Back to Journals » Journal of Multidisciplinary Healthcare » Volume 17
Towards Agility in Breast Cancer Treatment Principles as Adopted from Agile Software Engineering
Authors Odeh Y , Al-Balas M
Received 12 November 2023
Accepted for publication 14 March 2024
Published 23 March 2024 Volume 2024:17 Pages 1315—1341
DOI https://doi.org/10.2147/JMDH.S449465
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Pavani Rangachari
Yousra Odeh,1 Mahmoud Al-Balas2
1Software Engineering Department, Faculty of Science and Information Technology, Al Zaytoonah University of Jordan, Amman, 11733, Jordan; 2Department of General Surgery, Anesthesia and Urology, Faculty of Medicine, The Hashemite University, Zarqa, 13133 Jordan
Correspondence: Yousra Odeh, Software Engineering Department, Faculty of Science and Information Technology, Al Zaytoonah University of Jordan, Amman, 11733, Jordan, Tel +9626-429151100962, Fax +9626- 4291432, Email [email protected]; [email protected]
Purpose: The complex nature of breast cancer demands flexible and adaptable principles that can account for the diverse characteristics and evolving conditions of each patient. However, there are no common breast cancer treatment agility principles that can influence policies and direct breast cancer professionals and healthcare providers into enhancing the delivery of health outcomes to patients under these conditions along with continuous rapid improvements in breast cancer treatment plan design. The incorporation of agile principles from software engineering offers a promising avenue for enhancing patient care. This research is conducted to identify breast cancer treatment agility principles adopted from the software engineering field and to validate their conformance to agility through work reported from literature in breast cancer treatment context.
Material and Methods: The authors applied a structured research methodology that involved interviews for eliciting and validating twelve agility principles from oncologists. Discussion of each principle is reflected using work reported from literature as a form of validation. Finally, a domain expert reviewed the literature-driven validation for each of the twelve identified breast cancer treatment agile principle to finally validate their conformance to agility and provide results.
Results: This work resulted twelve validated agility principles for breast cancer treatment and classified whether they are meeting, partially-(hybrid), or not meeting agility. Seven out of the twelve agile principles resulted as meeting agility, where the remaining five principles resulted as partially meeting agility. None of them is recorded as not meeting agility.
Conclusion: The work contributes to forming an agile mindset that can empower breast cancer professionals to optimize treatment plans, enhance patient experiences, and continuously improve the quality of care. The twelve identified agile principles are anticipated to contribute to driving more efficient oncology practices, policies, and protocols. It is concluded that the breast cancer treatment agility principles are not limited to twelve.
Keywords: agile breast cancer, breast cancer, breast cancer treatment, agile, oncology, agile software engineering, agile healthcare, agile principles, multidisciplinary research, healthcare policy
Introduction
According to the World Health Organization, the most frequent cancer diagnosis and the main cause of cancer mortality in women globally is breast cancer.1 Traditional breast cancer therapy (BCT) has been the gold standard of care for the past 20 years, and it has significantly improved patient outcomes.2 Nonetheless, inability to take into account the distinct features of every patient’s cancer typically led to ineffective, nontargeted treatment with negative side effects that include toxicity, a reduced quality of life, and, in certain cases, a subsequent delay in the detection of progression.2–4 The lack of consideration for each patient’s tumor characteristics hinders the advancement of more effective treatments, as clinical trials and drug development efforts may not accurately represent treatment efficacy.3,5 Since breast cancer is described as a heterogeneous disease that affects a spectrum of patients from different age groups, cultures, religions, ages, health conditions, and genders, there is a need to deliver the treatment tailored to their characteristics.2,3 Although patients may share similar characteristics, each individual patient should have a respective personalized treatment.2 Evolution in precision medicine and personalized treatments aims to address some of the challenges associated with traditional approaches and offer more personalized effective options for breast cancer patients.6,7 Thus, today, BCT is shifting towards more personalized approaches that enhance patient experience considering diverse characteristics and evolving conditions.3,5 The authors interpret this shift as a transition towards agility.
Multidisciplinary research provided knowledge returns to people, motivated communities, and enhanced environmental consequences.8 It has shown that there is a more complex and larger collaborative world beyond their single discipline.8 The fields of software engineering and BCT may complement one another in a number of ways to advance BCT and research. High-quality useful software products are generated as outputs from good, designed software development processes. Two well-known development approaches are applied in the field of software engineering and in BCT: traditional and agile.9 The traditional, namely, the waterfall, development process is described as a linear structured approach. Each phase must be completed before moving on to the next, and there is little to no backtracking or iteration between phases.9 Incorporating user feedback into a project is frequently left until the latter phases.9 The result may be that the finished product falls short of user demands or expectations.9 This is because clients may modify their requirements after seeing functional software because they are unsure of what they originally wanted, which results in more costly redesign, rebuilding, and retesting. Traditional software development approach has negative characteristics including its low user involvement, limited flexibility, late detection of errors, long and delayed time-to-market cycle, and high cost.9 It is too rigid, thus, too risky.
In February 2001, the Agile Manifesto was created to promote agile practices in software development and other fields through four values and 12 principles.10 Agile is a mindset, considering that its four values and 12 principles are not firm rules to follow. The agile mindset has changed how software products are developed.11,12 Wherever user-centered experience and design is considered in the work of an agile team, value delivery to customer is enhanced.13 Agility has been adopted in the healthcare industry to transform traditional care delivery into personalized treatment that is patient-centric where the patient is involved in decision-making, enhances learning and continuous improvement, promotes individuals cooperation, enhances innovation, and flexibility of health service design.14–20
The founders of agile principles have prioritized individuals collaboration since they are the ones who will determine the success or failure of a software project, over the techniques, tools, or technology employed. Similarly, in the cancer treatment process, the patient is at the primary focus of care. This appears by developing a personalized treatment plan that takes the patient’s preferences, situation, and values into account and by working with their family to create precise and comprehensive strategies.21 However, many barriers occur while planning for treatment. These are related to the concurrent illness, patient’s health literacy, and emotional state.21 Overcoming these barriers could be through adopting an agile mindset.21–23
In this article, we aim to identify BCT agile principles that are adopted from the software engineering field. The translation of agility values is not in the scope of this work. Also, to investigate whether breast cancer therapy actions are carried out in an agile manner or if they still adhere to traditional forms in some aspects. This is crucial in order to inform decision-making and multidisciplinary collaboration, to respond to continuous changes and adjustments regarding the treatment plan, and to personalize care, increase treatment efficacy, innovate through clinical trials, and comprehend and reflect on possible treatment outcomes. Together, patients and breast cancer care professionals collaborate to choose the best course of action for each unique patient, increasing the opportunity for a good treatment outcome and a higher standard of living in terms of quality of life.
Material and Methods presents our conducted research methodology. This section presents a walkthrough validation in literature of the 12 agile breast cancer treatment principles, as identified by the domain expert. Results presents the results. Discussion is carried out in Discussion. Finally, we conclude the work in Conclusion.
Material and Methods
In this section, we present a multidisciplinary research methodology designed to identify the BCT agility principles that are adopted and adapted from the software engineering field. During the research, the authors validate whether the BCT identified principles conform to agility. This can bring several benefits to the research community and BCT process, professionals, and patients. The research methodology is shown in Figure 1.
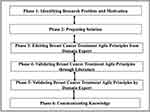 |
Figure 1 Research Methodology. |
The designed methodology encourages scientists to actively participate from different disciplines in the multidisciplinary process of problem-solving to produce outcomes and contribute to knowledge that can directly impact healthcare community. This contributes to enriching the aimed outcome and to bridge the gap between different disciplines.
The process directs the researcher through six phases of a structured overlapping process focusing on identifying and validating BCT agile principles. The six phases are identifying the research problem and motivation, proposing solution that addresses the problem, eliciting BCT agile principles from oncologists, validating the identified BCT agile principles through reporting literature, validating the BCT agile principles through domain experts, and, finally, communicating and disseminating knowledge. Iterations within phases are permitted for refinement needs.
Phase 1: Identifying Research Problem and Motivation
Breast cancer treatment lifecycle involves complex and dynamic actions and requirements. Well-defined principles for BCT may be necessary to ensure agility, efficiency, personalization, safe progress, and successful outcomes with minimum side effects. According to a literature review and to our knowledge, there is a lack of validated identification of BCT agile principles, although BCT professionals strive to adhere to agility as reported in the literature. For instance, personalized medicine is widely adopted in BCT and conforms to agility in one way or another.3 In the field of software engineering, agile principles have shown promise in enhancing productivity, adaptability, customer satisfaction, and collaboration in software development. This has motivated the authors to integrate knowledge and expertise from software engineering discipline that can lead to enhancing knowledge exchange that contribute to the BCT field.
Herein, we describe our research motivation. Multidisciplinary research efforts in cancer care can lead to better patient care, improved treatment options, and disease prevention strategies. Further, this work paves the way for future collaboration of researchers and individuals from academia, software engineering and technology, economics, healthcare, and government.8 Nevertheless, this multidisciplinary research can lead to personal and professional growth for the individuals involved.8
It is essential for patients to have open, transparent, and personalized communication with their care team professionals and health providers about their treatment approach and whether it is agile or not. Patients are empowered when they are aware of how BCT is delivered, particularly if agile mindsets are leading the journey. This encourages them to take an active role in making decisions about their care, asking pertinent questions, and advocating for appropriate treatment options that meet their individual needs and circumstances. Having an initial manifesto for agile BCT in means of validated principles would pave the way to lead to the identification of more personalized and flexible BCT policies, procedures, and protocols. Moreover, specifying quality assurance processes that indicate the extent of conformance to agility is another motivation to bring to BCT policy-makers. Hence, this motivates healthcare organizations to improve their care delivery considering higher patient satisfaction.
Phase 2: Proposing Solution
Based on the identified research problem and associated motivation, the authors seek to identify BCT agile principles. The twelve BCT agile principles are adopted and adapted from the software engineering field. Also, the authors aim to investigate their conformance to agility in BCT context through a walkthrough validation in literature by referencing respective works for each identified BCT agile principle.
Phase 3: Eliciting Twelve BCT Agile Principles from Oncologist
Through three interview elicitation sessions with a BC oncologist, the 12 agile principles of software engineering were reviewed and rearticulated into language that is tailored to BCT practitioners. To validate these identified BCT agility principles, each of them is presented in the next phase and discussed through work reported from the literature. In the discussion below, the reader can observe reflection, based on the literature, that describes the extent of the agility conformance of the proposed identified BCT agile principle. The output here is the 12 identified BCT agility principles as follows:
Principle 1: Our highest priority is to satisfy the patient and family through early and continuous delivery of effective safe treatment. Principle 2: Welcome changing requirements, even late in treatment lifecycle. Agile treatment cycles harness change for the patient’s benefit regarding cure, treatment side effects, and quality of life. Principle 3: Deliver effective and safe treatment frequently with a preference for short timescales without contradicting the principles of effective and safe treatment. Principle 4: Patients, their families, and treatment team must work together regularly throughout the treatment lifecycle. Principle 5: Develop cancer treatment plans and solutions around motivated individuals. Give them the environment and support they need and trust them to get the job done. Principle 6: The most efficient and effective method of conveying information to patients and within treatment teams is a combination of face-to-face conversation, digital tools, and online community groups. Principle 7: Overall patient survival, QoL, disease-free survival, and progression-free survival are the ultimate measures of progress. Principle 8: Cancer care institutional policies, breast cancer team, and patients should sustain a constant rhythm in breast cancer treatment life cycle. Principle 9: Continuous attention to technical excellence and appropriate design of BCT plan according to available data enhances agility. Principle 10: It is essential to meet patient needs through a simple treatment plan. Principle 11: The best breast cancer treatment plans, treatment requirements, and tests emerge from self-organizing teams. Principle 12: At regular intervals, the MD treatment team reflects on how to become more effective, and then tunes and adjusts its behavior accordingly.
Phase 4: Validating BCT Agile Principles Through Literature Walkthrough
In this phase, the authors present work reported from literature for each identified BCT agile principle to validate its extent of conformance to agility. Each principle is discussed below with work referenced from literature.
The First Principle
The first software engineering agile principle states that “our highest priority is to satisfy the customer through early and continuous delivery of valuable software”.10 As the primary focus of the agile mindset is the customer, customer satisfaction is considered the ultimate measure of success.10 Instead of waiting for the entire product to be developed, the team works to deliver it in an incremental manner to investigate if it meets their needs and expectations and promptly respond accordingly if any improvements are required. Incremental delivery allows customers to feel tangible benefits of the products and, thus, increases satisfaction. This also enables a quicker response to changing market demands and customer needs.10 This principle can be translated into BCT language as follows: “our highest priority is to satisfy the patient and family through early and continuous delivery of effective safe treatment”.
In BCT, a patient and the family are also the focus. The treatment delivery pattern is incremental and observed through the patient’s continuous visits, where visit feedback is looped into the overall cycle to improve the next treatment cycle in the next visit. Patient satisfaction could be addressed through Shared Decision-Making (SDM), where professionals strive to implicate their preferences in the treatment plan.24–26 In SDM, patients and others participate in providing valuable information that supports the complex therapy process.25 By doing this, the mismatch between the information people require and what is provided is reduced.25 Incorporating a patient into the SDM process allows tailoring the treatment plan to the patient’s preferences, which improves patient satisfaction and makes the treatment process more patient-centered.26
Regarding the early and continuous treatment delivery aspects of this principle, studies reported higher survival rate with early detection through screening and timely diagnosis, which are fundamental to an effective BCT.27,28 Research consistently shows that early-stage breast cancer has a higher likelihood of successful treatment and improved survival rates.27,28 For example, widespread screening program participation and compliance in western nations has resulted in early detection and higher survival rates for women.28 With an early focus on quality of life, ongoing specific strategies are needed to manage the long-term effects of breast cancer therapies as survival rates and the number of patients who are cured increase.27 It is essential to provide uninterrupted therapy to ensure optimal outcomes, minimize the risk of disease progression, and enhance patients’ quality of life. This should not be carried out without considering the management of the side effects according to the patient’s desires and characteristics.29
There is no commonly agreed definition of effective, safe BCT. Timely and continuous delivery of BCT is crucial for improving patient outcomes and ensuring their safety. Early diagnosis, followed by a well-structured treatment plan delivered without delays, significantly contributes to the effectiveness and safety of BCT.30 Moreover, early diagnosis allows for the development of a comprehensive and personalized treatment plan. Tailoring treatment to an individual patient’s specific cancer characteristics contributes to the effectiveness and safety of therapy.31 Initiating treatment promptly after diagnosis helps prevent the progression of the disease. Delayed treatment may lead to the advancement of the cancer, which can decrease treatment effectiveness and pose greater risks to the patient.32 Effective SDM contributes to effective and safe BCT and improved quality of life requirements, where patients are fully informed about all treatment tradeoffs and risks when participating in decision-making.25 Finally, continuous and consistent treatment management reduces the risk of complications. Careful monitoring and adherence to treatment protocols ensure that patients receive the necessary interventions to manage side effects and complications promptly, leading to increasing BCT safety and effectiveness.33
The Second Principle
In software engineering, this principle states that “Welcome changing requirements, even late in development. Agile processes harness change for the customer’s competitive advantage”.10 An agile team understands that changes are always expected to happen at any point of the project lifecycle.10 Therefore, the team remains open and responsive to changes that may entail emerging customer needs, market conditions, or even new trends and insights.10 This principle can be translated into BCT language as:
Welcome changing requirements, even late in treatment lifecycle. Agile treatment cycles harness change for the patient’s benefit regarding cure, treatment side effects and quality of life.
Advancements in BCT are essential to improve patient outcomes, reduce side effects, and enhance overall quality of life. These advancements come in the form of changes that occur regularly based on the patient’s response to therapy, diagnostic information, emerging clinical data, evolving treatment guidelines, and changes in disease status. The team relies on evidence-based medicine to make informed decisions. They review clinical trials, research papers, and evidence-based guidelines to assess the efficacy and safety of new treatments. Continuous changes may emerge in BCT in the following aspects:
- Continuous assessment and monitoring: the patient’s response to treatment is regularly monitored after the initial treatment plan is designed. Imaging tests, pathology reports, and other diagnostic tools are used to assess the effectiveness of the applied treatment approach. For example, MRI assists the oncologist by providing frequent information that influences the decision of surgical management.34–36
- Adjusting dosages and treatment intensity: BCT professionals may change the amount or intensity of chemotherapy, radiation therapy, or targeted therapy based on the patient’s reaction and side effects to improve treatment outcomes while controlling adverse side effects. For example, new risk factors can be identified from low relative dose intensity (RDI) that may enhance radiotherapy treatment delivery.37 Changes may involve suggesting medium-to-high intensity physical fitness exercises to improve chemotherapy treatment completion.38 This has resulted in a lower risk of not attaining RDI.38 Another dimension in this perspective can be considered through combining different therapy approaches representing promising results in comparison with other approaches. For example, the work of combining metronomic chemo-endocrine therapy with a FulVEC regimen resulted in a high rate of clinical benefits, particularly for patients with endocrine treatment (ET) and metronomic chemotherapy (CT) resistance.39 The literature contains numerous studies concerning dose adjustment or treatment combinations recommended according to patient health characteristics and response.40,41
- Incorporating new research findings: as new research and clinical trial results emerge, the treatment team may consider incorporating promising new medicines or treatment techniques into the patient’s plan, particularly if they have the potential to be more effective and safer than existing approaches. Metronomic chemo-endocrine therapy with the FulVEC regimen is a novel approach that compares favorably to other approaches in patients who are resistant to endocrine therapies.39 Similarly, clinical findings proved that medium-to-high physical fitness exercise improves chemotherapy treatment completion.38 Another finding is that radiotherapy could be reduced from about 4–6 weeks to 3 weeks for early-stage breast cancer women who have had surgery to remove a tumor with a high risk of recurrence.42 This short radiation course contributed to reducing the chance that cancer would recur in the same breast.42,43 Recently, researchers have investigated the role of artificial intelligence and deep learning in detecting BC.44,45 Innovation is becoming compulsory in the BCT field.46 For all promising research findings, researchers propose their recommendations to professionals who work in the field, where these findings could lead to changes in the form improvements for their patients’ cases.
- Surgical decision adjustment: the surgical approach that is part of a treatment plan may be incorporated based on the response to neoadjuvant therapies or changes in tumor size or characteristics. In recent studies, if negative margins are obtained, then multifocal and multicentric breast cancers can be surgically treated with breast-conserving surgery with no change in oncologic outcomes.47
- Personalized treatment and medicine: the treatment plan may be personalized based on the patient’s unique characteristics such as age, gender, genetic factors, overall health, and preferences. Whenever BCT personalization is required, whether early or late in the BCT lifecycle, this is another means for welcoming changes. Indicating the molecular characteristics of breast cancer subtypes is necessary to create a tailored therapy and diagnosis.48 It is necessary to establish molecular profiles and metrics in order to tailor an appropriate treatment and evaluate its benefits and risks. Dose ratios and regimens are likely to change according to the identified combination therapy.49 Personalized treatment has the potential to reduce not just the treatment burden and adverse side effects but also recurrence rates. This is reported in personalized radiotherapy dosing in BCT based on genomic data instead of delivering uniform doses.50
- Treatment side effect management: if the patient experiences significant adverse side effects from a treatment, the medical team may modify the treatment plan to manage these side effects effectively. Studies have reported that a large proportion of patients suffer from adjuvant ET adverse side effects, such as musculoskeletal, vulvovaginal, and vasomotor symptoms, that compromise adherence to oncological treatment outcomes and quality of life. These side effects can be mitigated through pharmacological and nonpharmacological approaches and integrated into a treatment plan.51,52 Pharmacological approaches are primary but only for short-term management without addressing the underlying causes.52,53 Exercise and psychological therapies have demonstrated potential in alleviating symptoms and providing long-term advantages.53 Recently, the effectiveness of mobile and web apps has been investigated in managing the side effects of BCTs for breast cancer survivors. Studies show that mobile apps were more frequently used and that nurses more frequently referred breast cancer survivors to mobile apps to obtain information regarding symptom management and treatment side effects.54
- Addressing disease progression or recurrence: in cases where the cancer progresses or recurs, the treatment plan will be re-evaluated, and the team may incorporate changes through involving different therapies or combinations of treatments. For example, sequential single-agent chemotherapy could be designed in a treatment plan for less toxicity, but with longer treatment time. However, when there is rapid disease progression, the treatment plan is recommended to change into combination chemotherapy instead of single-agent chemotherapy.55 Studies show that combination chemotherapy resulted in significant improvement in tumor response and time for metastatic breast cancer.56 Clinical data reported a significant reduction of breast cancer recurrence when statins are integrated into the treatment plan postdiagnosis.57 Statin types and the schedule of their use according to time of diagnosis, treatment duration, follow-up timeframe, and characteristics of the patient all vary from one case to another.57 These factors can all influence changes in a BCT plan.57
- Cost-effectiveness analysis: recent developments in novel BCTs have greatly improved clinical results and significantly raised the cost of treatment-related healthcare.58 Cost-effectiveness analysis is a form of systematic economic evaluation that compares health outcomes of different strategies of chemotherapy and targeted therapies in terms of cost. Cost-effectiveness evaluation depends on factors such as drug price, administration schedule, and the extent of survival rate improvement.58 The cost of cancer treatment can be a significant financial burden for patients and their families. A more cost-effective treatment option may be preferred to ensure that the patient can afford the therapy without compromising their quality of life, financial well-being, or facing severe financial strain. For example, recent studies have reported a cost-effective screening method for women with extremely dense breasts, namely, contrast-enhanced breast MRI.59 This approach is not only cost effective but has reduced breast cancer mortality for these women.59
The cancer treatment team should remain open and responsive to help the patient stay ahead of the halting of a treatment that could improve the patient’s quality of life. Changes always occur in the cancer treatment lifecycle. This principle confirms that treatment requirements or results are not always fully known or predictable. The team is required not to resist any change that is significant for the benefit of the patient. Instead, they encourage changes and implement plans for continuous improvement. Iterations of diagnosis and evaluation can be repeated for the purpose of change. BCT involves regular assessments and adjustments to the treatment plan. These changes happen based on the patient’s response to therapy, diagnostic information, emerging clinical data, evolving treatment guidelines, and changes in disease status. This principle permits for personalized modifications to optimize treatment outcomes.
In conclusion, and after validation with the domain expert, this BCT principle does fully conform, in terms of its compatibility, with the respective software engineering agile principle, but it does so in BCT terms. This leads us to classify this principle as achieving agility.
The Third Principle
In software engineering, this principle states the following: “Deliver working software frequently, from a couple of weeks to a couple of months, with a preference for a shorter timescale”.10 This principle promotes frequent incremental software delivery within a specified timeframe instead of delivering it after a long period where errors or faults are likely to happen, which may add further delay.10 Agile teams strive to allocate small timeframes to receive feedback quickly and respond to changes accordingly.10 The software should be fully functional and usable, where the customer can feel the tangible results and benefit from its use in the environment, if possible.10 This principle can be translated into BCT language as follows: “Deliver effective and safe treatment frequently with a preference for short timescales without contradicting the principles of effective and safe treatment”.
The literature reports that cancer care teams strive to deliver the required treatment within the shortest and earliest timeframes, depending on the conditions.32,42,43,60 Recent studies recommend short timeframes for radiation, especially for early-diagnosed patients.42 Therapy could be reduced from about 4–6 weeks to 3 weeks for early-stage breast cancer women who had surgery that removed a tumor with a high risk of recurrence.42 This short radiation course contributed to reducing the chance that cancer will recur in the same breast.42,43 According to experts, “This shorter course of treatment makes radiation therapy less burdensome for patients”.42 The delivery of radiation therapy for breast cancer and other malignancies is still being improved by radiation oncologists, who are also looking at ways to minimize treatment periods without sacrificing the treatment’s overall effectiveness.42,43,60 The optimal buffer of time following diagnosis is 90 days for surgery, 120 days for chemotherapy, and 365 days for radiation, if chemotherapy is administered.32 In breast cancer care guidelines, some timeframes are identified as below in:61
- Within 2 weeks, a patient with symptoms and indications that might indicate breast cancer should consult a doctor.
- The ideal time frame for testing is 2 weeks.
- Within 2 weeks after the patient’s cancer diagnosis, the surgeon should ideally see them.
- Within 2 weeks following the initial consultation appointment, diagnostic tests should be finished.
- For invasive breast cancer, surgery should preferably take place 4–6 weeks following the decision to use neoadjuvant systemic therapies or within 5 weeks of the decision to treat.
- After deciding to treat, neoadjuvant chemotherapy should start within 4 weeks.
- Within 4 weeks following surgery, adjuvant chemotherapy should start for breast cancer that is HER2-positive and triple-negative.
- As soon as possible after chemotherapy, radiation therapy, and/or surgery is finished, endocrine therapy should commence (and in some situations, will start during neoadjuvant treatment).
- For patients who did not receive adjuvant chemotherapy, radiation therapy should start 3–4 weeks after chemotherapy or no later than 8 weeks following surgery.
While radiotherapy offers substantial benefits, it can also impose a time burden on breast cancer patients due to the need for multiple treatment sessions over several weeks, because of the effect that associated psychological distress and side effects have on quality of life.62 Considerable time burdens for treatment have been reported by radiotherapy patients.63 The extended treatment duration can present challenges for patients, particularly those who face geographical, financial, or transportation barriers.64,65 Moreover, workers with breast cancer experienced stress from time burdens associated with returning to work.66,67 Thus, it is crucial to consider supportive care measures that might significantly lessen side effects and the treatment timescale.63
Healthcare professionals may strike a time balance between treatment efficacy and maintaining high-quality care by putting into practice strategies like hypofractionation, APBI, telemedicine, and holistic patient support.68
To deliver more effective radiation for breast cancer and to improve the entire patient experience within the shortest timeframe, collaboration between patients, medical personnel, and researchers is crucial.
According to clinical outcomes in adjuvant endocrine therapy—a form of treatment used in hormone receptor-positive breast cancer patients after primary treatments such as surgery, chemotherapy, or radiation therapy—studies reported a disease-free survival benefit of extended endocrine therapy of 10 years versus 5 years.69
Thus, in BCT, the literature lacks an optimal uniform time frame for each modality.32 It may not always be ideal to deliver treatment within a couple of weeks or months. This timeframe varies depending on several factors that are considered for each patient’s case.32 These factors are not limited to patient circumstances and overall health, cancer type and stage, and availability of healthcare system resources. Numerous risk factors for treatment delay exist and doubling tumor times make it difficult to evaluate outcomes and impact of this long period of treatment.32 This is subjective. For example, in some cases, a treatment may not be initiated until the patient recovers from some symptoms—symptoms for which the recovery time is unknown.
In conclusion, this BCT principle does not fully conform, in terms of its compatibility, with the respective software engineering principle. This leads us to classify this principle as partially meeting agility.
The Fourth Principle
This agile software engineering principle states that “Business people and developers must work together daily throughout the project”. This principle emphasizes the significance of continuous active involvement and collaboration of both business stakeholders and developers throughout the entire software development project lifecycle. Business people are the customer’s representatives.10 They could be product owner, business analyst, manager, domain experts, or developer stakeholders who possess technician skills that are crucial for the project success.10 They include programmers, designers, architects, and testers. Their daily collaboration minimizes ambiguity, misunderstanding, risks, and delays. This principle can be translated into BCT language as follows: “Patients, their families, and treatment team must work together regularly throughout the treatment lifecycle”.
In the context of BCT, the term “regular meetings” usually refers to planned gatherings or consultations involving the patient, their healthcare team, their families if needed, and other pertinent healthcare specialists. These meetings are held periodically over the breast cancer therapy lifecycle to review the patient’s progress, discuss the treatment plan and strategies, resolve any issues, and present alternatives and decisions regarding the best course of action. For a successful BCT and management, it is essential to have collaboration and communication between patients, their families, and the treatment team.25,70 Solid collaboration ensures that everyone is informed and participating in decision-making and provides the required support throughout the treatment lifecycle. More actively engaged care partners as family members have significantly increased care benefits, showing an increase in patients’ access to portals, ability to view clinician visit notes, and understanding of illness.25,70 Herein, we present two perspectives that operationalize successful regular collaboration that are translated into the SDM and multidisciplinary (MD) approaches considering openness communication during the BCT lifecycle.
- Shared decision-making: patients and their families share the decision-making responsibility with the BCT team in the treatment journey.25 Their collaboration is not necessarily daily but likely within short timeframes to provide quick feedback and act, accordingly, as discussed in the first principle. When a patient is involved in the decision-making, this permits the consideration of the patient’s preferences in the treatment and the increase in his/her satisfaction.26 The National Health Service in the UK defines the multidisciplinary team (MDT) as “a group of health and care staff who are members of different organizations and professions (eg GPs, social workers, nurses, anesthesiologist, radiologist, pathologist, etc.) that work together to make treatment and related services decisions of individual patients. MDTs are used in both health and care settings”.71 MDT individuals and their interventions can enhance the BCT planning.72–76 MDT meetings with patients also involve understanding treatment goals.25 In short, involving patients in MDT communication has enhanced and improved their quality of life and survival rate.76–80 A patient-centric process empowers BCT, and this supports addressing the agility perspective in BCT. Good, shared decisions are made based on good collaborations.26 SDM communication and collaboration start early in the breast cancer life cycle and continues after the cancer. This is seen in early diagnosis, treatment planning, treatment evaluation outcomes, managing treatment side effects, long-term management for survivorship, follow-up, palliative care, and end-of-life discussion.81 A major obstacle to SDM implementation is the lack of time and necessary training for both staff and patient.26
- Regular open communication: it is crucial to maintain open regular communication, whether it is through face-to-face, email, telephone, or online channels.82 This not only involves therapeutic communication regarding sharing treatment progress, treatment strategies, changes on treatment plan, and continuous monitoring and evaluation of results. Studies have reported that women with BC communication needs were also involved in “facilitating empathy” and five associated subcategories: “trust-building therapist”, “crying out to be heard”, “seeking a soothing presence”, “sharing knowledge”, and “supportive peers”.83 This shows how providing openness and emotional support is also crucial in BCT. Tailoring psychological support for BC patients after diagnosis and treatment has demonstrated positive impact in reducing distress and depression in daily activities and patients experiencing balanced wellness.84 Communication openness implies a BCT team that does not rely exclusively on words to describe treatment risks, benefits, complications, strategies, alternatives, and side effects. Studies recommended employing other forms of communication through numbers, graphic displays, or charts.85 A recent study recommended communicating treatment risks to patients through numbers or visualizations.85 Using the effects of negative and positive framing is recommended while discussing treatment choices.85
In conclusion, at each point of communication during the BCT lifecycle, the BCT team is responsible for following an SDM approach, with openness, and using the appropriate methods of communication according to the aspect and stage in the BCT lifecycle. This is vital to enhance patient understandability in order to attain an effective participation in decision-making. However, collaboration in the BCT lifecycle does not happen daily but regularly. Hence, this leads us to classify this principle as partially meeting agility.
The Fifth Principle
The fifth agile principle states the following: “Build projects around motivated individuals. Give them the environment and support they need and trust them to get the job done”.10 This principle highlights autonomy, ownership, and accountability of team members. It is important to empower individuals and place them in the environment they require in order to excel.10 This involves accessing a positive environment atmosphere, enabling effective communication, ensuring availability of resources, having the information they need, and removing obstacles. A supportive environment is a community that encourages growth and learning. When a customer and team members are engaged, enthusiastic, and invested in their roles, they are more likely to contribute to the project.10 Trusting team members means involving them in decision-making and trusting their capabilities in carrying out their tasks without strict control. This principle can be translated into BCT language as follows: “Develop cancer treatment plans and solutions around motivated individuals. Give them the environment and support they need and trust them to get the job done”.
In this principle, we present the means for motivating individuals in terms of the patient, family, and BCT team. The motivation of BC patients is described as personal and influenced by each individual journey’s circumstances. Patient motivation evolves up and down during the BC lifecycle. However, knowing the patient’s motivation contributes to promoting their quality of life by implementing useful personalized support programs.86 Healthcare practitioners should educate women more thoroughly about breast cancer and screening services. By increasing awareness of the benefits of mobile services, mammography screening should be more widely available and accessible. In order to motivate women’s involvement in screening, national and institutional policies should be developed and implemented to address their concerns and sociocultural barriers.87
Psychological therapies frequently call for the patient’s participation and dedication. For this kind of participation, it is required that the patient be motivated.88 Therefore, prior to a patient’s participation, it is useful to analyze his/her motivations, in terms of personal goals, expectations, and needs, in order to forecast both their adherence to the intervention and the results that will be obtained.88 This facilitates useful tailoring for a targeted environment and support that they need.
Exercise is effective for reducing the burden of adverse side effects resulting from BCT. Medical oncologists should motivate survivors to engage them in performing exercises.89 The treatment team can encourage engagement in these activities through pairing enjoyment, self-monitoring, setting goals, and social support. They should also motivate survivors to exercise through using evidence-based tools and referring patients to hospital services and community activities.89 Socio-relational activities have proven to be an important source of motivation for women with cancer.88 It is recommended to focus on developing patients’ motivation and engagement in SDM by improving patients’ elicitation skills and clarification exercises of their preferences and following both formal and informal coaching approaches. The biggest barriers are lack of time and resources.26 Return to work, for women with breast cancer, can be significant and one of the biggest motivations.90 A clinical framework was developed specifically for BC patients to promote quality of life and acts as a practical guide for health professionals to help in understanding the process of returning to work based on expert consensus.90 Being around family and loved ones is another significant motivation for BC patients.
Family support is needed and can be empowered to improve the quality of BCT for patients. Patients felt motivated to undergo and recover from chemotherapy when they had received various kinds of support from their families, such as emotional, appreciation, physical, financial, instrumental, and informational support.91 There is a strong correlation between family support and a patient’s motivation to comply with chemotherapy treatment instructions.91 A family is motivated when one of its members is receiving support from other members in another family.91 Engaging a patient’s family in a BC family community group as a social network will motivate them to continue their support and social networking.92 Educating and enriching family members’ knowledge through open communication and sharing reliable information about breast cancer life cycle stages, treatment, side effects, and risks contributed to enhancing family motivation. This helps family members understand the situation and provide the appropriate kind of support required for the patient. In some countries, sociocultural values motivate and influence the role of the family to support the patient as a caregiver.93
Before the introduction of multidisciplinary care in 1995, the mortality rate was higher (11%),78 as the management complexity of BCT prevents a single specialty from covering all treatment needs for a patient. Promoting the MDT approach toward BCT is the cornerstone to enhanced treatment care outcomes for BC patients, improving their quality of life and attaining a better management outcome. The key factors that were reported to motivate MDT and address efficacy are organization structure, data availability, preparation, and meeting discipline.94 In addition, it is required to actively involve all stakeholders, such as institutions, patients, and health professionals, to develop appropriate solutions.71,78 Facilitating MDT tasks can motivate them, and this is accomplished by providing a supportive environment that enables international collaborations and operationalizes key tools such as telemedicine and electronic medical records.95 Recently, clinical decision trees have been used as a supportive tool for MDTs in their decision-making.96 MDT participation and decision-making is increased by allocating smaller caseloads, recording higher attendance at meetings, having leadership support, supportive regulations, participating in strong and continuous communication, identifying clearly involved roles and responsibilities, and considering the patient’s holistic needs.76
However, there are some barriers that constitute a challenge to addressing this principle for an MDT in its environment, including lack of financial support, lack of resources, excessive caseload, and absence of leadership.26 Patients’ physical and social barriers are additional obstacles faced by this principle.90,95
In conclusion, this BCT principle does not fully conform, in terms of its compatibility, with the respective software engineering principle. In fact, the complexity of BCT, the unpredicted outcomes, and the personalized journey of this disease do not always support providing the needed motivation and the appropriate environment. This leads us to classify this principle as partially meeting agility. However, in the optimal case, if all elements in this principle are addressed, then developing an effective and safe BCT plan is likely delivered.
The Sixth Principle
This agile principle states that “The most efficient and effective method of conveying information to and within a development team is face-to-face conversation”.10 Face-to-face communication builds stronger relationships between project members. It allows openness, real-time communication, an effective way of understanding complex ideas and solving problems, and instant feedback.10 Direct communication facilitates quick response to feedback and change.10 Body language and verbal cues enrich communication experience and skills. Global teams can simulate face-to-face communication through video conferencing tools.10 This principle can be translated into BCT language as
The most efficient and effective method of conveying information to patient and within treatment team is combination of face-to-face conversation, digital tools, and online community groups.
It is compulsory to provide a patient with all reasonable treatment options, including none.97 However, in communicating evidence, there is no correct way of communication. A picture is worth a thousand words, as they say. It conveys a lot. Thus, it is recommended to use images if there is an opportunity to reduce any bias based on emotional perspectives in decision-making.98
It is important to address patients’ needs by paying particular attention to the nonverbal aspects of communication that convey empathy and respect toward patients, as well as allowing patients to ask questions.99 Even though there are many websites dedicated to breast cancer, most of them do a poor job of providing women the crucial knowledge they need to fully engage in the decision-making process for breast cancer surgery.100 Providing patients quick access to high-quality online information has the potential to greatly enhance their decision-making experiences.100 Efficient information is conveyed through a conversation that provides benefits for patients that are revealed through real-time interaction and demonstrate emotional personalized support.
Electronic medical record (EMR) systems and electronic messages have been utilized by patients with breast cancer and contributed to improved screening behaviors and clinical benefits.101 In a recent review, the quality of life and physical health of women benefited from internet-based support treatments, but these treatments resulted in psychological distress, symptoms of anxiety and/or depression, social support, and self-efficacy.102 Online storytelling support groups were arranged to break social barriers after the end of breast cancer for women. Findings reported that the information and experience-sharing within the support group empowered these women.103,104 However, online digital tools and websites to facilitate communication between patients and healthcare providers in the cancer context are still limited.105,106 Online communities could be considered as a supplemental resource playing a significant role in filling the gap of unmet needs for breast cancer survivors.107 Online communities were used primarily during BCT for information needs and symptom management (daily or weekly) and were used less often for emotional support. Reasons provided by patients for this reduced use include lack of trust, need, and awareness.107 Patients with BC who permitted e-Health intervention reported improvement in QOL, distress, self-efficacy, and fatigue.82
Due to the specificity of BCT as reported in the literature, the appropriate information conveying approach for breast cancer patients is a combination of online resources, digital tools, and face-to-face conversations. Comparing the support received from online communities or face–face groups, patients who participated in both received the most social support.108 Patients can utilize digital tools and internet resources such as websites to educate themselves, keep updated with the latest research, and connect with international support networks that involve supporting groups.106 Patient can benefit from internet’s high availability and accessibility, a variety of valuable information and survival stories that it provides, and global networking. However, the internet is limited in terms of emotional support and personalized treatment. Regarding the face-to-face approach, patients can speak with medical staff, neighborhood support groups, and family members for individualized guidance and emotional support.
In conclusion, this BCT principle does not fully conform, in terms of its compatibility, with the respective software engineering principle. This leads us to classify this principle as partially meeting agility.
The Seventh Principle
This agile principle states that “Working software is the primary measure of progress”.10 In agile software development, real progress relies on the continuous incremental delivery of tangible outputs, that is, a working software that meets customer requirements.10 This is a priority over documentation or prototypes.10 This can be translated into BCT language as “Overall patient survival, QoL, disease free survival, and progression free survival are the ultimate measure of progress”.
Writing this principle required determining what constitutes progress in BCT. According to a BC oncologist domain expert, the following four perspectives shape the measure of progress in BCT:
- Patient survival: higher survival rates are often associated with advancements in research findings, early detection, supportive care, personalized treatments, education and awareness, and long-term monitoring. Recent reports and statistics have shown that women who detected breast cancer early through national programs had a higher survival rate compared to decades ago. This is considered as progress in national and global health.109–111 Early breast cancer survivors who had a late diagnosis were more likely to have a poor quality of life because they had more unmet supportive care demands in the physical and psychological domains.112 Treatments are carried out to increase survival opportunities. Personalized treatment for breast cancer can significantly increase survival rates with tailored therapeutic approaches based on an individual patient’s preferences, specific characteristics, tumor stage, and treatment response.6,48,113 For example, it is a problem when a BC therapy lacks compliance with patient preferences and characteristics, especially in endocrine therapies. Lifestyle changes are integrated to increase the efficiency of treatment.113,114 Breast cancer education and awareness play a crucial role in increasing survival rates by promoting early detection and improving treatment outcomes.115,116 Follow-up after BCT also enhances survival opportunities.117
- Patient QoL: studies show that women with a higher QoL recorded 38% lower risk of mortality and 48% lower risk of cancer recurrence.118 Social well-being is the most significant QoL factor to consider in the first year after cancer diagnosis to improve survival outcomes.118,119 Compared to women with better prospects, those who reported having a negative outlook on the future had a greater likelihood of mortality.119 According to recent results, women with breast cancer who were active or moderately active had a lower risk of mortality from the disease than those who were completely inactive. Therefore, physical exercise should be incorporated into the care management plans for women with breast cancer in order to enhance their overall quality of life and increase their chance of survival.120
- Disease-free survival (DFS): this is an essential metric used to evaluate the effectiveness of BCT and advancements in breast cancer care. It is a critical measure in BCT management, where it describes the period that a patient remains free of BC symptoms.121 Positive lifestyles and supportive care for BC positively impact DFS.114 For example, diet and vitamin D supplements improved DFS results.122 The main variables that have been reported as closely associated with DFS are tumor size, lymph node ratio, grade, education level, and hormone therapy.123 A well-organized MDT contributed to improving DFS for breast cancer patients undergoing neoadjuvant chemotherapy (NAC) and, particularly, to reducing therapy delay, addressing timely pathological assessment, higher overall survival, and conformance to treatment guidelines and recommendations.124,125
- Progression-free survival (PFS): this is defined as “the time from randomization to progression or death”.126 It refers to the length of time during which a patient’s cancer does not show signs of progressing or getting worse. This is a crucial measure in treatment management to determine treatment efficacy. Personalized medicine contributed to a better PFS through profiling genomics, where gene changes are identified and optimized drug dosages are chosen within the target therapy.127 Lifestyle changes produced a significant increase in overall survival rates in terms of both PFS and DFS.128 Lifestyle changes include changes in diet, physical exercise, smoking cessation, and integrating vitamins and supplements.128
In conclusion, this BCT principle does fully conform, in terms of its compatibility, with the respective software engineering principle, but it does so in BCT terms and translated into the four aspects discussed above. This leads us to classify this principle as achieving agility.
The Eighth Principle
This agile software principle states that “Agile processes promote sustainable development. The sponsors, developers, and users should be able to maintain a constant pace indefinitely”.10 Instead of focusing on the achievement of milestones, this agile principle aims to balance between the well-being of individuals and the quality of the product through maintaining a constant pace of the development work.10 Sustainable development is addressed through incremental and iterative delivery, timeframe, continuous improvement, and team collaboration.10 This principle can be translated into BCT language as “Cancer care institutional policies, breast cancer team, and patients should sustain constant rhythm in breast cancer treatment life cycle”.
Ensuring a constant and sustainable rhythm in the BCT lifecycle involves aligning institutional policies, the cancer care team, and patients in a way that promotes consistent and effective care delivery over the long term. According to a domain expert, this principle considers various factors including clinical practices, patient engagement, and healthcare system efficiency.
Setting the framework for sustainable rhythm in breast cancer care requires the enactment of institutional policies. These policies should emphasize evidence-based practices, quality improvement, and resource allocation. To measure and improve the quality of care, the “American Society of Clinical Oncology’s Quality Oncology Practice Initiative” (QOPI) provides guidelines for oncology practices. Implementing such initiatives ensures that care practices are standardized, leading to consistent and effective care delivery over time.129
Regarding BCT team collaboration, effective teamwork and collaboration among various healthcare professionals, including oncologists, radiologists, anesthesiologists, nurses, social workers, and administrators, is crucial for sustaining a constant pace in BCT.71 The MDT model emphasizes interdisciplinary collaboration to enhance patient outcomes, improve quality of life, and increase survival rate and experiences.76,78–80,130
Patient engagement and SDM contribute to sustaining constant rhythm and quality of care in the BCT life cycle.25 Working together is much more than “policies, strategies, structures and processes”.24 Therefore, engaging BC professionals, patients, their families, and everyone related in this complex therapy process enhances positive delivery of valuable information and contributes to addressing this principle. Hence, this contributes to reducing any mismatch between the information that is delivered and the information they need, permitting more sustained delivery of care.25
Ensuring the efficiency of healthcare systems is vital for maintaining a constant pace in breast cancer care. In practice, the “Lean Six Sigma” methodology and LEAN principles have been applied in healthcare settings to streamline processes and reduce waste. By improving operational efficiency, healthcare organizations can consistently deliver high-quality care.131,132
Maintaining a constant pace in BCT requires a combination of evidence-based institutional policies, interdisciplinary collaboration, patient engagement, and healthcare system efficiency. The literature supports the idea that well-structured policies, MDT teamwork for patient-centered treatment, and efficient processes contribute to sustained quality in cancer care delivery.
In conclusion, this BCT principle does fully conform, in terms of its compatibility, with the respective software engineering principle, but it does so in BCT terms translated into the three aspects that support sustainable constant rhythm. This leads us to classify this principle as achieving agility.
The Ninth Principle
In software engineering, this principle states that “Continuous attention to technical excellence and good design enhances agility”.10 This principle aims to achieve long-term agility by paying attention to the time and effort invested in building solid technical skills and employing effective design principles.10 This principle can be translated into BCT language as “Continuous attention to technical excellence and appropriate design of BCT plan according to available data enhances agility”.
According to a domain expert, technical excellence and appropriate design of BCT can be enhanced by the following:
- Data-informed decision-making: BCT professionals can make well-informed decisions when designing BCT plans when they are able to continuously analyze available data related to treatments’ response rate, patient outcomes, and research findings. One future perspective proposes involving real-world data to help begin treatment more quickly and improve understanding of advanced BC.133 Facilitating personalized patient-centered decision-making for women undergoing treatment can be addressed using machine learning algorithms where patient-reported outcome can be accurately predicted.134 Researchers incorporated a business intelligence data-driven approach to support decision-making in diagnosis, treatment, and even in complex situations where unpredictable and interactive variables are involved.135 The work resulted in higher financial reduction with more accurate results compared to the conservative experience-driven approach.
- Iterative treatment adjustments: BCT plans frequently require modifications and refinement to align with the patient’s response to therapy and evolving medical circumstances. This iteration cycle of modifications or refinements is based on re-evaluations and reassessments. This agility can lead to better treatment outcomes and potentially improved PFS. For example, a surgery is followed by systemic chemotherapy and enables evaluation of the histopathologic response. Only 3–27% of patients show a full histopathologic response, even though 70% of patients exhibit a clinical response during a physical examination or upon anatomic imaging.136 This degree of response reveals important prognostic information.136 Patients who have a full pathologic response have considerably longer overall and disease-free lifetimes than non-responders.136 In another example, dose ratios and regimens are likely to change according to the identified combination therapy.49 A change may require a halt in the treatment to recover from the side effects.137 Treatment decisions for breast cancers are based not only on the assessment of prognostic factors but also on the assessment of pathological and clinical aspects. Changes to alternative BC therapies are reconsidered depending on tumor size, age, stage, genomic tests, and response rate.136,137 The reader can learn more about how therapies are adjusted in a summative form in.137.
- Using technology: decision support systems (DSSs) contributed to providing alternative cost-saving treatment strategies when optimal treatment is absent. The application of a DSS on a large scale would improve healthcare processes’ agility, especially in areas with low resources and facing economic challenges.135 However, physicians and oncologists do not and should not be replaced by technology as the full control of decision-making should be held by the physicians and oncologists. Disease diagnosis based on AI, machine learning algorithms, and biosensors has shown promising results in accurate prediction.134,138,139 In BC screening, AI is a technology that is undergoing development with the aim of improving efficiency and efficacy of image interpretation.140 In the MDT context, a virtual MDT was developed to support the agility, availability, and accessibility of the MDT.141 The literature contains numerous researches reporting technology incorporated into the BCT lifecycle to improve decision-making in all BCT phases, treatment planning, and management.142–144
- Collaboration of multidisciplinary teams: in cancer treatment, an MDT is described as the “gold standard” by some healthcare authorities.145 Practicing a multidisciplinary approach in BCT has shown strong clinical evidence in improving BC diagnosis accuracy, BCT plan design, and patient health outcomes, resulting in higher survival rates.76,78,79,146 Accuracy in diagnosis is higher and patient outcomes are improved when patient information is examined by multidisciplinary specialists with diverse backgrounds of knowledge and experience, particularly in the field of breast cancer.76,78,79,146 Due to economic challenges, MDT workload, and the continuous need for MDTs, a virtual MDT has been developed.141 This supports agility in BCT. To increase utilization and optimization of MDTs, it is necessary to develop a standardized approach, protocols, frameworks, and improve team performance and outcomes for patients.146
- Improvement of learning: BC professionals become closer and more coherent through face-to-face communication, discussing care aspects that focus on solving a problem and engaging in continuous collaboration in different contexts more frequently than isolated members.25,146 These meetings and discussions empower their communication skills with other members and patients. Members in an MDT become more educated and experienced through their participation in regular meetings and interactions by sharing their knowledge, expertise, and feedback.25 An MDT meeting is a platform to discuss the latest research findings, technological advancements in the field, treatment innovation, and clinical trial opportunities.25,146 This increases the participants’ learning curve and efficiency throughout the course of time; this, in turn, is reflected in their decisions regarding BCT plan design and patient outcomes.79 An MD approach is widely and internationally adopted due to its benefits for both patient health outcomes and MDT members.146
In conclusion, this BCT principle does fully conform, in terms of its compatibility, with the respective software engineering principle, but it does so in BCT terms translated into the continuous attention to technical excellence and appropriate design of a BCT plan with respect to available data for enhancing agility. This leads us to classify this principle as achieving agility.
The Tenth Principle
In software engineering, this agile principle states that “Simplicity—the art of maximizing the amount of work not done—is essential”.10 This principle focuses on delivering the essential features and functionality required to meet customer needs. Any unnecessarily complex features should be avoided. This can be achieved through minimizing waste, prioritizing the essential features, and delivering them in short iterations.10 This principle can be translated into BCT language as “It is essential to meet patient needs through a simple treatment plan”.
BC professionals strive to design a simple BCT plan by reducing unnecessary complexity without sacrificing quality.147 Studies in the literature have reported work that focuses on and discusses the importance and need for complexity in BCT plans.148–150 However, according to domain expert, meeting BC patient needs through BCT plan design that is as simple as possible is essential for many reasons including attaining clear understanding and effective communication, continuity of care, adhering to policies and standards, and facilitating reach to support communities. Each is summarized below from the literature.
- Clarity of understanding and effective communication: when the simplicity factor is considered in the BCT plan design, this facilitates and encourages patient engagement in meetings, treatment sessions, and even after survival, in support groups to reduce post-treatment psychological barriers.151 Hence, this positively contributes to patient-centered care.18,25 A simple treatment plan reduces the gap between the BC patient, family, and BCT professionals and helps BC patients increase awareness gradually. A clear understanding facilitates communicating their preferences and values in SDM meetings to incorporate them into the tailored treatment plan.18,25,152 Hence, this effective communication increases patient satisfaction.18,152
- Continuity of care: At primary and secondary care centers, simple surgery techniques may be conducted by general surgeons to reduce the burden on specialist surgeons in primary cancer center.151 Continuity of care reduces patients’ physical and mental burdens and, hence, improves patient satisfaction.
- Adherence to policies and standards: simple treatment plans facilitate oncologists and BCT professionals linking their treatment decisions and evaluations to respective policies and standards.153,154 This contributes to reducing barriers in relation to further decisions.
- Reaching out to support communities: when a simple BCT plan and lifecycle is communicated to the patient, s/he is motivated to reach out to supporting communities and to share with them, and with their loved ones, their experience, concerns, and challenges.155,156 On the other hand, this facilitates comprehension of the patient’s case by their loved ones and facilitates these communities’ ability to deliver the needed support.156 Hence, this also increases patients’ or survivors’ satisfaction and improves their QoL.
Although BC professionals strive to design a simple BCT plan, this may not be possible. This is because many clinical and nonclinical factors are considered in BCT plan design that play a crucial role in the degree of complexity.148,150 Due to the complexity of BC, especially with aggressive BC, complexity in the BCT plan is inevitable. The complexity of a BCT plan varies according to tumor level, stage of disease, treatment modalities,149 patient characteristics and preferences, lifestyle, treatments’ physical and physiological side effects,51,53,67,157 personalized medicine, genetic-related and molecular-related factors,158 availability and accessibility of resources, financial support, clinical trials, research findings, MDT decisions and recommendations, and QoL.148
In conclusion, this BCT principle does not fully conform, in terms of its compatibility, with the respective software engineering principle in addressing simplicity. In BCT, it is essential to address simplicity; however, the complexity of this disease does not usually permit the appropriate BCT to be simple. This leads us to classify this principle as partially meeting agility.
The Eleventh Principle
This agile software engineering principle states that “The best architectures, requirements, and designs emerge from self-organizing teams”.10 This principle emphasizes empowering self-organizing teams through their autonomy and responsibility for decision-making. This promotes ownership, creativity, innovation, and faster decision-making. This results in the development of high-quality solutions.10 This principle can be translated into BCT language as “The best breast cancer treatment plans, treatment requirements, and tests emerge from self-organizing teams.
This principle specifies the benefit of having a self-organizing team concept in BCT, as this enhances the delivery of an appropriate BCT plan and a well-defined description of its needs. This also involves conducting the appropriate diagnosis, assessment, and tests. Thus, a self-organizing team concept is centered in this agile principle. According to domain expert, MDT collaboration, SDM, and a personalized BCT plan and care can all operationalize this self-organizing team concept, we report on the findings of related work in the literature below:
- MDT collaboration: An MDT consists of medical and relevant experts from various fields who regularly meet to review a list of assigned patients and develop an accurate, coordinated diagnosis and treatment plan.159 Experts’ collaboration involves an oncologist, radiologist, histopathologist, surgeon, specialized nurse, allied health specialists, and an administrator.159 BC MDT collaboration has been proven to produce improved quality of treatment, clinical decisions, survival rate, and evidence-based practice.77–80,160 A well-organized MDT contributed to facilitating a clear definition of patient needs related to diagnosis, treatment, surveillance, and monitoring, allowing for dynamic decisions to be made. Planning for BCT care has significantly improved by taking into account all SDM perspectives such as scheduling appointment, handling referral processes, and assigning roles and duties for patient care. The research community reports the priority of having self-organized MDT; however, some factors may still hinder the effectiveness of these interactions, and these are reported to include leadership, individuals personality, cultural and belief systems, the need for regular clinical meeting, healthcare workers with double positions, availability of workforce, goals of care, implementation of national health insurance, hospital bureaucracy, issues with hospital infrastructure, patients, and high turnover.80,161,162
- Shared decision-making: as mentioned earlier, working together is much more than “policies, strategies, structures and processes”.24 This is observed in involving patients, their families, BC experts, and other relevant parties in the exchange of significant information to assist in managing this complex therapy process.25 This contributes to reducing any mismatch between the information that is delivered and the information the stakeholders need in order to make dynamic decisions.25 When a patient participates in the SDM process, their preferences for their care may be taken into account, which in result increases their satisfaction.26 To enhance patients’ participation in the SDM process, it is advised to use both formal and informal coaching, as well as elicitation and clarifying activities on their preferences. Nonetheless, some major obstacles to the SDM are a lack of time and resources.26 Through their interactions, this facilitates self-organization to implement the appropriate patient-centered treatment plan. This comes from sharing the BCT plan to provide a roadmap for everyone regarding how the plan will be navigated.18 However, in practice, some obstacles may hinder self-organizing team emergence. Some of these obstacles may stem from the physician or patient.18 Physicians may have difficulty in explaining complex information and providing tools that facilitate treatment.18 Patients’ lack of health literacy, emotional obstacles, and illness obstacles may hinder a treatment plan. Good decisions are made based on good collaborations, namely, through shared decision-making.26
- Personalized BCT plan and care: benefiting from the role of an MDT and SDM, BC professionals are capable of developing a personalized treatment plan and care, resulting in a better care coordination and high patient and provider satisfaction.160 The integration of different professionals from different departments provides continued and comprehensive support for patients during their BCT lifecycle. This is seen in close management of BC patients’ symptoms and personalized follow-up.163 The involvement of a self-organized MDT in a personalized BCT plan and care results in improved quality of life, better treatment adherence, reduction in long-term side effects, and, ultimately, in improved treatment outcome and survival rates.163
In conclusion, this BCT principle does fully conform, in terms of its compatibility, with the respective software engineering principle, but it does so in BCT terms translated into showing that self-organizing teams come from effective MDT collaboration, SDM, and their contribution in personalizing the BCT plan. This leads us to classify this principle as achieving agility.
The Twelfth Principle
This is the final agile principle and states that “At regular intervals, the team reflects on how to become more effective, then tunes and adjusts its behavior accordingly”.10 This principle emphasizes the importance of continuous reflection for continuous improvement within agile teams. This involves regular assessment of their processes, interactions, and overall performance to identify areas for enhancement and make necessary adjustments.10 This principle can be translated into BCT language as “At regular intervals, the MD treatment team reflects on how to become more effective, then tunes and adjusts its behavior accordingly”.
At present, breast cancer serves as the most important example of the need for and benefit of MDTs.164 An MDT is considered the gold standard in BCT and is widely adopted internationally.165 Regarding this agile principle, members of an MDT grow and develop as individuals throughout their engagement in the regularly conducted face-to-face meetings that involve debates, discussions, and decision-making in different aspects of care.76,146 In these meetings, they discuss recent findings in the field and the challenges they face in the assigned cases and share feedback to see how to improve a situation. Moreover, MDT effectiveness depends on factors related to the team, individuals, environment, and patient.166
The regular reflection in BC MDT work to improve their effectiveness comes from the regular evaluation meetings of treatment outcomes, data analysis, and incorporating new evidence-based practices into clinical care. Regular team meetings should be held in an environment of cooperation with the absence of competition or little competition between specialists.164 These regular cooperative meetings open a space to discuss the anticipated improvements, risks, and challenges of adopting novel treatments and using emerging technologies.76,167 Therefore, the organization should provide a protected time to allow the MDT to prepare for these meetings.166 A lack of protected time is a barrier for achieving an effective MDT meeting and increases the likelihood of poor attendance.168 Effective MDT meetings happen when MDT members are dedicated in preparation and attendance. This also contributes to developing knowledge and expertise of MDT members when they share their prepared insights and opinions, on one table, from different specialties. MDT meetings are considered ineffective when information is inadequate or unavailable at decision-making time.166 This highlights the necessity for allocating protected time for MDT preparation. Otherwise, this would negatively impact the management of the BCT plan and lower team morality and attendance.166 Regularly, the MDT should be active in clinical trials, empowering and encouraging the patient to engage in meetings and all treatment needs, reviewing their quality of decisions and research to improve patient care outcomes.164 Consequently, the MDT can improve their insights through patient-reported outcomes and become effective in managing patients’ problems and symptoms.164 Effective MDTs work together, optimize resources and time, and manage each patient case individually to contribute to developing strategies to improve MDT policies locally, regionally, and internationally.164 An MDT needs properly prepared clinical cases that are provided promptly to each team member, permitting them time to review and reflect for further information.164
In MDT meetings, critical thinking is stimulated whenever disagreement is recorded and discussed with the patient. This also enhances decision-making evaluation.166 “Group think” is a negative risk for MDT effectiveness where no member in the MDT dissents.166 This indicates poor communication and a lack of open communication, resulting in poor decision-making. An open culture for discussion increases MDT effectiveness, reduces risks and errors, and facilitates the delivery of an optimal treatment plan.166
Beyond MDT meetings and collaboration, their effectiveness can be promoted through the environment. This can be seen in changing their meeting room layout by arranging the chairs into a U shape instead of rows169 or in using a checklist to consider some patient care aspects that are usually omitted, such as emotional support and patients’ psychology and preferences.168 Assigning a rotational chairperson to ensure all necessary information is transformed and all voices are heard, ensuring equality, is one of the recommended strategies to attain better MDT decision-making.166,167 This contributed to reduced interprofessional conflicts and increased morale and effectiveness.166 Good informatics can improve MDT duties.
Hence, it is possible to conclude that ineffective MDT teamwork comes from low participation at meetings, excessive workload, a lack of leadership, unclear roles, inaction in research and clinical trials, and pitfalls in considering patients’ holistic needs.76 Due to the fact that an MDT is an integral part in BC care, studies have reported approaches for evaluating the performance effectiveness of MDTs.166
In conclusion, this BCT principle does fully conform, in terms of its compatibility, with the respective software engineering principle, but it does so within BCT terms translated into the fact that MDT reflection effectiveness occurs regularly during the BCT lifecycle and, accordingly, the team strives to adjust their behavior. This leads us to classify this principle as achieving agility.
Phase 5: Validating Breast Cancer Treatment Agile Principles by Domain Expert
In this phase, the output generated from the earlier phase is reviewed by the domain expert to validate that the identified BCT agile principles in the third phase are consistent with the reported work from literature as presented in phase 4.
Phase 6: Communicating Results and Knowledge
This phase involves documenting and publishing the results and knowledge obtained from this research to enrich further multidisciplinary collaboration, engagement, and communication in promoting agility in healthcare to increase healthcare delivery satisfaction for patient, their families, and healthcare professionals. Results are shown in the next section.
Results
A summary of this study results regarding identifying BCT agility principles and investigating the extent of their conformance to agility in comparison to principles in software engineering is shown in Figure 2. The work results 12 BCT agility principles validated by domain experts and supported through work reported from literature. They are categorized into two categories. Seven principles are classified as meeting agility, while the rest are classified as partially meeting agility. None of the identified principles recorded as not meeting agility. They are specified as below:
- Seven BCT principles resulted as meeting agility:
Principle 1: Our highest priority is to satisfy the patient and family through early and continuous delivery of effective safe treatment.
Principle 2: Welcome changing requirements, even late in treatment lifecycle. Agile treatment cycles harness change for the patient’s benefit regarding cure, treatment side effects, and quality of life.
Principle 7: Overall patient survival, QoL, disease-free survival, and progression-free survival are the ultimate measures of progress.
Principle 8: Cancer care institutional policies, breast cancer team, and patients should sustain constant rhythm in breast cancer treatment life cycle.
Principle 9: Continuous attention to technical excellence and appropriate design of BCT plan according to available data enhances agility.
Principle 11: The best breast cancer treatment plans, treatment requirements, and tests emerge from self-organizing teams.
Principle 12: At regular intervals, the MD treatment team reflects on how to become more effective, and then tunes and adjusts its behavior accordingly.
- Five BCT principles resulted as partially meeting agility:
Principle 3: Deliver effective and safe treatment frequently with a preference for short timescales without contradicting the principles of effective and safe treatment.
Principle 4: Patients, their families, and treatment team must work together regularly throughout the treatment lifecycle.
Principle 5: Develop cancer treatment plans and solutions around motivated individuals. Give them the environment and support they need and trust them to get the job done.
Principle 6: The most efficient and effective method of conveying information to patient and within treatment team is combination of face-to-face conversation, digital tools, and online community groups.
Principle 10: It is essential to meet patient needs through a simple treatment plan.
 |
Figure 2 Breast Cancer Treatment Agile Principles. |
Discussion
The concept of agility in healthcare revolves around the ability to continuously adapt rapidly to changing circumstances, incorporate clinical trials, evidence-based and innovative therapies, provide personalized care to patients, and collaborate closely with patient and family. In this research, agility was focused around the principles where agility values are identified as out of the scope, as it has been already addressed in recently published work.170
Referring to the results in Results, it is apparent that the proposed 12 BCT agility principles conform to the agility notion as overall (ie, shown in resulting seven principles meeting agility where the five partially do). None of the proposed principles is recorded as not meeting agility. However, we cannot claim that any additional BCT agility principle should be fully or partially meeting agility.
In the first principle, the agility focus is around the early and continuous delivery of effective and safe treatment. The literature supports this in the incremental delivery through continuous feedback during BCT lifecycle. In the second principle, the agility is focused on accepting changes for the patient’s benefit. Literature reports the significant need for change in agility in different aspects in BCT lifecycle. In the third principle, the fact of delivering BCT in a short period is not always possible, although it is desired, as it depends on several factors that are not limited to patient’s circumstances or overall health. This marks this principle as partially meeting agility. The fourth principle states the need for BCT team, patient, and family to communicate regularly with no pre-identified timeframe. This is reported through SDM and regular open communication. This marks this principle as partially meeting agility. In the fifth principle, providing a motivated environment is very challenging for BC as a complex disease, although the literature reports approaches for motivating patients, families, and MDT in the BCT lifecycle. This marks this principle as partially meeting agility. In the sixth principle, it is not always the face-to-face appropriate approach for communication. Literature reports that the most effective way of communication with patient is the combination of face-to-face, digital tools, and online community groups. And this varies according to patient’s circumstances and BCT stage making this principle partially meeting agility. In the seventh principle, the ultimate progress of BCT is reported through patient’s QoL, disease free survival, and progression-free survival. These three aspects have been reported from literature and thus made this principle meeting agility. The literature has shown that institutional policies, BCT, and patient shall sustain constant rhythm in BCT lifecycle in the eighth agile principle. Also, literature is full of work that reports the necessity of continuous attention to utilize data for enhancing the agility of BCT plan design and for technical excellence. This makes the ninth principle meeting agility. The tenth principle focuses on delivering simple treatment plan for many benefits as reported in literature. However, the complexity and unpredicted nature of BC does not always support this objective. Therefore, this marks this principle as partially meeting agility. The 11th principle focused on self-organizing teams as the cornerstone for best treatment plans. Literature work supported validating the agility of this principle. Finally, the 12th principle is marked as meeting agility through the continuous need for adjusting MDT behavior to become more effective at regular intervals.
After identifying and discussing the 12 BCT agility principles using work reported from literature in Material and Methods, we identify areas that need improvement. The current principles lack the consideration of designing BCT processes that methodologically and comprehensively reflect and implement these principles. Also, the design of a BCT process shall be more reflective and innovative in putting forward new principles for adoption. This in turn shall reflect on the MDT and patient behaviour and adjust it accordingly. In the context of information science, incorporating ontology can provide a formal representation of knowledge in a structured and organized manner for BCT agility. This would enhance information sharing, common understandability among different healthcare domains, and traceability to continuous changes.
Incorporating agile principles into BCT contributes to enhance BCT plan, patient outcomes, streamline healthcare processes, collaboration among cross-functional healthcare professionals, feedback response, information sharing and prioritization, and improve the overall continuous experience for both healthcare professionals and patients. However, it is essential to consider the specific context and challenges of the healthcare system when implementing agile principles to ensure successful adoption. This work highlights the need to identify agility principles not only for BCT but also in any healthcare domain such as in nursing, labs, dentistry, public health, emergency department, etc. This research is anticipated to open a workspace for the healthcare community to participate not only in amending or enriching the BCT agility principles in this article but also in proposing further principles that are necessary to implement agile approaches to health care, ultimately leading to more patient-centered care with better outcomes, higher quality of life for patients, longer-term survivorship, and more effective MDT. This work contributes further to bridge the gap between BCT agile principles and respective agile software services, which shall be developed to enact the BCT agile practices and processes. Having agile software services that highly meet BCT agile principles is anticipated to enhance patient-centered care, collaboration, and continuous improvement in BCT and overall patient care.
Implementing agile principles in the context of BCT involves several practical challenges due to the unique nature of healthcare and the complexities associated with breast cancer care. According to the domain expert, these challenges involve compliance with regulations, interdisciplinary collaboration, data security and privacy in the open communications for information sharing, integrating existing technology or implementing new technology that address agility principles, training healthcare culture for mind shift, and identifying metrics to measure BCT agility. Implementation challenges could also include agility aspects of recruiting big data approach for personalized medicine prediction.171 Prediction could not be possible without big data and artificial intelligence.171 Innovating agile BCT protocols is challenging and may require accommodating artificial intelligence considering intellectual property and legal content generation.172 Addressing these challenges requires an interdisciplinary tailored approach that considers the specific needs of the healthcare environment, regulatory requirements, and the quality of life of patients. Thus, a successful implementation of agile principles in BCT may require close collaboration between healthcare professionals, regulatory bodies, and technology experts to implement systems that conform to the proposed BCT agility principles.
Conclusion
The integration of 12 agile principles derived from software engineering into breast cancer treatment represents a transformative approach to healthcare delivery. By embracing these principles, healthcare professionals, researchers, policy-makers, and patients stand to benefit significantly in their collective pursuit of improved outcomes, patient-centered care, and continuous innovation.
The adaptation of these 12 agile principles from software engineering to the realm of breast cancer treatment has brought a paradigm shift in the way healthcare is conceived, developed, and executed, as was shown in the discussion of each principle. The literature work and domain expert validation show that the 12 breast cancer treatment principles tend towards meeting agility rather than partially meeting agility or not meeting it. This is represented in the seven principles infront the five principles that were validated as partially meeting agility. None of the 12 principles were recorded as not meeting agility. We do not claim that these are the only breast cancer treatment agility principles. The work delivers the first version of breast cancer treatment agile principles manifesto.
As the healthcare landscape evolves, the identification and application of agile principles will continue to be a driving force in advancing the quality and effectiveness of breast cancer care.
Abbreviations
BCT, breast cancer treatment; CT, metronomic chemotherapy; DSS, decision support systems; DFS, disease-free survival; ET, endocrine treatment; MDT, multidisciplinary team; PFS, progression-free survival; QoL, quality of life; RDI, relative dose intensity.
Informed Consent Statement
There is no need to obtain informed written consent as no participants were involved in this study.
Institutional Review Board Statement
No review or approval was required for this research by an institutional review board or ethics committee.
Acknowledgments
The authors are thankful to Al Zaytoonah University of Jordan, Amman, Jordan, and the Hashemite University, Zarqa, Jordan, for research fund support.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. 2023 Breast Cancer. Available From: https://www.who.int/news-room/fact-sheets/detail/breast-cancer.
2. Olopade OI, Grushko TA, Nanda R, Huo D. Advances in breast cancer: pathways to personalized medicine. Clin Cancer Res. 2008;14:7988–7999. doi:10.1158/1078-0432.CCR-08-1211
3. Sarhangi N, Hajjari S, Heydari SF, Ganjizadeh M, Rouhollah F, Hasanzad M. Breast cancer in the era of precision medicine. Molec Biol Rep. 2022;49:10023–10037. doi:10.1007/s11033-022-07571-2
4. Segota E, Bukowski RM. The promise of targeted therapy: cancer drugs become more specific. Cleve Clin J Med. 2004;71:551–560. doi:10.3949/ccjm.71.7.551
5. Gambardella V, Tarazona N, Cejalvo JM, et al. Personalized medicine: recent progress in cancer therapy. Cancers. 2020;12:1009. doi:10.3390/cancers12041009
6. Khoury T. The evolving approach to breast cancer: moving toward de-escalating treatment and personalized. Cancers. 2023;15:3502. doi:10.3390/cancers15133502
7. Rodrigues R, Duarte D, Vale N. Drug repurposing in cancer therapy: influence of patient’s genetic background in breast cancer treatment. Int J Mol Sci. 2022;23:4280. doi:10.3390/ijms23084280
8. Uiterkamp AJMS, Vlek C. Practice and outcomes of multidisciplinary research for environmental sustainability. Journal of Social Issues. 2007;63:175–197. doi:10.1111/j.1540-4560.2007.00502.x
9. Sommerville I. Software Engineering.
10. Beck K, Beedle M, van Bennekum A, et al. Agile Manifesto; 2022. Available from: https://www.agilealliance.org/agile101/the-agile-manifesto/.
11. Highsmith J, Cockburn A. Agile software development: the business of innovation. Computer. 2001;34:120–127. doi:10.1109/2.947100
12. Fowler M. The Agile Manifesto: where it came from and where it may go; 2002. Available from: http://martinfowler.com/articles/agileStory.html.
13. Zaina LAM, Sharp H, Barroca L. UX information in the daily work of an agile team: a distributed cognition analysis. Int J Hum Comput Stud. 2021;147:102574. doi:10.1016/j.ijhcs.2020.102574
14. Sindhwani R, Singh PL, Prajapati DK, Iqbal A, Phanden RK, Malhotra V. Agile System in Health Care: literature Review. Advan Indust Prod Eng. 2019;1:643–652.
15. Kokol P, Blažun H, Kokol M, Završnik J. Role of agile in digital public health transformation. Front Public Health. 2022;10:1111. doi:10.3389/fpubh.2022.899874
16. Ivanova D, Kadurin V. A New Proposed Software Development Methodology for Healthcare Industry. In:
17. Goodison R, Borycki EM, Kushniruk AW. Use of agile project methodology in health care IT implementations: a scoping review. Stud Health Technol Inform. 2019;257:140–145.
18. Thomas A, Suresh M. Readiness for agility adaptability and alignment in healthcare organizations. IISE Trans Healthc Syst Eng. 2023;13(2):161–174. doi:10.1080/24725579.2022.2144966
19. Chen WS, Leung CY, Kamath JRC. Advancing change agility in healthcare. Manag Healthcare. 2023;7(2):102–111.
20. Broekharst DS, van de Wetering R, Ooms W, Helms RW, Roijakkers N. Deploying predictive analytics to enhance patient agility and patient value in hospitals: a position paper and research proposal. Health Anal. 2023;3:100141. doi:10.1016/j.health.2023.100141
21. Balogh E, Ganz P, Murphy S, Nass S, Ferrell B, Stovall E. Patient‐centered cancer treatment planning: improving the quality of oncology care. Summary of an institute of medicine workshop. Oncologist. 2011;16:1800–1805. doi:10.1634/theoncologist.2011-0252
22. Malik JA, Ahmed S, Jan B, et al. Drugs repurposed: an advanced step towards the treatment of breast cancer and associated challenges. Bio Pharmac. 2022;145:112375. doi:10.1016/j.biopha.2021.112375
23. Taefehshokr S, Parhizkar A, Hayati S, et al. Cancer immunotherapy: challenges and limitations. Pathol Res Pract. 2022;229:153723. doi:10.1016/j.prp.2021.153723
24. Schot E, Tummers L, Noordegraaf M. Working on working together: a systematic review on how healthcare professionals contribute to inter-professional collaboration. J Interprof Care. 2020;34:332–342. doi:10.1080/13561820.2019.1636007
25. Katz SJ, Belkora J, Elwyn G. Shared decision making for treatment of cancer: challenges and opportunities. J Oncol Pract. 2014;10:206–208. doi:10.1200/JOP.2014.001434
26. Maes-Carballo M, Martín-Díaz M, Mignini L, Khan KS, Trigueros R, Bueno-Cavanillas A. Evaluation of the use of shared decision making in breast cancer: international survey. Int J Environ Res Public Health. 2021;18:2128. doi:10.3390/ijerph18042128
27. Nardin S, Mora E, Varughese FM, et al. Breast cancer survivorship, quality of life, and late toxicities. Front Oncol. 2020;10:864. doi:10.3389/fonc.2020.00864
28. Marmot MG, Altman DG, Cameron DA, Dewar JA, Thompson SG, Wilcox M. The benefits and harms of breast cancer screening: an independent review. Br J Cancer. 2013;108:2205–2240. doi:10.1038/bjc.2013.177
29. Gennari A, D’amico M, Corradengo D. Extending the duration of first-line chemotherapy in metastatic breast cancer: a perspective review. Therapeut Adv Med Oncol. 2011;3:229–232. doi:10.1177/1758834011413423
30. Basu P, Tripathi R, Mehrotra R, Ray K, Srivastava A, Srivastava A. Role of integrative medicine in the continuum of care of breast cancer patients in the Indian context. Cancer Causes Control. 2021;32:429–440. doi:10.1007/s10552-021-01399-0
31. Hudis CA, Gianni L. Triple‐negative breast cancer: an unmet medical need. Oncologist. 2011;16:1–11. doi:10.1634/theoncologist.2011-S1-01
32. Bleicher RJ, Ruth K, Sigurdson ER, et al. Time To surgery and breast cancer survival in the United States. JAMA Oncol. 2016;2:330–339. doi:10.1001/jamaoncol.2015.4508
33. Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, Asnis-Alibozek A. Adherence To Initial Adjuvant Anastrozole Therapy Among Women With Early-Stage Breast Cancer. J Clin oncol. 2008;26:556–562. doi:10.1200/JCO.2007.11.5451
34. McLaughlin R, Hylton N. MRI In Breast Cancer Therapy Monitoring. NMR Biomed. 2011;24:712–720. doi:10.1002/nbm.1739
35. Duygulu G, Oktay A, Bilgen IG, Kapkaç M, Zekioğlu O. The role of breast MRI in planning the surgical treatment of breast cancer. Diagn Interv Radiol. 2012;18:460–467. doi:10.4261/1305-3825.DIR.5429-11.2
36. Christensen D, Shehata M, Javid S, Rahbar H, Lam D. Preoperative breast MRI: current evidence and patient selection. J Br Imag. 2023;5:112–124. doi:10.1093/jbi/wbac088
37. Schauer T, Henriksson A, Strandberg E, et al. Pre-treatment levels of inflammatory markers and chemotherapy completion rates in patients with early-stage breast cancer. Internat J Clin Oncol. 2023;28:89–98. doi:10.1007/s10147-022-02255-0
38. Groen WG, Naaktgeboren WR, Van Harten WH, et al. Physical fitness and chemotherapy tolerance in patients with early-stage breast cancer. Med Sci Sport Exer. 2022;54:537–542. doi:10.1249/MSS.0000000000002828
39. Buda-Nowak A, Kwinta Ł, Potocki P, et al. Metronomic chemo-endocrine therapy (FulVEC) as a salvage treatment for patients with advanced, treatment-refractory ER+/HER2-breast cancer—a retrospective analysis of consecutive patients data. J Clin Med. 2023;12:1350. doi:10.3390/jcm12041350
40. Bonilla L, Ben-Aharon I, Vidal L, Gafter-Gvili A, Leibovici L, Stemmer SM. Dose-dense chemotherapy in nonmetastatic breast cancer: a systematic review and meta-analysis of randomized controlled trials. J National Cancer Inst. 2010;102:1845–1854. doi:10.1093/jnci/djq409
41. Chikermane SG, Sharma M, Abughosh SM, Aparasu RR, Trivedi MV, Johnson ML. Dose-dependent relation between metformin and the risk of hormone receptor-positive, Her2-negative breast cancer among postmenopausal women with type-2 diabetes. Br Can Res Treat. 2022;195:421–430. doi:10.1007/s10549-022-06706-0
42. Ben-Ari E. Shorter course of radiation is effective, safe for some with early-stage breast cancer; 2022. Available from: https://www.cancer.gov/news-events/cancer-currents-blog/2022/early-breast-cancer-shorter-radiation-therapy.
43. Jones B, Green S. Modern radiation techniques in early stage breast cancer for the breast radiologist. Clin Imaging. 2021;80:19–25. doi:10.1016/j.clinimag.2021.06.035
44. Nassif AB, Talib MA, Nasir Q, Afadar Y, Elgendy O. Breast Cancer Detection Using Artificial Intelligence Techniques: a Systematic Literature Review. Artif. Intell. Med. 2022;127:102276. doi:10.1016/j.artmed.2022.102276
45. Yusoff M, Haryanto T, Suhartanto H, Mustafa WA, Zain JM, Kusmardi K. Accuracy analysis of deep learning methods in breast cancer classification: a structured review. Diagnostics. 2023;13:683. doi:10.3390/diagnostics13040683
46. Chopra S, Khosla M, Vidya R. Innovations and challenges in breast cancer care: a review. Medicina. 2023;59:957. doi:10.3390/medicina59050957
47. Sabih QA, Lupinacci KM, Diego EJ. Current recommendations and role of breast conservation surgery for multifocal or multicentric breast cancer. Curr Br Can Rep. 2023;1:1–9.
48. Sabatier R, Gonçalves A, Bertucci F. Personalized medicine: present and future of breast cancer management. Crit Rev Oncol/Hematol. 2014;91:223–233. doi:10.1016/j.critrevonc.2014.03.002
49. Jun Z, Yu X, Xiaomei Z, et al. Current landscape of personalized clinical treatments for triple-negative breast cancer. Front Pharmacol. 2022;13:1.
50. Ahmed KA, Grass GD, Orman AG, et al. Personalizing radiation treatment delivery in the management of breast cancer. Internat J Br Can. 2018;2018. doi:10.1155/2018/6729802
51. Condorelli R, Vaz-Luis I. Managing side effects in adjuvant endocrine therapy for breast cancer. Expert Rev Anticancer Ther. 2018;18:1101–1112. doi:10.1080/14737140.2018.1520096
52. Odle TG. Adverse effects of breast cancer treatment. Radiol Technol. 2014;85:297M–319M.
53. Palesh O, Scheiber C, Kesler S, Mustian K, Koopman C, Schapira L. Management of side effects during and post‐treatment in breast cancer survivors. Br J. 2018;24:167–175. doi:10.1111/tbj.12862
54. Wanchai A, Anderson EA, Armer JM. A systematic review of m-health apps on managing side effects of breast cancer treatment. Support Care Cancer. 2023;31:86. doi:10.1007/s00520-022-07464-x
55. Dear RF, McGeechan K, Jenkins MC, Barratt A, Tattersall MH, Wilcken N. combination versus sequential single agent chemotherapy for metastatic breast cancer. Cochrane Database Syst Rev. 2013;12:1.
56. Carrick S, Parker S, Thornton C, Ghersi D, Simes J, Wilcken N. Single agent versus combination chemotherapy for metastatic breast cancer. Cochrane Database Syst Rev. 2009;2:1.
57. Van Wyhe RD, Rahal OM, Woodward WA. Effect of statins on breast cancer recurrence and mortality: a review. Br Can. 2017;1:559–565.
58. Blank PR, Dedes KJ, Szucs TD. Cost effectiveness of cytotoxic and targeted therapy for metastatic breast cancer. Pharmacoeconomics. 2010;28:629–647. doi:10.2165/11535560-000000000-00000
59. Mann RM, Athanasiou A, Baltzer PAT, et al. Breast cancer screening in women with extremely dense breasts recommendations of the European society of breast imaging (EUSOBI). Eur Radiol. 2022;32:4036–4045. doi:10.1007/s00330-022-08617-6
60. Offersen BV, Alsner J, Nielsen HM, et al. Hypofractionated versus standard fractionated radiotherapy in patients with early breast cancer or ductal carcinoma in situ in a randomized Phase III trial: the DBCG HYPO Trial. J Clin oncol. 2020;38:3615–3625. doi:10.1200/JCO.20.01363
61. Cancer Council Australia. Optimal care pathway for people with breast cancer: quick reference guide; 2021. Available from: https://www.cancer.org.au/assets/pdf/breast-cancer-quick-reference-guide.
62. Aitken LA, Hossan SZ. The psychological distress and quality of life of breast cancer survivors in Sydney, Australia. In Healthcare. 2022;2017:10.
63. Henry DH, Viswanathan HN, Elkin EP, Traina S, Wade S, Cella D. Symptoms and treatment burden associated with cancer treatment: results from a cross-sectional national survey in the US. Support Care Cancer. 2008;16:791–801. doi:10.1007/s00520-007-0380-2
64. Perry LM, Hoerger M, Seibert K, Gerhart JI, O’Mahony S, Duberstein PR. Financial strain and physical and emotional quality of life in breast cancer. J Pain Sympt Manage. 2019;58:454–459. doi:10.1016/j.jpainsymman.2019.05.011
65. Spence D, Morstyn L, Wells K. The support and information needs of women with secondary breast cancer (Breast Cancer Network Australia); 2015. Available from: https://www.bcna.org.au/media/2936/bcn1166-sbc-report-2015.pdf.
66. Mehnert A. Employment And Work-Related Issues In Cancer Survivors. Crit Rev Oncol Hematol. 2011;77:109–130. doi:10.1016/j.critrevonc.2010.01.004
67. Schmidt ME, Scherer S, Wiskemann J, Steindorf K. Return to work after breast cancer: the role of treatment‐related side effects and potential impact on quality of life. European Journal of Cancer Care. 2019;28:e13051. doi:10.1111/ecc.13051
68. Al-Rashdan A, Roumeliotis M, Quirk S, et al. Adapting radiation therapy treatments for patients with breast cancer during the COVID-19 pandemic: hypo-fractionation and accelerated partial breast irradiation to Address world health organization recommendations. Adv Radiat Oncol. 2020;5:575–576. doi:10.1016/j.adro.2020.03.011
69. Li L, Chang B, Jiang X, et al. Clinical outcomes comparison of 10 years versus 5 years of adjuvant endocrine therapy in patients with early breast cancer. BMC Cancer. 2018;18:1–11. doi:10.1186/s12885-018-4878-4
70. Wolff JL, Aufill J, Echavarria D, et al. A randomized intervention involving family to improve communication in breast cancer care. NPJ Breast Cancer. 2021;14.
71. National Health Service (NHS). Information sharing in multidisciplinary teams (MDTs); 2022. Available from: https://transform.england.nhs.uk/information-governance/guidance/information-governance-guidance-support-multidisciplinary-teams-mdts/.
72. Miller R. Implementing a survivorship care plan. Clin J Oncol Nurs. 2007;12:759–767.
73. Houserková D, Zlámalová N, Spáčilová K, et al. Role of the radiologist during neoadjuvant systemic therapy for breast cancer. Rozhl Chir. 2021;100:285–294. doi:10.33699/PIS.2021.100.6.285-294
74. Carroll-Johnson R. Redefining interdisciplinary practice. Oncol nurs forum. 2001;28:619.
75. Hewitt M, Greenfield S, Stovall E. Institute of Medicine & National Research Council (IOM & NRC). In: From Cancer Patient to Cancer Survivor: Lost in Transition. Washingon, DC: National Academies Press; 2006.
76. Taylor C, Shewbridge A, Harris J, Green JS. Benefits of multidisciplinary teamwork in the management of breast cancer. Breast Cancer. 2013;30:79–85.
77. Spalluto L, Bonnet K, Sonubi C, et al. Barriers to implementation of breast cancer risk assessment: the health care team perspective. J Am College Radiol. 2023;20:342–351.
78. Kesson EM, Allardice GM, George WD, Burns HJG, Morrison DS. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13,722 women. BMJ. 2012;344:e2718. doi:10.1136/bmj.e2718
79. Pangarsa EA, Rizky D, Tandarto K, et al. The effect of multidisciplinary team on survival rates of women with breast cancer: a systematic review and meta-analysis. Ann Med Surg Lond. 2023;85:2940–2948. doi:10.1097/MS9.0000000000000914
80. Kurniasih DAA, Setiawati EP, Pradipta IS, Subarnas A. Interprofessional collaboration in the breast cancer unit: how do healthcare workers see it? BMC Womens Health. 2022;22:1–9. doi:10.1186/s12905-022-01818-7
81. Van Veenendaal H, Voogdt-Pruis HR, Ubbink DT, Hilders CGJM. Effect of a multilevel implementation programme on shared decision-making in breast cancer care. BJS Open. 2021;5:zraa002. doi:10.1093/bjsopen/zraa002
82. Singleton AC, Raeside R, Hyun KK, et al. electronic health interventions for patients with breast cancer: systematic review and meta-analyses. J Clin Oncol. 2022;40:2257.
83. Khoshnazar TAK, Rassouli M, Akbari ME, et al. Communication needs of patients with breast cancer: a qualitative study. Indian J Palliat Care. 2016;22:402. doi:10.4103/0973-1075.191763
84. Di Giacomo D, Ranieri J, Donatucci E, et al. Emotional “patient-oriented” support in young patients with i–ii stage breast cancer: pilot study. Front Psychol. 2018;9:2487. doi:10.3389/fpsyg.2018.02487
85. Van de Water LF, Van Kleef JJ, Dijksterhuis WPM, et al. Communicating treatment risks and benefits to cancer patients: a systematic review of communication methods. Qual Life Res. 2020;29:1747–1766. doi:10.1007/s11136-020-02503-8
86. Sebri V, Durosini I, Mazzoni D, Pravettoni G. Breast cancer survivors’ motivation to participate in a tailored physical and psychological intervention: a qualitative thematic analysis. Behav Sci. 2022;12:271. doi:10.3390/bs12080271
87. Luleci D, Kilic B. Factors affecting women’s participation in breast cancer screening in Turkey. Asian Pac J Cancer Prev. 2022;23:1627. doi:10.31557/APJCP.2022.23.5.1627
88. Durosini I, Savioni L, Triberti S, Guiddi P, Pravettoni G. The motivation journey: a grounded theory study on female cancer survivors’ experience of a psychological intervention for quality of life. Int J Environ Res Public Health. 2021;18:950. doi:10.3390/ijerph18030950
89. Coletta AM, Basen-Engquist KM, Schmitz KH. Exercise across the cancer care continuum: why it matters, how to implement it, and motivating patients to move. Am Soc Clin Oncol Educat Book. 2022;42:932–938. doi:10.1200/EDBK_349635
90. Porro B, Campone M, Moreau P, Roquelaure Y. Supporting the return to work of breast cancer survivors: from a theoretical to a clinical perspective. Int J Environ Res Public Health. 2022;19:5124. doi:10.3390/ijerph19095124
91. Karim UN, Setiyadi A, Efrida Y. Family support on motivation of compliance and chemotherapy protocol among patients with breast cancer. J Holis Nurs Sci. 2023;10:22–26. doi:10.31603/nursing.v0i0.7792
92. Aprilianto E, Lumadi SA, Handian FI. Family social support and the self-esteem of breast cancer patients undergoing neoadjuvant chemotherapy. J Publ Health Res. 2021;10:jphr–2021. doi:10.4081/jphr.2021.2234
93. Kusi G, Boamah Mensah AB, Boamah Mensah K, Dzomeku VM, Apiribu F, Duodu PA. Caregiving motivations and experiences among family caregivers of patients living with advanced breast cancer in Ghana. PLoS One. 2020;15:e0229683. doi:10.1371/journal.pone.0229683
94. Kočo L, Weekenstroo HH, Lambregts DM, et al. The effects of multidisciplinary team meetings on clinical practice for colorectal, lung, prostate and breast cancer: a systematic review. Cancers. 2021;13:4159. doi:10.3390/cancers13164159
95. Barrios H. Global challenges in breast cancer detection and treatment. Breast. 2022;62:S3–S6. doi:10.1016/j.breast.2022.02.003
96. Ebben KC, Hendriks MP, Markus L, et al. Using guideline-based clinical decision support in oncological multidisciplinary team meetings: a prospective, multicenter concordance study. Int J Qual Health Care. 2022;34:mzac007. doi:10.1093/intqhc/mzac007
97. Freeman ALJ. How To communicate evidence to patients. Drug Therap Bulletin. 2019;57:119–124. doi:10.1136/dtb.2019.000008
98. Ancker JS, Senathirajah Y, Kukafka R, Starren JB. Design features of graphs in health risk communication: a systematic review. J Am Med Inf Assoc. 2006;13:608–618. doi:10.1197/jamia.M2115
99. Shim EJ, Park J, Yi M, Jung D, Lee KM, Hahm BJ. Tailoring communications to the evolving needs of patients throughout the cancer care trajectory: a qualitative exploration with breast cancer patients. BMC Women’s Health. 2016;16:65. doi:10.1186/s12905-016-0347-x
100. Bruce JG, Tucholka JL, Steffens NM, Neuman HB. Quality of online information to support patient decision‐making in breast cancer surgery. J Surg Oncol. 2015;112:575–580. doi:10.1002/jso.24046
101. Conroy M, Powell M, Suelzer E, et al. Electronic medical record–based electronic messaging among patients with breast cancer: a systematic review. Appl Clin Inform. 2023;14:134–143. doi:10.1055/a-2004-6669
102. Huang Y, Li Q, Zhou F, Song J. Effectiveness of internet-based support interventions on patients with breast cancer: a systematic review and narrative synthesis. BMJ open. 2022;12:e057664. doi:10.1136/bmjopen-2021-057664
103. Høybye MT, Johansen C, Tjørnhøj-Thomsen T. Online Interaction. Effects of storytelling in an internet breast cancer support group. Psycho-Oncology. 2005;14:211–220. doi:10.1002/pon.837
104. Orgad S. Storytelling Online: Talking Breast Cancer on the Internet. New York. USA: Peter Lang Publishing; 2005.
105. Hong YA, Hossain MM, Chou WYS. Digital interventions to facilitate patient‐provider communication in cancer care: a systematic review. Psycho Oncol. 2020;29:591–603. doi:10.1002/pon.5310
106. Triberti S, Savioni L, Sebri V, Pravettoni G. Ehealth for improving quality of life in breast cancer patients: a systematic review. Cancer Treat Rev. 2019;74:1–14. doi:10.1016/j.ctrv.2019.01.003
107. Bender JL, Katz J, Ferris LE, Jadad AR. What is the role of online support from the perspective of facilitators of face-to-face support groups? A multi-method study of the use of breast cancer online communities. Pat Educat Couns. 2013;93:472–479. doi:10.1016/j.pec.2013.07.009
108. Setoyama Y, Yamazaki Y, Nakayama K. Comparing support to breast cancer patients from online communities and face-to-face support groups. Pat Educ Couns. 2011;85:e95–e100. doi:10.1016/j.pec.2010.11.008
109. Mayor S. Five year survival rate of women whose cancer is detected by screening has risen to 96.4%. BMJ. 2008;336:1398–1399. doi:10.1136/bmj.a373
110. The Australian Institute of Health and Welfare. Breast cancer survival very high with early detection; 2007. Available from: https://www.aihw.gov.au/news-media/media-releases/2007/oct/breast-cancer-survival-very-high-with-early-detect.
111. Office on Women Health (OWH). 99% percent survival rate for breast cancer caught early; 2022. Available from: https://www.womenshealth.gov/blog/99-percent-survival-rate-breast-cancer-caught-early.
112. Edib Z, Kumarasamy V, Binti Abdullah N, Rizal AM, Al-Dubai SAR. Most prevalent unmet supportive care needs and quality of life of breast cancer patients in a tertiary hospital in Malaysia. Health Qual Life Outc. 2016;14:1–10. doi:10.1186/s12955-016-0428-4
113. Scharl A, Kühn T, Papathemelis T, Salterberg A. The right treatment for the right patient–personalised treatment of breast cancer. Geburtshilfe Frauenheilkd. 2015;75:683–691. doi:10.1055/s-0035-1546270
114. Selvan P, Hriso C, Mitchell J, Newberg A. Systematic review of yoga for symptom management during conventional treatment of breast cancer patients. Complement Therap Clin Pract. 2022;48. doi:10.1016/j.ctcp.2022.101581
115. Al-Hosni K, Chan MF, Al-Azri M. Effectiveness of an educational program on awareness of breast cancer risk factors, symptoms, and barriers to seeking medical help among adolescent Omani school students—an interventional study. Current Oncol. 2023;30:4126–4138. doi:10.3390/curroncol30040314
116. Rakhshani T, Dada M, Kashfi SM, Kamyab A, Jeihooni AK. The effect of educational intervention on knowledge, attitude, and practice of women towards breast cancer screening. Internat J Br Can. 2022;2022. doi:10.1155/2022/5697739
117. Sisler J, Chaput G, Sussman J, Ozokwelu E. Follow-up after treatment for breast cancer: practical guide to survivorship care for family physicians. Can Family Phys. 2016;62:805–811.
118. Epplein M, Zheng Y, Zheng W, et al. Quality of life after breast cancer diagnosis and survival. J Clin oncol. 2011;29:406. doi:10.1200/JCO.2010.30.6951
119. De Aguiar SS, Bergmann A, Mattos IE. Quality of life as a predictor of overall survival after breast cancer treatment. Qual Life Res. 2014;23(2):627–637. doi:10.1007/s11136-013-0476-8
120. Chen LH, Irwin MR, Olmstead R, Haque R. Association of physical activity with risk of mortality among breast cancer survivors. JAMA Network Open. 2022;5:e2242660–e2242660. doi:10.1001/jamanetworkopen.2022.42660
121. Saad ED, Katz A, Hoff PM, Buyse M. Progression-free survival as surrogate and as true end point: insights from the breast and colorectal cancer literature. Ann Oncol. 2010;21:7–12. doi:10.1093/annonc/mdp523
122. Zeichner SB, Koru-Sengul T, Shah N, et al. Improved clinical outcomes associated with vitamin d supplementation during adjuvant chemotherapy in patients With HER2+ nonmetastatic breast cancer. Clin Br Can. 2015;15:e1–e11. doi:10.1016/j.clbc.2014.08.001
123. Diniz RW, Guerra MR, Cintra JRD, Fayer VA, Teixeira MTB. Disease-Free Survival In Patients With Non-Metastatic Breast Cancer. Rev Assoc Med Bras. 2016;62:407–413. doi:10.1590/1806-9282.62.05.407
124. Bhardwaj PV, Mason H, Kaufman SA, Visintainer P, Makari-Judson G. Outcomes of a multidisciplinary team in the management of patients with early-stage breast cancer undergoing neoadjuvant chemotherapy at a community cancer center. Current Oncol. 2023;30:4861–4870. doi:10.3390/curroncol30050366
125. Lu J, Jiang Y, Qian M, Lv L, Ying X. The improved effects Of A multidisciplinary team on the survival of breast cancer patients: experiences from China. Int J Environ Res Public Health. 2020;17:277. doi:10.3390/ijerph17010277
126. Niederhuber J, Armitage J, Doroshow J, Kastan M, Tepper J. Abeloff’s Clinical Oncology.
127. Chan CWH, Law BMH, So WKW, Chow KM, Waye MMY. Novel strategies on personalized medicine for breast cancer treatment: an update. Int J Mol Sci. 2017;18:2423. doi:10.3390/ijms18112423
128. Hamer J, Warner E. Lifestyle modifications for patients with breast cancer to improve prognosis and optimize overall health. Cmaj. 2017;189:E268–E274. doi:10.1503/cmaj.160464
129. Neuss MN, Desch CE, McNiff KK, et al. A process for measuring the quality of cancer care: the quality oncology practice initiative. J Clin Oncol. 2005;23:6233–6239. doi:10.1200/JCO.2005.05.948
130. Fayanju O, Mayo TL, Spinks TE, et al. Value-based breast cancer care: a multidisciplinary approach for defining patient-centered outcomes. Ann Surg Oncol. 2016;23:2385–2390. doi:10.1245/s10434-016-5184-5
131. Ebben RH, Vloet L, Verhofstad MH, Meijer S, Groot JA, van Achterberg T. Adherence to guidelines and protocols in the prehospital and emergency care setting: a systematic review. Scand J Trauma Resusc Emerg Med. 2013;21:1–16. doi:10.1186/1757-7241-21-9
132. Hung DY. lean practices for resource use, timeliness, and coordination of care in breast cancer navigation. Number 5. 2022;26:503–509.
133. Cottu P, Ramsey SD, Sola-Morales O, Spears PA, Taylor L. The emerging role of real-world data in advanced breast cancer therapy: recommendations for collaborative decision-making. Breast. 2022;61:118–122. doi:10.1016/j.breast.2021.12.015
134. Pfob A, Mehrara BJ, Nelson JA, Wilkins EG, Pusic AL, Sidey-Gibbons C. Towards patient-centered decision-making in breast cancer surgery: machine learning to predict individual patient-reported outcomes at 1-year follow-up. Ann Surg. 2023;277:e144. doi:10.1097/SLA.0000000000004862
135. Basile LJ, Carbonara N, Pellegrino R, Panniello U. Business intelligence in the healthcare industry: the utilization of a data-driven approach to support clinical decision making. Technovation. 2023;120:102482. doi:10.1016/j.technovation.2022.102482
136. Avril N, Sassen S, Roylance R. Response to therapy in breast cancer. J Nucl Med. 2009;50:55S–63S. doi:10.2967/jnumed.108.057240
137. Cancer.net. Breast cancer: types of treatment; 2022. Available from: https://www.cancer.net/cancer-types/breast-cancer/types-treatment.
138. Jayaraman M, Shanmugavadivu P, Riyaz NK. Multimodal data-driven intelligent systems for breast cancer prediction–a review. Eur Chem Bull. 2023;12:4349–4357.
139. Amethiya Y, Pipariya P, Patel S, Shah M. Comparative analysis of breast cancer detection using machine learning and biosensors. Intel Med. 2022;2:69–81. doi:10.1016/j.imed.2021.08.004
140. Baughan N, Douglas L, Giger MLP. Present, and future of machine learning and artificial intelligence for breast cancer screening. J Br Imag. 2022;4:451–459. doi:10.1093/jbi/wbac052
141. Munro AJ, Swartzman S. What Is A virtual multidisciplinary team (vMDT)? Br J Cancer. 2013;108:2433–2441. doi:10.1038/bjc.2013.231
142. Nemade V, Pathak S, Dubey AK. A systematic literature review of breast cancer diagnosis using machine intelligence techniques. Arch. Comput. Methods Eng. 2022;29:4401–4430. doi:10.1007/s11831-022-09738-3
143. Chowdhary CL, Khare N, Patel H, Koppu S, Kaluri R, Rajput DSP. Present and future of gene feature selection for breast cancer classification–a survey. Internat J Engin Syst Mod Simul. 2022;13:140–153. doi:10.1504/IJESMS.2022.123355
144. Van Baelen K, Geukens T, Maetens M, et al. Current and future diagnostic and treatment strategies for patients with invasive lobular breast cancer. Ann Oncol. 2022;33:769–785. doi:10.1016/j.annonc.2022.05.006
145. Cancer Research UK. Achieving world-class cancer outcomes; 2015. Available from: https://www.cancerresearchuk.org/sites/default/files/achieving_world-class_cancer_outcomes_-_a_strategy_for_england_2015-2020.pdf.
146. Blackwood O, Deb R. Multidisciplinary team approach in breast cancer care: benefits and challenges. Indian J Pathol Microbiol. 2020;63:105. doi:10.4103/IJPM.IJPM_885_19
147. Scaggion A, Fusella M, Agnello G, et al. Limiting treatment plan complexity by applying a novel commercial tool. J Appl Clin Med Phys. 2020;21:27–34. doi:10.1002/acm2.12908
148. Lenti MV, Sottotetti F, Corazza GR. Tackling the clinical complexity of breast cancer. Drugs in Context. 2022;11. doi:10.7573/dic.2022-2-3
149. Kimbung S, Loman N, Hedenfalk I. Clinical And Molecular Complexity Of Breast Cancer Metastases. Semi Cancer Biol. 2015;35:85–95.
150. Sumpio C, Knobf MT, Jeon S. Treatment complexity: a description of chemotherapy and supportive care treatment visits in patients with advanced-stage cancer diagnoses. Support Care Cancer. 2016;24:285–293. doi:10.1007/s00520-015-2775-9
151. Pan American Health Organization (PAHO). Planning: improving access to breast cancer care; 2016. Available from: https://www.paho.org/en/documents/planning-improving-access-breast-cancer-cure.
152. Dobkowski D. The complexity of decision-making for early-stage breast cancer treatment; 2021. Available from: https://www.curetoday.com/view/the-complexity-of-decision-making-for-early-stage-breast-cancer-treatment.
153. American Society of Clinical Oncology (ASCO). ASCO Guidelines; 2023. Available from: https://old-prod.asco.org/practice-patients/guidelines.
154. National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology; 2021. Available from: https://www.nccn.org/guidelines/guidelines-process/about-nccn-clinical-practice-guidelines.
155. Van Eenbergen MC, Van de Poll-Franse LV, Heine P, Mols F. The impact of participation in online cancer communities on patient reported outcomes: systematic review. JMIR Cancer. 2017;3:e7312. doi:10.2196/cancer.7312
156. Guan M, Han JY, Shah DV, Gustafson DH. Exploring the role of social support in promoting patient participation in health care among women with breast cancer. Health Communication. 2021;36:1581–1589. doi:10.1080/10410236.2020.1773704
157. Conner K. Treatment side effects depression; 2023. Available from: https://www.breastcancer.org/treatment-side-effects/depression.
158. Feng Y, Spezia M, Huang S, et al. Breast cancer development and progression: risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018;5:77–106.
159. Rankin NM, Lai M, Miller D, et al. Cancer multidisciplinary team meetings in practice: results from a multi‐institutional quantitative survey and implications for policy change. Asia Pacific J Clin Oncol. 2018;14:74–83. doi:10.1111/ajco.12765
160. Haq R, Kong A, Gulasingam P. A multidisciplinary approach to implement personalized breast cancer treatment and care plans. Current Oncol. 2021;28:767–782.
161. Lee PY, Cheong AT, Ghazali SS, et al. Barriers of and strategies for shared decision-making implementation in the care of metastatic breast cancer: a qualitative study among patients and healthcare professionals in an Asian country. Health Expect. 2022;25:2837–2850. doi:10.1111/hex.13590
162. Gravel K, Légaré F, Graham ID. Barriers and facilitators to implementing shared decision-making in clinical practice: a systematic review of health professionals’ perceptions. Implementation Sci. 2006;1:16.
163. Taberna M, Gil Moncayo F, Jane-Salas E, Antonio M, Arribas L, Vilajosana E. The multidisciplinary team (MDT) approach and quality of care. Front Oncol. 2020;10. doi:10.3389/fonc.2020.00085
164. Selby P, Popescu R, Lawler M, Butcher H, Costa A. The value and future developments of multidisciplinary team cancer care. Am Soc Clin Oncol Educat Book. 2019;39:332–340. doi:10.1200/EDBK_236857
165. Cancer Research UK. Improving the effectiveness of multidisciplinary team meetings in cancer services; 2017. Available from: https://www.cancerresearchuk.org/about-us/we-develop-policy/our-policy-on-cancer-services/improving-The-effectiveness-of-mdts-in-cancer-services.
166. Gandamihardja TAK, Soukup T, McInerney S, Green JSA, Sevdalis N. Analysing breast cancer multidisciplinary patient management: a prospective observational evaluation of team clinical decision-making. World J Surg. 2019;43:559–566. doi:10.1007/s00268-018-4815-3
167. Cancer Australia. All About Multidisciplinary Care; 2023. Available from: https://www.canceraustralia.gov.au/clinicians-hub/multidisciplinary-care/all-about-multidisciplinary-care.
168. Lamb BW, Sevdalis N, Vincent C, Green JS. Development and evaluation of a checklist to support decision making in cancer multidisciplinary team meetings: MDT-quic. Ann Surg Oncol. 2012;19:1759–1765. doi:10.1245/s10434-011-2187-0
169. McCain DV. Facilitation Basics. Alexandria, USA: The Association for Talent Development; 2015.
170. Odeh Y, Al-Balas M. Implications of agile values in software engineering for agility in breast cancer treatment: protocol for a comparative study. JMIR Res Prot. 2023;12:e53124. doi:10.2196/53124
171. Alzyadat W, Muhairat M, Alhroob A, Rawashdeh T. A recruitment big data approach to interplay of the target drugs. Internat J Adv Soft Comput. 2022;14:02–13. doi:10.15849/ZUJIJASACA.220328.01
172. Abdallah M, Salah M. Artificial Intelligence and Intellectual properties: legal and ethical considerations. Int J Intell Syst and Appl Eng. 2024;12:368–376.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
