Back to Journals » Hepatic Medicine: Evidence and Research » Volume 14
Undiagnosed Seroprevalence of Hepatitis B and C Virus Infections in the Community of Wolaita Zone, Southern Ethiopia
Authors Kumalo A , Teklu T , Demisse T, Anjulo A
Received 9 May 2022
Accepted for publication 29 July 2022
Published 9 August 2022 Volume 2022:14 Pages 111—122
DOI https://doi.org/10.2147/HMER.S374029
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Gerry Lake-Bakaar
Abera Kumalo,1 Takele Teklu,1 Tigistu Demisse,1 Abebe Anjulo2
1School of Medical Laboratory Sciences, College of Health Sciences and Medicine, Wolaita Sodo University, Sodo, Ethiopia; 2Department of Medical Laboratory, Wolaita Sodo Blood Bank, Sodo, Ethiopia
Correspondence: Abera Kumalo, School of Medical Laboratory Sciences, College of Health Sciences and Medicine, Wolaita Sodo University, P. O. Box: 138, Sodo, Ethiopia, Tel +251906548012; +251911807270, Email [email protected]
Background: Despite Ethiopia’s hepatitis endemic status with intermediate to hyperendemic level, there is no national strategy for monitoring, preventing, and controlling viral hepatitis. In order to advise community-based intervention programs, studies on the magnitude, determinant factors, and understanding of indigenous social organization are important. Thus, this study examined undiagnosed seroprevalence and associated factors for HBV and HCV infections among community members in Wolaita Zone, Southern Ethiopia.
Methods: A cross-sectional study was conducted on 320 individuals from randomly selected two woredas in the Wolaita Zone to determine the magnitude of HBV and HCV. Multistage sampling was used to select participants. Relevant clinical and sociodemographic data were collected using a structured questionnaire. One test strip technique was used for the screening of hepatitis B surface antigen and for antibodies against HCV. Both tests were confirmed by ELISA methods. The associated factors were assessed using bivariate and multivariate logistic regression analyses. P-values less than 0.05 were considered statistically significant.
Results: The seroprevalence for HBV infection was 6.6% (95% CI: 4.22%, 8.69%) using a one-step HBsAg test strip and 5.6% (95% CI: 3.47%, 8.58%) using confirmatory test (ELISA). The two tests had a very good agreement (K = 0.918; SE = 0.047; P < 0.001). The overall seroprevalence for HCV infection was 1.9% (95% CI: 0.9%, 3.0%). All four of the one-step HCV test strip positives were also positive by ELISA. One (0.3%) of the participants was co-infected with HBV and HCV. Hospital admission (AOR = 0.22; 95% CI = 0.5– 0.95) and needle stick (AOR = 0.15; 95% CI = 0.07– 0.72) were independently associated with HBV infections.
Conclusion: According to the current study, in Wolaita community, there is endemic to HBV at a higher-intermediate level and to HCV at a low level. It would be imperative to increase awareness of transmission modes and prevention of infection, as well as vaccination, in order to reduce the burden of both HBV and HCV.
Keywords: hepatitis B virus, hepatitis C virus, seroprevalence, Wolaita, Southern Ethiopia
Introduction
Hepatitis is an inflammation of the liver caused by hepatitis viruses such as hepatitis A, B, C, D, and E, of which hepatitis B virus (HBV) and hepatitis C virus (HCV) are the two most common causes.1 HBV and HCV infections are common diseases in the world, infecting about 2 billion and 3.9 million people, including about 350 million chronic cases.2 HBV and HCV are hepatotropic virus whose primary replication occurs in the liver. These infections have a high rate of development of liver cirrhosis and can cause serious mortality, raising a major concern for global health.2
Both HBV and HCV can share the same mode of transmission via sexual contact, blood, or vertical transmission.3 An HBV infection can resolve spontaneously, leading to protective immunity, chronic infection, or acute liver failure with a high mortality rate. Contrary to HBV, HCV infection is usually chronic. The worst-case scenario for people with chronic HBV and/or HCV infection remains infectious to others and is at risk of serious liver diseases, such as liver cirrhosis or hepatic cell carcinoma later in life.4,5 HCV infects 170 million people around the world, of whom 55% to 80% have chronic infections.6 Similar to HBV, it causes liver abnormalities and cirrhosis, liver damage, and hepatocellular carcinoma.7 Besides hepatic manifestations, HCV infection can also cause extrahepatic manifestations, such as insulin resistance, mixed cryoglobulinemia, glomerulonephritis, porphyria cutaneous tarda, and benign monoclonal gammopathy.8
There are 350 million chronic HBV infections and 170 million chronic HCV infections worldwide, according to the World Health Organization (WHO). Globally, hepatitis B surface antigen (HBsAg) seroprevalence ranges from 0.1% to 20%. Approximately 5% of people worldwide have chronic HBV infection, but this figure varies widely by region. The United States and Western Europe have low infection rates (2.0%), Mediterranean countries and Japan have intermediate infection rates (2.8%), and South-East Asia and sub-Saharan Africa have high infection rates (8.0–20.0%). There is an epidemic of HCV infection around the world.9 Chronic infection rates are high in Egypt (22%), Pakistan (4.8%), and China (3.2%).10 A prevalence of 43 (35.8%) for HBsAg and 27 (22.5%) for anti-HCV-Ab was found in Ethiopia for chronic liver disease.11 HBV infection can be prevented with a vaccine, thus reducing liver cancer incidence.11,12
As a result of social, demographic, and migrant trends, the prevalence of hepatitis will continue to rise. A greater burden of disease is expected to be felt by developing nations, although industrialized nations will also experience an increase.13 Though Ethiopia has an intermediate to hyperendemic level of hepatitis endemicity,14 there is no national strategy for surveillance, prevention, and control. In the country, chronic HBV and HCV infections are responsible for more than 60% of chronic liver disease and nearly 80% of hepatic cell carcinoma.15 In some areas, however, even children have not been vaccinated against HBV. In addition, community-level data on HBV and HCV infection epidemiology are rare, principally for HCV, which is underdiagnosed and under-reported. In this way, the lack of adequate epidemiological data may compromise HBV and HCV at the community level on hepatitis. As a result, it is crucial to determine HBV and HCV infections at the local level in order to set plans to reduce spread within members of the community. In the context of the concentrated epidemic phase in Wolaita and the existing mass-scale mobility of its people, studies on determinant factors and understanding indigenous social organizations are of the utmost importance in order to advise community-based interventions programs. Therefore, this study was aimed to investigate the undiagnosed seroprevalence and associated factors for HBV and HCV infections among community members in Wolaita Zone, Southern Ethiopia.
Materials and Methods
Study Area
This project was conducted in the Wolaita zone, Southern Nations, Nationalities Peoples’ Region of Ethiopia. The Zone is located 320 kilometers south of Ethiopia’s capital, Addis Ababa. According to the 2007 census of the Central Statistical Agency of Ethiopia16 the Zone has a total population of 1,721,339; with an area of 4208.64 square kilometers and a population density of 356.67. A total of 310,454 households were counted in the Zone in 2007, which results in an average of 5 persons per household, and 297,981 housing units. The Zone is divided into sixteen districts and seven towns. There are five hospitals, 69 health centers, 372 health posts and 98 private clinics in the Zone. Two woredas/districts of the Wolaita Zone were studied.
Study Population, Study Design and Sampling Procedure
We conducted a cross-sectional study among randomly selected two woredas (Duguna Fango & Kindo Koysha) in the Wolaita Zone to determine the magnitude of HBV and HCV. For sample size calculation was made using a single population proportion formula by assuming 95% confidence interval, 5% margin of error, the prevalence (P) of assumed prevalence of 10.5%17 and 10% for possible non-response. In addition, this study was conducted in heterogonous groups that might result in design effect. To comprise it we used a design effect of 2 which was given a final sample size of 320. A multi-stage sampling method was used to select study participants. Population size based proportional allocation to each of woredas was applied to increase the efficiency of sampling and precision. From each woreda, a number of kebele/s containing the amount of people allotted to that respective area was sampled using a lottery method and a sampling frame was taken from each woreda health facilities. After selecting the first household from the first sampling interval randomly, a systematic random sampling technique was followed to select the next households from each study kebeles subsequent the nth value (sampling interval) that was obtained by dividing the total number of households by the sample size allocated to each Kebele. One eligible individual was recruited from each household randomly using a draw system.
To be eligible for the study, age ≥18 years, volunteered to participate and able to provide five milliliters of blood were minimum criteria. Respectively selected individuals underwent clinical and physical examinations. Face-to-face interviews were conducted with study members individually to identify risk factors for hepatitis infection. Participants’ sociodemographic characteristics were also included in the Supplementary Questionnaire.
Data Collection
The participants provided written consent, and a structured questionnaire was used to interview them about their sociodemographic characteristics (age, sex, marital status, and educational status), as well as their exposure to potential risk factors of HBV and HCV infections, such as sharing sharp materials, tattooing, and other factors. Those who were unable to communicate in English were assisted in translating the questionnaire by supervisors. For the development and adaptation of the questionnaire, a comprehensive literature review was conducted.11,18–21
Laboratory Analysis
A blood sample of 5 milliliters was collected from each participant after obtaining their written consent. All blood samples were allowed to clot, and serum was separated by centrifugation at 3000 rpm for 5 minutes before testing for HBsAg and anti-HCV rapid test strips (Eco test) according to the manufacturer’s instructions. Enzyme-Linked Immunosorbent Assay (ELISA) tests were conducted at the Ethiopian Blood Bank, Wolaita Sodo branch on all serum samples, which were positive for HBV or HCV. As a control, known positive and negative serums were used for HBV and HCV testing during testing according to standard operating procedures (SOPs).
Data Analysis
The data were entered into Epi Data 3.1, cleaned, and processed, and analyzed using SPSS version 21. Using binary logistic regression analysis, sociodemographic factors and other potential risk factors were analyzed. In the multivariate logistic regression model, all variables that had a P < 0.05 were included. To measure the strength of the association, odds ratios (ORs) at 95% confidence intervals (CIs) were computed. In all cases, P-values of 0.05 were considered significant.
Ethical Clearance
The study was ethically cleared and approved by the Ethical Review Committee (ERC) of College of Health Science and Medicine, Wolaita Sodo University. Then, the official ethical approval letter was written in Protocol No: CRSCD 16/01/13 in date of 17–10-2013 E.C with a supporting letter to district health offices. Participants were informed in their local languages about the objectives and potential risks and benefits of the study after the respective health facilities leaders approved the study. Interviews and blood samples were collected entirely on a voluntary basis. Data confidentiality was maintained by avoiding personal identifiers and anonymity of personal data records. During the study period, hepatitis-positive subjects were linked to the respective health facilities clinics to be treated with the supplements, according to guidelines.
Data Quality Assurance
The questionnaire was pretested among 5% of the sample size in a similar set-up before the actual data collection period. The coherence and skipping pattern of the questionnaire were corrected after the pretest. We made proper training of the data collectors on the data collection procedures. To get information from respondents, we selected data collectors who are fluent speakers of the respondents’ local language. We stored the serum samples at −70℃ until we processed them. Laboratory tests were conducted in accordance with the manufacturer’s directions and SOPs. ELISA test cut points were interpreted in accordance with the manufacturers. During each run, internal control kits with negative, low-positive, and high-positive results were used to ensure that the reagents were of high quality.
Results
Participants’ Sociodemographic Characteristics
This study included 320 participants (52.3% males, average age ±SD = 34.20 ±12.95 years) with a non-respondent rate of 12 (3.5%). Most of the study groups were between 36 and 45 years old. Study participants were overwhelmingly urban (87.7%); diploma holders (75.5%); single (46.2%); and employed by the government (60.4%) (Table 1).
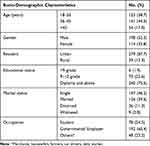 |
Table 1 Socio-Demographic Characteristics of Participants Investigated for Undiagnosed HBV and HCV in Wolaita Community (August–October, 2021) |
Seroprevalence of HBV and HCV Infections Among the Study Participants
The overall HBV seroprevalence was 6.6% (95% CI: 4.22%, 8.69%) using a one-step HBsAg test strip and 5.6% (95% CI: 3.47%, 8.58%) using a confirmatory test (ELISA). One-step HBsAg test strips found 21 samples positive for HBV infection, while ELISA found three samples negative. The two tests had a very good agreement (K = 0.918; SE = 0.047; P < 0.001). Hepatitis B virus infection prevalence was almost comparable in all age groups (6.5%, 7.1% and 5.6%; х2= 0.153; P = 0.957 in age of 18–35, 36–45 and >45, respectively), and in males and females (6.1 vs 7.0; x2=0.110; P = 0.456). However, the prevalence was higher in Urban than in Rural (7.2% vs 2.5%), less educated, widowed, and other than students and governmental employed, but in all cases not statistically significant (P > 0.05). All of the 12 individuals with a history of HBV vaccine were tested negative for HBV infection. Meanwhile, all six individuals who took HBV examinations in the past were found to be negative. Except for one infected individual, the rest of 20 multi-partner sex used condoms during sex got positive for HBV infection (Table 2). Three of the five risk factors associated with HBV infection (dental extract, needle stick, and hospital admission) are directly or indirectly associated with medical transmission.
 |
Table 2 Bivariate Analysis of Factors for HBsAg Seropositivity Among Participants Investigated for Undiagnosed HBV and HCV in Wolaita Community (August–October, 2021) |
Overall, 1.9% of people had HCV infection (95% CI: 0.9%, 3.0%) (Table 3). All four of the one-step HCV test strip positives were also positive by confirmatory test (ELISA). One participant (0.3%) had both HBV and HCV coinfections.
 |
Table 3 Bivariate Analysis of Factors for HCV Seropositivity Among Participants Investigated for Undiagnosed HBV and HCV in Wolaita Community (August–October, 2021) |
Multivariate Analysis on Factors Associated with Hepatitis B Virus Infection
The association between marital status, dental extraction, hospital admission, needle stick, and tattoo in the community was significant when the bivariate analysis was performed. The multivariable analysis found that both hospital admission AOR = 0.22 [95% CI 0.5–0.95] and needle stick AOR = 0.15 [95% CI 0.07–0.72] remained statistically significant factors associated with HBsAg positivity (Table 4).
 |
Table 4 Multiple Logistic Regressions for Factors Analysis of HBsAg Seropositivity Among Participants Investigated for Undiagnosed HBV and HCV in Wolaita Community (August–October, 2021) |
Discussion
In this study, the prevalence of HBV infection was 5.6% (95% CI: 3.47%, 8.58%) which showed higher-intermediate endemicity level according to WHO classification criteria22,23 in the general population in Wolaita Zone community. HBsAg seroprevalence was similar to that of 6.1% of the entire African region9 and within the range of 5–10% reported in sub-Saharan African countries.24 Similarly, the prevalence was 7.4% for the previous national pooled prevalence and 8.0% for the community-based prevalence,17 7.2% for the South Omo Zone community prevalence,20 and 6.9% for Madagascar.25 However, the prevalence in our study is lower than 9.5% in the community of Southern Ethiopia,19 9% in the adult community of Southwest Ethiopia,21 17.6% in a community-based study of Northern Uganda26 and similar study population in Pakistan (9.33%).27 The prevalence of HBsAg in the current study is much higher than the 3.5% prevalence findings reported in a community in Gojjam, Ethiopia,28 2.1% in Kenya,29 3.5% worldwide magnitude in the general population9 and similar research conducted in developed countries, eg, 1.4% in Brazil,30 1.6% in Europe,31 2.5% in South Korea32 and 2.8% in Turkey.33 This variation between Ethiopia and the others might be the study population difference, study duration, geographical factors, and knowledge differences about the transmission. In another way, the relative increase in the prevalence of HBsAg indicates that the area should be one of the priority target areas for the prevention and control of hepatitis, as well as screening for care and treatments.
Overall anti-HCV seroprevalence was 1.9% (95% CI: 0.9%, 3.0%), which is considered low endemicity by WHO.22,23 The results were consistent with the results of the previous studies conducted on the general population in the South Omo Zone, 1.9%,20 the recent pooled national prevalence of 2%,34 the 2% prevalence reported from Gambella, Ethiopia18 and 1.4% among community members in southern Ethiopia19 However, the seroprevalence was less than that of 3.1% of the previous national pooled prevalence,17 4.3% in previous studies reported from Northwest Ethiopia,35 3.0% prevalence found in sub-Saharan Africa,36,37 3.4% Africa, 4.8% Somalia, 6.2% Pakistan, 6.5% Cameron, and 11.9% Egypt.38–42 On the contrary, it is greater than the 1.0% prevalence reported in Gojjam, Ethiopia;28 0.64% prevalence reported in Nekemite, Ethiopia,43 0.4% in Iraq,44 0.3% in Djibouti, 0.9% in Somalia, and 1.0% in Sudan36 among the general populations. This might be due to differences in different risky behaviors at different geographical locations, study period, design, population, health programs, and sociocultural practices that contributed to disease transmission. Mostly, in Ethiopia, community practices, such as tattooing and medical injections administered by people other than health professionals, are widely practiced by the Ethiopian community.
The two viruses share a similar way of transmission, and coinfection is common, especially in high-endemic areas and among people at high risk for parenteral infection.45–48 In current study, coinfection was 0.3% among the general population in the community of Wolaita. This was in line with 0.3% prevalence among community members in South Omo Zone,20 0.2% prevalence in Hawassa University Comprehensive Specialized Hospital, Hawassa City, Southern Ethiopia,49 0.4% prevalence among hemodialysis patients in Addis Ababa, Ethiopia50 and 0.43% prevalence among surgical patients in Lahore.51 HBV seroprevalence was determined by using viral antigen (HBsAg) as a serologic marker, and HCV infection by antibody (anti-HCV antibody), both confirmed by ELISA. Accordingly, recent viral transmission may result in higher HBsAg seroprevalence (5.6%) than anti-HCV prevalence (1.9%), thereby diluting the effect of coinfection.20 Besides geographic variation, the more recent transmission of HBV might explain the low coinfection rate in the current study area through the sexual route. The study also assessed risk factors associated with HBsAg and anti-HCV infections to identify the adult populations with the risks in a community. In this study, we found that the prevalence did not vary significantly according to socio-demographic variables including age, gender, marital status, educational status, residence, and occupation. Similarly, other studies conducted in Ethiopia previously21,28 and elsewhere25 documented that those socio-demographic factors could not significantly influence the prevalence of HBsAg.
Multivariate analysis controlling for potential confounders showed, needle stick (AOR = 0.15; 95% CI: 0.073, 0.72) and history of hospital admission (AOR = 0.22; 95% CI: 0.5, 0.95) significantly associated with HBV infection. This may indicate that patients coming to hospitals for healthcare services may be at risk of contracting infections from hospital practices and other practices. Health bureaus and health facilities need to pay special attention to these situations because the patients may get infections other than the diseases that bring them to health facilities.9,52 Similarly, studies previously reported that these variables were risk factors for hepatitis infection.11,35,49,51 A number of factors could contribute to this, including poor infection control practices in health facilities, poorly sterilized medical equipment, improper healthcare procedures, and bare hands in contact with blood.9,35,52,53 Hepatitis B virus can survive and keep its infectivity for more than a week on the surfaces of non-sterile materials. Thus, the sharing of non-sterile materials during hospital practices might contribute to the transmission of HBV in this study setup. In our study, the occurrence of all 12 vaccinated individuals was negative for HBV and 20 out of 21 multi-partner sex got positive for HBV infection, encouraging other HBV prevention and control measures to be promoted to reduce the transmission of HBV in the community.
Strengths and Limitations of the Study
The inclusion of community-based wide population group covering rural and urban populations and the use of fourth-generation ELISA to confirm the infection are the strengths of this study. However, the findings of this study should be interpreted considering the following potential limitations: firstly, due to financial reasons made studies impossible to perform all diagnostic markers of hepatitis, which would have been helpful to differentiate chronic infection from acute infections and to determine viral load. Another limitation was that this study also lacked an occult hepatitis B test in HBsAg ELISA negatives to estimate silent-transmission risks since polymerase chain reaction test was not carried out in this study. The small sample size in our study might limit the association between risk factors and HBV and HCV, and the cross-sectional nature of this study makes it difficult to attribute causality to the observed associations. Therefore, large-scale longitudinal study design is needed for the future. Despite these limitations, studies believe that the use of population-based data provides valuable information for determining the community burden of infection in countries where surveillance for these diseases is limited.
Conclusions
In general, the current study showed that the burden of HBV infection in adults at the community level revealed higher-intermediate endemicity and low prevalence of HCV among the general population in Wolaita community. Those individuals with modifiable risk factors, such as needle sticks and history of hospital admission, were identified as at higher risk of acquiring HBV. This indicates that there is a high risk of transmission of these infectious pathogens in the community and points to a need for special attention to be paid to HBV-linked risk factors. Therefore, increasing awareness of people on modes of transmission and prevention of infection as well as vaccination would have utmost importance that could help in reducing the burden of both HBV and HCV. Furthermore, research using molecular and other more sensitive and specific assays for detecting active HBV and HCV infections in larger population-based screening with a larger sample size is also needed in the future.
Data Sharing Statement
All relevant data are within the article, but any additional data required are available from the corresponding author upon request.
Acknowledgments
The authors thank the Health Professionals Education Partnership Initiative Ethiopia for their financial support. The authors thank Wolaita Sodo University and Wolaita Zone Health Bureau, for providing permission to conduct the study. The authors would also like to thank Wolaita Sodo Blood Bank for laboratory works, all study participants, data collectors and supervisors.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study was supported by a Health Professionals Education Partnership Initiative (HEPI) grant (grant number: R25TW011214) obtained from the US National Institutes of Health, Fogarty International Center. However, the funding organization had no interference in the overall study activities including data collection, interpretation and in writing the manuscript.
Disclosure
None of the authors declared any conflicts of interest in this work.
References
1. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264–73.e1. PMID: 22537432. Pubmed Cenntral PMCID: PMC3338949. doi:10.1053/j.gastro.2011.12.061
2. Ephraim R, Nsiah P, Osakunor D, Adoba P, Sakyi S, Anto E. Seroprevalence of hepatitis B and C viral infections among type 2 diabetics: a cross-sectional study in the Cape Coast Metropolis. Ann Med Health Sci Res. 2014;4(5):719–722. doi:10.4103/2141-9248.141529
3. Wasley A, Grytdal S, Gallagher K. Surveillance for acute viral hepatitis—United States, 2006. MMWR Surveill Summ. 2008;57(2):1–24. PMID: 18354374.
4. Sorrell MF, Belongia EA, Costa J, et al. National institutes of health consensus development conference statement: management of hepatitis B. Hepatology. 2009;49(S5):S4–S12. doi:10.1002/hep.22946
5. Eke AC, Eke UA, Okafor CI, Ezebialu IU, Ogbuagu C. Prevalence, correlates and pattern of hepatitis B surface antigen in a low resource setting. Virol J. 2011;8(1):12. doi:10.1186/1743-422X-8-12
6. Messina JP, Humphreys I, Flaxman A, et al. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61(1):77–87. doi:10.1002/hep.27259
7. Sangiovanni A, Prati GM, Fasani P, et al. The natural history of compensated cirrhosis due to hepatitis C virus: a 17‐year cohort study of 214 patients. Hepatology. 2006;43(6):1303–1310. doi:10.1002/hep.21176
8. Zignego A, Ferri C, Pileri S, Caini P, Bianchi F. Extrahepatic manifestations of Hepatitis C Virus infection: a general overview and guidelines for a clinical approach. Dig Liver Dis. 2007;39(1):2–17. doi:10.1016/j.dld.2006.06.008
9. World Health Organization. World Health Organization: global hepatitis report, Geneva; 2017. Available from: www.who.int/hepatitis/publications/globalhepatitis-report2017/en/.
10. Lavanchy D. Viral hepatitis: global goals for vaccination. J Clin Virol. 2012;55(4):296–302. doi:10.1016/j.jcv.2012.08.022
11. Ayele AG, Gebre-Selassie S. Prevalence and risk factors of hepatitis B and hepatitis C virus infections among patients with chronic liver diseases in public hospitals in Addis Ababa, Ethiopia. Int Sch Res Notices. 2013;2013. doi:10.1155/2013/563821
12. Rathmann W, Giani G. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(10):2568–2569. doi:10.2337/diacare.27.10.2568
13. Greco DB. A epidemia da AIDS: impacto social, científi co, econômico e perspectivas [The AIDS epidemic: social, scientific and economical impacts and perspectives]. Estud Av. 2008;22:73–94. Portuguese. doi:10.1590/S0103-40142008000300006
14. World Health Organization. Global policy report on the prevention and control of viral hepatitis; 2013: 536 Available from: http://www.who.int/hiv/pub/hepatitis/global_report/en/.
15. Bane A, Patil A. Healthcare cost and access to care for viral hepatitis in Ethiopia. IJIAS. 2014;9(4):1718–1723.
16. Central Statistical Agency (CSA). The 2007 population and housing census of Ethiopia: statistical report for Southern Nations, Nationalities and Peoples’ Region; part i: population size and characteristics; 2010.
17. Belyhun Y, Maier M, Mulu A, Diro E, Liebert UG. Hepatitis viruses in Ethiopia: a systematic review and meta-analysis. BMC Infect Dis. 2016;16(1):1–4. doi:10.1186/s12879-016-2090-1
18. Ayele A, Abera D, Hailu M, Birhanu M, Desta K. Prevalence and associated risk factors for Hepatitis B and C viruses among refugees in Gambella, Ethiopia. BMC Public Health. 2020;20:721. doi:10.1186/s12889-020-08893-1
19. Beykaso G, Mulu A, Giday M, et al. Burden and transmission risks of viral hepatitis in Southern Ethiopia: evidence needed for prevention and control measures. Risk Manag Healthc Policy. 2021;14:4843–4852. doi:10.2147/RMHP.S336776
20. Endale A, Erku W, Medhin G, Berhe N, Legesse M. Community-based seroprevalence of hepatitis B and C infections in South Omo Zone, Southern Ethiopia. PLoS One. 2019;14(12):1–12.
21. Sayih A, Derseh D, Shewasinad S, Mitiku K. Hepatitis B virus infection and associated factors among adults in Southwest Ethiopia: community-based cross-sectional study. Int J Gen Med. 2020;13:323–332. doi:10.2147/IJGM.S259375
22. Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30(12):2212–2219. PMID: 22273662. doi:10.1016/j.vaccine.2011.12.116
23. Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546–1555. PMID: 26231459. doi:10.1016/S0140-6736(15)61412-X
24. World Health Organization. World Health Organization: hepatitis B fact sheet; 2016. Available from: http://www.who.int/mediacentre/factsheets/fs204/en/#.
25. Andriamandimby SF, Olive -M-M, Shimakawa Y, et al. Prevalence of chronic hepatitis B virus infection and infrastructure for its diagnosis in Madagascar: implication for the WHO’s elimination strategy. BMC Public Health. 2017;17(1):636. doi:10.1186/s12889-017-4630-z
26. Ochola E, Ocama P, Orach CG, et al. High burden of hepatitis B infection in Northern Uganda: results of a population-based survey. BMC Public Health. 2013;13(1):727. doi:10.1186/1471-2458-13-727
27. Umar M, Tul Bushra H, Ahmad M, et al. Hepatitis C in Pakistan: a review of available data. Hepat Mon. 2010;10:205.
28. Abera B, Adem Y, Yimer M, Mulu W, Zenebe Y, Mekonnen Z. Community seroprevalence of hepatitis B, C and human immunodeficiency virus in adult population in gojjam zones, northwest Ethiopia. Virol J. 2017;14(1):21. doi:10.1186/s12985-
29. Ochwoto M, Kimotho JH, Oyugi J, et al. Hepatitis B infection is highly prevalent among patients presenting with jaundice in Kenya. BMC Infect Dis. 2016;16(1):1–4. doi:10.1186/s12879-016-1409-2
30. Scaraveli NG, Passos AM, Voigt AR, et al. Seroprevalence of hepatitis B and hepatitis C markers in adolescents in Southern Brazil. Cad Saude Publica. 2011;27:753–758.58. doi:10.1590/S0102-311X2011000400014
31. Hahné SJ, Veldhuijzen IK, Wiessing L, Lim TA, Salminen M, van de Laar M. Infection with hepatitis B and C virus in Europe: a systematic review of prevalence and cost-effectiveness of screening. BMC Infect Dis. 2013;13(1):1–6.55. doi:10.1186/1471-2334-13-181
32. Kang J, Cho JH, Suh CW, et al. High prevalence of hepatitis B and hepatitis C virus infections in Korean patients with hematopoietic malignancies. Ann Hematol. 2011;90(2):159–164.56. doi:10.1007/s00277-010-1055-5
33. Köse Ș, Mandıracıoğlu A, Çavdar G, et al. Seroprevalence of hepatitis B and hepatitis C: a community based study conducted in Izmir, Turkey. Prevalence. 2014;45(7):
34. Deress T, Million Y, Belachew T, Jemal M, Girma M. Seroprevalence of hepatitis C viral infection in Ethiopia: a systematic review and meta-analysis. Hindawi Sci World J. 2021;2021:12.
35. Andualem M. Epidemiology of hepatitis B and C virus infections among patients who booked for surgical procedures at Felege Hiwot referral hospital, Northwest Ethiopia. PLoS One. 2020;15(6):e0234822. doi:10.1371/journal.pone.0234822
36. Chaabna K, Mohamoud YA, Chemaitelly H, Mumtaz GR, Abu- Raddad LJ. Protocol for a systematic review and meta-analysis of hepatitis C virus (HCV) prevalence and incidence in the Horn of Africa sub-region of the Middle East and North Africa. Syst Rev. 2014;3. doi:10.1186/2046-4053-3-146
37. Chaabna K, Kouyoumjian SP, Abu-Raddad LJ. Hepatitis C virus epidemiology in Djibouti, Somalia, Sudan, and Yemen: systematic review and meta-analysis. PLoS One. 2016;11(2):e0149966. doi:10.1371/journal.pone.0149966
38. Mora N, Adams WH, Kliethermes S, et al. A synthesis of hepatitis C prevalence estimates in Sub-Saharan Africa: 2000–2013. BMC Infect Dis. 2016;16:283. PMID: 27296465. Pubmed Central PMCID: PMC4906983. doi:10.1186/s12879-016-1584-1
39. Hassan-Kadle MA, Osman MS, Ogurtsov PP. “Epidemiology of viral hepatitis in Somalia: systematic review and meta-analysis study”. World J Gastroenterol. 2018;24(34):3927–3957. doi:10.3748/wjg.v24.i34.3927
40. Al Kanaani Z, Mahmud S, Kouyoumjian SP, Abu- Raddad LJ. “The epidemiology of hepatitis C virus in Pakistan: systematic review and meta-analyses”. R Soc Open Sci. 2018;5(4):180257. doi:10.1098/rsos.180257
41. Bigna JJ, Marie A, Asangbeh SL, Kenne AM, Nansseu JR. “Seroprevalence of hepatitis C virus infection in Cameroon: a systematic review and meta-analysis”. BMJ Open. 2017;7:e015748. doi:10.1136/bmjopen-2016-015748
42. Kouyoumjian SP, Chemaitelly H, Abu-Raddad LJ. Characterizing hepatitis C virus epidemiology in Egypt: systematic reviews, meta-analyses, and meta-regressions. Sci Rep. 2018;8:1661. doi:10.1038/s41598-017-17936-4
43. Abebe M, Alemnew B, Biset S. Prevalence of hepatitis B virus and hepatitis C virus among blood donors in nekemte blood bank, Western Oromia, Ethiopia: retrospective 5 years study. J Blood Med. 2020;11:543–550. doi:10.2147/JBM.S282099
44. Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17:107–115. doi:10.1111/j.1469-0691.2010.03432.x
45. Bigna JJ, Kenne AM, Hamroun A, et al. “Gender development and hepatitis B and C infections among pregnant women in Africa: a systematic review and meta-analysis”. Infect Dis Poverty. 2019;8:16. doi:10.1186/s40249-019-0526-8
46. Te HS, Jensen DM. Epidemiology of hepatitis B and C viruses: a global overview. Clin Liver Dis. 2010;14(1):1–21. doi:10.1016/j.cld.2009.11.009
47. Malhotra R, Soin D, Grover P, Galhotra S, Khutan H, Kaur N. Hepatitis B virus and hepatitis C virus coinfection in hemodialysis patients: a retrospective study from a tertiary care hospital of North India. J Nat Sci Biol Med. 2016;7(1):72–74. PMID: 27003974. Pubmed Central PMCID: PMC4780172. doi:10.4103/0976-9668.175076
48. World Health Organization. Combating hepatitis B and C to reach elimination by 2030: advocacy brief. World Health Organization; 2016.
49. Taye M, Daka D, Amsalu A, Hussen S. Magnitude of hepatitis B and C virus infections and associated factors among patients scheduled for surgery at Hawassa University comprehensive specialized Hospital, Hawassa City, southern Ethiopia. BMC Res Notes. 2019;12(412). doi:10.1186/s13104-019-4456-0
50. Kedir S, Ahmed N, Kebede S, Getahun M, Arega T, Abdi AM. Prevalence of hepatitis B and C viruses infections among hemodialysis patients in Addis Ababa, Ethiopia. J Int Nephrol. 2018;1(1):08–14.
51. Javed K, Ishrat Z, Sultana N. Hepatitis C is more common than hepatitis B among surgical patients and previous surgery is the most common risk factor. Blood Transfus. 2006;50(4):32.
52. European Centre for Disease Prevention and Control. Hepatitis B and C Epidemiology in Selected Population Groups in the EU/EEA. Stockholm: ECDC; 2018.
53. Ford DA. Implementing AORN recommended practices for sharps safety. AORNJ. 2014;99:106–120. doi:10.1016/j.aorn.2013.11.013
54. Kumar KPS, Bhowmik D. Jaundice-review of clinical features, differential diagnosis and remedies. Pharm Res. 2010;4(2):241–252.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
