Back to Journals » Neuropsychiatric Disease and Treatment » Volume 20
Vitamin D Deficiency Participates in Depression of Patients with Diabetic Peripheral Neuropathy by Regulating the Expression of Pro-Inflammatory Cytokines
Received 30 September 2023
Accepted for publication 19 February 2024
Published 27 February 2024 Volume 2024:20 Pages 389—397
DOI https://doi.org/10.2147/NDT.S442654
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yuping Ning
Jingjing Zhou,1,2 Dongfeng Li,2 Youmin Wang1
1Department of Endocrinology, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, 230001, People’s Republic of China; 2Department of Endocrinology, The Second People’s Hospital of Lu’an, Affiliated Hospital of West Anhui Health Vocational College, Lu’an, Anhui, 237000, People’s Republic of China
Correspondence: Youmin Wang, Email [email protected]
Objective: Vitamin D deficiency is associated with patients with diabetic peripheral neuropathy (DPN), and low levels of vitamin D are common in patients with depression. Depression is common in DPN patients and the definite pathogenesis remains unclear. This study aimed to determine vitamin D deficiency in the onset of depression in DPN and evaluate the effect of vitamin D supplementation on depression.
Methods: A total of 192 patients with DPN were enrolled in this study. Clinical and laboratory information was collected. Chemiluminescent immunoassay was used to measure the level of 25(OH)D. Enzyme-linked immunosorbent assay was employed to measure the concentrations of interleukin (IL)-1β, tumor necrosis factor-α (TNF-α), and IL-17A. Subjects with low 25(OH)D received 5000IU vitamin D daily for 12 weeks. Depression scores and levels of 25(OH)D, IL-1β, TNF-α, and IL-17A were re-evaluated after supplementation.
Results: The incidence of vitamin D deficiency and depression was high in DPN patients. Compared with vitamin D sufficient participants, Hamilton Depression Rating Scale (HAMD) scores and the levels of inflammatory markers IL-1β, TNF-α, and IL-17A were significantly higher in insufficient group (all p< 0.05). HAMD score, IL-1β, TNF-α, and IL-17A were negatively correlated with 25(OH)D (all p< 0.05). A linear relationship existed among IL-1β, TNF-α, IL-17A, and 25(OH)D (p< 0.05). HAMD scores, IL-1β, TNF-α, and IL-17A were all reduced significantly after supplementation of vitamin D (p< 0.05). Binary logistic analysis revealed that vitamin D insufficiency was an independent risk factor for depression in patients with DPN. Receiver operating characteristic (ROC) curve analysis showed a high sensitivity (87.20%) of 25(OH)D in discriminating DPN patients with depression.
Conclusion: Vitamin D deficiency participated in occurrence of depression in DPN patients and could be mediated, at least in part, by upregulation of pro-inflammatory cytokines. Vitamin D supplementation may be effective in improving depressive symptoms in DPN patients.
Keywords: vitamin D, depression, diabetic peripheral neuropathy, interleukin-1 beta, tumor necrosis factor-alpha, interleukin-17A
Introduction
Diabetic peripheral neuropathy (DPN) is a very frequent complication of diabetes, affecting up to 50% of diabetes subjects over their lifetime. Patients with DPN may suffer from neuropathic pain, diabetic foot ulceration, and are at high risk of lower-extremity amputations. Older age, duration of diabetes, poor glycaemic control, patient’s height, male gender, hypertension, hyperlipidaemia, and certain ethnicities are the major risk factors for DPN.1 Neuropathic pain may lead to the deterioration of the general health status of diabetic patients, resulting in functional and emotional restrictions, alteration of physical activity and social implications.2 The most common mental disorder among diabetic patients is low mood, ultimately leading to depression. Significant depressive symptoms are found in patients with DPN compared to subjects without neurological complications.3 Clinical measurements of DPN, for instance, the neuropathy disability score and the vibration perception threshold, have been proven to be independently associated with Hospital Anxiety and Depression Scale (HADS).4 Furthermore, symptoms of DPN, including reduced foot sensation, unsteadiness, and pain, are also related to depression, and severe symptoms are associated with more significant depression scores.4,5
Vitamin D is known as a steroid hormone that plays an important role in bone mineral metabolism, participating in calcium, phosphorus metabolism, and skeletal homeostasis. In addition to bone metabolism-related diseases, vitamin D deficiency also participates in the pathologies of many clinical conditions such as cancer, infectious diseases, multiple sclerosis, and cardiovascular diseases.6 Vitamin D deficiency is also considered to be a potential modifiable risk factor in DPN patients. Recent studies suggest that vitamin D deficiency is an independent risk factor for painful DPN, and vitamin D supplementation can improve pain scores in patients with painful DPN.7,8 On the other hand, vitamin D deficiency is related to brain function and development, and is associated with mental and neuropsychiatric disorders, such as autism, psychotic and mood disorders, and cognitive decline.9,10 Deficiency of vitamin D is common in individuals with depression.11 Recently, several studies have indicated that vitamin D participated in the depression of T2DM patients, and supplementation of vitamin D may protect them against the onset of depressive symptoms.12–14
Cytokines have been studied in association with stress, anxiety, and depression. Compared to non-depressed individuals, individuals with depression have higher levels of circulating pro-inflammatory cytokines.15 In the case of depression, various cytokines may be transported to effector cells of the brain, where they affect the neurotransmitter systems.16 Serum pro-inflammatory cytokine levels are elevated in patients with DPN, which reveals that cytokines may be involved in the pathogenesis of diabetic neuropathy.7
Vitamin D deficiency is closely related to DPN, and low levels of vitamin D are common in patients with depression. At the same time, there are disorders in the expression of cytokines in patients with DPN and individuals with depression. However, the potential mechanism of comorbidity between depression and DPN remains unclear.
We speculate that vitamin D deficiency may participate the pathogenesis of depression in DPN patients by regulating changes in cytokine levels. In the present study, we measure the serum concentrations of vitamin D and pro-inflammatory cytokines in DPN patients, and assess the effectiveness of vitamin D supplementation on depression in patients with DPN. We hope to provide more evidence and shed new light for the treatment of comorbid depression and DPN.
Patients and Methods
Patients
A prospective study was carried out, and patients with DPN were recruited from the department of endocrinology at the affiliated Hospital of West Anhui Health Vocational College. T2DM was diagnosed on the basis of the World Health Organization criteria in 1999. The diagnosis of DPN was based on related neurological symptoms and electrophysiological test. Neurological symptoms included feelings of burning, numbness, tingling, fatigue, cramping, and aching. Electrophysiological test was carried out to measure the nerve conduction examinations (NCVs) on both sides, including median nerve, ulnar nerve, peroneal nerve, tibial nerve, superficial peroneal nerve, and sural nerve. If three or more of the results were abnormal, then nerve conduction was considered abnormal and diagnosed on the basis of reference values for the Chinese population.17 The inclusion criteria include 1) T2DM patients with DPN; 2) Age ≥18 years old. The exclusion criteria include 1) type 1 DM; 2) suffering from inflammatory, infectious, mental, or nervous disease; 3) suffering from cardiac or respiratory illness, hepatic or renal failure, or malignancy, or blindness, or amputation; 4) intake of cognition-impairing drugs or vitamin D in the previous 3 months. Written informed consent was obtained from all participants. Our study complied with the Declaration of Helsinki. The research was approved by the Ethical Committee of Affiliated Hospital of West Anhui Health Vocational College (No LAEY-2021-013).
Clinical Assessment and Biochemical Measurements
Detailed data on gender, age, smoking, diabetes duration, hypertension, and antidiabetic drugs utilization were obtained from patients’ medical records. The BMI was calculated by the formula: BMI = weight (kg)/height (m2). Education, marital status, and family income were collected through a questionnaire. Other diabetes complications were diagnosed based on clinical manifestations, laboratory, and related examinations.
Fasting plasma glucose (FPG), total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) were measured on an automatic biochemical analyzer. Glycosylated hemoglobin (HbA1c) was measured by high-pressure liquid chromatography. Automated competitive chemiluminescent immunoassay was employed for assessing the serum level of 25(OH)D. Vitamin D status was determined and categorized based on concentrations of 25(OH)D as follows: sufficiency (≥30ng/mL), insufficiency (20–29ng/mL), and deficiency (<20ng/mL).18 In order to guide the further investigation and vitamin D supplementation, all patients in our research were classified as vitamin D sufficiency (serum 25(OH)D ≥30ng/mL) and vitamin D insufficiency [serum 25(OH)D < 20 ng/mL and serum 25(OH)D 20–29 ng/mL].
Depression Assessment
To detect and evaluate the severity of depression, the HAMD scale, a scale considered to be the “gold standard” in our clinical practice and research settings, was used before and after the intervention. The Hamilton depression-17 (HAMD-17) scale used in our study is a predominant tool to screen, diagnose, and measure the severity of depression worldwide. The scale consisted of 17 items in a multiple-choice format. The total score of this scale ranges from 0 to 52, with higher scores indicating higher levels of depression. A score of ≤7 indicates no depression, and a score >7 is considered as depression.19
Serum Cytokines Measurement
Morning fasting blood samples were collected before and after the supplementation. The specimens were quickly centrifuged, and the serum was then stored at −80°C immediately for cytokines measurement. IL-1β, TNF-α, and IL-17A concentrations were measured using enzyme-linked immunosorbent assay kits (R&D Systems). Minimal detectable concentrations were 0.1 pg/mL for IL-1β, TNF-α, and IL-17A. All serum samples were measured in duplicates.
Statistical Analysis
The SPSS 13.0 software (Chicago, IL, USA) was used for statistical analysis. Data with normal distribution were presented as mean ± standard deviation. Differences between the two groups were tested using independent sample t-test or Chi-square test. The Spearman correlations were used to determine the correlation between two variables. Multiple linear regression analysis was applied to investigate the association among IL-1β, TNF-α, IL-17A, and 25(OH)D. The logistic regression analysis was used to identify independent variables for depression. Receiver operating characteristic (ROC) curve analysis was used to determine the overall prognostic accuracy of serum 25(OH)D for depression. For all tests, p < 0.05 was considered statistically significant.
Results
From January 2020 to March 2023, 192 patients with DPN were enrolled in our study. Of 192 recruited, 158 patients (82.29%) had vitamin D insufficiency. The average serum 25(OH)D concentration was 21.49 ± 7.88 ng/mL. The percentage of patients with depression was 77.08% (148/192), and the mean HAMD score of the participants was 17.28 ± 11.36.
Based on serum 25(OH)D levels, subjects can be categorized as vitamin D sufficient group (serum 25(OH)D≥30 ng/mL, n=34) and vitamin D insufficient group (serum 25(OH)D≤29 ng/mL, n=158). Table 1 reports the demographic characteristics, HAMD scores, levels of 25(OH)D and concentrations of inflammatory markers (IL-1β, TNF-α, and IL-17A) in two groups. No significant difference was found in gender, age, diabetes duration, and the proportions of hypertension and smokers between the two groups (all p > 0.05). No significant difference in the levels of HbA1c, TC, TG, and LDL-C was observed between the two groups (all p > 0.05) and the level of HDL-C was lower in the vitamin D insufficient group (p<0.05). Symptoms of depression, measured as HAMD score, were significantly higher in the vitamin D insufficient group. Compared with sufficient participants, the levels of inflammatory markers IL-1β, TNF-α, and IL-17A were significantly higher in vitamin D insufficient subjects.
 |
Table 1 Baseline Characteristics of Vitamin D Sufficient Subjects and Vitamin D Insufficient Participants with DPN |
In all patients with DPN, HAMD score, IL-1β, TNF-α, and IL-17A were negatively correlated with 25(OH)D (Figure 1). When each of the variables was entered into a multiple regression model, a linear regression relationship existed among IL-1β, TNF-α, IL-17A, and 25(OH)D (p< 0.05) (Table 2).
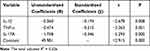 |
Table 2 Multiple Linear Regression Analysis of the Effects of 25(OH)D of Multiple Cytokines (IL-1β, TNF-α, and IL-17A) |
 |
Figure 1 Spearman correlations between serum 25(OH)D level and HAMD score, inflammatory cytokines (IL-1β, TNF-α, and IL-17A) in patients with DPN. |
Patients with vitamin D insufficiency were given a 125ug (5000 IU) vitamin D soft gel daily for 12 weeks. HAMD scores were re-evaluated, and laboratory assessments of 25(OH)D, IL-1β, TNF-α, and IL-17A were completed after 12 weeks of supplementation. Table 3 shows the comparison of the HAMD scores, the levels of serum 25(OH)D and cytokines at the beginning and the end of supplementation. Following intervention, serum 25(OH)D concentrations were increased significantly from baseline; a significant reduction was observed in HAMD score (p<0.001). Furthermore, the levels of IL-1β, TNF-α, and IL-17A were all reduced significantly at the end of intervention (p<0.01). For 158 patients with vitamin D intervention, 31 patients were treated with oral hypoglycemic drugs alone, while the remaining patients were treated with insulin-containing regimens. There was no significant change in the level of HbA1c (Table 3).
 |
Table 3 Comparison of the HAMD Score, the Levels of Serum 25(OH)D, Cytokines and HbA1c Before and After the Supplementation of Vitamin D |
Binary logistic regression analysis results revealed that vitamin D insufficiency, marital status, and family income were independent risk factors for depression in DPN patients (p<0.05) (Table 4). ROC curve analysis was used to determine the value of serum 25(OH)D in diagnosing depression in DPN patients. The optimal cutoff value for the level of 25(OH)D was 29 ng/mL, which yielded a higher sensitivity [Figure 2, the sensitivity and specificity were 87.20% and 34.10%, respectively; area under the curve (AUC) was 0.580, 95% CI: 0.507–0.651].
 |
Table 4 Univariate and Multivariate Logistic Regression for Depression in DPN Patients |
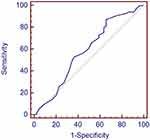 |
Figure 2 ROC curve analysis was used to evaluate the significance of serum 25(OH)D to diagnose depression in patients with DPN. |
Discussion
There is increasing evidence suggesting that depression exists more frequently in patients with DPN. We should pay attention to the fact that depression is common in diabetes patients presenting with DPN as this condition can complicate conditions and impair outcomes if overlooked and not addressed timely in the management of DPN. Results have reported that vitamin D supplementation can improve pain scores in painful DPN and generate beneficial effects on depression in depressed patients.8,20,21 Nevertheless, the underlying mechanisms of vitamin D supplementation on these two conditions comorbid remain unclear. Our findings demonstrate a significant improvement in depression symptoms in DPN patients following vitamin D supplementation. We also discover that this intervention significantly affects the inflammatory state of isolated subjects, as judged by serum cytokines measurement. Our results firstly reported that low vitamin D might participate in the pathogenesis of depression in DPN by regulating the cytokines implicated in depression.
The co-existence of DPN and depression in patients with diabetes is being increasingly recognized. Patients with DPN were more likely to have depression symptoms than their counterparts with T2DM only.3 In agreement with previous results, our study showed a high proportion of depression in DPN patients. Several studies have reported that the level of 25(OH)D was lower in T2DM patients in the DPN, and 25(OH)D was significantly correlated with DPN.22–24 In line with previous studies, our results also revealed a high frequency of vitamin D insufficiency in DPN patients. It has been reported that a deficiency of 25(OH)D in patients with T2DM was associated with poor glycemic control and dyslipidemia.25 In our study, subjects with vitamin D insufficiency had a higher HbA1c and a lower HDL-C, which indicated that vitamin D deficiency was closely related to the occurrence of diabetic chronic complications.
Previous studies have demonstrated that low serum 25(OH)D had bidirectional association between T2DM and depression. A low serum 25(OH)D was a risk factor for developing T2DM and was also related to depression.14,26 In a longitudinal study in Italy, low serum 25(OH)D increased progression of depressive symptoms with time.27 Another study reported that T2DM with cognitive impairment was at higher risk of serum 25(OH)D deficiency as compared to healthy controls.28 Consistently, our research demonstrated that the HAMD scores were higher in the vitamin D insufficient group. Correlation analysis showed a negative correlation between 25(OH)D and depression score. Our findings suggest that vitamin D deficiency participated in the onset of depression in DPN patients.
Previous studies have reported the effect of vitamin D supplementation on depression.29 However, no study has been carried out to elucidate the potential mechanism of vitamin D intervention on depression in DPN patients. The relationship between cytokines and depression has been studied for a long time.30 In the case of depression, various cytokines produced in the body were transported to effector cells of the brain and affect the neurotransmitter systems.31,32 Compared with non-depressed individuals, patients with depression were more likely to have higher levels of pro-inflammatory cytokines and lower levels of anti-inflammatory cytokines.33,34 Medications which were used to treat depression and schizophrenia significantly decreased the serum levels of IL-1β and IFN-γ and stabilized the overproduced inflammatory cytokines.35,36 Growing evidence showed that T helper 17 (Th17) cells played a crucial role in depression and other central nervous system (CNS) diseases.37 Th17 cells were characterized by the production of Th17-associated cytokines (IL-17A to IL-17F), of which interleukin-17A (IL-17A) was a signature cytokine.38 A variety of other pro-inflammatory cytokines were considered to promote Th17 cell differentiation, such as TNF-α, IL-1β, IL-21, or IL-23. To explore the Th17/IL-17A pathway in the pathogenesis of depression in DPN patients, pro-inflammatory cytokines including IL-1β, TNF-α, and IL-17A were selected in our study. Our results indicated that the levels of IL-1β, TNF-α, and IL-17A of DPN patients in the vitamin D insufficient group were significantly higher than in the sufficient group, and a negative correlation was identified between the vitamin D level and the cytokines. In addition, multiple regression analysis showed a linear regression relationship existed between IL-1β, TNF-α, IL-17A, and 25(OH)D. Furthermore, vitamin D supplementation significantly decreased HAMD scores and reduced the levels of pro-inflammatory cytokines. Therefore, vitamin D may be associated with the mechanism of inflammatory processes, which could contribute to the progression of depression in DPN patients.
ROC curve analysis was used in our study to determine the potential value of serum 25(OH)D in diagnosing depression in patients with DPN. Due to the high sensitivity but relatively low specificity, it suggested that 25(OH)D had a certain diagnostic value, but a more reliable indicator was needed to diagnose depression in patients with DPN. Other factors, such as age, sex, family income, and marital status, may also have a potential impact on depression. Therefore, we need to consider other factors in the exploration of 25(OH)D as a basis for diagnosis.
A few limitations exist in the present study. Firstly, this was a single-center study with a relatively small number of cases. Secondly, no placebo-controlled group existed for vitamin D supplementation, and short-term intervention for depression might not reflect the long-term effects. Finally, only pro-inflammatory cytokines were studied, and further research should examine whether anti-inflammatory cytokines, such as IL-2, IL-4, and IL-10, implicated in the pathogenesis of depression.
Conclusion
In conclusion, our study indicated that vitamin D deficiency was involved in the occurrence of depression by upregulating of pro-inflammatory factors in DPN patients. Serum 25(OH)D might play a predictive role in determining whether DPN patients are comorbid with depression. Vitamin D supplementation could prevent the occurrence of depression in DPN patients.
Abbreviation
25(OH)D, 25-hydroxyvitamin D.
Consent for Publication
All authors and patients agree to publish this article.
Acknowledgments
The authors would like to acknowledge all study participants and researchers who have offered support throughout the project.
Author Contributions
All authors made a great contribution to the work, whether that is in the conception, design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising or reviewing the article, gave final approval of the version to be published, have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The work was supported by the Natural Science Foundation of the Higher Education Institutions of Anhui Province (Grant No. KJ2021B005).
Disclosure
All authors declared no conflicts of interest in this work.
References
1. Papanas N, Ziegler D. Risk factors and comorbidities in diabetic neuropathy: an update 2015. Rev Diabet Stud. 2015;12(1–2):48–62. doi:10.1900/RDS.2015.12.48
2. Gore M, Brandenburg NA, Dukes E, Hoffman DL, Tai KS, Stacey B. Pain severity in diabetic peripheral neuropathy is associated with patient functioning, symptom levels of anxiety and depression, and sleep. J Pain Symptom Manage. 2005;30(4):374–385. doi:10.1016/j.jpainsymman.2005.04.009
3. Alghafri RM, Gatt A, Formosa C. Depression symptoms in patients with diabetic peripheral neuropathy. Rev Diabet Stud. 2020;16(1):35–40. doi:10.1900/RDS.2020.16.35
4. Vileikyte L, Leventhal H, Gonzalez JS, et al. Diabetic peripheral neuropathy and depressive symptoms: the association revisited. Diabetes Care. 2005;28(10):2378–2383. doi:10.2337/diacare.28.10.2378
5. Barnard KD, Skinner TC, Peveler R. The prevalence of co-morbid depression in adults with Type 1 diabetes: systematic literature review. Diabet Med. 2006;23(4):445–448. doi:10.1111/j.1464-5491.2006.01814.x
6. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281.
7. Xiaohua G, Dongdong L, Xiaoting N, et al. Severe vitamin D deficiency is associated with increased expression of inflammatory cytokines in painful diabetic peripheral neuropathy. Front Nutr. 2021;8:612068. doi:10.3389/fnut.2021.612068
8. Yammine K, Wehbe R, Assi C. A systematic review on the efficacy of vitamin D supplementation on diabetic peripheral neuropathy. Clin Nutr. 2020;39(10):2970–2974. doi:10.1016/j.clnu.2020.01.022
9. Harms LR, Eyles DW, McGrath JJ, Mackay-Sim A, Burne TH. Developmental vitamin D deficiency alters adult behaviour in 129/SvJ and C57BL/6J mice. Behav Brain Res. 2008;187(2):343–350. doi:10.1016/j.bbr.2007.09.032
10. Eyles DW, Burne TH, McGrath JJ. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol. 2013;34(1):47–64. doi:10.1016/j.yfrne.2012.07.001
11. Khan B, Shafiq H, Abbas S, et al. Vitamin D status and its correlation to depression. Ann Gen Psychiatry. 2022;21(1):32. doi:10.1186/s12991-022-00406-1
12. Wang Y, Yang H, Meng P, Han Y. Association between low serum 25-hydroxyvitamin D and depression in a large sample of Chinese patients with type 2 diabetes mellitus. J Affect Disord. 2017;224:56–60. doi:10.1016/j.jad.2016.10.040
13. Omidian M, Mahmoudi M, Abshirini M, et al. Effects of vitamin D supplementation on depressive symptoms in type 2 diabetes mellitus patients: randomized placebo-controlled double-blind clinical trial. Diabetes Metab Syndr. 2019;13(4):2375–2380. doi:10.1016/j.dsx.2019.06.011
14. Sun HM, Yu Y, Gao XR, et al. Potential role of 25(OH)D insufficiency in the dysfunction of glycolipid metabolism and cognitive impairment in patients with T2DM. Front Endocrinol. 2022;13:1068199. doi:10.3389/fendo.2022.1068199
15. Chistyakov DV, Astakhova AA, Sergeeva MG. Resolution of inflammation and mood disorders. Exp Mol Pathol. 2018;105(2):190–201. doi:10.1016/j.yexmp.2018.08.002
16. Miller AH. Norman Cousins Lecture. Mechanisms of cytokine-induced behavioral changes: psychoneuroimmunology at the translational interface. Brain Behav Immun. 2009;23(2):149–158. doi:10.1016/j.bbi.2008.08.006
17. Jin J, Wang W, Gu T, et al. Low serum bilirubin levels contribute to the presence and progression of distal symmetrical polyneuropathy in Chinese patients with type 2 diabetes. Diabetes Metab. 2019;45(1):47–52. doi:10.1016/j.diabet.2018.02.007
18. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline [published correction appears in J Clin Endocrinol Metab. 2011 Dec;96(12):3908]. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi:10.1210/jc.2011-0385
19. Ballesteros J, Bobes J, Bulbena A, et al. Sensitivity to change, discriminative performance, and cutoff criteria to define remission for embedded short scales of the Hamilton depression rating scale (HAMD). J Affect Disord. 2007;102(1–3):93–99. doi:10.1016/j.jad.2006.12.015
20. Alavi NM, Khademalhoseini S, Vakili Z, Assarian F. Effect of vitamin D supplementation on depression in elderly patients: a randomized clinical trial. Clin Nutr. 2019;38(5):2065–2070. doi:10.1016/j.clnu.2018.09.011
21. Kaviani M, Nikooyeh B, Etesam F, et al. Effects of vitamin D supplementation on depression and some selected pro-inflammatory biomarkers: a double-blind randomized clinical trial. BMC Psychiatry. 2022;22(1):694. doi:10.1186/s12888-022-04305-3
22. Ahmadieh H, Azar ST, Lakkis N, Arabi A. Hypovitaminosis d in patients with type 2 diabetes mellitus: a relation to disease control and complications. ISRN Endocrinol. 2013;2013:641098. doi:10.1155/2013/641098
23. Esteghamati A, Fotouhi A, Faghihi-Kashani S, et al. Non-linear contribution of serum vitamin D to symptomatic diabetic neuropathy: a case-control study. Diabet Res Clin Pract. 2016;111:44–50. doi:10.1016/j.diabres.2015.10.018
24. Pang C, Yu H, Cai Y, et al. Vitamin D and diabetic peripheral neuropathy: a multi-centre nerve conduction study among Chinese patients with type 2 diabetes. Diabetes Metab Res Rev. 2023;39(7):e3679. doi:10.1002/dmrr.3679
25. Darraj H, Badedi M, Poore KR, et al. Vitamin D deficiency and glycemic control among patients with type 2 diabetes mellitus in Jazan City, Saudi Arabia. Diabetes Metab Syndr Obes. 2019;12:853–862. doi:10.2147/DMSO.S203700
26. Pittas AG, Dawson-Hughes B, Li T, et al. Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care. 2006;29(3):650–656. doi:10.2337/diacare.29.03.06.dc05-1961
27. Liu E, Meigs JB, Pittas AG, et al. Predicted 25-hydroxyvitamin D score and incident type 2 diabetes in the Framingham Offspring Study. Am J Clin Nutr. 2010;91(6):1627–1633. doi:10.3945/ajcn.2009.28441
28. Parveen R, Kapur P, Venkatesh S, Agarwal NB. Attenuated serum 25-hydroxyvitamin D and vitamin D binding protein associated with cognitive impairment in patients with type 2 diabetes. Diabetes Metab Syndr Obes. 2019;12:1763–1772. doi:10.2147/DMSO.S207728
29. Xie F, Huang T, Lou D, et al. Effect of vitamin D supplementation on the incidence and prognosis of depression: an updated meta-analysis based on randomized controlled trials. Front Public Health. 2022;10:903547. doi:10.3389/fpubh.2022.903547
30. Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimäki M. Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav Immun. 2015;49:206–215. doi:10.1016/j.bbi.2015.06.001
31. Müller N, Ackenheil M. Psychoneuroimmunology and the cytokine action in the CNS: implications for psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22(1):1–33. doi:10.1016/s0278-5846(97)00179-6
32. Dunn AJ, Wang J, Ando T. Effects of cytokines on cerebral neurotransmission. Comparison with the effects of stress. Adv Exp Med Biol. 1999;461:117–127. doi:10.1007/978-0-585-37970-8_8
33. Song C, Halbreich U, Han C, Leonard BE, Luo H. Imbalance between pro- and anti-inflammatory cytokines, and between Th1 and Th2 cytokines in depressed patients: the effect of electroacupuncture or fluoxetine treatment. Pharmacopsychiatry. 2009;42(5):182–188. doi:10.1055/s-0029-1202263
34. Chen MH, Chang WC, Hsu JW, et al. Correlation of proinflammatory cytokines levels and reduced gray matter volumes between patients with bipolar disorder and unipolar depression. J Affect Disord. 2019;245:8–15. doi:10.1016/j.jad.2018.10.106
35. Hiles SA, Baker AL, de Malmanche T, Attia J. Interleukin-6, C-reactive protein and interleukin-10 after antidepressant treatment in people with depression: a meta-analysis. Psychol Med. 2012;42(10):2015–2026. doi:10.1017/S0033291712000128
36. Tourjman V, Kouassi É, Koué MÈ, et al. Antipsychotics’ effects on blood levels of cytokines in schizophrenia: a meta-analysis. Schizophr Res. 2013;151(1–3):43–47. doi:10.1016/j.schres.2013.10.011
37. Beurel E, Lowell JA. Th17 cells in depression. Brain Behav Immun. 2018;69:28–34. doi:10.1016/j.bbi.2017.08.001
38. Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–1132. doi:10.1038/ni1254
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
