015;9(3):174–190. doi:10.1002/term.1635
207. Bouten CVC, Ramakrishna S, Narayan R. Additive manufacturing for regenerative medicine: where do we go from here? Curr Opin Biomed Eng. 2017;2:iii–v. doi:10.1016/j.cobme.2017.07.002
208. Youssef A, Hollister SJ, Dalton PD. Additive manufacturing of polymer melts for implantable medical devices and scaffolds. Biofabrication. 2017;9(1):012002. doi:10.1088/1758-5090/aa5766
209. Hassan M, Dave K, Chandrawati R, Dehghani F, Gomes VG. 3D printing of biopolymer nanocomposites for tissue engineering: nanomaterials, processing and structure-function relation. Eur Polym J. 2019;121:109340. doi:10.1016/j.eurpolymj.2019.109340
210. Li X, Cui R, Sun L, et al. 3D-printed biopolymers for tissue engineering application. Int J Polym Sci. 2014. doi:10.1155/2014/829145
211. Valino AD, Dizon JRC, Espera AH, Chen Q, Messman J, Advincula RC. Advances in 3D printing of thermoplastic polymer composites and nanocomposites. Prog Polym Sci. 2019;98:101162. doi:10.1016/j.progpolymsci.2019.101162
212. Mano JF, Sousa RA, Boesel LF, Neves NM, Reis RL. Bioinert, biodegradable and injectable polymeric matrix composites for hard tissue replacement: state of the art and recent developments. Compos Sci Technol. 2004. doi:10.1016/j.compscitech.2003.09.001
213. Vladkova TG. Surface engineered polymeric biomaterials with improved biocontact properties. Int J Polym Sci. 2010;2010:1–22. doi:10.1155/2010/296094
214. Simionescu BC, Ivanov D. Natural and synthetic polymers for designing composite materials. In: Antoniac IV, editor. Handbook of Bioceramics and Biocomposites. Springer International Publishing Switzerland; 2016:233–286. doi:10.1007/978-3-319-12460-5_11
215. Tamay DG, Usal TD, Alagoz AS, Yucel D, Hasirci N, Hasirci V. 3D and 4D printing of polymers for tissue engineering applications. Front Bioeng Biotechnol. 2019;7. doi:10.3389/fbioe.2019.00164
216. Carrow JK, Di Luca A, Dolatshahi-Pirouz A, Moroni L, Gaharwar AK. 3D-printed bioactive scaffolds from nanosilicates and PEOT/PBT for bone tissue engineering. Regen Biomater. 2019;6(1):29–37. doi:10.1093/rb/rby024
217. Liu A, Xue GH, Sun M, et al. 3D printing surgical implants at the clinic: a Experimental Study on anterior cruciate ligament reconstruction. Sci Rep. 2016. doi:10.1038/srep21704
218. Yeon YK, Park HS, Lee JM, et al. New concept of 3D printed bone clip (polylactic acid/hydroxyapatite/silk composite) for internal fixation of bone fractures. J Biomater Sci Polym Ed. 2018;29(7–9):894–906. doi:10.1080/09205063.2017.1384199
219. Masaeli R, Jafarzadeh Kashi TS, Dinarvand R, et al. Efficacy of the biomaterials 3 wt%-nanostrontium-hydroxyapatite-enhanced calcium phosphate cement (nanoSr-CPC) and nanoSr-CPC-incorporated simvastatin-loaded poly(lactic-co-glycolic-acid) microspheres in osteogenesis improvement: an explorative multi-phas. Mater Sci Eng C. 2016;69:171–183. doi:10.1016/j.msec.2016.06.033
220. Hirenkumar M, Steven S. Poly Lactic-co-Glycolic Acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers (Basel). 2012. doi:10.3390/polym3031377.
221. Yoshioka T, Kawazoe N, Tateishi T, Chen G. In vitro evaluation of biodegradation of poly(lactic-co-glycolic acid) sponges. Biomaterials. 2008;29(24–25):3438–3443. doi:10.1016/j.biomaterials.2008.04.011
222. Loo SC, Ooi CP, Wee SH, Boey YC. Effect of isothermal annealing on the hydrolytic degradation rate of poly(lactide-co-glycolide) (PLGA). Biomaterials. 2005;26(16):2827–2833. doi:10.1016/j.biomaterials.2004.08.031
223. Rasoulianboroujeni M, Fahimipour F, Shah P, et al. Development of 3D-printed PLGA/TiO 2 nanocomposite scaffolds for bone tissue engineering applications. Mater Sci Eng C. 2019;96:105–113. doi:10.1016/j.msec.2018.10.077
224. Khoshroo K, Jafarzadeh Kashi TS, Moztarzadeh F, Eslami H, Tahriri M. The influence of calcination temperature on the structural and biological characteristics of hydrothermally synthesized TiO2 nanotube: in Vitro Study. Synth React Inorg Met Nano-Metal Chem. 2016;46(8):1189–1194. doi:10.1080/15533174.2015.1004438
225. Gao G, Huang Y, Schilling AF, Hubbell K, Cui X. Organ bioprinting: are we there yet? Adv Healthc Mater. 2018. doi:10.1002/adhm.201701018
226. LeGeros RZ, Daculsi G, LeGeros JP. Bioactive bioceramics. In: Pietrzak WS, editor. Musculoskeletal Tissue Regeneration. Humana Press; 2008:153–181. doi:10.1007/978-1-59745-239-7_8
227. Wang X, Jiang M, Zhou Z, Gou J, Hui D. 3D printing of polymer matrix composites: a review and prospective. Compos Part B Eng. 2017. doi:10.1016/j.compositesb.2016.11.034
228. An J, Teoh JEM, Suntornnond R, Chua CK. Design and 3D printing of scaffolds and tissues. Engineering. 2015;1(2):261–268. doi:10.15302/J-ENG-2015061
229. Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26(27):5474–5491. doi:10.1016/j.biomaterials.2005.02.002
230. Masaeli R, Zandsalimi K, Rasoulianboroujeni M, Tayebi L. Challenges in three-dimensional printing of bone substitutes. Tissue Eng Part B Rev. 2019;25(5):387–397. doi:10.1089/ten.teb.2018.0381
231. Sobral JM, Caridade SG, Sousa RA, Mano JF, Reis RL. Three-dimensional plotted scaffolds with controlled pore size gradients: effect of scaffold geometry on mechanical performance and cell seeding efficiency. Acta Biomater. 2011;7(3):1009–1018. doi:10.1016/j.actbio.2010.11.003
232. Zeltinger J, Sherwood JK, Graham DA, Müeller R, Griffith LG. Effect of pore size and void fraction on cellular adhesion, proliferation, and matrix deposition. Tissue Eng. 2001;7(5):557–572. doi:10.1089/107632701753213183
233. Haleem A, Javaid M, Khan RH, Suman R. 3D printing applications in bone tissue engineering. J Clin Orthop Trauma. 2020;11:S118–S124. doi:10.1016/j.jcot.2019.12.002
234. Mercado-Pagán ÁE, Stahl AM, Shanjani Y, Yang Y. Vascularization in bone tissue engineering constructs. Ann Biomed Eng. 2015;43(3):718–729. doi:10.1007/s10439-015-1253-3
235. Ma J, Lin L, Zuo Y, et al. Modification of 3D printed PCL scaffolds by PVAc and HA to enhance cytocompatibility and osteogenesis. RSC Adv. 2019. doi:10.1039/c8ra06652c
236. Christenson EM, Anseth KS, Van den Beucken JJJP, et al. Nanobiomaterial applications in orthopedics. J Orthop Res. 2007;25(1):11–22. doi:10.1002/jor.20305
237. In: Bean AC, Tuan RS. Stem cells and nanotechnology in tissue engineering and regenerative medicine. Ramalingam M, Jabbari E, Ramakrishna S, Khademhosseini A, editors. Micro and Nanotechnologies in Engineering Stem Cells and Tissues. John Wiley & Sons, Inc; 2015:1–26. doi:10.1002/9781118574775
238. O’Brien C, Holmes B, Zhang LG. Nanotechnology: a toolkit for cell behavior. In: Zhang LG, Fisher JP, Leong KW, editors. 3D Bioprinting and Nanotechnology in Tissue Engineering and Regenerative Medicine. Elsevier Inc; 2015:1–24. doi:10.1016/B978-0-12-800547-7.00001-1
239. Srivatsan TS. Biomaterials: a nano approach, by Sreeram Ramakrishna, Murugan Ramalingam, T. S. Sampath Kumar, and Winston O. Soboyejo. Mater Manuf Process. 2014;29(11–12):1510–1511. doi:10.1080/10426914.2014.950068
240. Owen A, Dufès C, Moscatelli D, et al. The application of nanotechnology in medicine: treatment and diagnostics. Nanomedicine. 2014;9(9):1291–1294. doi:10.2217/nnm.14.93
241. Smith WR, Hudson PW, Ponce BA, Rajaram Manoharan SR. Nanotechnology in orthopedics: a clinically oriented review. BMC Musculoskelet Disord. 2018;19(1). doi:10.1186/s12891-018-1990-1
242. Salata OV. Applications of nanoparticles in biology and medicine. J Nanobiotechnology. 2004;2(1):3. doi:10.1186/1477-3155-2-3
243. Sharma B, Malik P, Jain P. Biopolymer reinforced nanocomposites: a comprehensive review. Mater Today Commun. 2018;16:353–363. doi:10.1016/j.mtcomm.2018.07.004
244. Chan CK, Sampath Kumar TS, Liao S, Murugan R, Ngiam M, Ramakrishnan S. Biomimetic nanocomposites for bone graft applications. Nanomedicine. 2006;1(2):177–188. doi:10.2217/17435889.1.2.177
245. Maleki A. One-pot multicomponent synthesis of diazepine derivatives using terminal alkynes in the presence of silica-supported superparamagnetic iron oxide nanoparticles. Tetrahedron Lett. 2013;54(16):2055–2059. doi:10.1016/j.tetlet.2013.01.123
246. Maleki A. Green oxidation protocol: selective conversions of alcohols and alkenes to aldehydes, ketones and epoxides by using a new multiwall carbon nanotube-based hybrid nanocatalyst via ultrasound irradiation. Ultrason Sonochem. 2018;40:460–464. doi:10.1016/j.ultsonch.2017.07.020
247. Mani MP, Jaganathan SK, Md Khudzari AZ, Ismail AF. Green synthesis of nickel oxide particles and its integration into polyurethane scaffold matrix ornamented with groundnut oil for bone tissue engineering. Int J Polym Anal Charact. 2019;24(7):571–583. doi:10.1080/1023666X.2019.1630930
248. Iravani S, Varma RS. Plants and plant-based polymers as scaffolds for tissue engineering. Green Chem. 2019;21(18):4839–4867. doi:10.1039/c9gc02391g
249. Wu F, Wei J, Liu C, O’Neill B, Ngothai Y. Fabrication and properties of porous scaffold of zein/PCL biocomposite for bone tissue engineering. Compos Part B Eng. 2012;43(5):2192–2197. doi:10.1016/j.compositesb.2012.02.040
250. Mututuvari TM, Harkins AL, Tran CD. Facile synthesis, characterization, and antimicrobial activity of cellulose-chitosan-hydroxyapatite composite material: a potential material for bone tissue engineering. J Biomed Mater Res Part A. 2013. doi:10.1002/jbm.a.34636
251. Wang L, Lu C, Li Y, Wu F, Zhao B, Dong X. Green fabrication of porous silk fibroin/graphene oxide hybrid scaffolds for bone tissue engineering. RSC Adv. 2015. doi:10.1039/c5ra12173f
252. Zhou Y, Yao H, Wang J, Wang D, Liu Q, Li Z. Greener synthesis of electrospun collagen/hydroxyapatite composite fibers with an excellent microstructure for bone tissue engineering. Int J Nanomedicine. 2015. doi:10.2147/IJN.S79241
253. Potamianos P, Amis AA, Forester AJ, McGurk M, Bircher M. Rapid prototyping for orthopaedic surgery. Proc Inst Mech Eng Part H J Eng Med. 1998;212(5):383–393. doi:10.1243/0954411981534150
254. Zou Y, Han Q, Weng X, et al. The precision and reliability evaluation of 3-dimensional printed damaged bone and prosthesis models by stereo lithography appearance. Med (United States). 2018. doi:10.1097/MD.0000000000009797
255. Maini L, Sharma A, Jha S, Sharma A, Tiwari A. Three-dimensional printing and patient-specific pre-contoured plate: future of acetabulum fracture fixation? Eur J Trauma Emerg Surg. 2018;44(2):215–224. doi:10.1007/s00068-016-0738-6
256. Ackland D, Robinson D, Lee PVS, Dimitroulis G. Design and clinical outcome of a novel 3D-printed prosthetic joint replacement for the human temporomandibular joint. Clin Biomech. 2018;56:52–60. doi:10.1016/j.clinbiomech.2018.05.006
257. Jalbert F, Boetto S, Nadon F, Lauwers F, Schmidt E, Lopez R. One-step primary reconstruction for complex craniofacial resection with PEEK custom-made implants. J Craniomaxillofac Surg. 2014;42(2):141–148. doi:10.1016/j.jcms.2013.04.001
258. Kang J, Wang L, Yang C, et al. Custom design and biomechanical analysis of 3D-printed PEEK rib prostheses. Biomech Model Mechanobiol. 2018;17(4):1083–1092. doi:10.1007/s10237-018-1015-x
259. Kanno Y, Nakatsuka T, Saijo H, et al. Computed tomographic evaluation of novel custom-made artificial bones, “CT-bone”, applied for maxillofacial reconstruction. Regen Ther. 2016;5:1–8. doi:10.1016/j.reth.2016.05.002
260. Hirsch E, Nacsa M, Ender F, Mohai M, Nagy ZK, Marosi GJ. Preparation and characterization of biocompatible electrospun nanofiber scaffolds. Period Polytech Chem Eng. 2018;62(4). doi:10.3311/PPch.12854
261. Vance A, Bari K, Arjunan A. Compressive performance of an arbitrary stiffness matched anatomical Ti64 implant manufactured using direct metal laser sintering. Mater Des. 2018;160:1281–1294. doi:10.1016/j.matdes.2018.11.005
262. Jaivignesh M, Suresh Babu A, Arumaikkannu G. In-vitro analysis of titanium cellular structures fabricated by direct metal laser sintering. Mater Today Proc. 2020;22:2372–2377. doi:10.1016/j.matpr.2020.03.360
263. Guo Y, Wu J, Xie K, et al. Study of bone regeneration and osteointegration effect of a novel selective laser-melted titanium-tantalum-niobium-zirconium alloy scaffold. ACS Biomater Sci Eng. 2019;5(12):6463–6473. doi:10.1021/acsbiomaterials.9b00909
264. Van der Stok J, Van der Jagt OP, Amin Yavari S, et al. Selective laser melting-produced porous titanium scaffolds regenerate bone in critical size cortical bone defects. J Orthop Res. 2013;31(5):792–799. doi:10.1002/jor.22293
265. Shuai C, Yang W, Yang Y, et al. Selective laser melted Fe-Mn bone scaffold: microstructure, corrosion behavior and cell response. Mater Res Express. 2020;7(1):15404. doi:10.1088/2053-1591/ab62f5
266. Schmidleithner C, Malferrari S, Palgrave R, Bomze D, Schwentenwein M, Kalaskar DM. Application of high resolution DLP stereolithography for fabrication of tricalcium phosphate scaffolds for bone regeneration. Biomed Mater. 2019;14(4):45018. doi:10.1088/1748-605x/ab279d
267. Liu Z, Liang H, Shi T, et al. Additive manufacturing of hydroxyapatite bone scaffolds via digital light processing and in vitro compatibility. Ceram Int. 2019;45(8):11079–11086. doi:10.1016/j.ceramint.2019.02.195
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
