Back to Journals » Pediatric Health, Medicine and Therapeutics » Volume 14
Treatment Outcome of Jaundice and Its Associated Factors Among Neonates Treated in Neonatal Intensive Care Unit of Comprehensive and Specialized Hospitals of Southern Nations Nationalities and Peoples Region, Ethiopia 2022
Authors Kebede C , Fentie B, Tigabu B
Received 28 February 2023
Accepted for publication 19 July 2023
Published 26 July 2023 Volume 2023:14 Pages 237—247
DOI https://doi.org/10.2147/PHMT.S405453
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Roosy Aulakh
Christian Kebede,1 Beletech Fentie,2 Bethelihem Tigabu2
1Department of Pediatrics and Neonatology Nursing, School of Nursing, College of Health Science and Medicine, Wolaita Sodo University, Wolaita Sodo, Ethiopia; 2Department of Pediatrics and Child Health Nursing, School of Nursing, College of Health science and Medicine, University of Gondar, Gondar, Ethiopia
Correspondence: Christian Kebede, Department of Pediatrics and Neonatology Nursing, School of Nursing, College of Health science and Medicine, Wolaita Sodo University, P.O. Box 138, Wolaita Sodo, Ethiopia, Tel +251924741458, Email [email protected]
Introduction: Jaundice is a common problem that affects up to 50– 60% of full-term babies and 80% of preterm babies. It is a benign condition, but sometimes it can cause profound complications and eventually death. Identifying the treatment outcome of jaundice and the factors affecting it is crucial to preventing the death associated with it.
Objective: To determine the treatment outcome of jaundice and its associated factors among neonates treated in neonatal intensive care unit of comprehensive and specialized hospitals of SNNPR, Ethiopia, 2022.
Methods: An institution-based retrospective follow up study was conducted from April 2018 to April 2022. The data was checked for completeness and then entered into Epi-data version 4.6 and exported to STATA version 17. The results were presented in frequencies and percentages for categorical variables as well as mean and median for continuous variables. A binary logistic regression model was used to estimate the effect of an independent variable and the outcome variable.
Results: A total of 423 charts were reviewed, and 416 (98%) were included in the final analysis. Almost 91.3% (95% CI: 88.2, 93.7) of neonates had improved. Factors significantly associated with the treatment outcome were: residence (AOR = 0.36, 95% CI: 0.2, 0.8); origin of admission (AOR = 0.35, 95% CI: 0.2, 0.8); gestational age (AOR = 0.26, 95% CI: 0.1, 0.8); and total serum bilirubin level (AOR = 0.40, 95% CI: 0.2, 0.9).
Conclusion and Recommendation: Improvement was lower compared to other low and middle-income countries; more emphasis should be given to improving treatment outcomes in hospitals.
Keywords: hyperbilirubinemia, jaundice, neonate, treatment outcome
Introduction
Neonatal jaundice refers to when an infant’s skin, mucous membranes, and whites of eyes become yellow within the early neonatal period due to a rise of bilirubin in the blood and a then accumulation of it on the skin and mucous membranes.1–3 Bilirubin is produced from the breakdown of senescent red blood cells during the early neonatal period.4,5 Jaundice is a frequent disorder that affects up to 50–60% of full-term and 80% of preterm newborn babies in their first week of life. It is a benign condition in most cases, but sometimes it results in profound complications such as bilirubin-induced neurologic dysfunction and death.6
Neonatal jaundice has been identified as a problem that requires increased global health attention, particularly in sub-Saharan Africa and Southeast Asia.7–10 In high-income countries, mortality due to neonatal jaundice is reported as 1 in 100,000 live births. In these countries, infants are routinely screened during their birth hospitalization, and monitored for the risk of subsequent severe hyperbilirubinemia and treatment is initiated early. So, this system facilitated timely referral for treatment and significantly improved treatment outcomes, resulting in low mortality due to jaundice.11,12 Since the 1990s, the number of cases of severe hyperbilirubinemia and associated morbidity and mortality has decreased in high-income countries due to advances in diagnosis and treatment.13
When compared to developed countries, the treatment outcome of jaundice is poor in developing countries, with mortality rate of 119 in 100,000 live births.14,15 Sub-Saharan Africa and Southeast Asia account for 70% of all cases of severe neonatal jaundice occurring worldwide each year, with a death rate of 16–35%.16 This was due to a failure to initiate appropriate treatment early due to constrained resources, such as devices for measuring bilirubin as well as effective phototherapy (PT).16 However, the true dimension of the problem was unknown in Ethiopia, where there was high neonatal mortality and a high prevalence of neonatal jaundice.17,18
During the period between the 3rd and 6th postnatal days, the risk of severe neonatal jaundice is highest and the serum bilirubin level reaches its peak, so timely detection and initiation of treatment within this critical period is important in improving the treatment outcome of jaundice.19,20 Nowadays, most parts of the world use various types of effective phototherapies and adjunctive treatment as well as exchange blood transfusion (EBT) severe cases to reduce jaundice-related morbidity and mortality.21 In developing countries factors such as socio-economic, delayed seeking of health care, health facility-related factors, home deliveries, and late presentation to the hospital led to severe jaundice and resulted in higher death rates among neonates as compared to affluent countries.6,12,14,22–24 Neonatal mortality due to jaundice can be reduced by determining magnitude of mortality due to jaundice and factors that affects treatment outcome. The principal investigator used different searching techniques to find studies on the treatment outcome of jaundice among neonates treated in the NICU. But in Ethiopia, no study has been found on this topic area. So, this study aims to identify the treatment outcome of jaundice and factors associated with it among neonates admitted to neonatal intensive care units. It is important for healthcare professionals and other stakeholders to give special emphasis to developing action plans and strategies for improving the treatment outcome.
Methods
Study Design, Area, and Period
An institution-based retrospective follow-up study was conducted in two University comprehensive and specialized hospitals of the SNNPR, Ethiopia, namely, Wolaita Sodo University comprehensive and specialized hospital (WSUCSH) and Wachamo University comprehensive and specialized hospital (WCSH) from April to May 2022.
Wolaita Sodo University Comprehensive and Specialized Hospital (WSUCSH) is found in the Wolaita zone, Sodo town, and is about 300 kilometers away to the south of Addis Ababa. It serves people in a catchment area of about 3.5–5 million, including the neighboring Dawuro Zone, Gamo Zone, Gofa Zone, and Kambata Tambaro Zone. There are three senior physicians, five neonatology nurses, eleven BSc nurses, two general practitioners, and four interns working in the neonatal intensive care unit. There are 22 beds in the ward, which has three phototherapy units and is an exchange transfusion referral center for the Wolaita zone and surrounding neighborhoods. The second one was Wachamo University comprehensive and specialized hospital (WCSH), which is found in Hadiya Zone, Hosaena town. It is located 230.9 kilometers to the south of Addis Ababa. There are two senior physicians, two general practitioners, three neonatal nurses, and fifteen BSc nurses working in the neonatal intensive care unit. The hospital also has a total of 19 beds in the ward, three phototherapy units, and a referral center for exchange transfusions in Hadiya Zone and surrounding neighborhoods. The region has no standardized guidelines for the treatment of neonatal jaundice, but hospitals use their own guidelines depending on the availability of healthcare professionals and diagnostic and treatment tools.
Population
Neonatal clinical charts and registries were reviewed for neonates treated for jaundice in the neonatal intensive care unit. The study population consisted of neonates treated for jaundice in a neonatal intensive care unit from April 2018 to April 2022 in two University comprehensive and specialized hospitals in SNNPR, Ethiopia.
Inclusion Criteria and Exclusion Criteria
All neonates treated for jaundice in the neonatal intensive care unit from April 2018 to April 2022 were included in the study, and neonates who left the hospital against medical orders by family wish, were referred to another center, or had physician-confirmed conjugated hyperbilirubinemia were excluded.
Sample Size and Sampling Procedure
The sample size was calculated using the assumption of a single population proportion formula using a 50% proportion because no similar previous study was found in the study area. The lists of neonates treated for neonatal jaundice in the neonatal intensive care unit from April 2018 to April 2022 were extracted from the patients’ registration books in each hospital. Then a proportionate number of study participants was allocated to each hospital. Finally, a simple random sampling method was used to choose participants by using a computer-generated random number from the extracted cases using the medical registration number as a sampling frame.
Variables of the Study
The treatment outcome of neonatal jaundice was defined based on the patient’s status during discharge from the hospital: either improved or dead. Neonates were said to be improved when an improvement report was recorded on the patient’s registration book. Independent variables of the study were socio-demographic factors of mothers and neonates, maternal factors, neonatal factors, and treatment-related factors.
Data Collection Tool and Procedure
Data was collected by using a data extraction tool. It has four components, such as socio-demographic, maternal, neonatal, and treatment-related factors, which were adapted from reviewing different literature.25–28 Since data collectors were nurses working in the hospital, an English-version data extraction tool was used. After obtaining permission, four BSc nurses working in the adult surgical ward and two NICU ward heads were assigned for data collection and supervision, respectively. To conduct sampling, a medical registration number was obtained from the patient’s registration book. Then a total of 423 sampled charts were extracted from the patients’ chart store according to inclusion and exclusion criteria. Then, incomplete charts were excluded, and if the neonate died, a death summary for the neonate was extracted from the codes in the patient’s registration book. If the cause of death was not due to neonatal jaundice, the charts were excluded and replaced with the next jaundice case chart.
To maintain confidentiality and privacy, only the patient chart room coordinator extracted the patient chart from the store, and the names of the participants were not mentioned during data collection.
Data Quality Control
The data extraction tool was pretested with 5%20 of the total sample size at the WSU comprehensive and specialized hospital to check for the clarity and consistency of the information. Tool face validation was performed by a senior physician working in the NICU at WSU’s comprehensive and specialized hospital and an assistant professor in pediatrics and child health nursing working at WSU. A one-day data collection training was given for data collectors and supervisors, emphasizing the objectives of the study and the data collection procedure. During data collection, close supervision was carried out by the supervisor and the principal investigator. The supervisor and principal investigator checked the data for completeness and consistency regularly until data collection was completed.
Data Processing and Analysis
The collected data was checked for completeness manually. Then it was entered into EP-data version 4.6 and exported to STATA version 17 for further cleaning, recoding, and analysis. Results were presented in frequency tables and charts, descriptively. Categorical variables were computed for their frequencies and percentages. Continuous variables were compiled as mean, median, standard deviation (SD), and interquartile range (IQR). The chi-square assumption test was checked for categorical variables. Multicollinearity was checked for categorical variables (rho = 1.15), and for continuous variables using a variation inflation factor (VIF =1.25). A binary logistic regression model was used to estimate the strength of the association of an independent variable with an outcome variable. Variables that were statistically significant with p<0.25 in the bivariable analysis were included in the multivariable analysis to control confounders. Model fitness was checked with the Hosmer-Lemeshow goodness-of-fit test for logistic regression (X² = 8.6 and p = 0.51). The results were interpreted in terms of an adjusted odds ratio (AOR) with 95% confidence intervals, and a p-value less than 0.05 is considered statistically significant.
Results
Socio-Demographic Characteristics
A total of 423 neonatal charts were reviewed; 416 (98%) were used in the final analysis. More than half of the neonates, 233 (56%) were male, and nearly half, 215 (51.7%) were urban residents. The median post-natal age of the neonates at admission was 2 (IQR ±3) days (Table 1).
Maternal and Neonatal Characteristics
Overall, 347 (83.4%) of mothers were under 35 years old, with a mean age of 29 (SD±4.5) years. Almost 240 (57.7%) of neonates were gestational ages of 37 weeks or more, and the median gestational age was 37 (IQR ± 4) weeks. The median neonatal birth weight was 2.7 (IQR±1.2) kg (Table 2).
 |
Table 2 Maternal and Neonatal Characteristics Among Neonates Treated in Neonatal Intensive Care Unit in University Comprehensive Specialized Hospitals of SNNPR Ethiopia, 2022 |
Treatment and Outcome
In the current study, 316 (76%) neonates were treated with phototherapy, and 100 (24%) neonates needed exchange transfusions. In addition to phototherapy and exchange transfusions, 30 (7.2%) neonates were treated with phenobarbitone.
Overall, 91.3% (95% CI: 88.2%, 93.7%) neonates were discharged home with improvement, and 8.7%, (95% CI CI: 6%, 12%) were dead (Figure 1).
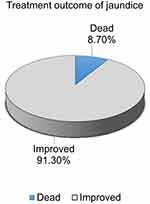 |
Figure 1 Treatment outcome of jaundice among neonates treated in neonatal intensive care unit of university comprehensive and specialized hospitals of SNNPR, Ethiopia 2022. |
Factors Associated with Treatment Outcome of Jaundice
In bi-variable analysis, residence, origin of admission, length of hospital stay, gestational age, birth weight, first minute Apgar score, fifth minute Apgar score, treatment type, and total serum bilirubin level at admission were significantly associated with the treatment outcome of jaundice. In multivariable analysis, residence, origin of admission, gestational age, and total serum bilirubin level at admission were significantly associated with the treatment outcome of neonatal jaundice.
In terms of residence, neonates who came from rural areas were 64% (AOR = 0.36, 95% CI: 0.2, 0.8) less likely to improve than their urban counterparts. Meanwhile, neonates who were admitted from outside (out born) were almost 65% (OR = 0.35, 95% CI: 0.2, 0.8) less likely to improve as compared to those who were born inside.
Compared to treatment outcome in terms of gestational age, babies who were born <37 weeks of gestation were 74% (AOR = 0.26, 95% CI: 0.1, 0.8) less likely to improve than those who were born ≥37 weeks of gestation, keeping other variables constant. When compared to neonates with a TSB level of <20 mg/dl at admission, those with a TSB level of ≥20 mg/dl were 60% (AOR = 0.4, 95% CI: 0.2, 0.9) less likely to improve (Table 3).
Discussion
This study aimed to identify the magnitude and factors associated with the treatment outcome of neonatal jaundice. Treatment outcomes were assessed in terms of improvement and mortality in the hospital.
Overall, 91.3% (95% CI: 88.2%, 93.7%) of neonates treated for jaundice were discharged home with improvement. This finding was in line with studies conducted in Bangladesh,29 north India,26 and Nigeria30 that revealed that 91.7%, 91.7%, and 91% of neonates were discharged home with improvement, respectively.
The treatment outcome of the current study was higher than studies conducted in western Nepal,25 South-east Nigeria31 and Benin City32 which revealed 86.2%, 83.3%, and 78.6% of neonates admitted to NICU were discharged home with improvement, respectively. The possible reason might be the variation in the definition of treatment outcome in the study. In the previous studies neonates discharged against medical orders were included under those who were not improved, which accounted for a large proportion in study. In the current study, those who left the hospital against a medical order were excluded because treatment outcome was not well determined for those who left the hospital without completing treatment.
In addition, as compared with the previous study conducted in western Nepal,25 neonates in the current study were admitted earlier, on the 2nd day of median postnatal age. But in the previous study conducted in western Nepal, the neonates were admitted on the 6th day of postnatal age. Early admission and treatment are important for good outcomes in every case. So in the current study, earlier admission might have helped to have a comparatively good outcome as compared to a study conducted in western Nepal.
In the current study, the number of neonates discharged home with improvement was lower as compared with reports in Baghdad,33 Egypt,34 India,35 and south-west Iran.27 In studies conducted in those countries, 99.3%, 99.6%, 96%, and 99.6% of neonates were discharged home with improvement, respectively. This difference might be due to the differences in standards of the neonatal intensive care unit in the setting and the difference in management protocols for neonatal jaundice.
In the current study setting, advanced diagnostic tools and treatments, such as advanced phototherapy units and other standard neonatal care equipment’s are less consolidated36 as compared to those study setting. So, standards of diagnostic tools and treatment might affect treatment outcomes in the neonatal intensive care unit.
Also, the number of neonates discharged home with improvement was lower as compared with outcomes reported in south-west Nigeria37 and Uganda,38 which revealed 96% and 97% of neonates discharged home with improvement, respectively. The possible reason might be due to a difference in the selection of study participants. In the previous studies, all cases of jaundice, regardless of severity, were selected, whereas in the current study, mild cases that were not candidates for phototherapy were not included in the study participants.
Among factors, residence was significantly associated with the treatment outcome of neonatal jaundice. Neonates who came from rural areas were 64% (AOR = 0.36, 95% CI: 0.2, 0.8) less likely to improve as compared to their urban counterparts. This finding was consistent with a study conducted in western Nepal.25 The possible reason might be that rural mothers have less knowledge of neonatal jaundice, its danger signs, consequences, and treatment modalities, so they might not seek care immediately when the condition occurs.39 In general, rural mothers have lower health-seeking behavior40,41 and have less access to appropriate health institutions and transportation nearby to seek care early so that neonates might be admitted and treated late.
In regard to origin of admission, out-born neonates (who were born in other health facilities and referred to this site) were 65% (AOR = 0.35, 95% CI: 0.2, 0.8) less likely to improve as compared to neonates born inside the hospital. This finding was consistent with studies conducted in South East Nigeria,42 Western Nepal,43 and Benin City.32 This might be due to out-born babies presented with severe illness and a higher total serum bilirubin level than in-born babies. They were also presented later than inborn babies because they might be referred from a longer distance, so jaundice was detected later after it reached a severe stage. But in-born babies were diagnosed and treated earlier due to the available equipment and health professionals in those hospitals, so that they were less severely ill and eventually more likely to improve.
In the case of gestational age, neonates with a gestational age of <37 weeks were 74% (AOR = 0.26, 95% CI: 0.1, 0.8) less likely to improve as compared to those with a gestational age of ≥37 completed weeks. A study conducted on the outcome of phototherapy among neonates treated for jaundice in India44 and a similar study on the efficacy of intensive phototherapy in the treatment of hyperbilirubinemia conducted in Egypt45 supports this finding, where premature babies were less responsive to treatment and less likely to improve than mature babies. And other studies conducted in south eastern Nigeria and Benin City32 revealed decreased improvement of neonates who had sepsis and prematurity.
This may be due to the fact that preterm neonates are prone to severe neurotoxicity of free bilirubin due to an immature blood-brain barrier and the liver as compared to mature neonates.46,47 For that reason, they have a decreased response to treatment and are less likely to improve than mature neonates. They are also prone to different phototherapy48 and exchange transfusion related complication,49 such as electrolyte imbalance, thrombocytopenia, dehydration, and even death, which further influence the treatment outcome of jaundice.
In the case of TSB level at admission, neonates with TSB level ≥20mg/dl were 60% (AOR = 0.40, 95% CI: 0.2, 0.9) less likely to improve as compared to neonates with TSB level <20mg/dl. This finding was consistent with studies conducted in China.50 The possible reason might be due to the fact that unconjugated bilirubin is neurotoxic at a high level that can lead to ABE and eventually death.51
Limitation
Since this study was built upon secondary data, some important variables, such as family income and the educational level of mothers, were not included in the study.
The long-term outcome was not considered in the current study.
This finding might not be representative for neonates treated in lower level health facilities, such as general and primary hospitals.
Conclusion and Recommendation
This study aimed to assess the treatment outcome of jaundice and its associated factors among neonates treated in the neonatal intensive care unit. Improvement upon treatment is still low because if neonates are admitted and treated early, jaundice will have good improvement. So, health professionals and other stakeholders need to emphasize improving the treatment outcome of neonatal jaundice. Special care needs to be given to low-gestational-age babies with jaundice. Priority needs to be given to those babies who come from rural areas and who have elevated serum bilirubin levels.
Abbreviations
AAP, American Academy of Pediatrics; ABE, Acute Bilirubin Encephalopathy; AOR, Adjusted Odds Ratio; BSc, Bachelor of Science; COR, Crude Odds Ratio; EBT, Exchange Blood Transfusion; G6PD, Glucose-6-Phosphate Dehydrogenase; IQR, Interquartile Range; LED, Light Emitting Diode; LMICs, Low and Middle Income Countries; MSc, Master of Science; NICE, National Institute for Health and Care Excellence; NICU, Neonatal Intensive Care Unit; PCHN, Pediatrics and Child Health Nursing; PDA, Patent Ductus Arteriosus; P.O, Post Office; PT, Phototherapy; SD, Standard Deviation; SNJ, Severe Neonatal Jaundice; SNNPR, Southern Nations Nationalities and Peoples Region; TcB, Transcutaneous Bilirubin; TSB, Total serum Bilirubin; UK, United Kingdom; WCUSH, Wachamo University Comprehensive Specialized Hospital; WSUCSH, Wolaita Sodo University Comprehensive Specialized Hospital.
Data Sharing Statement
Data that supports the study’s findings is available upon reasonable request of the corresponding author.
Ethical Consideration and Patient Privacy
The study was carried out after getting ethical clearance from the ethical clearance committee of the school of nursing on behalf of the Ethical Review Board of the University of Gondar (PCHN/720). After reviewing the ethical clearance, the chief clinical director of each hospital ordered to contact with the NICU head. Data collection began after getting permission from the neonatal intensive care unit head.
By removing names and other identifiers from the data extraction tool, patients’ information was kept private. So this work complied with the Declaration of the Helsinki.
Acknowledgment
I am grateful to the University of Gondar, the College of Medicine and Health Science, the School of Nursing, and the Department of Pediatrics and Child Health Nursing for their guidance and willingness to provide me with the opportunity to conduct research.
I would like to express my gratitude to my advisors, Mrs. Beletech Fentie and Mrs. Bethelihem Tigabu, for their invaluable advice and assistance throughout the research process.
I would also like to express my appreciation to Wolaita Sodo and Wachamo University comprehensive specialized hospitals for their willingness to accept my request to collect data in their hospitals. I would also like to thank the clinical directors, NICU ward head, and data collectors who were working in the institutions for their invaluable support.
Finally, I’d like to express my heartfelt gratitude to my family for their appreciation and support, which gave me the strength to pursue this wonderful opportunity.
Disclosure
The authors report no conflicts of interest in this work.
References
1. American College of Gastroenterology AG. Improving patient care. Neonatal Jaundice; 2022.
2. Bizuneh AD, Alemnew B, Getie A, Wondmieneh A, Gedefaw G. Determinants of neonatal jaundice among neonates admitted to five referral hospitals in Amhara region, Northern Ethiopia: an unmatched case-control study. BMJ Paediatr Open. 2020;4(1):e000830–e. doi:10.1136/bmjpo-2020-000830
3. Mir SE, van der Geest BAM, Been JV. Management of neonatal jaundice in low- and lower-middle-income countries. BMJ Paediatrics Open. 2019;3(1):e000408. doi:10.1136/bmjpo-2018-000408
4. Mitra S, Rennie J. Neonatal jaundice: aetiology, diagnosis and treatment. Br J Hosp Med. 2017;78(12):699–704. doi:10.12968/hmed.2017.78.12.699
5. Amos RC, Jacob H, Leith W. Jaundice in newborn babies under 28 days: NICE guideline 2016 (CG98). Arch Dis Child Educ Pract. 2017;102(4):207–209. doi:10.1136/archdischild-2016-311556
6. Greco C, Arnolda G, Boo NY, et al. Neonatal jaundice in low- and middle-income countries: lessons and future directions from the 2015 Don Ostrow Trieste Yellow Retreat. Neonatology. 2016;110(3):172–180. doi:10.1159/000445708
7. Ullah S, Rahman K, Hedayati M. Hyperbilirubinemia in neonates: types, causes, clinical examinations, preventive measures and treatments: a narrative review article. Iran J Public Health. 2016;45(5):558–568.
8. Olusanya BO, Osibanjo FB, Slusher TM. Risk factors for severe neonatal hyperbilirubinemia in low and middle-income countries: a systematic review and meta-analysis. PLoS One. 2015;10(2):e0117229. doi:10.1371/journal.pone.0117229
9. Olusanya BO, Ogunlesi TA, Kumar P, et al. Management of late-preterm and term infants with hyperbilirubinaemia in resource-constrained settings. BMC Pediatr. 2015;15(1):1–12. doi:10.1186/s12887-015-0358-z
10. Hyperbilirubinemia Guideline. Dell Children’s Medical Center E-Boc. Hyperbilirubinemia Guideline; 2021:11.
11. Olusanya BO, Teeple S, Kassebaum NJ. The contribution of neonatal jaundice to global child mortality: findings from the GBD 2016 study. Pediatrics. 2018;141(2). doi:10.1542/peds.2017-1471
12. Blencowe H, Vos T, Lee ACC, et al. Estimates of neonatal morbidities and disabilities at regional and global levels for 2010: introduction, methods overview, and relevant findings from the Global Burden of Disease study. Pediatr Res. 2013;74(1):4–16. doi:10.1038/pr.2013.203
13. Bhutani VK, Vilms RJ, Hamerman-Johnson L. Universal bilirubin screening for severe neonatal hyperbilirubinemia. J Perinatol. 2010;30(1):S6–S15. doi:10.1038/jp.2010.98
14. Bhutani VK, Zipursky A, Blencowe H, et al. Neonatal hyperbilirubinemia and Rhesus disease of the newborn: incidence and impairment estimates for 2010 at regional and global levels. Pediatr Res. 2013;74(1):86–100. doi:10.1038/pr.2013.208
15. Olusanya BO, Imam ZO, Emokpae AA, Iskander IF. Revisiting the criteria for exchange transfusion for severe neonatal hyperbilirubinemia in resource-limited settings. Neonatology. 2016;109(2):97–104. doi:10.1159/000441324
16. Slusher TM, Zamora TG, Appiah D, et al. Burden of severe neonatal jaundice: a systematic review and meta-analysis. BMJ Paediatr Open. 2017;1(1):e000105. doi:10.1136/bmjpo-2017-000105
17. Birhanu MY, Workineh AA, Molla Y, Abebaw E, Arora A, Bazezew Y. Rate and predictors of neonatal jaundice in northwest Ethiopia: prospective cohort study. J Multidiscip Healthc. 2021;14:447–457. doi:10.2147/JMDH.S298034
18. Lake EA, Abera GB, Azeze GA, Gebeyew NA, Demissie BW. Magnitude of neonatal jaundice and its associated factor in neonatal intensive care units of Mekelle City public Hospitals, Northern Ethiopia. Int J Pediatr. 2019;2019:1054943. doi:10.1155/2019/1054943
19. Hyperbilirubinemia AAoPSo. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114(1):297–316. doi:10.1542/peds.114.1.297
20. Olusanya BO, Ogunlesi TA, Slusher TM. Why is kernicterus still a major cause of death and disability in low-income and middle-income countries? Arch Dis Child. 2014;99(12):1117–1121. doi:10.1136/archdischild-2013-305506
21. Al-Lawama M, Badran E, Ala’Elrimawi ABM, Alkhatib H, Alkhatib H. Intravenous Immunoglobulins as adjunct treatment to phototherapy in isoimmune hemolytic disease of the newborn: a retrospective case-control study. J Clin Med Res. 2019;11(11):760. doi:10.14740/jocmr4003
22. Olusanya B, Osibanjo F, Mabogunje C, Slusher T, Olowe S. The burden and management of neonatal jaundice in Nigeria: a scoping review of the literature. Niger J Clin Pract. 2016;19(1):1–17. doi:10.4103/1119-3077.173703
23. Dennery PA. Pharmacological interventions for the treatment of neonatal jaundice. Semin Neonatol. 2002;7(2):111–119. doi:10.1053/siny.2002.0098
24. Slusher TM, Vaucher YE. Management of neonatal jaundice in low- and middle-income countries. Paediatr Int Child Health. 2020;40(1):7–10. doi:10.1080/20469047.2019.1707397
25. Sharma S. Neonatal hyperbilirubinemia: hospital based study in western region, Nepal. Janap J Interdiscip Stud. 2016;5:75–82. doi:10.3126/jjis.v5i0.17841
26. Kumar M, Tripathi S, Singh SN, Anand V. Outcome of neonates with severe hyperbilirubinemia in a tertiary level neonatal unit of North India. Clin Epidemiology Glob Health. 2016;4(2):51–56. doi:10.1016/j.cegh.2015.05.003
27. Shitran RF, Abed MY. Risk factors and outcomes of neonatal jaundice at al-Ramadi teaching hospital for maternity and childhood. Ann Trop Med PH. 2020;23:231. doi:10.36295/ASRO.2020.231228
28. Zhang M, He Y, Tang J, et al. Intensive phototherapy vs. exchange transfusion for the treatment of neonatal hyperbilirubinemia: a multicenter retrospective cohort study. Chin Med J. 2022;135(5):598–605. doi:10.1097/CM9.0000000000001962
29. Zabeen B, Nahar J, Nabi N, et al. Risk factors and outcome of neonatal jaundice in a tertiary hospital. Ibrahim Med Coll J. 2010;4(2):70–73. doi:10.3329/imcj.v4i2.6500
30. Mukoro G, Omekwe D, Kennis B, et al. Survey and management outcome of neonatal jaundice from a developing tertiary health centre, Southern Nigeria. IOSR J Dent Med Sci. 2014;13:35–39. doi:10.9790/0853-13413539
31. Osuorah CD, Ekwochi U, Asinobi IN. Clinical evaluation of severe neonatal Hyperbilirubinaemia in a resource-limited setting: a 4-year longitudinal study in south-East Nigeria. BMC Pediatr. 2018;18(1):1–7. doi:10.1186/s12887-018-1174-z
32. Israel-Aina Y, Omoigberale A. Risk factors for neonatal jaundice in babies presenting at the University of Benin teaching hospital, Benin City. Niger J Paediatr. 2012;39(4):159–163. doi:10.4314/njp.v39i4.2
33. Marzoog AS, Mohammed HN, Habib KD. Effectiveness of conventional phototherapy, intensive phototherapy and exchange transfusion in treating neonatal jaundice at Fatima Al-Zahra Hospital for maternity and children in Baghdad. AL-Kindy Coll Med J. 2020;16(2):25–29. doi:10.47723/kcmj.v16i2.262
34. Meslhy MF, El-Mohsen MM A, Hammad KS, Ahmed HH. Aetiological profile and outcome of jaundiced neonates admitted to Bab Alsharyia university hospital. Al-Azhar Journal of Pediatrics. 2020. doi: 10.21608/azjp.2020.128813.
35. Lakshmi S. Neonatal Hyper Bilirubinemia in Level II NICU And Its Outcome-A Tertiary Care Centre Experience, Hyderabad, India. IOSR J Dent Med Sci. 2017;16(11):12–18. Available from: https://www.iosrjournals.org/iosr-jdms/papers/Vol16-issue11/Version-4/C1611041218.pdf
36. Demtse A, Sebsibie G, Godie Y, Birhan Y, Nesru A. Clinical audit on neonatal care unit structure in five selected governmental hospitals of Addis Ababa, Ethiopia 2019. Int Arch Nurs Health Care. 2020;6:140.
37. Oluwafemi RO, Abiodun MT, Owa JA. Prevalence, risk factors and short-term outcome of babies with Neonatal Jaundice in a secondary facility with free-health services in South-West, Nigeria. Borno Med J. 2019;2019:1–9.
38. Nyangabyaki-Twesigye C, Mworozi E, Namisi C, et al. Prevalence, factors associated and treatment outcome of hyperbilirubinaemia in neonates admitted to St Francis hospital, Nsambya, Uganda: a descriptive study. Afr Health Sci. 2020;20(1):397–405. doi:10.4314/ahs.v20i1.46
39. Demis A, Getie A, Wondmieneh A, Alemnew B, Gedefaw G. Knowledge on neonatal jaundice and its associated factors among mothers in northern Ethiopia: a facility-based cross-sectional study. BMJ Open. 2021;11(3):e044390. doi:10.1136/bmjopen-2020-044390
40. Islam MM, Masud MS. Health care seeking behaviour during pregnancy, delivery and the postnatal period in Bangladesh: assessing the compliance with WHO recommendations. Midwifery. 2018;63:8–16. doi:10.1016/j.midw.2018.04.021
41. Jain A, Singh S, Choudhary A, Jain A, Chouudhary A. Maternal health-care seeking behavior in North India. Fam Med Prim Care Rev. 2017;6(2):265–269. doi:10.4103/2249-4863.219999
42. Onyearugha C, Onyire B, Ugboma H. Neonatal jaundice: prevalence and associated factors as seen in Federal medical centre Abakaliki, Southeast Nigeria. J Clin Med Res. 2011;3(3):40–45.
43. Kafle SP, Bhatta M, Shrestha R, Sitaula S, Koirala N, Koirala A. Outcome of Neonatal Hyperbilirubinemia from a Tertiary Care Hospital in Eastern Nepal: a Cross-sectional Study. J BP Koirala Inst Health Sci. 2021;4(1):37–42. doi:10.3126/jbpkihs.v4i1.36324
44. Rao AK, SaiRam A, Seethamahalakshmi K. Maternal and Child factors associated with Neonatal jaundice influencing the outcome of Phototherapy in Karimnagar district. MRIMS J Health Sci. 2017;5(4):124. doi:10.4103/2321-7006.302553
45. Abdelazeem K, Soliman A, Askar E. Efficacy of intensive phototherapy in management of neonatal hyperbilirubinemia inneonatal unit of assiut university children Hospital. J Neonatal Biol. 2017;6:266.
46. Amin SB, Wang H. Unbound unconjugated hyperbilirubinemia is associated with central apnea in premature infants. J Pediatr. 2015;166(3):571–575. doi:10.1016/j.jpeds.2014.12.003
47. Wang J, Guo G, Li A, Cai W-Q, Wang X, Chen J. Challenges of phototherapy for neonatal hyperbilirubinemia. Exp Ther Med. 2021;21(3):1. doi:10.3892/etm.2020.9464
48. Wang J, Guo G, Li A, Cai W-Q, Wang X. Challenges of phototherapy for neonatal hyperbilirubinemia (Review). Exp Ther Med. 2021;21(3):231. doi:10.3892/etm.2021.9662
49. Wolf MF, Childers J, Gray KD, et al. Exchange transfusion safety and outcomes in neonatal hyperbilirubinemia. J Perinatol. 2020;40(10):1506–1512. doi:10.1038/s41372-020-0642-0
50. Zhang R, Kang W, Zhang X, et al. Outcome Analysis of Severe Hyperbilirubinemia in Neonates Undergoing Exchange Transfusion. Neuropediatrics. 2022;53:257–264. doi:10.1055/s-0041-1742156
51. Qian S, Kumar P, Testai FD. Bilirubin Encephalopathy. Curr Neurol Neurosci Rep. 2022;2022:1–11.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


