Back to Journals » Clinical Interventions in Aging » Volume 14
Wearable Technology To Reduce Sedentary Behavior And CVD Risk In Older Adults: A Pilot Randomized Clinical Trial
Authors Roberts LM, Jaeger BC, Baptista LC , Harper SA , Gardner AK, Jackson EA , Pekmezi D, Sandesara B , Manini TM, Anton SD, Buford TW
Received 10 July 2019
Accepted for publication 26 September 2019
Published 23 October 2019 Volume 2019:14 Pages 1817—1828
DOI https://doi.org/10.2147/CIA.S222655
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Walker
Lisa M Roberts,1,2 Byron C Jaeger,3 Liliana C Baptista,1,2 Sara A Harper,1,2 Anna K Gardner,4 Elizabeth A Jackson,5 Dorothy Pekmezi,6 Bhanuprasad Sandesara,4 Todd M Manini,4 Stephen D Anton,4 Thomas W Buford1,2
1Department of Medicine, Division of Gerontology/Geriatrics/Palliative Care, University of Alabama at Birmingham, Birmingham, AL, USA; 2Center for Exercise Medicine, University of Alabama at Birmingham, Birmingham, AL, USA; 3Department of Biostatistics, University of Alabama at Birmingham, Birmingham, AL, USA; 4Department of Aging and Geriatric Research, University of Florida, Gainesville, FL, USA; 5Department of Medicine, Division of Cardiovascular Disease, University of Alabama at Birmingham, Birmingham, AL, USA; 6Department of Health Behavior, School of Public Health, the University of Alabama at Birmingham, Birmingham, AL, USA
Correspondence: Thomas W Buford
Department of Medicine, Division of Gerontology/Geriatrics/Palliative Care, University of Alabama at Birmingham, 1313 13th Street S., Birmingham, AL 35205, USA
Tel +1 205 996 3008
Fax +1 205 996 3110
Email [email protected]
Background: Physical exercise is associated with decreased cardiovascular disease (CVD) risk, but recent large-scale trials suggest that exercise alone is insufficient to reduce CVD events in high-risk older adults.
Purpose: This pilot randomized clinical trial aimed to collect critical data on feasibility, safety, and protocol integrity necessary to design a fully powered randomized controlled trial (RCT) and evaluate the impact of combining structured exercise with an intervention designed to enhance non-exercise physical activity (EX+NEPA) compared to EX alone.
Methods: Forty participants aged ≥60 years with moderate-to-high risk of coronary heart disease events were randomly assigned to either the EX+NEPA or EX groups and followed for 20 weeks. Both groups underwent a twice-weekly, 8-week center-based exercise intervention with aerobic and resistance exercises. EX+NEPA group also received a wearable activity tracking device along with behavioral monitoring and feedback throughout the study. Study outcomes were evaluated at 8 and 20 weeks.
Results: Data are presented as adjusted mean change of the differences over time with 95% confidence intervals at 20 weeks. Relative to EX, the change in steps/day at 20 weeks was 1994 (−40.27, 4028) higher for EX+NEPA. For sedentary time at close-out, the EX+NEPA group was −6.8 (−45.2, 31.6) min/day relative to EX. The between-group differences for systolic and diastolic blood pressure were −9.9 (−19.6, −0.3) and −1.8 (−6.9, 3.3) mmHg, respectively.
Conclusion: The addition of wearable technology intervention appeared to positively influence daily activity patterns and changes in blood pressure – potentially improving risk factors for CVD. A fully powered randomized trial is needed to ultimately test this hypothesis.
Keywords: aging, cardiovascular, exercise, physical activity, activity monitor
Introduction
Cardiovascular disease (CVD) is the leading cause of death in the US and worldwide. Given the rapid aging of the population and older adults account for the majority of CVD-related deaths.1 Thus, finding methods to reduce CVD risk among older adults is a critical public health priority. Although physical activity is one of the best-known interventions for reducing CVD-related incidents,2,3 recent data from clinical trials suggest that activity alone is not enough to reduce the risk of CVD in older adults.4,5 Independent of physical activity, sedentary behavior has been recently identified as having negative impacts on health status in older adults.6,7 Specifically, sedentary behavior has been associated with metabolic disorders, CVD, cancer, mortality, and psychological distress.8–13 Therefore, reducing sedentary activity provides an alternative strategy to reduce the risk of CVD and CVD-related mortality among older adults.
Recent evidence suggests that older adults spend more than half of their day (approximately 62%) engaging in sedentary behavior.14–16 Chronic sedentary behavior has also been related to CVD risk factors, such as increased waist circumference, BMI, systolic blood pressure (SBP), fasting triglycerides, HDL cholesterol, and insulin levels.10,17,18 As previously described,19 data regarding interventions for reducing sedentary behavior are currently sparse.20 Bravata et al showed that wearing an activity monitor increased daily step counts among sedentary adults by more than 2000 steps/day compared to adults without an activity monitor.21 Further, a recent pilot trial evaluated the impact of providing wearable activity trackers to adult populations with chronic medical conditions (ie, hypertension, type 2 diabetes, hyperlipidemia) and showed that the addition of activity tracking resulted in decreased low-density lipoprotein (LDL) and weight loss.22 To date, the use of wearable, physical activity monitoring technologies combined with structured exercise intervention to increase non-exercise physical activity (NEPA) has not been evaluated in older adults.
Therefore, the purpose of this study was to collect critical data on feasibility, safety, and protocol integrity necessary to design a fully powered randomized controlled trial (RCT) evaluating the combination of a traditional, structured exercise intervention with an innovative, technology-based intervention to decrease sedentary behavior and increase NEPA among older adults with elevated CVD risk.
Methods
Overview
The study design was described in detail previously.19 Briefly, the study was a two-arm randomized pilot trial evaluating the feasibility and efficacy of utilizing wearable technology to reduce sedentary behavior and CVD risk among older adults. Participants were randomly assigned to either the exercise and technology intervention (EX+NEPA) or the exercise only (EX) group by a random number generator. Both groups underwent an 8-week, twice-weekly, center-based exercise intervention. Participants were then assessed for changes in daily NEPA and cardiovascular risk factors at 8- and 20-week post-randomization (Figure 1). A comprehensive study team oversaw participant safety – including the principal investigator, study physician, study staff, and an appointed Data and Safety Monitoring Board. All study procedures were approved by the University of Florida Institutional Review Board and were in accordance with the Declaration of Helsinki. The study was registered at www.clinicaltrials.gov (NCT02632487).
Study design followed the Consolidated Standards of Reporting Trials (CONSORT) Group23,24 and study staff conducting the assessments were masked to intervention randomization. To ensure masking, study intervention and study assessments occurred in separate physical locations and the intervention groups were conducted at different times to prevent contamination bias between groups. All statistical analyses were performed by a biostatistician who was masked to the intervention throughout, providing a double-masked (assessors and biostatistician) design.
Participants
Community-dwelling, sedentary, older adults from the Gainesville, FL area were recruited between 2016 and 2017 for the study using a targeted approach of direct mailings, newspaper advertisements, and other community approaches. Eligible participants met inclusionary criteria as follows: 1) ≥60 years old, 2) inactive lifestyle, defined as <150 min/week of moderate physical activity by the Community Health Activities Model Program for Seniors (CHAMPS) questionnaire,25 and 3) moderate-to-high risk of CHD events according to the National Cholesterol Education Program’s Adults Treatment Panel (ATP-III) risk classification algorithm.26 Participants were excluded based on the following: 1) absolute contraindication to exercise training according to the American College of Sports Medicine (ACSM) guidelines,27 2) SBP of >180 mmHg or DBP >110 mmHg, or 3) any other medical conditions that impair the ability to participate in the exercise intervention.
Interventions
All participants participated in a structured exercise program twice weekly for 8 weeks designed to meet exercise and physical activity guidelines for older adults from ACSM and the American Heart Association (AHA).28 The exercise program consisted of a brief warmup, followed by 30 mins of moderate-intensity walking, 30 mins of light lower- and upper-body resistance training, and ended with balance and stretching exercises. Intensity was determined by a subjective 0–10 scale (Borg category ratio 10 scale)29 and with a heart rate monitor (Polar Ft2, Lake Success, NY). Participants were instructed to walk at a moderate intensity (5–6 on CR10) with periods of vigorous walking (7–8 on CR10). Following the 8 weeks of exercise intervention, all participants were given instructions to achieve 150 mins of moderate to vigorous activity per week for the remainder of the study (12 weeks). Additionally, all participants received cognitive-behavioral counseling that focused on reducing sedentary behavior and increasing NEPA in their daily life. The behavioral counseling occurred in person at the start of exercise intervention and at the beginning of each exercise session for the duration of the intervention phase. Behavioral counseling was individualized to each participant on how they could strategize an increase in NEPA. Participants in the EX+NEPA group were provided a Fitbit Zip® (San Francisco, CA, USA) activity tracker and asked to wear it during all waking hours with the exception of when they were at an exercise session during the intervention phase. Further, participants were instructed to record any time without wearing the tracker via a log given to them. The Fitbit Zip monitor was used to track NEPA for the duration of the study (20 weeks). To encourage NEPA in the EX+NEPA group, the study team monitored participants’ daily NEPA and communicated weekly (at the exercise intervention visits or by phone, depending on phase of the study) to provide additional motivation and individual goal-based strategies for increasing NEPA. Lastly, any difficulty with syncing the device or battery replacement were addressed in person at the intervention or by the weekly phone calls.
Assessments
Assessments were conducted at baseline, week 8, and week 20. Daily activity patterns were quantified with a hip worn, solid-state triaxial accelerometer (Actigraph GT3X).15,16 Participants were instructed to wear the accelerometer for seven consecutive days before or after each assessment visit. The accelerometer provided no feedback to participants on their daily activity and data were sampled at 1-min epochs over 24-hr periods to estimate average daily expenditure and total minutes of activity.30 Sedentary behavior was defined as <100 counts/min30 and non-wear time was defined at a 60-min window of zero counts in all three axes, allowing a 2-min interval of non-zero counts for artificial movement detection. For data to be included in this study, participants had to wear the accelerometer for a minimum of three consecutive days for 10 hrs per day. Both systolic and diastolic blood pressure were evaluated as a study outcome at every assessment using standard clinical procedures. Briefly, participants were instructed to sit quietly for a minimum of 5 mins then, blood pressure was measured in the right arm with an automated monitor which utilized a triplicate mode of measurement and averages the values to closely resemble clinical measurements (Microlife®, FL). Exercise capacity was assessed with the 6-min walk test, as previously described.19 Briefly, participants walked as far and fast as safely possible for 6 mins and total distance was measured at the end. Waist circumference was measured according to the National Health and Nutrition Examination Survey (NHANES) Anthropometry Procedures Manual protocol.31
Blood samples were collected after a minimum of an 8 hrs fast at each study visit (baseline, 8weeks, 20weeks) to assess blood lipids, glucose, and hemoglobin A(1c) levels. In addition, blood samples were also assayed using commercially available enzyme-linked immunosorbent assays (ELISAS) kits for prominent biomarkers of inflammation and oxidative stress including tumor necrosis factor α (TNF-α), IL-6, vascular cell adhesion molecule-1 (VCAM-1), endothelium selectin (E-selectin), oxidized low-density lipoprotein (oxLDL), and myeloperoxidase (MPO). Concentrations of target proteins were identified using the colorimetric method at an optical density of 450 nm with a microplate reader (Biotek, Winooski, VT, USA). Intra-assay coefficients of variation for each assay were determined for each duplicate for all participants and resulted in a mean coefficient of variation of 3.4%.
Lastly, several secondary outcome measures were assessed during the study because of their association with CVD risk or potential benefits from exercise intervention. As an important factor in risk of CVD, a 3-day diet recall was analyzed using commercial software (ESHA, Salem, OR). Additionally, physical function has been associated with CVD risk and therefore usual-paced walking (4-m walk) and grip strength (Jamar Hydraulic Hand Dynamometer, Fred Sammons, Inc. Burr Ridge, IL) were assessed at all study visits. While lower-extremity function via the Short Physical Performance Battery32 was assessed at baseline and week 20 study assessments.
Adherence And Safety
Recruitment success and retention rates were measured by the number of participants recruited, the number of withdrawals, and losses to follow-up throughout the intervention. Exercise adherence was assessed by study staff for compliance and quantified by the number of attended sessions. Adherence to wearing the Fitbit Zip activity tracker was self-reported by participants as time they put the device on upon waking and when they removed the tracker prior to sleep, along with any time during the day they removed the device.
Safety of the participants was measured by the number and/or seriousness of adverse events attributable to the intervention. Study staff monitored safety during all study visits and every intervention session. Participants were also encouraged to report any adverse experiences that occurred during the study to study staff. Further, clinical blood tests (ie, comprehensive metabolic panel, coagulation markers) were conducted at every assessment visit to monitor potential hematologic and metabolic abnormalities in response to the intervention.
Statistical Analysis
Analyses were performed in R (version 3.5.1) following a protocol described previously.19 The primary statistician was blinded to treatment assignment throughout the analysis. As recommended by CONSORT, estimates of change over time with 95% confidence intervals (CIs) were reported and formal hypothesis testing was not conducted.33,34 Participant characteristics were tabulated overall and by treatment group. Data from all randomized participants were analyzed, regardless of adherence (ie, the intent-to-treat principle). Linear mixed models were applied to estimate change over time, adjusted for age, sex, and baseline outcome measures, in primary and secondary outcomes overall and by treatment group35,36 Heterogeneity between subjects and serial correlation of observations within subjects was modeled using subject-specific random intercepts. Normality of the distribution of residual error and random intercept terms were assessed. Log-transformation was applied to outcomes as needed to achieve normally distributed distributions of residual errors and random effects. A secondary analysis was conducted on participant outcome measures not adjusted for baseline values (see Supplementary Figures 1–3).
Results
Participants
A total of 275 individuals were initially assessed for eligibility via a telephone pre-screening beginning in April 2016 and the last participant was screened in April 2017. Of those, 58 individuals were assessed during an in-person screening visit for eligibility for the study. Forty participants met the required eligibility criteria and were randomized into the study. Participants were randomized to either the EX+NEPA (n=20) or EX (n=20) group (Figure 2). At baseline, groups had generally similar demographics and characteristics (Table 1).
 |
Table 1 Participant Demographic Baseline Characteristics |
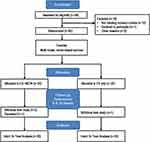 |
Figure 2 Flow diagram of study progress in the Consolidated Standards of Reporting Trials (CONSORT) Group. |
Retention, Adherence, And Safety
In total, n=36 (90%) participants were retained and completed the study. Two participants withdrew from the EX+NEPA group and one participant withdrew from the EX group. The EX+NEPA group had one participant's death that was unrelated to the study. Adherence to the center-based exercise intervention (ie, attendance) was 82.2% for EX+NEPA and 77.5% for EX. Adherence to wearing the Fitbit Zip device was determined by self-reported wear time. Participants in the EX+NEPA group reported an average wear time of 829.85 (±184.64) min/day. Overall, both groups performed similarly during the exercise sessions. Mean walking time for both groups was 28.26 (±2.87) mins and average heart rate during walking was 101.78 (±17.3) beats/min. Finally, mean walking distance and speed for both groups were 1981.21 (±512.31) meters and 1.14 (± 0.22) m/s, respectively.
Regarding safety, one serious adverse event was reported and deemed unrelated to the study (one participant was admitted to the hospital for digestive issues). A total of 18 unanticipated adverse events were reported after randomization. Both groups had a similar number of adverse events with the most common being musculoskeletal issues (n=8), flu-like symptoms (n=3), elevated blood pressure (n=3), lightheadedness (n=1), fall (n=1), chest pain (n=1), and toothache (n=1). Total events reported by group were: 9 EX + NEPA and 9 EX.
Daily Activity Patterns, Blood Pressure, Exercise Capacity, And Waist Circumference
The 20-week adjusted mean difference in the change in steps/day between groups (EX+NEPA relative to EX) was 1994 (−40.27, 4028) steps/day (Figure 3). The between-groups difference in total sedentary time/day was −6.79 (−45.17, 31.59) min/day. Blood pressure changes between groups were −9.94 (−19.57, −0.31) mmHg for systolic and −1.77 (−6.89, 3.34) mmHg for diastolic. Finally, the mean difference for 6-min walk test was −15.08 (−40.44, 10.29) meters and for waist circumference was 1.15 (−1.98, 4.28) cm.
Clinical Metabolic Profiles
The 20-week adjusted mean changes in 1) total, 2) LDL, and 3) HDL cholesterol between groups (EX + NEPA relative to EX) were 1) 4.99 (−10.74, 20.72), 2) 4.47 (−9.10, 18.04), and 3) −2.09 (−8.02, 3.85) mg/dL, respectively (Figure 4). Between groups, the difference for triglyceride levels was −5.53 (−58.63, 47.57) mg/dL. The between-groups difference in change in blood glucose was 0.70 (−15.90, 17.31) mg/dL and 0.11 (−0.13, 0.35) % for hemoglobin A1C.
Inflammatory And Oxidative Stress Biomarkers
Between groups, the 20-week adjusted mean change for TNF-α was 0.01 (−0.14, 0.16) log pg/mL and in IL-6 the mean difference was 0.15 (−0.16, 0.46) log pg/mL (Figure 5). The adjusted between-group mean change for E-selectin was −1.40 (−6.50, 3.70) log ng/mL. Between groups, the mean difference VCAM at week 20 were and 0.07 (−0.04, 0.18) log ng/mL. Lastly, mean changes between groups for 1) MPO and 2) oxLDL at week 20 were 1) −0.01 (−0.27, 0.26) log μg/mL and 2) −0.01 (−0.19, 0.18) log mU/L.
Supportive Outcomes
Overall, there appeared to be similar mean changes in both groups for secondary outcomes related to functional status (Table S1). Of note, a positive change pattern was observed in the SPPB for the EX+NEPA group (20 weeks relative to baseline) 0.41 (0.05, 0.76) points. Additionally, both groups had generally similar dietary intake (ie, total calories and macronutrients) across all time-points (Table S2).
Regarding the data from the Fitbit Zip device, average daily step data in the EX+NEPA group was similar to the accelerometer used for study outcomes. The Fitbit Zip data (20 weeks relative to baseline) indicated steps/day as 4563.66 (±2674.0) steps/day (accelerometer data: 4818.0±2697.31). Active time (was determined as 10 or more consecutive minutes of movement) was also recorded from the Fitbit Zip device and mean active time was 128.62 (±136.35) min/day.
Discussion
This pilot RCT investigated the safety and feasibility of adding a technology-based intervention designed to decrease sedentary behavior and increase NEPA to a traditional, structured exercise intervention among older adults with elevated CVD risk. Congruent with best practice for pilot studies37 and as described previously,19 our primary goal was to assess and refine the study protocol to inform a fully powered RCT to test the effectiveness of wearable activity trackers combined with exercise in older adults with elevated risk of CVD. Our preliminary data showed high retention rates, satisfactory adherence to center-based exercise intervention, and both groups had similar anticipated adverse events suggesting both EX+NEPA and EX were safe for participants. Further, our results suggest the addition of a wearable activity tracker may increase NEPA and decrease blood pressure. Therefore, this 20-week exercise and technology intervention is safe and feasible and suggests that a fully powered RCT can be implemented.
Determining safety and feasibility were two of the primary objectives of the trial. Overall the study protocol appeared safe as adverse events – both in frequency and severity – were in line with reasonable expectations given the study populations and interventions delivered. Regarding feasibility, one promising aspect was the relative lack of difficulty recruiting into the trial. Recruitment began in April 2016 and the last participant was screened the following April in 2017 and 58 individuals were screened for the study with a total randomization of 40 participants (69% of individuals assessed for eligibility qualified and were interested). An important consideration for recruitment in this trial was the relatively short exercise phase requiring participants to attend exercise intervention two days/week (8 weeks total). The remaining 12 weeks of the study had low (EX+NEPA) to no (EX) direct involvement with study staff. Although recruitment is often one of the most challenging aspects in clinical trials,38,39 a strength of the study design was that the current study did not experience difficulty recruiting participants.
Another important aspect for feasibility of the study involved the potential for compliance and usage of the technology in the EX+NEPA group. Usage of the activity tracker was self-reported by participants in time worn per day. Participants reported wearing the device for an average of 829.85 min/day (~14 hrs/day). Additionally, technological issues with syncing the device or batteries were addressed at the twice-weekly intervention or by weekly phone calls, depending on which phase of the study participants were in – importantly, weekly contact may have aided in adherence to using the Fitbit Zip tracker. Our results support recent evidence suggesting older adults are interested and able to use wearable activity trackers.40,41 McMahon et al reported that after 8 months of usage, 68% older adults found a wearable tracker to be useful and 82% had positive perceptions of the ease-of-use of a wearable tracker.40 Currently, this study had a total retention of 90% and adherence to supervised exercise of >77%, which further support the feasibility of the pilot trial.
An encouraging result from this study was the potential impact of the wearable activity tracker on changes in SBP. Although the pilot trial was not powered to detect significance, the observed directional change between the EX+NEPA and EX groups indicate the addition of a wearable activity tracker might improve SBP outcomes. Blood pressure is a well-established risk factor for CVD and reductions as small as 2 mmHg in SBP are associated with decreased risk of CVD events and cardiovascular death.42 Our results indicate a potential 3-month impact of EX+NEPA >9 mmHg. Though this difference is likely overestimated in the nature of a pilot trial, it still indicates substantial promise for further follow-up in a larger-scale trial.
Results from the current pilot trial suggest that daily activity patterns may be increased through the use of a wearable activity tracker. There were directional changes in average steps/day between groups. At study close-out, the EX+NEPA group had approximately 2000 additional steps/day than the EX group. Our findings are in line with previous research,21 suggesting the wearable activity tracker influenced steps/day. Notably, an additional 500 steps taken per day has been associated with a 10% reduction in CVD event risk43 and in older women, there is evidence that increased light physical activity (measured by accelerometry) is related to a reduction in risk for CHD and CVD.44
Directional differences for total sedentary time per day were modest (EX+NEPA group had ~48 mins/week less than EX group). However, prior findings from our group suggested that every minute increase of sedentary time was associated with a 0.03–0.04% increase in risk in 10-year CHD and increased time spend sedentary predicts risk of major CVD events in older adults.16 Thus, even small differences in sedentary time may have important impact of CVD risk for older adults. Given that older adults spend approximately 60–80% of their waking hours being sedentary,15,16,45 interventions aimed at reduced sedentary behavior are becoming increasingly important for reducing risk of CVD events.
As this is a pilot trial, the study was not powered to detect statistically significant changes in clinical or behavioral outcome measures. Further, another important limitation to acknowledge is the continued behavioral counseling the EX+NEPA group received via weekly phone calls during the second phase of the trial. Since this was not quantified, it cannot be discounted that positive findings in the EX+NEPA group could have been influenced by the weekly behavioral counseling. Importantly, this will be accounted for in a fully powered RCT.
In summary, results from this pilot RCT suggest that an intervention with a combination of structured exercise and a wearable activity tracker in older adults with an elevated risk of CHD is safe and feasible. These data suggest the potential for wearable activity trackers, combined with behavioral feedback, to reduce increase NEPA and improve CVD risk factors among older adults. Although pilot data should not be over-interpreted, they do provide preliminary data necessary for the design of a fully powered RCT to ultimately address this hypothesis.
Ethics Approval And Informed Consent
All study procedures were approved by a university Institutional Review Board and all participants signed an approved Informed Consent prior to participating in the study.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgment
We would like to thank the research participants and other staff who helped to make this research possible.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Dr Elizabeth Jackson is a paid consultant for McKesson as well as for UpToDate, Inc. and has served as an expert witness for DeBlase Brown Everly LLP, all relationships are modest. Dr Jackson also received research funding from NIH, Amgen for epidemiology, medication utilization. She also served at the editorial board for American Heart Association (AHA) and served as editor/consultant for American College of Cardiology. Dr Todd M Manini reports grants from NIH and AHA, during the conduct of the study and grants from NIH and Regeneron, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56–e66. doi:10.1161/CIR.0000000000000659
2. Pescatello LS, Franklin BA, Fagard R, et al. American college of sports medicine position stand. Exercise and hypertension. Med Sci Sports Exerc. 2004;36(3):533–553. doi:10.1249/01.mss.0000115224.88514.3a
3. Pollock ML, Franklin BA, Balady GJ, et al. AHA Science Advisory. Resistance exercise in individuals with and without cardiovascular disease: benefits, rationale, safety, and prescription: an advisory from the Committee on Exercise, Rehabilitation, and Prevention, Council on Clinical Cardiology, American Heart Association; Position paper endorsed by the American College of Sports Medicine. Circulation. 2000;101(7):828–833. doi:10.1161/01.cir.101.7.828
4. Look ARG, Wing RR, Bolin P, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369(2):145–154. doi:10.1056/NEJMoa1212914
5. Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311(23):2387–2396. doi:10.1001/jama.2014.5616
6. Da Silva Coqueiro R, de Queiroz BM, Oliveira DS, et al. Cross-sectional relationships between sedentary behavior and frailty in older adults. J Sports Med Phys Fitness. 2017;57(6):825–830. doi:10.23736/S0022-4707.16.06289-7
7. Ekelund U, Tarp J, Steene-Johannessen J, et al. Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: systematic review and harmonised meta-analysis. BMJ. 2019;366:l4570. doi:10.1136/bmj.l4570
8. Matthews CE, George SM, Moore SC, et al. Amount of time spent in sedentary behaviors and cause-specific mortality in US adults. Am J Clin Nutr. 2012;95(2):437–445. doi:10.3945/ajcn.111.019620
9. Owari Y, Miyatake N. Long-term relationship between psychological distress and continuous sedentary behavior in healthy older adults: a three panel study. Medicina (Kaunas). 2019;55(9):324.
10. Thorp AA, Healy GN, Owen N, et al. Deleterious associations of sitting time and television viewing time with cardiometabolic risk biomarkers: Australian diabetes, obesity and lifestyle (ausdiab) study 2004-2005. Diabetes Care. 2010;33(2):327–334. doi:10.2337/dc09-0493
11. Sisson SB, Camhi SM, Church TS, et al. Leisure time sedentary behavior, occupational/domestic physical activity, and metabolic syndrome in U.S. men and women. Metab Syndr Relat Disord. 2009;7(6):529–536. doi:10.1089/met.2009.0023
12. Stamatakis E, Hamer M, Dunstan DW. Screen-based entertainment time, all-cause mortality, and cardiovascular events: population-based study with ongoing mortality and hospital events follow-up. J Am Coll Cardiol. 2011;57(3):292–299. doi:10.1016/j.jacc.2010.05.065
13. Katzmarzyk PT, Church TS, Craig CL, Bouchard C. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med Sci Sports Exerc. 2009;41(5):998–1005. doi:10.1249/MSS.0b013e3181930355
14. Schlaff RA, Baruth M, Boggs A, Hutto B. Patterns of sedentary behavior in older adults. Am J Health Behav. 2017;41(4):411–418. doi:10.5993/AJHB.41.4.5
15. Mankowski RT, Aubertin-Leheudre M, Beavers DP, et al. Sedentary time is associated with the metabolic syndrome in older adults with mobility limitations–the LIFE study. Exp Gerontol. 2015;70:32–36. doi:10.1016/j.exger.2015.06.018
16. Fitzgerald JD, Johnson L, Hire DG, et al. Association of objectively measured physical activity with cardiovascular risk in mobility-limited older adults. J Am Heart Assoc. 2015;4(2). doi:10.1161/JAHA.114.001288
17. Saunders TJ, Larouche R, Colley RC, Tremblay MS. Acute sedentary behaviour and markers of cardiometabolic risk: a systematic review of intervention studies. J Nutr Metab. 2012;2012:712435. doi:10.1155/2012/712435
18. Peddie MC, Bone JL, Rehrer NJ, Skeaff CM, Gray AR, Perry TL. Breaking prolonged sitting reduces postprandial glycemia in healthy, normal-weight adults: a randomized crossover trial. Am J Clin Nutr. 2013;98(2):358–366. doi:10.3945/ajcn.112.051763
19. Krehbiel LM, Layne AS, Sandesara B, Manini TM, Anton SD, Buford TW. Wearable technology to reduce sedentary behavior and CVD risk in older adults: design of a randomized controlled trial. Contemp Clin Trials Commun. 2017;6:122–126. doi:10.1016/j.conctc.2017.04.003
20. Aunger JA, Doody P, Greig CA. Interventions targeting sedentary behavior in non-working older adults: a systematic review. Maturitas. 2018;116:89–99. doi:10.1016/j.maturitas.2018.08.002
21. Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298(19):2296–2304. doi:10.1001/jama.298.19.2296
22. Gualtieri L, Rosenbluth S, Phillips J. Can a free wearable activity tracker change behavior? the impact of trackers on adults in a physician-led wellness group. JMIR Res Protoc. 2016;5(4):e237. doi:10.2196/resprot.6534
23. Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P, Group C. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med. 2008;148(4):295–309. doi:10.7326/0003-4819-148-4-200802190-00008
24. Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P, Group C. Methods and processes of the CONSORT group: example of an extension for trials assessing nonpharmacologic treatments. Ann Intern Med. 2008;148(4):W60–W66. doi:10.7326/0003-4819-148-4-200802190-00008-w1
25. Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33(7):1126–1141. doi:10.1097/00005768-200107000-00010
26. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the national cholesterol education program adult treatment panel III guidelines. Circulation. 2004;110(2):227–239. doi:10.1161/01.CIR.0000133317.49796.0E
27. Medicine ACoS. ACSM’s Guidelines for Exercise Testing and Prescription. Lippincott Williams & Wilkins; 2013.
28. Chodzko-Zajko WJ, Proctor DN, et al.; American College of Sports M. American college of sports medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41(7):1510–1530. doi:10.1249/MSS.0b013e3181a0c95c
29. Borg G. Borg’s Perceived Exertion and Pain Scales. Champaign, IL: Human kinetics; 1998.
30. Freedson PS, Melanson E, Sirard J. Calibration of the computer science and applications, inc. accelerometer. Med Sci Sports Exerc. 1998;30(5):777–781. doi:10.1097/00005768-199805000-00021
31. Lumley T, Kronmal RA, Cushman M, Manolio TA, Goldstein S. A stroke prediction score in the elderly: validation and web-based application. J Clin Epidemiol. 2002;55(2):129–136. doi:10.1016/s0895-4356(01)00434-6
32. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. doi:10.1093/geronj/49.2.m85
33. Horne E, Lancaster GA, Matson R, Cooper A, Ness A, Leary S. Pilot trials in physical activity journals: a review of reporting and editorial policy. Pilot Feasibility Stud. 2018;4:125. doi:10.1186/s40814-018-0317-1
34. Eldridge SM, Chan CL, Campbell MJ, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Pilot Feasibility Stud. 2016;2:64. doi:10.1186/s40814-016-0105-8
35. Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis.
36. Fitting Linear Mixed-Effects Models Using lme4 [computer program]. arXiv: arXiv:1406.5823; 2014.
37. Thabane L, Ma J, Chu R, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;10:1. doi:10.1186/1471-2288-10-1
38. Rajadhyaksha V. Conducting feasibilities in clinical trials: an investment to ensure a good study. Perspect Clin Res. 2010;1(3):106–109.
39. El-Kotob R, Giangregorio LM. Pilot and feasibility studies in exercise, physical activity, or rehabilitation research. Pilot Feasibility Stud. 2018;4:137. doi:10.1186/s40814-018-0326-0
40. McMahon SK, Lewis B, Oakes M, Guan W, Wyman JF, Rothman AJ. Older adults’ experiences using a commercially available monitor to self-track their physical activity. JMIR Mhealth Uhealth. 2016;4(2):e35. doi:10.2196/mhealth.5120
41. Mercer K, Giangregorio L, Schneider E, Chilana P, Li M, Grindrod K. Acceptance of commercially available wearable activity trackers among adults aged over 50 and with chronic illness: a mixed-methods evaluation. JMIR Mhealth Uhealth. 2016;4(1):e7. doi:10.2196/mhealth.4225
42. Turnbull F, Blood Pressure Lowering Treatment Trialists C. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362(9395):1527–1535. doi:10.1016/s0140-6736(03)14739-3
43. Cochrane SK, Chen SH, Fitzgerald JD, et al. Association of accelerometry-measured physical activity and cardiovascular events in mobility-limited older adults: the LIFE (Lifestyle Interventions and Independence for Elders) study. J Am Heart Assoc. 2017;6(12). doi:10.1161/JAHA.117.007215
44. LaCroix AZ, Bellettiere J, Rillamas-Sun E, et al. Association of light physical activity measured by accelerometry and incidence of coronary heart disease and cardiovascular disease in older women. JAMA Netw Open. 2019;2(3):e190419. doi:10.1001/jamanetworkopen.2019.5313
45. Matthews CE, Chen KY, Freedson PS, et al. Amount of time spent in sedentary behaviors in the United States, 2003-2004. Am J Epidemiol. 2008;167(7):875–881. doi:10.1093/aje/kwm390
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




