Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 14
Thymoquinone Preserves Pancreatic Islets Structure Through Upregulation of Pancreatic β-Catenin in Hypothyroid Rats
Authors Faddladdeen K, Ali SS, Bahshwan S, Ayuob N
Received 25 April 2021
Accepted for publication 11 June 2021
Published 29 June 2021 Volume 2021:14 Pages 2913—2924
DOI https://doi.org/10.2147/DMSO.S317417
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ming-Hui Zou
Khadija Faddladdeen,1 Soad Shaker Ali,2,3 Safia Bahshwan,4 Nasra Ayuob5,6
1Biology Department, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia; 2Anatomy Department, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia; 3Histology Department, Faculty of Medicine, Assuit University, Assuit, Egypt; 4Biology Department, College of Science and Arts in Al-Makhwah, Al-Baha University, Al-Baha, Saudi Arabia; 5Medical Histology and Cell Biology Department, Faculty of Medicine, Damietta University, Damietta, Egypt; 6Yousef Abdullatif Jameel, Chair of Prophetic Medical Applications (YAJCPMA), Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia
Correspondence: Nasra Ayuob
Medical Histology and Cell Biology Department, Faculty of Medicine, Damietta University, Damietta, Egypt
Tel +201066513745
Email [email protected]
Background: Altered status of thyroid hormones, which have a key role in regulating metabolism, was reported to affect glucose homeostasis and insulin secretion.
Objective: This study was designed to assess the impact of propylthiouracil (PTU)-induced hypothyroidism on the pancreatic islet cells and the efficacy of thymoquinone (TQ) in alleviating this impact and explore the mechanism behind it alleviating oxidative stress and affecting β-catenin expression.
Materials and Methods: PTU (6 mg/kg/body weight) was used to induce hypothyroidism in Wistar rats. Four groups of rats (n=6 each) were utilized in this study. Untreated hypothyroid and TQ-treated hypothyroid groups (50 mg/kg/body weight for 4 weeks) were included. Thyroid functions, antioxidant profile and pancreatic β-catenin and IL-10 mRNA were measured. Histopathological and immunohistochemical assessment of the pancreas was performed.
Results: PTU administration induced a hypothyroid status that was associated with a marked disturbed oxidant/antioxidant status and a significant hyperglycemia (p< 0:001), hypoinsulinemia (p=0.01) and decreased HOMA-β-cell (p< 0.001). Islet cells of hypothyroid pancreas showed many degenerative changes with increased apoptosis, reduced insulin β-catenin immunoexpression. Administration of TQ alleviated these effects on the thyroid function, antioxidants, structure of pancreatic islet cells. Up-regulation of β-catenin, IL-10 and CAT gene expression in pancreatic islets after treatment with TQ supported its antioxidant and preserving β-cell function and viability mechanistic action.
Conclusion: TQ alleviated PTU-induced hypothyroidism changes in insulin homeostasis and pancreatic β cells mostly through its antioxidant effect as well as up-regulation of pancreatic β-catenin expression.
Keywords: Nigella sativa, pancreas, hypothyroidism, insulin, glucose, caspase-3
Introduction
Thyroid hormone is considered a key regulator of metabolism in all tissues. It adjusts the endocrine function of the pancreas acting through the pancreatic receptors of thyroid hormone.1 It was reported that “thyroid hormone is a physiological stimulus for the postnatal maturation of the functional beta cells.”2 Altered status of thyroid hormones was reported to affect glucose homeostasis and insulin secretion.3
High prevalence of hypothyroidism and other thyroid disorders was reported previously in diabetic patients. Therefore, it was recommended to perform a routine screening for hypothyroidism in diabetic patient for early diagnosis and effective management.4 Type 2 diabetes mellitus (T2DM) and hypertension have an intersecting underlying pathology with hypothyroidism.5
The link between thyroid hormones and the antioxidant status was previously described and attributed to the unknown impacts on oxidative metabolism and mitochondrial respiration.6 It was reported that oxidative stress associated with hypothyroidism might lead to a progress in pancreatic β-cell dysfunction and reduce β-cell mass with subsequent impairment in glucose tolerance and insulin secretion.7
β-catenin plays an important role in controlling glucose homeostasis. It has been reported to have a role in enhancing insulin secretion by β-cell and modulating islet inflammation, thus helping in controlling hyperglycemia in response to diet-induced obesity.8 Alterations in β-catenin/Wnt signaling might increase diabetes susceptibility not only by altering the rate of β-cell proliferation and total mass but also by regulating other tissues involved in control of appetite, energy expenditure, and growth.9 Activation of β-catenin generates an immunosuppressive environment in the islet through IL-10 production and increased infiltration of T regulators (Tregs).8 IL-10 has well-characterized anti-inflammatory properties, and emerging evidence suggests the cytokine can exert direct effects on β-cell function and viability.10 In addition, Tregs are known to promote systemic insulin sensitivity.11 Because of that β-catenin expression was assessed in this study in the pancreatic tissue in order to explore its role in pancreatic islets cells of hypothyroid rats.
6-Propyl-2-thiouracil (PTU) is one of the antithyroid drugs in treating hyperthyroidism.12 It is used if methimazole or radioactive iodine treatment is contraindicated or as an alternative treatment option in a patient with Graves’ disease or toxic multinodular goiter or in the first trimester of pregnancy.13 PTU inhibits the production of new thyroid hormone in the thyroid gland. Peripherally, it acts by inhibiting the conversion of T4 to T3. It has effects on the existing thyroid hormones stored in the thyroid gland or circulating in the blood.14 It was repeatedly utilized to induce an animal model of hypothyroidism to be used in testing the efficacy of new treatments or drugs.15
Nigella sativa (NS) was described to significantly improve thyroid function either in experimental models of hypothyroidism16 or in patients with Hashimoto’s thyroiditis, the most common cause of hypothyroidism.17 A significant evidence also exists on the impact of NS and its active constituent thymoquinone (TQ) on reducing hyperglycemia and enhancing insulin secretion.18 However, these studies did not provide an in depth investigation of the mechanism behind these effects.
In this study, we hypothesized that PTU-induced hypothyroidism is associated with pancreatic injury which might be attributed to pancreatic oxidative stress so that TQ, due to its strong antioxidant activity, can alleviate this effect. Therefore, the current study aimed to evaluate the impact of PTU-induced hypothyroidism on pancreatic islet cells and the efficacy of TQ in alleviating this impact. Additionally, the mechanism behind TQ induced-effects was explored regarding alleviating oxidative stress and affecting β-Catenin expression.
Materials and Methods
Drugs
Propylthiouracil (Sigma-Aldrich Inc. Hainesport, USA) was used for induction of hypothyroidism. It was daily administrated using intragastric tube at a dose 6 mg/kg/body weight for 6 weeks.19
Thymoquinone (Sigma-Aldrich Inc. Hainesport, USA) was diluted with dimethyl sulfoxide (DMSO) (1:100), then daily administrated (50 mg/kg/body weight) using intragastric tube for 4 weeks.20
Animals
Twenty-four adult male Wister rats weighing from 180 to 200 g were obtained from the animal house at King Fahad Medical Research Center (KFMRC) and left to acclimatize for 14 days under the standard laboratory conditions. Rats were housed in plastic cages in an air-conditioned room at 22 ± 1°C. They were offered the standard animal chow and water ad libitum. They were divided, at random, into four equal groups (n=6); the control, TQ-treated, hypothyroid and hypothyroid+TQ. Rats of the control group were administrated the vehicle; DMSO using intragastric tube for 6 weeks, while those of the TQ-treated group received TQ for 6 weeks. The other two groups were administrated PTU for 6 weeks, in order to induce hypothyroidism. After two weeks, thyroid function included T3, T4 and TSH levels were assessed in order to confirm the occurrence of hypothyroidism. Hypothyroid rats were assigned into two groups; untreated hypothyroid group (Hypothyroid) that continued on PTU for a total 6 weeks and hypothyroid group treated with TQ (hypothyroid+TQ) that received PTU plus TQ daily for 4 weeks.20 Rats were fed the standard diet and water during the whole experiment.
Biochemical Assessment
In order to perform biochemical assessment during the experiment, blood samples were withdrawn from the intraorbital sinus and from the heart at the end of the experiment.21 The rats were fasted for 12 hours before obtaining the blood samples. The latters were centrifuged at “3000 rpm for 15 min at 4°C” and the obtained sera were kept at −18°C till the time of biochemical assessment.
Serum levels of T3, T4, and TSH were assessed using automated competitive chemiluminescence immunoassay (Kanawha River, Western West Virginia, USA). Malondialdehyde level (MDA, reduced glutathione (GSH), and nitric oxide (NO) were assessed in the plasma using kits purchased from Biodiagnostic (Giza-Egypt). Glutathione peroxidase (GPX), catalase (CAT) and superoxide dismutase (SOD) activities were assessed in the plasma using kits provided by Biodiagnostic (Giza-Egypt).
The enzymatic glucose kits are used to assess blood glucose level (BGL) (Human Gesellschaft für and Diagnostica mbH, Germany). Insulin ELISA kits were used to measure serum insulin levels (Cat. no. ezrmi-13kelisa, Billerica, MA, USA) according to the previously described method.22
Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) and Homeostatic Model Assessment for Insulin Resistance β-cells (HOMA- β-cells) were calculated in order to assess insulin resistance and β-cells function, respectively. They were calculated as was previously described23 based on these formulas; HOMA-IR = fasting serum glucose (mg/ld.) × fasting serum insulin (μU/mL)/405. HOMA-β cell function = 20 x fasting serum insulin (μU/mL)/fasting serum glucose (mg/dL)-3.5.
Gene Expression Using Quantitative Real Time Polymerase Chain Reaction (qRT-PCR)
RT-PCR system (Applied Biosystems, 7500, USA) and 2X SYBR Green PCR Master Mix (Applied Biosystems, USA) were used to perform qRT-PCR assay.24 The sequences of the primers used in this study were: β-catenin, forward 5′-TGCTGAAGGTGCTGTCTGTC-3′ and reverse: 5′-TCG GTA ATG TCC TCC CTG TC-3′, catalase, forward -GAATGGCTATGGCTCACACA, backward CAAGTTTTTGATGCCCTGGT, IL-10, forward 5′-ATG-CAG-GAC-TTT-AAG-GGTTAC-TTG-3′ and reverse: 5′-CTA-GAC-ACC-TTG-GTC-TTGGAG-CTT-A-3′and GAPDH, forward ATGGAGAAGGCTGGGGCTCACCT and backward AGCCCTTCCACGATGCCAAAGTTGT. The latter house-keeping gene was utilized as a control in order to normalize with the primer sequence. The result analysis was done using the LightCycler 480 software (version 1.5, Roche Applied Science, Mannheim, Germany). The CT values were figured out after that ΔCT for every example is computed and also linearized making use of 2-ΔCT. To help with the contrast in between the experimental as well as control group; ΔΔCT were computed and also linearized making use of 2-ΔΔCT, therefore making it possible for the normalization of the catalase gene to GAPDH (endogenous reference gene) and also to the control group.
Histological Techniques
At the end of the experiment, rats were anesthetized using halothane then decapitated. The abdomen was opened, and the pancreas was cautiously dissected out. Small specimens of the pancreas were fixed in 10% neutral buffered formalin and processed into paraffin blocks. The latters were sectioned at 4-μm thickness to be stained either with haematoxylin and Eosin or immunohistochemical stains.26 Anti-caspase-3 (Dako Company, Cairo, Egypt; at a dilution 1/200), anti-insulin (Dako Company, Cairo, Egypt; at a dilution 1/200), and β-catenin (Santa Cruz, USA; at a dilution 1/200) were utilized in this study. During immunohistochemical staining, the primary antibody was omitted, in few slides, and only the secondary antibody IgG was added to be used as a negative control. Counterstaining with haematoxylin was done and brown cytoplasmic staining was considered positive reaction in anti-caspase-3, insulin and β-catinin.
Olympus Microscope BX-51 (Olympus) connected to a digital camera and a computer was used for photographing. Pro Plus image analysis software (version 6.0) (Media Cybernetics, Inc. Rockville, MD 20850 USA) was used for semiquantitative analysis of antibody immunoreactivity. Area percentage, an indicator of the extension of the reaction was assessed in 30 fields using × 40 objective lens and ×10 ocular lens.27 The mean area of islets of Langerhans was also measured.
Ethical Considerations
This study was approved by “the Biomedical Research Ethics Committee” at the Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia (Reference number 46–20). The Guidelines of the Care and Use of Laboratory Animals set at KFMRC were followed during the experiment conduction.
Statistical Assessment
Statistical package for the social sciences (SPSS, version 16; SPSS Inc., Chicago, Illinois, USA) was used to analyze the raw data. The results were presented in the form of mean ± standard deviation (SD). The studied groups were compared using analysis of variance ANOVA (f-test), followed by Bonferroni post-hoc test to avoid a multiple comparison effect. For nonparametric data, Kruskal–Wallis ANOVA followed by a post-hoc test (based on Dunn procedure) was done. P value <0.05 was considered significant.
Results
Thyroid Function
It was found that T3 and T4 levels showed a significant decrease (p=0.02, p=0.001) after administration of PTU, while TSH level was significantly increased (p<0.001) when compared to the control group. Treatment with TQ, after administration of PTU, significantly increased (p=0.004) T3 and T4 levels and decreased (p<0.001) TSH level compared to the hypothyroid group (Figure 1A–C).
Fasting Blood Glucose, Insulin, HOMA-IR and HOMA-β
The effect of PTU-induced hypothyroid on glucose homeostasis was assessed. It was found that BGL showed no significant change (p=0.9) in TQ group, while it showed a significant increase (p<0.001) in hypothyroid group compared to the control. Administration of TQ resulted in a significant decrease (p=0.009) in the BGL compared to hypothyroid group (Figure 1D).
Although TQ, when administrated to the control rats, induced non-significant increase in insulin level, a significant reduction (p=0.01) in serum insulin was observed in untreated hypothyroid rats compared to the control. Treatment of hypothyroid rats with TQ resulted in a significant increase (p=0.02) in insulin levels compared to untreated hypothyroid rats (Figure 1E).
HOMA-IR level showed an insignificant increase (p=0.25) in hypothyroid group compared to the control as well as an insignificant decrease (p=0.9) in TQ-treated group compared to the hypothyroid group. On the other hand, HOMA-β-cell showed a significant decrease (p<0.001) in hypothyroid group compared to the control, while it showed a significant increase (p=0.02) in TQ-treated group compared to the hypothyroid group (Figure 1F).
Levels of MDA and NO in the Serum
Administration of TQ to the control rats induced a significant decrease (p=0.006) in MDA level in the serum, while its level significantly increased (p=0.01) in hypothyroid group compared to the control. Serum NO level significantly (p=0.01, p<0.001) increased in TQ- and PTU-treated rats compared to the control and further increased significantly in the rats treated with TQ after receiving PTU (Figure 2A and B).
Levels of SOD, GPX, GSH and CAT in the Serum
The hypothyroid state resulted after PTU administration was associated with a significant decrease in SOD activity (p<0.001) and an insignificant decrease in GSH (p=0.09) compared to the control group. TQ-treated rats showed significantly increased SOD (p<0.001) and GSH (p=0.02) levels, compared to the hypothyroid rats. Serum level of CAT showed a significant increase (p<0.001) while that of GPX showed insignificant increase (p=0.12) as a result of PTU administration, while both parameters did not show a significant change in the TQ-treated rats compared to the untreated ones (Figure 2C–F).
Effect of TQ on Gene Expression of Catalase, β-Catenin and IL-10
The level of pancreatic CAT and β-catenin gene expression showed no significant change in TQ-treated rats compared to the control, while it showed a significant down-regulation (p<0.001) in hypothyroid group compared to the control rats. However, both levels showed a significant up-regulation (p<0.001, p=0.001) in TQ-treated hypothyroid rats compared to the untreated rats (Figure 3A and B).
Regarding IL-10 mRNA, its level showed no significant change in TQ-treated group, while its level showed significant down-regulation (p=0.03) in Hypothyroid group compared to the control. On the other hand, IL-10 mRNA level significantly up-regulated in TQ-treated hypothyroid rats compared to the untreated rats, respectively (Figure 3C)
Histological Results
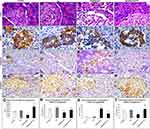
On histopathological examination of pancreas of the control and TQ-treated group, it was observed that many islets of Langerhans appeared between the exocrine pancreatic parenchyma with intact cells separated by blood capillaries. Pancreases of the hypothyroid group showed some islets with many degenerated cells that had vacuolated cytoplasm and dark nuclei as well as dilated congested blood capillaries. The pancreases of TQ-treated hypothyroid group showed more or less intact islets similar to those of the control apart from some congested blood capillaries and few degenerated cells (Figure 4A–D and Q).
Expression of pancreatic insulin was assessed immunohistochemistry. It was found the control, TQ-treated and TQ-treated hypothyroid groups showed strong to moderate positive insulin expression while hypothyroid group showed a weak reaction. Semi-quantitative assessment of insulin immunoexpression revealed that it was significant reduction (p<0.001) in hypothyroid group compared with the control, while it significantly increased (p=0.002) in TQ-treated hypothyroid group compared with hypothyroid group (Figure 4E–H and R).
Regarding Caspase-3 immunoexpression in pancreatic islet cells, it was significantly increased (p<0.001) in hypothyroid rats, while it showed a significant reduction (p<0.001) in TQ-treated hypothyroid group compared to hypothyroid group (Figure 4I–L and S).
Assessment of β-catenin immunoexpression in pancreatic islet cells showed that it was significantly decrease (p<0.001) in hypothyroid rats, while it showed a significant increase (p=0.001) in TQ-treated hypothyroid group compared to hypothyroid group (Figure 4M–P and T).
Discussion
Although impairment of glucose metabolism and insulin secretion was reported in hypothyroid rats, the exact mechanism behind this impairment still not fully explained.7 Several reports have also indicated a higher prevalence of thyroid diseases in T2DM patients, with hypothyroidism being the most common disorder. The coexistence of hypothyroidism and type 1 diabetes mellitus (T1DM) may be due to similar autoimmune pathogenesis28 or attributed to genetic, biochemical, or of hormonal origin.29
Nigella sativa thymoquinone was reported to possess significant beneficial effects on the health including antioxidant, hypoglycemic, anti-inflammatory and immune-modulatory properties with no reported side effects and with low cost.30,31 In a previous experimental study, Nigella Sativa induced a potent protective effect on the thyroid cell with subsequent relieve of the hypothyroidism-induced oxidative stress following PTU administration (Khalawi et al, 2013). Therefore, TQ was selected in this study to counteract the hyperglycemic effect and counteract the impact of PTU-induced hypothyroidism.
The present study demonstrates that PTU-induced hypothyroidism in rats was associated by hyperglycemia, hypoinsulinemia, increased oxidative markers and reduced enzymatic and non-enzymatic antioxidants in the serum. In addition, this study revealed that hypothyroidism induced a significant reduction in pancreatic β-catenin and IL-10 that was associated with degenerative changes in islet cells. TQ, due to its antioxidant effect as well as up-regulation of β-catenin, could modulate hypothyroidism associated negative impact on islets β-cell and enhanced insulin production.
In this study, PTU administration resulted in induction of hypothyroid condition evident by the significant increase in T3 and T4 and a reduction in TSH levels. It was noticed that TQ significantly alleviated these changes. In accordance with that, it was previously reported that TQ significantly increased the T4 level in PTU-induced juvenile hypothyroid rats.32
In a recent study conducted by Li et al33 on the general population and non-treated type 2 diabetes mellitus patients, it was concluded that thyroid hormone even in reference range could play an important role in the function of pancreatic islets. In this study, PTU-induced hypothyroidism was associated with significant hyperglycemia and hypoinsulinemia. This is in agreement with the study of Taguchi et al34 who reported that hypothyroidism resulted in impairment if glucose metabolism and attenuated capacity of insulin secretion. Godini et al35 also reported that reduced insulin in hypothyroidism was attributed to the abnormalities in some parts of the glucose sensor apparatus of the islets including glucokinase activity and glucose transporter 2 protein expressions.
Assessment of HOMA-IR, in this study, revealed insignificant change in Hypothyroid or TQ-treated groups indicating absence of insulin resistance in this model of hypothyroidism. This is supported by the recent study conducted by Kozacz et al.36 On the other hand, assessment of HOMA-β-cell revealed a reduction in β-cell mass in hypothyroid group. Affection of β-cell, confirmed histopathologically and immunohistochemistry in this study, might be induced by oxidative stress condition, observed also in this study. Kajimoto and Kaneto37 reported that excessive accumulation of the reactive oxygen species causes chronic oxidative stress, which is principally hazardous for the islet cells because they have the lowest levels of intrinsic antioxidant defenses.
Administration of TQ markedly reduced the FBG and increased insulin level. This is in agreement with Abdelraxek et al18 during their study of the effect of TQ in STZ-induced animal model of diabetes. Fararh et al38 reported that TQ reduces the blood glucose level due to its insulinotropic action. In addition, Mansi39 found that administration of TQ increased insulin production due to its ability to induce partial regeneration of pancreatic β cells. This was evident in this study as TQ administration was associated with increased immunohistochemical expression of insulin in the pancreas. It was also reported that TQ enhances insulin peripheral utilization,40 reduces hepatic glucose synthesis and absorption from the intestine, down-regulates expression of gluconeogenic enzymes,41 and inhibits gluconeogenesis.42 Although all these studies investigated the glucose lowering effect of TQ in diabetic animal models, they represented plausible explanations of FBG-lowering effect of TQ in hypothyroid animal model.
In this study, PTU-hypothyroidism was associated with a significant increase in MDA and NO, produced as a result of augmented lipid peroxidation. This observation was in accordance with some previous studies.7,43 Increased MDA and NO as well as reduced antioxidants enzymes, evident in this study, might be attributed to the associated hyperglycemia, recorded also in this study. This was supported by the previous research which revealed that persistent hyperglycemia promoted oxidative stress through the formation or release of reactive oxygen species (ROS) and depletion of antioxidant reserve.44 Treatment with TQ, used in this study, effectively restored GSH, CAT, GPX and SOD in the serum. This finding was supported by some previous studies.18,45
Serum CAT level was significantly increased, in this study, in PTU-induced hypothyroidism. This was unexpected finding so the level of the mRNA was assessed in the pancreas using qRT-PCR. A significant down-regulation in CAT gene expression was found in PTU-induced hypothyroid rats. Actually, these two findings are not considered conflicting as increased CAT activity in the serum of hypothyroid rat is considered a direct compensatory mechanism against hypothyroidism-induced oxidative stress and came from the already present CAT while hypothyroidism suppress CAT gene expression. So CAT level in the serum is expected to be down-regulated after some time in hypothyroid rats. These findings are in line with those of Bunker et al and Chattopadhyay et al.24,46 TQ significantly up-regulated CAT gene expression in pancreatic tissue, in this study and this was in concordance with the previous studies conducted on the liver and kidney.25,47,48
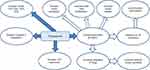
In this study, PTU-induced hypothyroidism was associated with a significant down-regulation of β-catenin and IL-10 gene expression as well as β-catenin immunoexpression in the pancreatic tissue, while they were significantly up-regulated in TQ-treated hypothyroid rats. The link between β-catenin and IL-10 in the pancreatic tissue was previously described. It was described that activation of β-catenin in conventional dendritic cells (cDCs) improves glucose homeostasis by increased insulin production, increased proliferation of islet cells and generating an immunosuppressive environment for increased insulin release.8 β-catenin activation enhances IL-10 production and increases infiltration of Regulatory T Cells (Tregs). IL-10 has proved anti-inflammatory effect and was suggested to exert direct effects on β-cell function and viability.49 Tregs are also known to promote systemic insulin sensitivity.11 Treatment with TQ either in low or high dose was reported to increase total β-catenin protein as well as cytoplasmic β-catenin expression.50 Therefore, up-regulating β-catenin expression could be a proposed mechanism through which TQ improve insulin hemostasis and alleviated hypothyroidism-induced hyperglycemia. This proposed mechanism is shown in Figure 5.
In this study, PTU induced degeneration and apoptotic changes of the islet cells that were confirmed immunohistochemistry by increased Caspase-3 immunoexpression in the islets. A significant reduction in insulin-positive β-cell was also observed. This indicated a reduction in β-cell mass in hypothyroid rats. Safayee et al previously reported that hypothyroidism resulted in oxidative stress in pancreatic islets with subsequent β-cells dysfunction and reduced β-cell mass in hypothyroid pancreas.7 Administration of TQ to hypothyroid rats, in the current study, reduced PTU-induced degenerative changes and up-regulated insulin expression in the pancreatic islet cells. In agreement with that Abdelrazek et al reported that TQ improved STZ-induced diabetic degenerative changes in islet cells of male Wistar rat and significantly increased insulin immunoexpression.18 Nigella sativa oil (NSO) exerts its effect through thymoquinone antioxidant potential, which improves pancreatic and hepatic integrity, increases pancreatic islet immunoreactivity and therefore, increases serum insulin level and increases hepatic glycogen content and reduces the elevated blood glucose level.18
Limitations and Future Perspectives
The primary research objective in this study was to evaluate of the efficacy of thymoquinone in preserving the structural integrity of pancreatic islets in PTU-induced hypothyroidism and restoring glucose homeostasis, while the secondary research objective was to explore the mechanism behind thymoquinone-induced changes. Although the involvement of β-catenin signaling and apoptosis was investigated in this study, a more comprehensive assessment of the multiple signaling molecules involved in that pathway is necessary and recommended in a coming study.
Conclusion
Thymoquinone administrated to PTU-induced hypothyroidism effectively alleviated the hyperglycemia, hypoinsulinemia, increased oxidative markers and reduced enzymatic and non-enzymatic antioxidants in the serum. This subsequently improved degenerative changes in β islet cells. These effects might be mediated through its antioxidant effect as well as up-regulation of pancreatic β-catenin expression.
Data Sharing Statement
Data will be made available by the corresponding author upon request.
Acknowledgments
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, Saudi Arabia, under grant no. G: 1608-247-1440. The authors, therefore, acknowledge with thanks DSR for technical and financial support.
Disclosure
The authors declare no conflicts of interest.
References
1. Zinke A, Schmoll D, Zachmann M, et al. Expression of thyroid hormone receptor isoform α1 in pancreatic islets. Exp Clin Endocrinol Diabetes. 2003;111(04):198–202. doi:10.1055/s-2003-40463
2. Mastracci TL, Evans-Molina C. Pancreatic and islet development and function: the role of thyroid hormone. J Endocrinol Diabetes Obes. 2014;2.
3. Kapadia KB, Bhatt PA, Shah JS. Association between altered thyroid state and insulin resistance. J Pharmacol Pharmacother. 2012;3(2):156–160. doi:10.4103/0976-500X.95517
4. Stojanovich L, Marisavljevich D. Stress as a trigger of autoimmune disease. Autoimmun Rev. 2008;7(3):209–213. doi:10.1016/j.autrev.2007.11.007
5. Khurana A, Dhoat P, Jain G. Prevalence of thyroid disorders in patients of type 2 diabetes mellitus. J Indian Acad Clin Med. 2016;17:12–15.
6. Campos C, Casado Á. Oxidative stress, thyroid dysfunction & down syndrome. Indian J Med Res. 2015;142(2):113. doi:10.4103/0971-5916.164218
7. Safayee S, Karbalaei N, Noorafshan A, Nadimi E. Induction of oxidative stress, suppression of glucose-induced insulin release, ATP production, glucokinase activity, and histomorphometric changes in pancreatic islets of hypothyroid rat. Eur J Pharmacol. 2016;791:147–156. doi:10.1016/j.ejphar.2016.08.024
8. Macdougall CE, Wood EG, Solomou A, et al. Constitutive activation of β-catenin in conventional dendritic cells increases the insulin reserve to ameliorate the development of type 2 diabetes in mice. Diabetes. 2019;68(7):1473–1484. doi:10.2337/db18-1243
9. Elghazi L, Gould AP, Weiss AJ, et al. Importance of β-catenin in glucose and energy homeostasis. Sci Rep. 2012;2(1):1–12. doi:10.1038/srep00693
10. Russell MA, Morgan NG. The impact of anti-inflammatory cytokines on the pancreatic β-cell. Islets. 2014;6(3):e950547. doi:10.4161/19382014.2014.950547
11. Mukherjee R, Chaturvedi P, Qin H-Y, Singh B. CD4+ CD25+ regulatory T cells generated in response to insulin B: 9–23 peptide prevent adoptive transfer of diabetes by diabetogenic T cells. J Autoimmun. 2003;21(3):221–237. doi:10.1016/S0896-8411(03)00114-8
12. Boas M, Feldt-Rasmussen U, Main KM. Thyroid effects of endocrine disrupting chemicals. Mol Cell Endocrinol. 2012;355(2):240–248. doi:10.1016/j.mce.2011.09.005
13. Allelein S, Schott M. Update graves’ disease 2019. Dtsch Med Wochenschr (1946). 2019;144:152–155.
14. Propylthiouracil. Drugs and Lactation Database (Lactmed). Bethesda (MD): National Library of Medicine (US); 2006.
15. Das K, Chainy G. Modulation of rat liver mitochondrial antioxidant defence system by thyroid hormone. Biochim Biophys Acta Mol Basis Dis. 2001;1537(1):1–13. doi:10.1016/S0925-4439(01)00048-5
16. Khalawi AA, Al-Robai AA, Khoja SM, Shaker A. Can Nigella sativa oil (NSO) reverse hypothyroid status induced by PTU in rat? Biochemical and histological studies. Life Sci J. 2013;10:1–5.
17. Farhangi MA, Dehghan P, Tajmiri S, Abbasi MM. The effects of Nigella sativa on thyroid function, serum vascular endothelial growth factor (VEGF)–1, Nesfatin-1 and anthropometric features in patients with Hashimoto’s thyroiditis: a randomized controlled trial. BMC Complement Altern Med. 2016;16(1):1–9. doi:10.1186/s12906-016-1432-2
18. Abdelrazek HMA, Kilany OE, Muhammad MAA, Tag HM, Abdelazim AM. Black seed thymoquinone improved insulin secretion, hepatic glycogen storage, and oxidative stress in streptozotocin-induced diabetic male Wistar rats. Oxid Med Cell Longev. 2018;2018:8104165. doi:10.1155/2018/8104165
19. Villar D, Rhind S, Dicks P, McMillen S, Nicol F, Arthur J. Effect of propylthiouracil-induced hypothyroidism on thyroid hormone profiles and tissue deiodinase activity in cashmere goats. Small Rumin Res. 1998;29(3):317–324. doi:10.1016/S0921-4488(97)00130-2
20. Seeram NP, Adams LS, Henning SM, et al. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J Nutr Biochem. 2005;16(6):360–367. doi:10.1016/j.jnutbio.2005.01.006
21. Parasuraman S, Raveendran R, Kesavan R. Blood sample collection in small laboratory animals. J Pharmacol Pharmacother. 2010;1(2):87. doi:10.4103/0976-500X.72350
22. Gurudeeban S, Kaliamurthi S, Thirugnanasambandam R. Positive regulation of Rhizophora mucronata poir extracts on blood glucose and lipid profile in diabetic rats. Herb Med. 2016;2.
23. Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi:10.1007/BF00280883
24. Bunker SK, Dandapat J, Sahoo SK, Roy A, Chainy GB. Neonatal persistent exposure to 6‐propyl‐2‐thiouracil, a thyroid‐disrupting chemical, differentially modulates expression of hepatic catalase and C/EBP‐β in adult rats. J Biochem Mol Toxicol. 2016;30(2):80–90. doi:10.1002/jbt.21766
25. Ayuob N, Balgoon MJ, El-Mansy AA, Mubarak WA, Firgany AE-DL. Thymoquinone upregulates catalase gene expression and preserves the structure of the renal cortex of propylthiouracil-induced hypothyroid rats. Oxid Med Cell Longev. 2020;2020:1–15. doi:10.1155/2020/3295831
26. Bancroft JD, Gamble M. Theory and Practice of Histological Techniques. Elsevier health sciences; 2008.
27. Leslie K, Taatjes D, Schwarz J, vonTurkovich M, Low RB. Cardiac myofibroblasts express alpha smooth muscle actin during right ventricular pressure overload in the rabbit. Am J Pathol. 1991;139(1):207.
28. Kadiyala R, Peter R, Okosieme OE. Thyroid dysfunction in patients with diabetes: clinical implications and screening strategies. Int J Clin Pract. 2010;64(8):1130–1139. doi:10.1111/j.1742-1241.2010.02376.x
29. Wang C. The relationship between type 2 diabetes mellitus and related thyroid diseases. J Diabetes Res. 2013;2013.
30. Salem ML. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int Immunopharmacol. 2005;5(13–14):1749–1770. doi:10.1016/j.intimp.2005.06.008
31. Omar NM, Atia GM. Effect of Nigella sativa on pancreatic β-cell damage in streptozotocin-induced diabetic rats: Histological and Immunohistochemical Study. Egypt J Histol. 2012;35(1):106–116. doi:10.1097/01.EHX.0000411475.79484.90
32. Baghcheghi Y, Hosseini M, Beheshti F, Salmani H, Anaeigoudari A. Thymoquinone reverses learning and memory impairments and brain tissue oxidative damage in hypothyroid juvenile rats. Arq Neuropsiquiatr. 2018;76(1):32–40. doi:10.1590/0004-282x20170182
33. Li Q, Lu M, Wang NJ, et al. Relationship between free thyroxine and islet beta-cell function in euthyroid subjects. Curr Med Sci. 2020;40(1):69–77. doi:10.1007/s11596-020-2148-6
34. Taguchi Y, Tasaki Y, Terakado K, Kobayashi K, Machida T, Kobayashi T. Impaired insulin secretion from the pancreatic islets of hypothyroidal growth-retarded mice. J Endocrinol. 2010;206(2):195. doi:10.1677/JOE-09-0465
35. Godini A, Ghasemi A, Zahediasl S, Maedler K. The possible mechanisms of the impaired insulin secretion in hypothyroid rats. PLoS One. 2015;10(7):e0131198. doi:10.1371/journal.pone.0131198
36. Kozacz A, Assis GGD, Sanocka U, Ziemba AW. Standard hypothyroid treatment did not restore proper metabolic response to carbohydrate. Endocrine. 2021;71(1):96–103. doi:10.1007/s12020-020-02334-0
37. Kajimoto Y, Kaneto H. Role of oxidative stress in pancreatic β-cell dysfunction. In: Mitochondrial Pathogenesis. Springer; 2004:168–176.
38. Fararh K, Atoji Y, Shimizu Y, Takewaki T. Isulinotropic properties of Nigella sativa oil in Streptozotocin plus Nicotinamide diabetic hamster. Res Vet Sci. 2002;73(3):279–282. doi:10.1016/S0034-5288(02)00108-X
39. Mansi KMS. Effects of oral administration of water extract of nigella sativa on serum concentrations of insulin and testosterone in alloxan-induced diabetic rats. Pak J Biol Sci. 2005;8(8):1152–1156. doi:10.3923/pjbs.2005.1152.1156
40. Krishnamurthy B, Chee J, Jhala G, et al. Complete diabetes protection despite delayed thymic tolerance in NOD8. 3 TCR transgenic mice due to antigen-induced extrathymic deletion of T cells. Diabetes. 2012;61(2):425–435. doi:10.2337/db11-0948
41. Meddah B, Ducroc R, Faouzi MEA, et al. Nigella sativa inhibits intestinal glucose absorption and improves glucose tolerance in rats. J Ethnopharmacol. 2009;121(3):419–424. doi:10.1016/j.jep.2008.10.040
42. Coughlan KA, Valentine RJ, Ruderman NB, Saha AK. AMPK activation: a therapeutic target for type 2 diabetes? Diabetes Metab Syndr Obes. 2014;7:241. doi:10.2147/DMSO.S43731
43. Yilmaz S, Ozan S, Benzer F, Canatan H. Oxidative damage and antioxidant enzyme activities in experimental hypothyroidism. Cell Biochem Funct. 2003;21(4):325–330. doi:10.1002/cbf.1031
44. Sturza A, Duicu OM, Vaduva A, et al. Monoamine oxidases are novel sources of cardiovascular oxidative stress in experimental diabetes. Can J Physiol Pharmacol. 2015;93(7):555–561. doi:10.1139/cjpp-2014-0544
45. Adewole SO, Caxton-Martins EA, Ojewole JA. Protective effect of quercetin on the morphology of pancreatic β-cells of streptozotocin-treated diabetic rats. Afr J Tradit Complement Altern Med. 2007;4(1):64–74. doi:10.4314/ajtcam.v4i1.31196
46. Chattopadhyay S, Zaidi G, Das K, Chainy G. Effects of hypothyroidism induced by 6-n-propylthiouracil and its rever sa l by T 3 on rat heart s up eroxide di s muta se, catalase and lipid peroxidation. 2003.
47. Mohebbati R, Hosseini M, Haghshenas M, Nazariborun A, Beheshti F. The effects of Nigella sativa extract on renal tissue oxidative damage during neonatal and juvenile growth in propylthiouracil-induced hypothyroid rats. Endocr Regul. 2017;51(2):105–113. doi:10.1515/enr-2017-0010
48. Ayuob NN, Abdel-Hamid A, Helal GMM, Mubarak WA. Thymoquinone reverses nonalcoholic fatty liver disease (NAFLD) associated with experimental hypothyroidism. Rom J Morphol Embryol. 2019;60(2):479–486.
49. Dirice E, Kahraman S, Jiang W, et al. Soluble factors secreted by T cells promote β-cell proliferation. Diabetes. 2014;63(1):188–202. doi:10.2337/db13-0204
50. Lang M, Borgmann M, Oberhuber G, et al. Thymoquinone attenuates tumor growth in ApcMin mice by interference with Wnt-signaling. Mol Cancer. 2013;12(1):41. doi:10.1186/1476-4598-12-41
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.