Back to Journals » Journal of Hepatocellular Carcinoma » Volume 11
Treatment Patterns and Recommendations for Improving the Management of Hepatocellular Carcinoma in Saudi Arabia
Authors Alolyan A, Alshammari K, Arabi M, Alshehri A, Alsuhaibani H, Ibnshamsah F, Alsharm A, Mahrous M, Al Zanbagi A, Hassanain M, Bazarbashi S
Received 1 November 2023
Accepted for publication 17 January 2024
Published 16 February 2024 Volume 2024:11 Pages 349—362
DOI https://doi.org/10.2147/JHC.S442842
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr David Gerber
Ashwaq Alolyan,1 Kanan Alshammari,1 Mohammad Arabi,1 Ahmed Alshehri,2 Hamad Alsuhaibani,3 Fahad Ibnshamsah,4 Abdullah Alsharm,5 Mervat Mahrous,6,7 Adnan Al Zanbagi,8 Mazen Hassanain,9 Shouki Bazarbashi10
1Department of Medical Oncology, Ministry of National Guard Health Affairs, Riyadh, Saudi Arabia; 2Department of Oncology, King Khalid National Guard Hospital Abdulaziz Medical City, Jeddah, Saudi Arabia; 3Department of Radiology, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia; 4Department of Medical Oncology, King Fahad Specialist Hospital, Dammam, Saudi Arabia; 5Department of Medical Oncology, King Fahad Medical City, Riyadh, Saudi Arabia; 6Department of Oncology, Prince Sultan Military Medical City Hospital, Riyadh, Saudi Arabia; 7Department of Medicine, Minia University of Egypt, Faculty of Medicine, Minia, Egypt; 8Department of Gastroenterology and Hepatology, King Abdullah Medical City, Makkah, Saudi Arabia; 9Department of Surgery, King Saudi University, Riyadh, Saudi Arabia; 10Department of Oncology, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia
Correspondence: Shouki Bazarbashi, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia, Email [email protected]
Abstract: Hepatocellular carcinoma (HCC) is the sixth most common type of cancer in the world associated with high morbidity and mortality. Despite being a significant healthcare burden there is limited information on the unmet needs and current treatment practices for intermediate and advanced-stage HCC in Saudi Arabia. This article analyzes the gaps and provides expert consensus on the management strategies for unresectable HCC in Saudi Arabia. A pre-meeting online questionnaire, comprising 20 objective questions about the treatment landscape and diagnosis of HCC in Saudi Arabia, was distributed to experts in the field of HCC management. An advisory board meeting including a panel of 13 experts was held in September 2022 where the responses to the survey questionnaire were reviewed and discussed. The survey results and experts’ discussion highlighted the growing incidence of liver cancer in Saudi Arabia. HCC comprised the majority of all liver cancer cases due to rising rates of chronic viral infections and lifestyle-related risk factors. Most physicians in Saudi Arabia follow the Barcelona Clinic Liver Cancer guidelines as a prognostic tool for the detection and staging of patients with HCC. Most of the patients with HCC in Saudi Arabia are diagnosed in the intermediate or advanced stages with poor prognoses and limited therapeutic options. Establishing evidence-based surveillance techniques, a multidisciplinary approach to diagnosis, and better accessibility of treatment options is vital for the management of HCC in Saudi Arabia.
Keywords: hepatocellular carcinoma, treatment patterns, multidisciplinary
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common type of cancer and the third most common cause of cancer-related mortality in the world.1,2 Risk factors for HCC vary according to geographic location and individual susceptibility. Major risk factors include chronic infection with hepatitis B (HBV) or hepatitis C (HCV) virus, and nonalcoholic fatty liver disease (NAFLD) which can be further categorized as nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH) based on histopathological criteria.3 While NAFL is generally nonprogressive and characterized by the presence of ≥5% hepatic steatosis without evidence of hepatocellular injury, NASH is characterized by the presence of >5% hepatic steatosis in conjunction with hepatocellular injury (such as hepatocyte ballooning) that often progresses to fibrosis, cirrhosis, and eventually HCC.3 Other risk factors for HCC include alcoholic liver disease, consumption of aflatoxins, obesity associated with metabolic syndrome, type 2 diabetes (T2D), and smoking.1,3,4
Temporal trends indicate a steady increase in the prevalence of liver cancer in the Arabian Gulf region with most cases reported from Saudi Arabia (83.4%) then Oman (6.6%), Kuwait (4.3%), Bahrain (2.4%), United Arab Emirates (1.8%), and Qatar (1.6%).5,6 According to the most recent Saudi Cancer Registry 2018, liver cancer accounts for 3.1% of all newly diagnosed cancer cases ranking eighth among men and fourteenth among women. HCC comprises more than three-fourth of the liver cancer cases in Saudi Arabia with an age-standardized incidence rate (ASIR) of 5.2 per 100,000 people as compared with an ASIR of 4.7 per 100,000 people for the Western Asia region.1,2,7–10
The major obstacle to early diagnosis and effective treatment of patients with HCC in Saudi Arabia is the fact that most patients with HCC remain asymptomatic until advanced stage.4 As such, screening for high-risk individuals is imperative for early identification. However, there is a dearth of information on the epidemiological factors, clinicopathological characteristics, and screening practices for high-risk individuals in Saudi Arabia. Additionally, information on the management, referral, and follow-up of patients with intermediate and/or advanced-stage HCC is also lacking. To address these gaps, an advisory board meeting (ABM) was convened on 30 September 2022 with a panel of 13 expert medical oncologists, hepatologists, surgeons, and interventional radiologists who were invited to review the current practices of screening, diagnosis, and treatment for HCC and discuss the challenges and unmet needs for the management of HCC in Saudi Arabia. A pre-meeting online survey comprising 20 multiple-choice questions about the treatment landscape and diagnosis of HCC in Saudi Arabia was administered to the key external experts and responses were collected between 10 September 2022 and 28 September 2022, followed by a descriptive analysis of the data. The results of the survey were summarized and presented before the experts during the ABM. The experts discussed HCC in Saudi Arabia under the following broad topics: (1) epidemiology and risk factors, (2) screening and diagnosis, and (3) available treatment options. This expert opinion paper describes the discussions in the ABM, along with a comprehensive literature review on the topic, and sets the foundation for analyzing gaps and providing recommendations for practicing physicians, regulators, stakeholders, and decision-makers to improve the screening practices and management of unresectable HCC in Saudi Arabia.
Epidemiology and Risk Factors for HCC in Saudi Arabia
The epidemiology of HCC in Saudi Arabia is evolving with trends reflecting differing exposure to “traditional” (viral hepatitis) and “new” (NAFLD) risk factors and differences in surveillance.11 In our expert survey results, while HCV (32%) infection was considered the primary risk factor for HCC in Saudi Arabia, NAFLD and NASH were a close second (30%) (Figure 1A). The results were reflective of earlier studies where viral-associated HCC was responsible for majority (77%) of the deaths due to HCC and was comparable to the global average.5 The pathophysiology of viral-associated HCC includes liver inflammation, oxidative stress, and deregulation of cell signaling pathways. While HCV promotes cellular proliferation, steatosis, inflammatory processes, mitochondrial dysfunction, and insulin resistance, all leading to oxidative stress, genetic instability, and DNA damage, with liver cirrhosis and HCC as the likely outcomes, HBV infection is more oncogenic due to the integration of the viral and cellular DNA resulting in persistence of the virus despite virological suppression by nucleotide analogs.4,11,12 Thus, individuals with chronic HBV infection are at higher risk of developing end-stage liver disease, including cirrhosis, liver failure, and HCC.4,11,12 Patients with chronic HBV infection may develop HCC even without liver cirrhosis while those with advanced chronic liver disease remain at risk of HCC despite virological suppression.4,11,12 Multiple scores have been developed in patients with chronic HBV infection and may be used to stratify an individual’s risk of developing HCC.11,12
Our survey results showed that HBV infection was responsible for about 26% of HCC cases in Saudi Arabia, which was an overestimation as per the experts’ opinion since the number of HBV-related HCC cases had reduced in the region following the initiation of the universal childhood HBV vaccination program in 1989. After the vaccination program, the HBV prevalence reduced from 6.7% in 1989 to 0.3% in 1997 and 0% in 2008.13 However, since the initial epidemiological studies showing a high prevalence of HBV were conducted on children who are now adults, it is reasonable to assume that about 20% of these individuals will probably develop cirrhosis with an annual risk of 1% to 4% for HCC.9,13 Additionally, the prevalence of HCV infection in Saudi Arabia ranges from 0.4% to 1.1% with an average prevalence of around 0.3% thereby further increasing the cases of HCC and related morbidity and mortality.13–15 Between 1996 and 2006, there has been 437,000 confirmed HCV cases in Saudi Arabia, estimated to increase to 103,000 cases by 2030, with a corresponding increase in HCC, decompensated and compensated liver cirrhosis, and liver‑related mortality.16,17 Individuals with HBV and HCV co-infection have a worse prognosis than infection with either virus alone as it is associated with more comorbidities, increased severity of liver disease, and a higher risk of HCC.5
The rise in HCC cases in Saudi Arabia is also attributed to the growing incidence of obesity and T2D.7,18 Obesity is an etiological factor for NAFLD.5,18 Obesity associated with T2D in patients infected with HCV increases the risk of HCC up to 100-fold.19 It is estimated that approximately 12 million individuals will be diagnosed with NAFLD in Saudi Arabia by 2030 with a 3-fold increase (580 to 1790) in NAFLD-related HCC cases and an annual incidence of HCC‑related deaths of 4800.7,13,20–24 Furthermore, with the reduced burden of viral hepatitis among the resident nationals, NASH is likely to be the leading cause of liver transplantation (LT) in Saudi Arabia due to the rapidly increasing obesity rates.5 Prevalence of NASH is expected to rise 96% by 2030.3,25 Additionally, the percentage of patients with F3/F4 fibrosis or advanced liver disease (decompensated cirrhosis or HCC) is anticipated to increase to 21.8% (13.5% in 2017) of the NASH cases in Saudi Arabia.3,25 Thus, early diagnosis involving routine screening protocols in high-risk patients is crucial for managing the additional demands on the healthcare system.
Screening and Diagnosis of HCC in Saudi Arabia
The prognosis of individual patients with HCC is dependent not only on the etiology of the tumor but also on the degree of functional failure of the liver due to the presence of cirrhosis.26,27 The 2022 Barcelona Clinic Liver Cancer (BCLC) guidelines highlighted the different concepts and parameters that physicians and multidisciplinary tumor boards should integrate for a personalized approach to treating patients with HCC.27 According to the BCLC 2022 guidelines, the Child-Pugh score, albumin-bilirubin (ALBI) score, and alpha-fetoprotein (AFP) levels may be used for the classification of HCC diagnosed using non-invasive radiological/imaging techniques (computerized tomography [CT] or magnetic resonance imaging [MRI]) and/or laboratory investigation with or without a liver biopsy.27,28 While decompensated liver cirrhosis emulated non-preserved liver function irrespective of the Child-Pugh score, compensated liver cirrhosis could be classified with additional granularity by using the ALBI score along with AFP concentration irrespective of the tumor burden.27,29,30 The BCLC stratified patients with HCC into BCLC-A, B, C, and D stages where stage D indicated terminal disease.5,27 Liver function for early stage (BCLC-A) solitary HCC was stratified as per the degree of portal hypertension indicating a higher rate of postoperative complications and lower long-term survival.
The American Association for the Study of Liver Diseases (AASLD) recommended the surveillance of adults with cirrhosis with follow-up ultrasound every 3 to 6 months with or without AFP.31 The AASLD also recommended diagnostic evaluation for HCC with either multiphase CT or multiphase MRI for nodules >1 cm and routine biopsy for indeterminate nodules (≤1 cm).31 The surveillance of patients with Child-Pugh C cirrhosis was not recommended by the AASLD unless they were on the transplant waiting list due to the low anticipated survival for these patients.31 Similar surveillance and diagnostic recommendations as the AASLD were made by the European Association for the Study of the Liver (EASL) and the Asia-Pacific Association for the Study of Liver.32,33 The Saudi Association for the Study of Liver Diseases and Transplantation (SASLT) practice guidelines are commonly used for the diagnosis in the Saudi population (Figure 2). To identify the HCC prevalence in Saudi Arabia, provide a definitive diagnosis, evidence-based management, and proper referral of HCC patients, the SASLT recently evaluated and updated the previously published guidelines by the Saudi Gastroenterology Association which were endorsed by the Saudi Oncology Society as its official HCC guidelines.13 During the discussion, most of the experts acknowledged depending on the SASLT guidelines for the management of HCC as these were evidence-based and specific for the Saudi Arabia region.
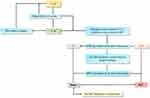 |
Figure 2 Algorithm for HCC diagnosis. Abbreviations: APHE, arterial phase hyperenhancement; CT, computed tomography; HCC, hepatocellular carcinoma; MRI, magnetic resonance imaging; USG, ultrasonography. Notes: Adapted from Alqahtani S, Sanai F, Alolayan A, et al. Saudi Association for the Study of Liver diseases and Transplantation practice guidelines on the diagnosis and management of hepatocellular carcinoma. Saudi J Gastroenterol. 2020;26(7):1. Creative Commons.7 |
The majority (82%) of the experts used the BCLC criteria for identifying the stage of patients with HCC and opined that most patients (44%) presented with advanced-stage HCC while approximately 26% presented with intermediate-stage HCC (Figure 1B). Since most patients presented with advanced or intermediate-stage disease, there were limited curative options available. Patients with intermediate-stage HCC, categorized as BCLC-B, are further classified into three groups according to tumor burden and liver function as per BCLC 2022.5,27,34
Available Treatment Options for HCC in Saudi Arabia
Curative Surgical Therapy in HCC Patients
Curative surgical therapies including liver resection and LT are recommended as first-line treatment in early-stage HCC patients (BCLC stage 0 and A) showing a 5-year survival of ~70–80%.26 However, according to the Milan criteria, several factors like performance status, liver function, portal pressure, and tumor characteristics (single tumor ≤5 cm or 2–3 tumors ≤3 cm without vascular invasion) are considered prior to resection or transplantation.26 The University of California in San Francisco (UCSF) criteria expanded the Milan criteria to include patients with a single nodule ≤6.5 cm or ≤3 nodules with the largest ≤4.5 cm and total sum of diameters ≤8 cm.7,35 For multifocal HCC lesions of ≤3 nodules, LT showed better outcomes as ablation and resection increase the risk of HCC recurrence.27 In addition to the Milan and UCSF criteria, the up-to-seven criteria further expanded the criteria for LT in patients with HCC by including a cutoff score of seven calculated by considering the total number of lesions plus the diameter (in cm) of the largest nodule.7,36 The up-to-seven score proved to be inadequate in patients with microvascular invasion, which limits its potential use in clinical practice.7,36 Another noteworthy method to select HCC candidates for LT combines the total tumor volume (TTV) with the AFP level. In a prospective study, it was shown that patients with a TTV ≤115 cm3 and an AFP level ≤400 ng/mL, without macrovascular invasion or extrahepatic disease could be safely selected for LT.37 Since most patients with HCC are diagnosed at the intermediate or advanced stages in Saudi Arabia, the majority of the experts (46%) agreed that curative surgical resection or LT could be recommended only in <5% of patients. Although less stringent options for LT have been proposed, there is no consensus on the expanded criteria for LT in patients with HCC. Additionally, lack of transplantation donors, sub-optimal donor management, the lack of deceased organ donors due to religious and cultural factors, and infections due to multi-drug resistant bacteria are the common difficulties faced during LT. In patients who do not meet the Milan criteria, a rescue LT after tumor down-staging, utilizing systemic therapy or locoregional therapy can enhance long-term outcomes.13,38–40 Hepatic resection is the treatment of choice for HCC patients without cirrhosis or with well-compensated cirrhosis. In patients with cirrhosis, it is recommended for single HCC tumors between 2 and 5 cm, and with sufficient liver volume.7 Suitability for resection is determined by assessing liver function, the extent of resection, and the presence or absence of major vascular invasion.
Locoregional Therapy in Patients with HCC
Locoregional treatment options include percutaneous ethanol ablation (PEA), radiofrequency ablation (RFA), microwave ablation (MWA), transarterial chemoembolization (TACE), transarterial radioembolization (TARE), cryoablation, and radiotherapy.13,27 (Figure 3)
Ablation Techniques
Ablation is a widely used therapy for early-stage HCC patients. It is often recommended in patients who are unsuitable for surgical therapies due to the progression of the disease, poor hepatic reserve, and co-morbidities. The most commonly preferred ablation techniques are PEA, RFA, and MWA.13,26 In specific instances where the tumor is inaccessible or unsuitable for procedures like RFA or MWA, physicians may opt for PEA. The use of PEA is less frequent compared to MWA or RFA.7,13 Radiofrequency ablation is considered a first-line therapy for early-stage HCC patients for tumors <2 cm and as an alternative to surgery for tumors of 3–4 cm or 2–3 tumors of <3 cm. As per the modified response evaluation criteria in solid tumors, complete response rate ranges from 70% to 90% which is significantly associated with better overall survival (OS).26 Studies have shown that the median OS with RFA was approximately 60 months and a 5-year recurrence rate was 50–70%.26 RFA is very effective with low recurrence rates and demonstrated superiority to PEI in objective response rates (ORR) and OS. MWA can achieve a larger ablation area than RFA as multiple needles can be used simultaneously.13,20 It is considered in early-stage HCC patients with lesions <4 cm.26,27 Studies have shown that MWA showed similar efficacy but higher complication rates in tumors >3 cm compared with RFA.26 While the OS, disease-free survival, and tumor recurrence are significantly better with resection, MWA can be an effective and safe alternative to liver resection for tumors that are not suitable for resection.13
TACE for Patients with HCC
Treating intermediate-stage HCC typically involves the utilization of TACE as the standard approach.7,26 Data from a trial on 112 patients randomized to arterial embolization only, TACE, or control treatment, TACE showed a survival benefit of 62% at 2 years versus 50% in the arterial embolization arm and 27% in the control arm.13,41 In a similar study among 80 Asian patients, TACE improved tumor response and showed significant improvement in 3-year survival compared with the control group (TACE 26% versus control 3%).13,41 Results from a meta-analysis of 7 randomized trials showed that TACE significantly reduced the 2-year mortality rate to 27% versus 41% in the control group.13,42 Another study has shown that median survival after TACE ranges from 16 to 45 months in the early stage (BCLC 0-A), 15.6 to 26.3 months in the intermediate-stage (BCLC B), and 6.8 to 13.6 months in the advanced stage (BCLC C) HCC.43 A retrospective study conducted on the Saudi population showed that survival with TACE at 1, 2, and 3 years was 69.2%, 41%, and 23%, respectively.44 TACE as a neoadjuvant therapy helps to reduce tumor progression of patients who are on the waiting list for LT (bridging therapy) or to downstage the tumor burden to within the Milan criteria.44
Guidelines recommend TACE as a first-line therapy for patients with intermediate-stage HCC and non-resectable HCC leading to a median survival of ≥2.5 years. It is also used as a palliative therapy.31–33 However, TACE is not recommended in advanced-stage HCC patients, main portal vein thrombosis, in porto-systemic shunts, either surgical or intrahepatic cases.13 As per the Asia-Pacific Primary Liver Cancer Expert 2019 Consensus, TACE is not suitable for three types of patients: (i) conditions that easily become refractory to TACE, (ii) conditions causing TACE to worsen the hepatic functional reserve in Child-Pugh class B, and (iii) conditions when tumors do not respond to TACE (TACE-resistant tumor).45,46 (Figure 4)
Two TACE techniques are mainly used in clinical practice: conventional TACE (cTACE) and TACE with drug-eluting beads (DEB-TACE).45 Studies have shown that DEB-TACE was non-inferior to cTACE and associated with a better ORR and disease control with fewer severe complications and all-cause mortality.13,26,45,47
As per the survey results, TACE was mostly used for small and, intermediate-sized (<8 cm) lesions irrespective of the number of lesions. Most of the experts (50%) seemed confident about repeating TACE at least twice while 30% agreed that it was safe to repeat TACE for three or more times. TACE was repeated depending on size of the tumor. The experts had differing opinions regarding the ideal interval before repeating TACE; 46% recommended <3 months as a minimum interval between repeated TACE, and 45% preferred 3–6 months. Data shows that only a few patients with HCC obtain complete responses after the first TACE treatment and most patients require repeated TACE therapy.7,27,45,48 There is no particular evidence about the optimal number of TACE treatments or predefined time intervals between the TACE sessions.7 However, based on the initial treatment outcomes, TACE can be repeated with a 1‑ or 2‑month gap between sessions.7,30 Suitable candidates for repeat TACE are identified based on several scores developed considering the changes in functional liver variables before and after TACE. These scores stratify candidates by anticipated survival, similar to those designed for identifying candidates for the first TACE.49 The EASL guidelines recommend that TACE should be ceased in cases where considerable necrosis of the tumor is not achieved after repeated TACE therapies.45 Several studies also suggest that repeated TACE therapy can damage liver function. Switching to systemic therapy helps to preserve liver function and improves ORR and OS.46 TACE can be considered for interdisciplinary treatment as it can be used in combination with other therapies like radiation therapy, PEI, RFA, and systemic therapy.48 TACE is accessible in most tertiary large centers of Saudi Arabia. However, there is a lack of trained interventional radiologists who can perform TACE.13
TARE in Patients with HCC
Transarterial radioembolization is often recommended for patients with intermediate-stage HCC. TARE indications are similar to TACE but unlike TACE, it can be safely used in patients with portal vein thrombosis.7,50,51 Based on this advantage, the SASLT guidelines recommend using TARE as an alternative to TACE for the treatment of intermediate HCC if associated with portal vein thrombosis.7 Also, the experts opined that TARE had the potential to be a bridging therapy for transplantation and also for down-staging the tumor before resection or transplantation therapy.50–53 Results from the TRACE and Premiere trials showed that TARE provided significantly longer progression free survival, better tumor control, and could reduce drop-out from transplant waitlists.54,55 In the SIRTACE trial, patients with unresectable HCC treated with either TACE or TARE showed similar efficacy outcomes. A retrospective study demonstrated a 2‑year OS advantage for TARE (59%) over TACE (47%) in patients with BCLC stage B‑C HCC.7
Radiation Therapy
The role of radiotherapy in the treatment of HCC has undergone significant evolution, driven by technological advancements and improved imaging techniques.56 It has been proven valuable particularly in cases of unresectable HCC, demonstrating high local control rates, even in instances with major vascular involvement. In case of metastatic HCC, radiotherapy plays a pivotal role in providing effective palliation, contributing to the management of symptoms and improving the quality of life for patients facing advanced disease stages.57 Furthermore, it offers as an alternative in situations where minimally invasive procedures like TACE (not recommended in case of malignant portal vein thrombosis, untreatable arteriovenous fistula, and impaired renal function), RFA, or MWA (to be avoided in patients with bleeding diathesis) may be contraindicated.57
Hepatic Arterial Infusion Therapy
Hepatic arterial infusion therapy (HAIC) is another locoregional treatment that uses a catheter technique to directly administer anti-cancer drugs into tumors through the hepatic artery. It has gained prominence particularly in East Asia as an effective treatment for HCC, for patients with more than four tumors and those who do not respond to TACE.58 Recent studies have shown superiority of HAIC over sorafenib in treating HCC with macroscopic vascular invasion.59,60 Moreover, patients with advanced HCC and portal vein tumor thrombosis demonstrated significantly longer OS (14.9 vs 2.7 months) and time-to-progression (7.2 vs 4.4 months) with HAIC compared to sorafenib.61 These findings indicate HAIC as a promising treatment strategy in HCC management.
Systemic Therapies
The experts’ opinion and survey results showed that patients with intermediate-stage HCC refractory to TACE and with a short time to TACE progression were the most eligible for systemic therapy followed by patients with intermediate-stage HCC (Child-Pugh A/B7, BCLC-B) (Figure 5A). However, the majority of experts agreed that systemic therapy was not to be recommended for patients with Child-Pugh C. The current systemic therapy landscape for intermediate and advanced-stage HCC in Saudi Arabia includes tyrosine kinase inhibitors (TKIs), vascular endothelial growth factor (VEGF) inhibitors, immune checkpoint inhibitors (ICIs), or a combination of two or more of these or with conventional chemotherapeutic agents.62 Currently, ICIs are the recommended first-line standard of care in Saudi Arabia followed by TKIs for patients ineligible to receive ICIs (Figure 5B). In a Phase 3 randomized (IMbrave 150) trial, the combination of the programmed cell-death ligand 1 (PDL-1) inhibitor atezolizumab and the anti-VEGF bevacizumab was found to improve 12 months’ OS and median progression free survival (PFS) versus sorafenib (OS: 67.2% vs 54.6%; PFS: 6.8 months vs 4.3 months).63 Following the positive safety and efficacy findings from the IMbrave150 trial, atezolizumab plus bevacizumab became the first-line treatment option for patients with unresectable HCC in Saudi Arabia.62–65 However, bevacizumab has the potential to increase the risk of hemorrhage, particularly in patients with HCC who often have cirrhosis, portal hypertension, or both, associated with esophageal or gastric varices and portal gastropathy.5,64–66 Studies have shown that patients most likely to have high-risk varices are those with decompensated cirrhosis, a platelet count ≤150x103/mm3, and liver stiffness ≥20 kPa (determined by transient elastography).65,67,68 Consequently, most of the experts (>75%) recommended upper gastrointestinal endoscopy within 6 months from study entry to assess the risk of hemorrhage from varices in all patients with HCC prior to initiation of atezolizumab plus bevacizumab therapy in line with published guidelines (Figure 5C). Additionally, atezolizumab could be immunogenic and induce undesirable anti-drug antibody (ADA) responses.63,69 A study by Kim et al on 132 patients with advanced HCC and high ADA levels (≥1000 ng/mL) at 3 weeks showed poor clinical outcomes. Compared with patients with low ADA levels, patients with high ADA levels exhibited reduced systemic exposure to atezolizumab which may interfere with drug efficacy, drug clearance and serum concentration, and/or induce antibody neutralization.70 Safety results from the IMbrave150 trial showed that adverse events (AEs) of particular relevance to atezolizumab (hepatitis, rash, hypothyroidism, infusion-related reaction, hyperthyroidism, pancreatitis, and T2D) occurred in 69% of patients receiving atezolizumab plus bevacizumab and in 82% of patients receiving sorafenib.63,69 Conversely, AEs of particular relevance to bevacizumab (hypertension, proteinuria, hemorrhagic as well as venous and arterial thromboembolic events) occurred in 58% of patients receiving atezolizumab plus bevacizumab and 49% of patients receiving sorafenib.64–66,71 Thus, prescribing physicians need to consider several important risks prior to initiating atezolizumab plus bevacizumab combination therapy in relation to an individual patient’s characteristics.
The HIMALAYA trial demonstrated the superiority of the STRIDE regimen (single tremelimumab [anti-cytotoxic T-lymphocyte–associated antigen-4; CTLA-4]) regular interval durvalumab [anti-PDL-1]; hazard ratio [HR], 0.78; 95% confidence interval [CI], 0.65–0.92; p = 0.0035) and non-inferiority of durvalumab monotherapy (HR, 0.86; 95.67% CI, 0.73–1.03) in terms of efficacy and safety versus sorafenib as the first-line treatment of patients with unresectable HCC.72–74 HIMALAYA was the first successful dual-immuno-oncology study in the treatment of unresectable HCC setting with median overall survival (mOS) for STRIDE 16.43 months (95% CI, 14.16–19.58) versus 13.77 months (95% CI, 12.25–16.13) for sorafenib.72 Survival rates at 36 months were 30.7% and at 48 months were 25.2% for the STRIDE regimen versus 20.2% and 15.1% respectively, for sorafenib. Durvalumab monotherapy was non-inferior to sorafenib with a mOS of 16.56 months (95% CI, 14.06–19.12).72,75 The data reinforce the sustained, long-term survival benefit of STRIDE regimen versus sorafenib with the unprecedented 3- and 4-year survival rates and the longest follow-up to date among phase 3 studies in unresectable HCC.75 Additionally, the incidence, frequency, and severity of the AEs for STRIDE and durvalumab were consistent with the known safety profiles of each agent, and no new safety signals were identified.75 Any grade treatment-related AEs (TRAEs) for the STRIDE, durvalumab, and sorafenib arms were 75.8%, 52.1%, and 84.8%, respectively. Grade 3/4 TRAEs occurred for 50.5% of patients with STRIDE, 37.1% with durvalumab, and 52.4% with sorafenib. Serious TRAEs were reported in 17.5% patients in STRIDE arm, 8.2% in durvalumab arm and 9.4% in sorafenib arm. TRAEs leading to death were 2.3% in STRIDE arm and 0.8% in sorafenib arm.72,74 The frequency and severity of grade 3/4 immune-mediated TRAEs, immune-mediated AEs requiring treatment with high-dose steroids, and immune-mediated AEs leading to discontinuation of treatment were higher in the STRIDE arm compared with durvalumab but did not raise any concerns about tolerability. The risk of treatment-related bleeding from gastroesophageal varices was considerably less with the STRIDE regimen due to the absence of anti-angiogenesis agents thus eliminating the pre-requisite of upper esophageal endoscopy prior to initiation of treatment.72,74 The HIMALAYA STRIDE regimen did not show the AEs such as proteinuria, hypertension, ascites, or encephalopathy associated with combination therapies with ICIs plus anti-VEGF/TKI related to the effect of molecular targeted agents.74 Most of the experts were willing to prescribe the HIMALAYA STRIDE regimen as first-line therapy for patients with unresectable HCC specifically since the STRIDE regimen had fewer restrictions for patient selection and could be used for treating a diverse patient population in addition to those ineligible for atezolizumab plus bevacizumab treatment that is mostly recommended for patients with Child-Pugh A. However, there is lack of data on the efficacy and outcomes of the HIMALAYA regimen among the Saudi population. Additionally, there is a lack of information on post-progression treatment after first-line therapy with atezolizumab plus bevacizumab or with the STRIDE regimen. A multidisciplinary approach may be the key to effective patient management and formulating an individualized treatment plan for patients with unresectable HCC in Saudi Arabia.76
The experts opined that patients with unresectable HCC in Saudi Arabia who were ineligible for atezolizumab plus bevacizumab, durvalumab plus tremelimumab, or durvalumab monotherapy, received TKIs sorafenib or lenvatinib as first-line therapy. In this regard, the selection of the appropriate option depended on careful analysis of the clinical, radiological, and biochemical profile of the patients, so that they fit into the target population enrolled in the trials where safety and efficacy were demonstrated. Thus, a personalized therapy for HCC recurrence post-LT was recommended with no evidence supporting the use of sorafenib in patients with disseminated HCC recurrence.77 The differences in outcomes among HCC patients who received sorafenib are often associated with their underlying hepatitis virus etiology at presentation.46,78–80 Our survey results showed that sorafenib was prescribed to only a quarter of patients ineligible for atezolizumab plus bevacizumab (Figure 5C). Lenvatinib was approved for advanced-stage HCC based on results from the REFLECT trial. Subgroup analyses on Asian patients have shown that lenvatinib was particularly efficacious in patients with underlying HBV infection, and high AFP serum concentrations (>200 ng/mL).78,81–83 TKIs, specially sorafenib, result in AEs that limit their clinical benefits in patients with HCC in Saudi Arabia. Although these toxicities are rarely associated with major morbidity or mortality, the complex molecular pathogenesis of HCC stimulated the investigation of combinations of sorafenib and other TKIs with molecular targeting drugs.84
Recent trials evaluated the efficacy of TKIs as second-line therapy in patients with advanced HCC who progressed on prior sorafenib as first-line treatment. The SASLT recommends regorafenib, cabozantinib, and ramucirumab are to be considered in this setting.14,67 Regorafenib is recommended for patients who progress after first-line treatment with sorafenib without any major safety concerns with studies demonstrating a clinical benefit regardless of the last dose, or the time-to-progression on sorafenib.85 The combination of nivolumab plus ipilimumab is recommended by the SASLT to be considered as second-line therapy for patients with HCC (Child-Pugh A) who were previously treated with sorafenib.22 It has been recently reported that metronomic capecitabine had anti-tumor efficacy in patients with advanced-stage HCC and liver cirrhosis after sorafenib failure.86 Subgroup analyses have shown that lenvatinib is particularly efficacious in Asian patients with underlying HBV infection and high AFP serum concentrations (>200 ng/mL).78,83 The search for more effective treatment of HCC continues, with several trials ongoing that combine an ICI with another agent such as a TKI or anti-VEGF agent. Patients with end-stage HCC were recommended to receive palliative support by the SASLT including management of pain, nutrition, and psychological support.7
Conclusion
HCC remains a devastating disease that has a ubiquitous and enormous impact on healthcare systems across Saudi Arabia considering the high prevalence of viral infections and lifestyle-related risk factors. Most of the patients with HCC are diagnosed in the intermediate or advanced stages with poor prognoses and limited therapeutic options. Only about half of the patients diagnosed with HCC qualify for systemic treatment, while the rest receive the best supportive care. Establishing evidence-based surveillance techniques, a multidisciplinary approach to diagnosis, and better accessibility of treatment options is vital for the management of patients with HCC in Saudi Arabia. Although there is an improved understanding of the HCC management guidelines, targeted trials need to be conducted specifically on the Saudi population to enable in-depth exploration of the association between HCC risk factors, prevalence, and the development of evidence-based strategies for improved patient outcomes. The currently available treatment options for unresectable HCC present opportunities to extend survival. Specifically, with the unprecedented 3- and 4-year survival rates seen for the STRIDE regimen, additional studies on the benefits of these therapies in the Saudi population is warranted.
Ethics Statement
Ethical approval was not required as this is an expert opinion paper.
Acknowledgments
Medical writing support was provided by Dr. Debasri Mukherjee and Dr. Chinmayee Joshi of Fortrea Scientific Pvt Ltd (formerly Labcorp Scientific Services & Solutions Pvt. Ltd) funded by AstraZeneca FZ LLC in accordance with GPP 2022 guidelines.
Author Contributions
All authors contributed equally to the conceptualization, literature review, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, reviewing and editing the manuscript. All the authors have read and approved the final version of the manuscript to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The preparation of this consensus manuscript and funding of the journal’s article processing charges was supported by AstraZeneca FZ LLC.
Disclosure
Dr Ashwaq Alolyan, Dr Kanan Alshammari, Dr Ahmed Alshehri, Dr Hamad Alsuhaibani, Dr Fahad Ibnshamsah, Dr Abdullah Alsharm, Prof Mervat Mahrous, Dr Adnan Al Zanbagi, Dr Mazen Hassanain declare no conflicts of interest in this work. Prof Shouki Bazarbashi received research support from Pfizer, BMS, Bayer, and Sanofi; speaker honorarium from Lilly, Roche, AstraZeneca, Merck Serono, Newbridge, Janssen, BMS, and Servier; and is an advisor to Roche, IPSEN, Astellas, AstraZeneca, Amgen, Bayer and Servier. Dr Mohammad Arabi reports personal fees from Sirtex Medical and travel expenses from Boston Scientific, outside the submitted work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. GLOBOCAN2020 I. Liver Fact Sheet 2020; 2020. Available from: https://gco.iarc.fr/today/home.
3. Sanai FM, Abaalkhail F, Hasan F, Farooqi MH, Nahdi NA, Younossi ZM. Management of nonalcoholic fatty liver disease in the Middle East. WJG. 2020;26(25):3528–3541. doi:10.3748/wjg.v26.i25.3528
4. Ogunwobi OO, Harricharran T, Huaman J, et al. Mechanisms of hepatocellular carcinoma progression. WJG. 2019;25(19):2279–2293. doi:10.3748/wjg.v25.i19.2279
5. Albarrak J, Al-Shamsi H. Current status of management of hepatocellular carcinoma in the Gulf region: challenges and recommendations. Cancers. 2023;15(7):2001. doi:10.3390/cancers15072001
6. Al-Hamdan N, Ravichandran K, Al-Sayyad J, et al. Incidence of cancer in Gulf Cooperation Council countries, 1998–2001. East Mediterr Health J. 2009;15(3):600–611.
7. Alqahtani S, Sanai F, Alolayan A, et al. Saudi Association for the Study of Liver diseases and Transplantation practice guidelines on the diagnosis and management of hepatocellular carcinoma. Saudi J Gastroenterol. 2020;26(7):1. doi:10.4103/sjg.SJG_477_20
8. Al-Nemari A, Al-Rawaji A, Al-Zamzani A, Al-Alyani S, Al-Shhrani Z. Cancer Incidence Report In Kingdom of Saudi Arabia 2018. Saudi Health Council; 2018.
9. Althubiti M, Alfayez M. Insights on hepatocellular carcinoma in Saudi Arabia. In: Carr BI, editor. Liver Cancer in the Middle East. Springer International Publishing; 2021:247–257. doi:10.1007/978-3-030-78737-0_16
10. Globocan I Estimated age-standardized incidence rates (World) in 2020, liver, both sexes, all ages, Northern Africa, Central and Western Asia Hub (Izmir); 2020. Available from: https://gco.iarc.fr/today/online-analysis-map.
11. Russo FP, Zanetto A, Pinto E, et al. Hepatocellular carcinoma in chronic viral hepatitis: where do we stand? IJMS. 2022;23(1):500. doi:10.3390/ijms23010500
12. Zamor PJ, deLemos AS, Russo MW. Viral hepatitis and hepatocellular carcinoma: etiology and management. J Gastrointest Oncol. 2017;8(2):229–242. doi:10.21037/jgo.2017.03.14
13. Abdo AA, Hassanain M, AlJumah A, et al. Saudi guidelines for the diagnosis and management of hepatocellular carcinoma: technical review and practice guidelines: created and endorsed by the Saudi Association for the Study of Liver Diseases and Transplantation and the Saudi Oncology Society. Ann Saudi Med. 2012;32(2):174–199. doi:10.5144/0256-4947.2012.174
14. Aljumah A, Babatin M, Hashim A, et al. Hepatitis B care pathway in Saudi Arabia: current situation, gaps and actions. Saudi J Gastroenterol. 2019;25(2):73. doi:10.4103/sjg.SJG_421_18
15. Alhuraiji A, Alaraj A, Alghamdi S, Alrbiaan A, Alrajhi AA. Viral hepatitis B and C in HIV-infected patients in Saudi Arabia. Ann Saudi Med. 2014;34(3):207–210. doi:10.5144/0256-4947.2014.207
16. Aljumah AA, Kuriry H, AlZunaitan M, et al. Clinical presentation, risk factors, and treatment modalities of hepatocellular carcinoma: a single tertiary care center experience. Gastroenterol Res Pract. 2016;2016:1–9. doi:10.1155/2016/1989045
17. Muallem Y. Epidemiology of HCV in Saudi Arabia and it’s Burden on the Health Care System. Med Case Rep. 2022;8(12):257. doi:10.36648/2471-8041.8.12.257
18. Alswat K, Aljumah A, Sanai F, et al. Nonalcoholic fatty liver disease burden – Saudi Arabia and United Arab Emirates, 2017–2030. Saudi J Gastroenterol. 2018;24(4):211. doi:10.4103/sjg.SJG_122_18
19. Li X, Wang X, Gao P. Diabetes mellitus and risk of hepatocellular carcinoma. Biomed Res Int. 2017;2017:1–10. doi:10.1155/2017/5202684
20. Al-Judaibi B, Dokus MK, Al-Hamoudi W, et al. Saudi association for the study of liver diseases and transplantation position statement on the hepatology workforce in Saudi Arabia. Saudi J Gastroenterol. 2022;28(2):101–107. doi:10.4103/sjg.sjg_576_21
21. Shaaban A, Salamah R, Abo Elseud Y, Mohanty A, Albarrak J. Presentation and outcomes of hepatocellular carcinoma in the arabian peninsula: a review of a single institution experience in the Sorafenib Era. J Gastrointest Cancer. 2021;52(1):85–89. doi:10.1007/s12029-019-00341-7
22. Cancer incidence report Saudi Arabia 2014. Saudi Health Council Saudi Cancer; 2022. Available from: https://www.nhic.gov.sa/eServices/Documents/2014.pdf.
23. Rasul KI, Al-Azawi SH, Chandra P, Abou-Alfa GK, Knuth A. Status of hepatocellular carcinoma in Gulf region. Chin Clin Oncol. 2013;2(4):42. doi:10.3978/j.issn.2304-3865.2013.11.02
24. Alkhammash M, Bugis B. A comparison review of the clinical healthcare services provided to liver disease patients in the GCC. J Basic Clin Health Sci. 2021. doi:10.30621/jbachs.854253
25. Sanai FM, Al Khathlan A, Al Fadhli A, et al. Clinical and economic burden of nonalcoholic steatohepatitis in Saudi Arabia, United Arab Emirates and Kuwait. Hepatol Int. 2021;15(4):912–921. doi:10.1007/s12072-021-10182-x
26. Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6. doi:10.1038/s41572-020-00240-3
27. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
28. Attwa MH, El-Etreby SA. Guide for diagnosis and treatment of hepatocellular carcinoma. World J Hepatol. 2015;7(12):1632–1651. doi:10.4254/wjh.v7.i12.1632
29. Singal AG, Hoshida Y, Pinato DJ, et al. International Liver Cancer Association (ILCA) white paper on biomarker development for hepatocellular carcinoma. Gastroenterology. 2021;160(7):2572–2584. doi:10.1053/j.gastro.2021.01.233
30. Cabibbo G, Maida M, Genco C, et al. Natural history of untreatable hepatocellular carcinoma: a retrospective cohort study. World J Hepatol. 2012;4(9):256–261. doi:10.4254/wjh.v4.i9.256
31. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi:10.1002/hep.29086
32. Omata M, Cheng AL, Kokudo N, et al. Asia–Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317–370. doi:10.1007/s12072-017-9799-9
33. European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406–460. doi:10.1016/j.jhep.2018.03.024
34. Mehta N, Bhangui P, Yao FY, et al. Liver transplantation for hepatocellular carcinoma. Working group report from the ILTS Transplant Oncology Consensus Conference. Transplantation. 2020;104(6):1136–1142. doi:10.1097/TP.0000000000003174
35. Yao FY, Xiao L, Bass NM, Kerlan R, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 2007;7(11):2587–2596. doi:10.1111/j.1600-6143.2007.01965.x
36. Pommergaard HC, Rostved AA, Adam R, et al. Vascular invasion and survival after liver transplantation for hepatocellular carcinoma: a study from the European Liver Transplant Registry. HPB. 2018;20(8):768–775. doi:10.1016/j.hpb.2018.03.002
37. Toso C, Meeberg G, Hernandez-Alejandro R, et al. Total tumor volume and alpha-fetoprotein for selection of transplant candidates with hepatocellular carcinoma: a prospective validation. Hepatology. 2015;62(1):158–165. doi:10.1002/hep.27787
38. Wayne JD, Lauwers GY, Ikai I, et al. Preoperative predictors of survival after resection of small hepatocellular carcinomas. Ann Surg. 2002;235(5):722–731.
39. Slotta JE, Kollmar O, Ellenrieder V, Ghadimi BM, Homayounfar K. Hepatocellular carcinoma: surgeon’s view on latest findings and future perspectives. World J Hepatol. 2015;7(9):1168–1183. doi:10.4254/wjh.v7.i9.1168
40. Lau W, Ho SKW, Yu SCH, Lai ECH, Liew C, Leung TWT. Salvage surgery following downstaging of unresectable hepatocellular carcinoma. Ann Surg. 2004;240(2):299–305. doi:10.1097/01.sla.0000133123.11932.19
41. Llovet JM, Real MI, Montaña X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359(9319):1734–1739. doi:10.1016/S0140-6736(02)08649-X
42. Llovet J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37(2):429–442. doi:10.1053/jhep.2003.50047
43. Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. doi:10.1038/nrdp.2016.18
44. Helmi H, Alharthi F, Madkhali A, et al. Outcome of transarterial chemoembolization in patients with unresectable hepatocellular carcinoma. J Nat Sci Med. 2020. doi:10.4103/JNSM.JNSM_30_19
45. Müller L, Stoehr F, Mähringer-Kunz A, Hahn F, Weinmann A, Kloeckner R. Current strategies to identify patients that will benefit from TACE treatment and future directions a practical step-by-step guide. J Hepatocell Carcinoma. 2021;8:403–419. doi:10.2147/JHC.S285735
46. Kudo M, Han KH, Ye SL, et al. A changing paradigm for the treatment of intermediate-stage hepatocellular carcinoma: Asia-Pacific Primary Liver Cancer Expert Consensus Statements. LIC. 2020;9(3):245–260. doi:10.1159/000507370
47. Bzeizi KI, Arabi M, Jamshidi N, et al. Conventional transarterial chemoembolization versus drug-eluting beads in patients with hepatocellular carcinoma: a systematic review and meta-analysis. Cancers. 2021;13(24):6172. doi:10.3390/cancers13246172
48. Ghanaati H, Mohammadifard M, Mohammadifard M. A review of applying transarterial chemoembolization (TACE) method for management of hepatocellular carcinoma. J Family Med Prim Care. 2021;10(10):3553–3560. doi:10.4103/jfmpc.jfmpc_2347_20
49. D’Avola D, Granito A, de la Torre-Aláez M, Piscaglia F. The importance of liver functional reserve in the non-surgical treatment of hepatocellular carcinoma. J Hepatol. 2022;76(5):1185–1198. doi:10.1016/j.jhep.2021.11.013
50. Sacco R, Conte C, Tumino E, et al. Transarterial radioembolization for hepatocellular carcinoma: a review. JHC. 2016;3:25–29. doi:10.2147/JHC.S50359
51. Kallini JR, Gabr A, Salem R, Lewandowski RJ. Transarterial radioembolization with Yttrium-90 for the treatment of hepatocellular carcinoma. Adv Ther. 2016;33(5):699–714. doi:10.1007/s12325-016-0324-7
52. She WH, Cheung TT. Bridging and downstaging therapy in patients suffering from hepatocellular carcinoma waiting on the list of liver transplantation. Transl Gastroenterol Hepatol. 2016;1:34. doi:10.21037/tgh.2016.03.04
53. Byrne TJ, Rakela J. Loco-regional therapies for patients with hepatocellular carcinoma awaiting liver transplantation: selecting an optimal therapy. WJT. 2016;6(2):306. doi:10.5500/wjt.v6.i2.306
54. Salem R, Gordon AC, Mouli S, et al. Y90 radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2016;151(6):1155–1163.e2. doi:10.1053/j.gastro.2016.08.029
55. Dhondt E, Lambert B, Hermie L, et al. 90 Y radioembolization versus drug-eluting bead chemoembolization for unresectable hepatocellular carcinoma: results from the TRACE phase II randomized controlled trial. Radiology.2022;303(3):699–710. doi:10.1148/radiol.211806
56. Kalogeridi MA, Zygogianni A, Kyrgias G, et al. Role of radiotherapy in the management of hepatocellular carcinoma: a systematic review. World J Hepatol. 2015;7(1):101–112. doi:10.4254/wjh.v7.i1.101
57. Chen CP. Role of radiotherapy in the treatment of hepatocellular carcinoma. J Clin Transl Hepatol. 2019;7(2):183–190. doi:10.14218/JCTH.2018.00060
58. Torimura T, Iwamoto H. Treatment and the prognosis of hepatocellular carcinoma in Asia. Liver Int. 2022;42(9):2042–2054. doi:10.1111/liv.15130
59. Iwamoto H, Niizeki T, Nagamatsu H, et al. Survival benefit of hepatic arterial infusion chemotherapy over sorafenib in the treatment of locally progressed hepatocellular carcinoma. Cancers. 2021;13(4):646. doi:10.3390/cancers13040646
60. Yan L, Lin J, Ke K, et al. A meta-analysis comparing hepatic arterial infusion chemotherapy and sorafenib for advanced hepatocellular carcinoma. Transl Cancer Res. 2022;11(1):99–112. doi:10.21037/tcr-21-1839
61. Choi JH, Chung WJ, Bae SH, et al. Randomized, prospective, comparative study on the effects and safety of sorafenib vs. hepatic arterial infusion chemotherapy in patients with advanced hepatocellular carcinoma with portal vein tumor thrombosis. Cancer Chemother Pharmacol. 2018;82(3):469–478. doi:10.1007/s00280-018-3638-0
62. Gordan JD, Kennedy EB, Abou-Alfa GK, et al. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J Clin Oncol. 2020;38(36):4317–4345. doi:10.1200/JCO.20.02672
63. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
64. Galle PR, Finn RS, Qin S, et al. Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): an open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22(7):991–1001. doi:10.1016/S1470-2045(21)00151-0
65. Hsu C, Rimassa L, Sun HC, Vogel A, Kaseb AO. Immunotherapy in hepatocellular carcinoma: evaluation and management of adverse events associated with atezolizumab plus bevacizumab. Ther Adv Med Oncol. 2021;13:17588359211031140. doi:10.1177/17588359211031141
66. Kulik L, da Fonseca LG, He AR, et al. Potential impact of IMbrave150 results in the evolving treatment landscape of advanced hepatocellular carcinoma: a multidisciplinary expert opinion. J Hepatocell Carcinoma. 2020;7:423–433. doi:10.2147/JHC.S274930
67. Alfadda A, Sherbeeni S, Alqutub A, et al. Transient elastography for the prevalence of non-alcoholic fatty liver disease in patients with type 2 diabetes: evidence from the CORDIAL cohort study. Saudi J Gastroenterol. 2022;28(6):426. doi:10.4103/sjg.sjg_73_22
68. Alswat K, Alanazi M, Bashmail A, et al. Validation of the EVendo score for the prediction of varices in cirrhotic patients. Saudi J Gastroenterol. 2022;28(5):378. doi:10.4103/sjg.sjg_624_21
69. Cheng AL, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76(4):862–873. doi:10.1016/j.jhep.2021.11.030
70. Kim C, Yang H, Kim I, et al. Association of high levels of antidrug antibodies against atezolizumab with clinical outcomes and T-Cell responses in patients with hepatocellular carcinoma. JAMA Oncol. 2022;8(12):1825. doi:10.1001/jamaoncol.2022.4733
71. Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646–649. doi:10.1002/bjs.1800600817
72. Abou-Alfa GK, Chan SL, Kudo M, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. JCO. 2022;40(4_suppl):379. doi:10.1200/JCO.2022.40.4_suppl.379
73. AstraZeneca. A randomized, open-label, multi-center Phase III study of durvalumab and tremelimumab as first-line treatment in patients with advanced hepatocellular carcinoma. clinicaltrials.gov; 2022. Available from: https://clinicaltrials.gov/ct2/show/NCT03298451.
74. Kudo M. Durvalumab plus tremelimumab: a novel combination immunotherapy for unresectable hepatocellular carcinoma. Liver Cancer. 2022;11(2):87–93. doi:10.1159/000523702
75. Sangro B, Chan S, Kelley R. SO-15 Four-year overall survival update from the phase 3 HIMALAYA study of tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. Ann Oncol. 2023;34(S1):168. doi:10.1016/j.annonc.2023.04.487
76. Siddique O, Yoo ER, Perumpail RB, et al. The importance of a multidisciplinary approach to hepatocellular carcinoma. J Multidiscip Healthc. 2017;10:95–100. doi:10.2147/JMDH.S128629
77. Abaalkhail FA, Al Sebayel MI, Shagrani MA, et al. Clinical practice guidelines for liver transplantation in Saudi Arabia. SMJ. 2021;42(9):927–968. doi:10.15537/smj.2021.42.9.20210126
78. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi:10.1016/S0140-6736(18)30207-1
79. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi:10.1016/S1470-2045(08)70285-7
80. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi:10.1056/NEJMoa0708857
81. Colagrande S, Inghilesi AL, Aburas S, Taliani GG, Nardi C, Marra F. Challenges of advanced hepatocellular carcinoma. World J Gastroenterol. 2016;22(34):7645–7659. doi:10.3748/wjg.v22.i34.7645
82. Llovet JM, De Baere T, Kulik L, et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18(5):293–313. doi:10.1038/s41575-020-00395-0
83. Gallage S, García-Beccaria M, Szydlowska M, et al. The therapeutic landscape of hepatocellular carcinoma. Medicine. 2021;2(5):505–552. doi:10.1016/j.medj.2021.03.002
84. Huang A, Yang XR, Chung WY, Dennison AR, Zhou J. Targeted therapy for hepatocellular carcinoma. Signal Transduct Target Ther. 2020;5(1):146. doi:10.1038/s41392-020-00264-x
85. Granito A, Forgione A, Marinelli S, et al. Experience with regorafenib in the treatment of hepatocellular carcinoma. Therap Adv Gastroenterol. 2021;14:175628482110169. doi:10.1177/17562848211016959
86. Trevisani F, Brandi G, Garuti F, et al. Metronomic capecitabine as second-line treatment for hepatocellular carcinoma after sorafenib discontinuation. J Cancer Res Clin Oncol. 2018;144(2):403–414. doi:10.1007/s00432-017-2556-6
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




