Back to Journals » OncoTargets and Therapy » Volume 17
Thyroid Metastases from Triple-Negative Breast Cancer with High PD-L1 Expression – A Rare Presentation
Authors Meng W, Guo Q, Tang G , Han G, Ma G, Zhang Q, Li R, Liu S, Yu G
Received 8 August 2023
Accepted for publication 27 November 2023
Published 14 February 2024 Volume 2024:17 Pages 103—107
DOI https://doi.org/10.2147/OTT.S428745
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Lukas Hawinkels
Wenjuan Meng,1,* Qingxia Guo,2 Gaoyan Tang,1 Guiyan Han,3 Guikai Ma,1 Qingyun Zhang,1 Rui Li,1 Shuzhen Liu,1,* Guohua Yu1
1Department of Oncology, Weifang People’s Hospital (The First Affiliated Hospital of Shandong Second Medical University), Weifang, Shandong, 261041, People’s Republic of China; 2Department of Oncology, Junan People’s Hospital, Junan, Shandong, 276600, People’s Republic of China; 3Department of Pathology, Weifang People’s Hospital (The First Affiliated Hospital of Shandong Second Medical University), Weifang, Shandong, 261041, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guohua Yu, Department of Oncology, Weifang People’s Hospital (The First Affiliated Hospital of Shandong Second Medical University), Weifang, Shandong, 261041, People’s Republic of China, Email [email protected]
Abstract: Thyroid metastases secondary to triple-negative breast cancer are sporadic. Diagnosis usually requires fine needle aspiration biopsy (FNAB) and immunohistochemistry. There are no treatment guidelines for this type of cancer, and to date, reports of chemotherapy combined with immunotherapy in thyroid metastases are very rare. Here, we first report the effectiveness of anti-PD-1 inhibitor in combination with chemotherapy for the treatment of metastatic thyroid cancer secondary to advanced triple-negative breast cancer with high expression of programmed cell death ligand 1 (PD-L1). Following six cycles of albumin paclitaxel (400mg d1/21 days) plus PD-1 antibody inhibitor (Sindilizumab 200mg d1/21 days), the patient experienced significant relief of neck swelling and obstructive feeding, both the thyroid metastases and the right breast lesion regressed completely following six cycles of treatment. Chemotherapy combined with immunotherapy may provide a new direction for unresectable advanced thyroid metastases.
Keywords: thyroid metastases, triple-negative breast cancer, albumin paclitaxel, anti-PD-1 inhibitor, effectiveness
Case Presentation
A 54-year-old middle-aged woman presented to our hospital with a swollen and uncomfortable neck with a feeling of obstruction to eating.
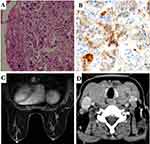
One week ago, she went to a local hospital where ultrasound of the neck showed diffuse enlargement of the thyroid gland, bilateral enlarged lymph nodes in the neck. The lymph nodes in the supraclavicular fossa were enlarged bilaterally. Ultrasound-guided fine-needle aspiration biopsy of the thyroid mass showed: invasive ductal carcinoma of mammary origin (Figure 1A), grade II, immunohistochemistry: ER (-), PR (-), GATA-3 (+), P120 (+), Ecad (+), TTF-1 (-), CK7 (+), CK20 (-), Villin (-), CK5/6 (-). PD-L1 expression was assessed by immunohistochemistry using a rabbit monoclonal anti-human PD-L1 antibody (Ventana SP142); the percentage of positive tumour cells was 26–49% (Figure 1B).
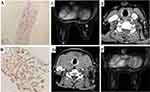
After admission, thyroid function tests: thyroglobulin autoantibodies (A-TG) 20.20 IU/mL (0–60); free triiodothyronine (FT3) 8.36 pmol/L (3.5–6.5); free thyroxine (FT4) 36.15 pmol/L (11.5–22.7); thyrotropin (TSH) 0.01 uIU/mL (0.55–4.78); thyroid peroxidase autoantibodies (A-TPO) <28.00 IU/mL (0–60). Breast MR showed a right breast nodule, BI-RADS category 4 (1.1cm × 0.8cm × 0.8cm) (Figure 1C). CT of the neck, chest and abdomen showed primary breast cancer with thyroid metastases (Figure 1D), multiple enlarged lymph nodes in the neck and bilateral axillae, solid occupancy in the upper lobe of the left lung, mediastinal and bilateral hilar lymph node metastases, left pleural effusion and increased density in the S1 vertebrae. Ultrasound-guided puncture biopsy of the right breast mass: invasive carcinoma of the breast (Figure 2A), NOS, grade III (3 points for glandular ducts + 2 points for nuclear grade + 3 points for nuclear schizophrenia = 8 points). Immunohistochemistry: GATA-3 (+), CK7 (+), CA153 (partial +), AR (-), ER (-), PR (-), C-erbB-2 (1+), CK5/6 (-), TTF-1 (-), GCDFP-15 (-), Ki-67 index (65%). Expression of the PD-L1 protein was evaluated with an immunohistochemical method using a mouse monoclonal anti-human PD-L1 antibody (Clone 22C3, Dako); PD-L1 positive tumor cells within the tumor tissue specimen were 80% (Figure 2B). The BRCA test germline was benign (BRCA1: C.4884T > C p.M1628T heterozygous mutation, germline, etc., benign mutation, BRCA2c.3396A > G p.K1132 = heterozygous mutation germline, etc., benign mutation). The diagnosis was triple negative adenocarcinoma of the right breast (cT1cN3M1, stage IV) according to the AJCC 8th edition staging criteria.
After a multidisciplinary consultation and discussion, the metastatic thyroid cancer was advanced and not indicated for surgery. We recommended albumin paclitaxel combined with anti-PD-L1 inhibitor as the first-line therapy for her, which the patient refused for financial reasons and requested chemotherapy alone. Then, we administered TE regimen chemotherapy (docetaxel 75mg/m² d1 + epirubicin 75mg/m² d1).
The neck symptoms were reduced following one cycle of treatment. Unfortunately, after three cycles, the patient again developed a sensation of neck swelling and a repeat breast MR showed a significantly enlarged right breast mass (1.6cm × 1.2cm × 1.1cm) (Figure 2C). CT scan of the neck, chest and abdomen showed significantly enlarged metastases in the thyroid gland (Figure 2D) and multiple lymph node metastases in the bilateral neck and clavicular region, which were larger than before; solid occupancy in the upper lobe of the left lung did not change significantly and the overall evaluation was progressive disease. Considering that the anti-PD-1 antibody was covered by medical insurance, we chose the second-line treatment of (Sindilizumab 200mg d1/21 days). Excitingly, both the thyroid metastases (Figure 2E) and the right breast lesion (Figure 2F) regressed completely following six cycles of treatment. In addition, the metastatic lymph nodes in the neck were significantly reduced. The patient felt numbness in the hands and feet (Grade 2) and myelosuppression (Grade 3), which was considered to be an adverse reaction to albumin paclitaxel, and was then given monotherapy with Sindilizumab (once every 3 weeks), and the patient now leads a high quality of life for seven months. The institution and the patient consented for the publication of the present case report.
Discussion
Up to now, we first reported thyroid metastases from triple-negative breast cancer with high PD-L1 expression. Because the thyroid is the second most arterialized organ in the body, metastases to the thyroid are rare. In recent years, cases of thyroid metastases have increased because of fine needle cytology and proton-emitting tomography of 18F-fluorodeoxyglucose.1,2 Large autopsy studies found that the incidence of thyroid metastases in patients with a history of cancer ranges from 1.9% to 24%.1,3–5 Two studies showed that thyroid metastases were more frequent than primitive thyroid cancer.1,3 In addition, the incidence of thyroid metastases in clinical and surgical series is 3%.6
Thyroid metastases are more common among women and appear independent of age.1 Time to detection and time from onset to death vary in different reports. The former ranged from 2 months to 15 years after the diagnosis of the primary cancer,3,7 while the latter ranged from 1 to 34 months.8 In one case, thyroid metastases were synchronized with the primary breast cancer.2
The clinical presentation of thyroid metastases is heterogeneous, with clinical manifestations in only a minority of patients and often incidentally detected by ultrasonography during postoperative follow-up. Thyroid metastases usually appear in the context of extensive metastatic disease, where the thyroid presentation is not clinically significant. On the other hand, when thyroid metastases are the first manifestation of recurrent disease, they usually present as a palpable painless neck mass or, although less frequently, as dysphagia, massive tracheal involvement, or dysphonia.9
In autopsy series, breast cancer, lung cancer and melanoma are the most frequent malignancies that metastasize to the thyroid gland.10 Clinical and surgical series of patients showed that breast cancer was the second most frequent primary tumor causing metastases to the thyroid after primary clear cell renal cancer.10,11 In reports containing histological information, the most prevalent breast cancer secondary to thyroid metastases was ductal invasive carcinoma.2,4,12 In six cases, the thyroid was the first and only site of recurrence.8 In 11 cases, breast cancer recurred at different sites, but metastatic sites other than the thyroid were not reported.13,14
In terms of diagnosis, thyroid ultrasound is currently considered difficult to distinguish between primary and secondary thyroid cancer, and ultrasonography generally shows irregular and heterogeneous lesions.9 In CT scans, thyroid metastases are hypodense, while in MR imaging, they appear isohyperintense compared to normal thyroid tissue.9 Fine needle cytology (FNA) is an effective method to diagnose benign and malignant thyroid nodules with an accuracy rate of more than 90%.14 Immunohistochemistry is another important diagnostic tool. GATA3 is a member of the important family of GATA transcription factors that mediates genetic differentiation of many cells and is a sensitive and specific immunomarker for breast cancer.15,16 GCDFP-15 is a highly specific and sensitive marker for breast cancer and can be used for differential diagnosis of breast cancer.17 It has been shown that GATA3 in combination with GCDFP-15 improves the diagnostic accuracy of metastatic breast carcinoma.18 TTF⁃1 is frequently expressed in thyroid cancer cells and lung adenocarcinoma cells. Although TTF⁃1 has been reported to be positively expressed in breast cancer,19,20 studies are still rare. PD-L1 expression in TNBC is approximately 20%.21 In our case, GATA3 and GCDFP-15 were positive, whereas TTF-1 was negative, which supports the diagnosis of secondary thyroid cancer; the expression of PD-L1 in tumor cells was 80%.
Treatment of thyroid metastases depends on the site of the primary tumor, the presence of other metastases, and the symptoms caused by the thyroid mass. Patients with single thyroid metastases should be treated surgically, whereas patients with multiple metastases from different organs should be given hormone therapy or chemotherapy for advanced breast cancer.22 Data on radiotherapy or chemotherapy for thyroid metastases are sporadic and limited.23 Atezolizumab, a PD-L1 inhibitor, combined with nab-paclitaxel, achieved remarkable success in a Phase III clinical study in advanced TNBC.24 In this patient, a PD-1/PD-L1 pathway inhibitor might lead to a good outcome given the high PD-L1 expression on the tumor cell surface. Currently, only the anti-PD-1 antibody Sintilimab is covered by medical insurance in our hospital. Considering the possible efficacy and the patients’ financial situation, we chose Sintilimab in combination with nab-paclitaxel. During the treatment, hyperthyroidism was present on admission and hypothyroidism developed after treatment was given, with possible causes being 1) destruction of thyroid tissue by the metastatic tumour, 2) effects of immunotherapy, and 3) effects of chemotherapeutic agents. Thyroid function tests were maintained in the normal range after adjustment of oral medication.
Conclusion
In our case, we first reported that nab-paclitaxel combined with anti-PD-1 inhibitor achieved complete response in the treatment of thyroid metastases secondary to breast cancer. We sincerely hope this case will draw more attention to researchers exploring the promise of immunotherapy in metastatic thyroid cancer and molecular indicators that may predict efficacy.
Ethics Statement
The study involving human participant was reviewed and approved by Weifang People’s Hospital. The patient provided her written informed consent to participate in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All authors declare that there is no conflicts of interest.
References
1. Chung AY, Tran TB, Brumund KT, Weisman RA, Bouvet M. Metastases to the thyroid: a review of the literature from the last decade. Thyroid. 2012;22(3):258–268. doi:10.1089/thy.2010.0154
2. Kim TY, Kim WB, Gong G, Hong SJ, Shong YK. Metastasis to the thyroid diagnosed by fine-needle aspiration biopsy. Clin Endocrinol. 2005;62(2):236–241. doi:10.1111/j.1365-2265.2005.02206.x
3. Wood K, Vini L, Harmer C. Metastases to the thyroid gland: the Royal Marsden experience. Eur J SurgOncol. 2004;30(6):583–588. doi:10.1016/j.ejso.2004.03.012
4. Gerges AS, Shehata SR, Gouda IA. Metastasis to the thyroid gland: unusual site of metastasis. J Egypt Natl Canc Inst. 2006;18(1):67–72.
5. Shimaoka K, Sokal JE, Pickren JW. Metastatic neoplasms in the thyroid gland. pathological and clinical findings. Cancer. 1962;15(3):557–565. doi:10.1002/1097-0142(196205/06)15:3<557::AID-CNCR2820150315>3.0.CO;2-H
6. Calzolari F, Sartori PV, Talarico C, et al. Surgical treatment of intrathyroid metastases: preliminary results of a multicentric study. Anticancer Res. 2008;28(5B):2885–2888.
7. Nakhjavani M, Gharib H, Coellner JR, van Heerden J. Metastases to the thyroid gland, a report of 43 cases. Cancer. 1997;79:574–578.
8. De Ridder M, Sermeus AB, Urbain D, Storme GA. Metastases to the thyroid gland-A report of six cases. Eur J Intern Med. 2003;14(6):377–379. doi:10.1016/S0953-6205(03)90005-7
9. Surov A, Machens A, Holzhausen HJ, Spielmann RP, Dralle H. Radiological features of metastases to the thyroid. Acta Radiol. 2016;57(4):444–450. doi:10.1177/0284185115581636
10. Molina Garrido MJ, Guillén Ponce C, Maciá Escalante S, Sevila MY, Carrato Mena C. Dysphagia and dysphonia in a woman with a previous breast cancer. Clin Transl Oncol. 2006;8(7):533–535. doi:10.1007/s12094-006-0054-4
11. Egaña N, Socias C, Matteucci T, Bilbao I, Alvarez-Coca M. Thyroid metastasis of lobular breast carcinoma. Endocrinol Nutr. 2012;59(3):219–220. doi:10.1016/j.endonu.2011.09.009
12. Papi G, Fadda G, Corsello SM, et al. Metastases to the thyroid gland: prevalence, clinicopathological aspects and prognosis: a 10-year experience. Clin Endocrinol. 2007;66(4):565–571. doi:10.1111/j.1365-2265.2007.02773.x
13. Wychulis AR, Beahrs OH, Woolner LB. Metastasis of carcinoma of the thyroid gland. Ann Surg. 1964;160(2):169–177. doi:10.1097/00000658-196408000-00001
14. Angorn IB, Baker LW, Baker LW. Tumour metastasis to the thyroid gland. S Afr Med J. 1977;51(15):509–512.
15. Miettinen M, A MCCUEP. SARLOMO⁃RIKALA M, et al.GATA3: a multi-specific but potentially useful marker in surgical pathology: a systematic analysis of 2500 epithelial and nonepithelial tumors[J]. Am J Surg Pathol. 2014;38(1):
16. Z CLARKB, Beriwal S, J DABBSD, et al. Semiquantitative GATA⁃3 immunoreactivity in breast, bladder, gynecologic tract, and other cytokeratin 7⁃positive carcinomas. Am J Clin Pathol. 2014;142(1):
17. Darb-Esfahani S, von Minckwitz G, Denkert C, et al. Gross cystic disease fluid protein 15 (GCDFP-15) expression in breast cancer subtypes. BMC Cancer. 2014;14(1):546. doi:10.1186/1471-2407-14-546
18. M GOWNA, S FULTONR, L KANDALAFTP. Markers of metastatic carcinoma of breast origin. Histopathology. 2016;68(1):
19. A KLINGENT, Chen Y, D GUNDERSENM, et al. Thyroid transcription factor⁃1 positive primary breast cancer: a case report with review of the literature. Diagn Pathol. 2010;5(1):37. doi:10.1186/1746-1596-5-37
20. Sakuraia SAKAIY. YATABEY.thyroid transcription factor⁃1 expression in rare cases of mammary ductal carcinoma. Histopathology. 2011;59(1):1.
21. Mittendorf EA, Philips AV, Meric-Bernstam F, et al. PD-L1 expression in triple negative breast cancer 2014. Cancer Immunol Res. 2014;2(4):361–370. doi:10.1158/2326-6066.CIR-13-0127
22. Available from: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
23. Pensabene M, Stanzione B, Cerillo I, et al. It is no longer the time to disregard thyroid metastases from breast cancer: a case report and review of the literature. BMC Cancer. 2018;18(1):146. doi:10.1186/s12885-018-4054-x
24. Schmid P, Adams S, Rugo HS, et al. IMpassion130 Trial Investigators. N Engl J Med. 2018;379(22):2108–2121.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.