Back to Journals » International Journal of Nanomedicine » Volume 14
The Toxicity Of Metallic Nanoparticles On Liver: The Subcellular Damages, Mechanisms, And Outcomes
Authors Yao Y, Zang Y, Qu J, Tang M, Zhang T
Received 19 April 2019
Accepted for publication 21 October 2019
Published 7 November 2019 Volume 2019:14 Pages 8787—8804
DOI https://doi.org/10.2147/IJN.S212907
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Mian Wang
Ying Yao,* Yiteng Zang,* Jing Qu, Meng Tang, Ting Zhang
Key Laboratory of Environmental Medicine and Engineering, Ministry of Education; School of Public Health, Southeast University, Nanjing 210009, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ting Zhang
Key Laboratory of Environmental Medicine and Engineering, Ministry of Education; School of Public Health, Southeast University, Nanjing 210009, People’s Republic of China
Tel +86-25-83272566
Fax +86-25-83272566
Email [email protected]
Abstract: Metallic nanoparticles (MNPs) are new engineering materials with broad prospects for biomedical applications; thus, their biosafety has drawn great concern. The liver is the main detoxification organ of vertebrates. However, many issues concerning the interactions between MNPs and biological systems (cells and tissues) are unclear, particularly the toxic effects of MNPs on hepatocytes and other liver cells. Numerous researchers have shown that some MNPs can induce decreased cell survival rate, production of reactive oxygen species (ROS), mitochondrial damage, DNA strand breaks, and even autophagy, pyroptosis, apoptosis, or other forms of cell death. Our review focuses on the recent researches on the liver toxicity of MNPs and its mechanisms at cellular and subcellular levels to provide a scientific basis for the subsequent hepatotoxicity studies of MNPs.
Keywords: metallic nanoparticles, hepatotoxicity, subcellular injury, dysfunctions, toxicity outcome
Introduction
With the rapid development of nanotechnology, nanomaterials (NPs) are considered to have enormous application potential due to their unique properties over the past few decades.1 Of all the NPs, metallic nanoparticles (MNPs) have generated considerable commercial interest owing to unique properties of NPs such as small size and the greater surface area to volume ratio as well as different electronic, magnetic, optical, and mechanical properties and also particle shape. MNPs mainly include metal nanoparticles and metal oxide nanoparticles, and MNPs have been widely included in a great diversity of products and the various fields, such as electronic devices, cosmetics, paints, additives in food, and biological and medical systems.2,3 With the widespread application of MNPs, it is inevitable that MNPs will be released into the environment or contact with humans directly. Therefore, their potential risks to human health and the environment have gained even more attention.4 MNPs can enter the body in various ways, for example, through the inhalation, gastrointestinal tract, or skin, and circulate via the blood or lymphatic system, eventually accumulating in various organs.5 Previous studies for metal nanoparticles and metal oxides nanoparticles, including nano-Cu, nano-Ag, nano-Ni, nano-TiO2, and nano- ZnO, have shown that MNPs reached the lung and gastrointestinal tract through the respiratory and digestive tract, and further translocated to the systemic circulation, and then accumulate the potential target organs such as the liver and the mononuclear phagocytic system.6 As a secondary exposure site, the liver is extremely important, as it has been shown to accumulate MNPs at much higher quantities compared with other organs.7 Meanwhile, MNPs accumulate the liver typically results in interaction with hepatic cells and the possibility of changing the structure and function of hepatic cells. The liver is a complex network of inter-related cells, including about 60–80% of the hepatocytes, and the additional cells include Kupffer cells, liver sinusoidal endothelial cells, hepatic stellate cells, and so on. The interactions of MNPs with liver cells determine the fate of administered MNPs in vivo and the results of hepatotoxicity. In vivo studies are mostly focused on the accumulation of the MNPs at the organ level, while most in vitro studies are focused on hepatic cells and do not summarize changes in subcellular levels and their relationship with hepatotoxicity. It is important, therefore, to summarize hepatotoxicity studies of MNPs on animals, the cell level and subcellular level and its molecular mechanism and outcomes. Furthermore, the physicochemical characteristics of MNPs, such as size, surface properties, and chemical nature would change and influence their potential toxicity. From these facts, the aim of this review is to compile and discuss the hepatotoxicity effects of MNPs both in vitro and in vivo, particularly those involved in subcellular levels as well as to highlight its molecular mechanism of action of these MNPs.
The Liver And Metallic Nanoparticles Toxicity
The liver is the primary organ for detoxification in human body. It possesses abilities of deoxidation, glycogen storage, and secreted protein synthesis. It acts as biological barriers by isolating and eliminating various exogenous compounds through phagocytosis. Previous in vivo studies have shown that different types of MNPs: nano-metal monomers and nano-metal oxides, tend to deposit in the liver with extensive toxic effects.8–10 As shown in Table 1, MNPs entering the body cause changes in inflammatory cytokines. NiO NP increased the concentrations of pro-inflammatory cytokines (IL-1β and IL-6) but decreased the levels of anti-inflammatory cytokines (IL-4 and IL-10).11 Liver dysfunction caused by MNPs leads to structural changes of liver. MNPs caused inflammation, which may lead to changes in liver coefficients.12–14 By analyzing blood serum, significant decrease of total bilirubin and increase of alkaline phosphatase (ALP) with aspartate aminotransferase (AST) indicated liver injury.11,15,16 The damage mainly manifested in liver structural changes causing metabolic dysfunction. AgNP caused the increase of relative spleen weight and affected diffuse and severe hepatocyte necrosis and hemorrhage, as well as multifocal peribiliary microhemorrhages, occasional portal vein endothelial damage, which in turn affects the liver.17 TiO2 NP induced alterations in the liver structure including hepatic inflammatory cell infiltration, increased density of liver tissue collagen, initiation of fibrosis and Glisson capsule thickness increase.18 AuNP was found to activate hepatic macrophages and then significantly aggravated the course of experimental immune hepatitis and liver injury.19
 |
Table 1 In Vivo Studies On The Liver Toxicity Of Metallic Nanoparticles (MNPs) |
The Hepatocytes And Metallic Nanoparticles Toxicity
Hepatocytes constitute the basic functional unit of the liver, the hepatic lobule, which contains 60% of the solid cells (hepatocytes) and 30% to 35% of the non-solid cells (hepatic stellate cells, Kupffer cells, and sinusoidal endothelial cells). NPs deposited in liver tissue may affect the normal physiological and biochemical functions of liver by affecting liver parenchymal cells and other cells along with important physiological functions in liver tissue. When studying the hepatotoxicity of MNPs, the effects on hepatocytes from different sources, including primary cells and cell lines, should be considered at the same time, so as to obtain more comprehensive information. It has been widely carried out in vitro toxicity research of MNPs, including the use of cell lines from different species and origins, as well as studies at the cellular, subcellular and molecular levels.. MNPs cause liver cell toxicity mechanism includeing triggering inflammation, oxidative stress, and possibly eventually leading to different types of cell death outcomes. As shown in Table 2, due to the role of MNPs, the decrease in survival rate of hepatocytes is common, accompanied by a time- and dose-dependent relationship. The sensitivity of hepatocytes from different sources to MNPs was different. Compared with normal cell lines, MNPs seem to have more obvious toxic effects on cancer cells. In the study of Mei-Lang et al,22 the IC50 for SK-Hep-1 and HepG2 cells were 25 and 85 ug/mL, respectively. Ali et al23 also found that HepG2 cells were more sensitive to rGO-Ag than human CHANG liver cells. The activity of lipid peroxide, superoxide dismutase, and catalase increased and glutathione decreased. Previous studies have shown that MNPs can destroy the function of mitochondria and cell respiratory transmission.24,25 MNPs induced decrease of ATP levels, activated the signaling pathway of inflammation, apoptosis, and autophagy.26–28 The changes in oxidative stress and inflammatory factors suggest the mechanism of cell fate induced by MNPS.24,29,30 In addition, MNPs can also damage DNA, which may explain the cause and mechanism of liver damage caused by MNPs at the organelle and molecular level.31
 |  |  |
Table 2 In Vitro Studies On The Liver Toxicity Of Metallic Nanoparticles (MNPs) |
Effects Of MNPs On Organelles
A small organ-like structure present inside the cell is called a cell organelle, which is the basic structural, functional, and biological unit of all known living organisms. The integrity of organelle determines the fate of the cell. Liver cell membrane is sensitive to free radical and lipid peroxidation injury. Liver cell membrane injury was characterized by decreased fluidity and increased permeability. Wang et al29 found that ZnO NPs were distributed in the nucleus or concentrated on the surface of primary hepatocytes membrane microvilli and other organelles in catfish. Under light micrographs, numerous MNPs caused changes in cell membrane permeability, as well as distinct damages under TEM. According to Vrček et al,24 treatment of Ag NPs and Ag ion on human hepatocytes both led to cell membrane damage, which was manifested as LDH leakage and decreased albumin synthesis with ALT activity inhibited. Changes in liver membrane fluidity can damage the enzyme activity, receptor and transportation function, and inhibit the function of liver cells. The internal structure of the membrane may be disturbed by MNPs, as they can cause changes in membrane permeability by causing the plasma membrane to partially dissolve and form pore structures. The exposure of MoS2 NPs made reduction of the phospholipid bilayer domain of the liver cancer cells and an increase in membrane fluidity.33
The nucleus controls the cellular genetic material and plays an important role in cell growth, metabolism, proliferation, and differentiation. MNPs reached the nucleus and affected genetic materials, thus destroying nuclear morphology, damaging DNA, and affecting gene expression concretely.34 The mouse hepatocyte exposed to ZnO NPs exhibited karyopyknosis, nuclear membrane irregularity with indentation, and chromatin fragmentation. Shrunken micronuclei of hepatocytes with reticular-pattern chromatin condensation and apoptotic activity were further observed.35 TiO2 NPs orally administered into C57/BL6 mice caused liver metabolic genes (Oatp1, Mrp3, Cyp2b10, Cyp2c37) to increase under high dose treatment.36 TiO2 NPs can also regulate the expression of mRNA p53 and the downstream genes regulating DNA damage response (p21 mdm2, gadd45) temporarily. Also, exposure of HepG2 cells to TiO2 NPs resulted in DNA strand breakage and sustained growth of purine oxide.37 Overall, MNPs could cause intracellular DNA damage, which induced different cell outcomes, for example, activating caspase-3 and caspase-7 mediated apoptosis,25,38,39 regulating relevant genes (Bax, Puma, Noxa),39 and promoting caspase-1 induced pyroptosis.38
Mitochondrial dysfunction enticed by MNPs included morphological changes, increased production of ROS, changes in calcium content, descending mitochondrial membrane potential, inhibition of various enzyme activities, inhibition of electron transport chains, inhibition of cellular respiration, decline of ATP synthesis, etc., which could further lead to insufficient energy supply and affect cell viability such as apoptosis and necrosis.40,41 ZnO NPs caused a series of morphological changes in mouse hepatocyte mitochondria, such as enlargement, elongation, angulations, swelling, cristolysis, lacked cristae, and ruptured membranes.35 Apart from morphological changes, three types of TiO2 NPs (commercially available rutile, anatase, P25) induced oxidative stress in primary rat hepatocyte, downregulated mitochondrial dynamin OPA-1 and mitochondrial fusion protein MFN-1 gene expression, significant loss of mitochondrial membrane potential (MMP), and decreased activity of mitochondrial Mn-SOD enzyme.30
Endoplasmic reticulum (ER) changes caused by MNPs include endoplasmic reticulum swelling, endoplasmic reticulum stress, misfolding of proteins, and increasing or decreasing protein synthesis.42,43 In liver, ER plays an important role in the synthesis of protein and steroid hormones, as well as promoting lipid metabolism and calcium storage. ER damage is related to the loss of protein synthesis initiation and liver detoxification function. The ER of mouse hepatocytes treated with ZnO NPs demonstrated ER pleomorphism in the form of dilatation, loss of parallel arrays, stacks shortening, vesiculation, upregulated transcription of genes encoding ER-resident molecular chaperones such as Grp78, Grp94, pdi-3 and xbp-1, and accelerated the process of protein kinase R-like reticulum kinase (PERK) and eukaryotic initiation factor 2α (eIF2α) phosphorylation. ER stress is considered to be one of the early sensitive indicators of cytotoxicity caused by MNPs.35,44 Chen et al showed that the level of xbp-1s and Chop mRNA elevated with mice exposed to Ag NPs. In addition, the upregulation of ER stress marker proteins (hsp70, bip, p-ire1, p-perk, and chop) was dose-dependent for Ag NPs exposure.34 Ultra-small superparamagnetic iron oxide nanoparticles (USPIO-NP) act on L02 cells, causing the expansion and vacuolation of ER, and increasing the level of calcium ions in ER cavity. ER stress and unfolded protein response to PERK/ATF4 signaling pathway were finally activated.45
MNPs enter lysosomes mainly through passive diffusion or endocytosis, causing changes in lysosomal structure.46 The destruction of lysosomal cristae in Kupffer cells was obviously observed in rats injected intraperitoneally with an interval of 48 hrs.47 ZnO NPs entered the lysosome mainly through endocytosis, leading to damage to lysosomal morphology during the interaction with the acidic environment, releasing a large amount of Zn2+ to the cytoplasm. And Zn2+ captured partially by mitochondria triggered the generation of ROS, causing mitochondrial dysfunction and apoptosis of cells.46 MNPs released ions under the lysosomal acidic environment, and then the lysosomal membranes were ruptured by MNPs or ions and the contents entering the cell lead to damage.38,48 Low-dose Ag-NPs (10 μg/mL) activated autophagic lysosome pathway in HepG2 cells and found increased level of lysosome activity, LC3-II protein expression, caspase-1, and IL-1 beta levels. Using ATG5 siRNA or chloroquine to destroy the autophagic pathway, Ag NPs induced increased caspase-1 activation and LDH release, suggesting that Ag NPs induced-cytotoxicity is associated with lysosomes damage and inflammatory bodies.26
Properties Of Metal Nanomaterials Affect Cell Absorption And Distribution Of MNPs
Toxicity of MNPs is largely dependent on cellular uptake and subcellular distribution. The size and surface properties of MNPs and the types of liver cells play a critical role in determining the outcome of interaction with the cells and other biological entities.49
Size
Size is a key factor determining the subcellular distribution. Numerous researches have indicated that MNPs, which mainly distributed in lysosome, cytoplasm, and nucleus, enter cells through endocytosis.50–52 When comparing three different sizes (8.9 nm, 27.6 nm, 56 nm) of gold NPs exposed HepG2 cells, the size of MNPs between 3 and 10 nm entered the nucleus, while the particles of 25 to 60 nm accumulated in the cytoplasm, which indicated the size is a key factor to determine the subcellular distribution.50 Another study showed that the shape of Au NPs affected the ratio of endocytosis. The highest cell uptake was triangular, followed by rod-shaped and star-shaped.53 It is worth noting that the phenotype, internalization, and dissociation kinetics of each type of cells in liver have impacts on the quantity and absorption rate to hepatocytes,38,54,55 which will ultimately determine the liver toxicity caused by MNPs. Previous studies have shown that the liver preferentially cleans larger nanomaterials.56,57 Because of their higher surface ligand density, they were more likely to be absorbed by primary rat Kupffer cells as well as immortalized mouse macrophages.55
Surface Modification
The interaction between nanomaterials and cells begins with the recognition of surface ligands and biofilm receptors. Current research is devoted to the surface modification of innovative materials to improve the specificity of cell recognition. Sykes et al56 studied the binding of nanoparticles to MDA-MB-435 cancer cells. It was found that within 60 nanometers, transferrin-modified ANP could be absorbed by cancer cells more quickly, while PEG-coated materials could penetrate into cancer cells more deeply, but the absorption rate was slow. Surface modification reduces the toxicity of some metal nanomaterials. Gao et al8,51,58synthesized a spherical silicon-coated gold nanomaterial (GNRS@SIO2), which was conjugated with amino terminus by folic acid as receptor, and finally produced GNRS@SiO2-FA. In the concentration range of 0–40 ppm, the composite has almost no toxic effect. Compared with unmodified GNRS@SiO2, the material can enter HepG2 cells quickly and distribute in cytoplasm and nucleus, while the internalization of unmodified nanomaterials is not obvious. Surface modification with enhanced biocompatibility can be used as an ideal material for targeted cancer therapy.59 Magnetic nanoparticle-aptamer probe demonstrates efficient in vitro MR imaging of the cancer cells and enhanced delivery of an anticancer drug into the cancer cell.60 Christopher et al61 modified NPs with bilayer nano-chitosan mercaptan and phosphatidylcholine and found that HepG2 cells consumed more new materials than gold polyvinyl glycol nanoparticles. Further studies showed that the structure of phosphatidylcholine-modified nanoparticles was similar to that of liposomes in hepatocytes, which enhanced the transport of gold nanoparticles. In vivo, the biodegradation and removal rate of PEG nanoparticles in liver and spleen is faster because PEG nanoparticles are more specific to tumors.62 Surface modification of metallic nanomaterials can enhance electrocatalytic activity. TiO263 and CeO264 nanocomposites modified by platinum nanoparticles enhanced the electrocatalytic activity of the materials for redox reaction. This may be due to the increase of oxygen capacity caused by strong electron coupling between composite structures.
Ion Release And Solubility
MNPs have the character of ion release. The toxicity of nano metallic monomer and metallic oxide not only comes from the NPs themselves but also from the release of metal ions or their interaction. Biological effects of MNPs in cells are shown to be mainly caused by the exposure to solubilized metallic ions. Han et al65 found that some MNPs decreased the activity of LDH. The similar deactivation mode of Cu2+ indicates that the decrease of LDH activity is mainly due to the dissolution of Cu NPs. Kinetic analysis showed that the Cu content in blood of Cu NPs exposed rats was 15–25% lower than that exposed by Cu2+. The Cu level in the organs (especially in the liver, kidney, and spleen) of the treated rats significantly increased. In the blood and organs of rats treated with Cu2+ and Cu, respectively, Cu reached the highest level later and lasted for a shorter time.66 Zn2+ and ZnO NPs can increase the Zn content of liver metallothioneins (MTs) and vitellogenin-like protein in plasma. It is noteworthy that MTs were upregulated by Zn2+ and ZnO NPs exposure, and the combination of Zn and Cu with MTs increased.67
The different results of subcellular distributions revealed that liver has different detoxification pathways for ZnO NPs and Zn2+. Metallothionein-like protein was the main effector of Zn2+, and ZnO NPs were mainly related to metal-rich granule.68 IvanaVinkovi Vrček et al24 compared the toxic effects of silver NPs and silver ions on HepG2 cells and found that the absorption of silver in the two forms was almost the same; the half-maximal inhibitory concentration (IC50) value of Ag NPs (50mg/L) was about 100 times higher than the corresponding value of Ag+ (0.5 mg•L−1). The possible reason was that Ag+ directly combined with SOD and GSH-Px and inhibited the enzyme activity.
Mechanisms of hepatotoxicity induced by metallic nanoparticles
Oxidative Stress
Reactive oxygen species (ROS) are active molecules produced during cell metabolism. In biology, ROS refers to superoxide anion radicals, hydroxyl radicals, and hydrogen peroxide.69 ROS are produced in the process of mitochondrial and cytoplasmic oxidation and help maintain cell function in the process of cell physiology. Excessive production of ROS can break the redox balance, resulting in oxidative stress, which leads to cell damage and cell death. Previous studies have shown that oxidative stress leads to lipid peroxidation and hepatocyte apoptosis, which is related to the occurrence and development of hepatitis, liver failure, ischemia-reperfusion injury, alcoholic liver disease, and other diseases.70
MNPs accumulated in the liver cause oxidative stress by altering the content and activity of antioxidant enzymes. The process of oxidative stress is accompanied by increased activity of antioxidant enzymes such as SOD, CAT, and glutathione peroxidase (GSH-px), as well as activity of non-enzymatic antioxidants such as ascorbic acid (ASA) and GSH.71 Due to the combined action of Ag NPs and Ti NPs, oral exposure in rats caused a strong level of oxidative stress in the liver. The endogenous antioxidant system showed decreased GSH/GSSG ratio and increased formation of reactive substances.72 After administration of polyvinylpyrrolidone-coated AgNPs (PVP-AgNPs) in male Sprague Dawley rats, the activities of SOD, CAT and TBARS increased and showed a dose-dependent effect.73 In addition, Fe3O4 NPs treatment caused significant increase in enzyme activities of GSH-Px, GR, and glutathione s-transferase (GST) with decrease in GSH content in Wistar rat organs.74
Oxidative stress injury is closely related to mitochondrial changes. TiO2 NPs can produce excessive ROS and reduce the antioxidant capacity of cells by destroying mitochondria. Further observations showed that TiO2 NPs could significantly reduce the mRNA levels of various detoxifying enzymes in the liver of mice, including SOD, CAT, GSH-px, and MT. Cytochrome P450, family 1, subfamily A, polypeptide 1 (CYP1A1), and heat shock protein 70 (HSP70) also came down by NPs and were involved, respectively, in toxic metabolism and DNA repair of hepatocyte damage.75
In vitro studies, Fe3O4 NPs exposure to primary rat hepatocytes showed that the excessive production of ROS was mainly due to the damage of mitochondria by MNPs.76The possible reason for excessive ROS production through mitochondria by MNPs was the accumulation of calcium ions, which interferes with the electron transport chain of mitochondria and makes mitochondria produce more oxygen-free radicals.77 Another study also confirmed that the effect of TiO2 NPs on HepG2 cells could activate NF-E2-related factor 2 (Nrf2) signals.78 In addition, the damage of ROS induced by MNPs to the production of endoplasmic reticulum cannot be ignored. ZnO NPs consumed antioxidants in the liver and induced ROS to affect the structure and function of the endoplasmic reticulum of mouse hepatocytes, which is believed to be related to apoptosis and autophagy.44
The results of ROS and antioxidant enzymes induced by MNPs are closely related to cell differentiation. Mei-Lang et al22 compared the effects of CuO NPs on different cells of cancer cell lines, showing that excessive CuO NPs can induce alter membrane permeability, damage the mitochondrial respiratory chain, and break DNA strands. Cells eventually died. SK-Hep-1 cells could not effectively remove the accumulated hydrogen peroxide due to low differentiation level and inadequate activity of CAT and GRx. SK-Hep-1 cells are more sensitive to oxidative stress induced by CuO NPs than HepG2 cells, and the cell damage is more serious.
Inflammation
Inflammation promotes the necrosis of parenchymal cells in organs and increases the accumulation of extracellular matrix in tissues. Mild damage leads to fibrosis while severe damage can lead to changes in the structure of organs and tissues. MNPs entering the body or liver cells induce inflammation. Liu et al showed that the liver of male Wistar rats was infiltrated by inflammatory cells because of exposure to ZnO NPs, TiO2 NPs, and Ag NPs. Steatosis of hepatocytes and necrosis of the central part of hepatic lobules were also observed. Serum IL-1β level increased significantly in MNP-exposed group, serum IFN-γ and TNF-α level decreased in ZnONP and TiO2 NP groups as well as the concentrations of TNF-α increased significantly in Ag NP groups.79 Kupffer cells, a kind of phenotype, are the resident macrophages in liver. MNPs accumulated in the liver are mainly ingested by Kupffer cells, with little uptake for hepatocytes, inflammation of the liver, Kupffer cells proliferation, and increased IL-1β release.38,55 Similar to the effect of Ag NPs on the expression of inflammation in vivo, the exposure of human hepatocyte line C3a to Ag NPs increased the expression of IL-8, macrophage inflammatory protein 2, IL-1RI, and tumor necrosis factor α (TNF-α).80 Ag NPs or AgNO3 contributed to the transition from hepatic steatosis to steatohepatitis. Ag NPs or AgNO3 acted on HFD mice caused the increase of serum total cholesterol, HDL, and LDL levels. More importantly, elevated levels of IL-6 and TNF in mouse liver suggested inflammation.13 The production of inflammation corpuscle NLRP3 is the core of inflammation induced by MNPs. The generation and activation of NLRP3 involve MAPK, NF-kB, and ROS signals. Manna et al81 showed that exposure to Cu NPs reduced liver index in a dose-dependent manner, resulting in oxidative stress and liver dysfunction. Cu NPs also increased the transcriptional activity of NF-kB. Ag NPs activated MAPK and PKB signaling pathways and induced ROS-mediated DNA damage in HepG2 cells.82 These signals are not only related to inflammation but also induce ROS to promote apoptosis. Cu NPs affect CYP450 activity and suppress some nuclear receptors through the NF-κB signaling pathway. In fact, the regulation of P450 is also related to ROS.12
The Outcomes Of Liver Cell Caused By MNPs
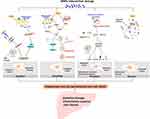
MNPs which reach the liver enter the cells and cause damage to the liver cells. As the basic unit of liver, different forms of cell death cause a series of damage, leading to liver dysfunction and pathological changes. Recent studies have highlighted the role of different death pathways in the pathogenesis of liver injury induced by MNPs as described in Figure 1.
Apoptosis
Apoptosis is a programmed process of cell death that is used to clear unwanted cells from the body and a safe and controllable process that does not affect surrounding cells.83 Apoptosis of hepatocytes leads to dysfunction, proliferation inhibition, cycle arrest, and decreased viability, thus causing liver fibrosis,84 nonalcoholic fatty liver diseases related to cirrhosis and hepatocellular carcinoma (HCC).85 Apoptosis is also regarded as the basis for chronic inflammation.86
Apoptosis is a prominent feature of hepatic damage of MNPs. Hepatocyte apoptosis is characterized by nuclear chromatin condensation, nuclear rupture, cell contraction, plasma membrane vacuolation, DNA damage, lack of nutrition and cytokine release, which reflect the activation of cell surface death receptors and apoptotic factors..87–90
Apoptosis is classified into endogenous apoptosis and exogenous apoptosis. Endogenous apoptosis, also known as mitochondrial pathway apoptosis, is a core event in which mitochondrial membrane permeability increases and mainly induced by activated BH3-only protein, which increases Bcl-2. Two proapoptotic molecules of the lymphoma 2 family, BAX (Bcl-2 related X protein) and BAK (Bcl-2 antagonist or killer), form oligomers in the outer membrane of mitochondria, which constitute a supramolecular channel-mediated cytochrome c,which causes other proteins to be released from the mitochondria into the cytoplasm, thereby activating Caspase 9 and the Caspase cascade, triggering endogenous apoptosis.91–93
For 40 adult male albino rats, histopathological examination of liver in the exposed group of TiO2 NPs showed that oral administration of TiO2 NPs caused obvious apoptotic damage, which is manifested in the increase of Bax gene and the decrease of anti-apoptotic Bcl-2 gene level.94 Ag NPs entering human liver cells induced ROS production, inhibited the production of reduced glutathione, caused DNA fragmentation, lipid membrane peroxidation, and protein carbonylation. In addition, the mechanism of cell damage caused by Ag NPs is mitochondrial-dependent endogenous apoptotic pathway. By regulating the expression of Bax and Bcl-2, Ag NPs destroyed mitochondrial membrane potential, induced cytochrome c release in cytoplasm, and activated caspase-9 and caspase-3.27 Exposure of the liver cells (HL7702 cells, CHANG cells, HepG2 cells) to MNPs (Cs-Ag NPs, rGO-Ag NPs, TiO2 NPs, Fe3O4-TiO2 NPs) has been proved to have the same damage effects.23,95,96
Xue et al97 found that Ag NPs acting on HepG2 cells not only caused mitochondrial-dependent apoptosis induced by ROS but also activated the Fas death receptor pathway by downregulation of NF-κB and activation of caspase-8 and caspase-3. This process illustrates the death receptor-mediated exogenous apoptosis pathway.
Another crucial mechanism involved in apoptosis is mediated by JNK-activated ER stress. Yang et al44 showed that ZnO NPs significantly reduced the expression of anti-apoptotic gene Bcl-2 in liver tissue of mice. The phosphorylation of JNK protein in mouse hepatocytes was activated, and the activities of caspase-3, caspase-9, and caspase-12 were observed.
Autophagic Cell Death
Autophagy, an important process of self-regulation and homeostasis of cells, is involved in cell cycle, cell death, self-renewal of stem cells, establishment of pluripotent-induced stem cells, and resistance to foreign pathogenic microorganisms.98 More and more studies have shown that autophagy, as a double-edged sword effect, plays a two-way regulating role in affecting cell survival and death.99 In liver metabolic diseases, autophagy is closely related to the occurrence of NAFLD, viral hepatitis, and even cancer.100
Numerous studies have shown that MNPs activate autophagy after entering the liver cells by endocytosis. MNPs (CTAB-GNR NPs, Ag NPs) entered the L02 cells and HepG2 cells, activated low levels of autophagy, increased protein expression of LC3-II, and observed double-layer membrane-coated autophagosomes under TEM.At this concentration, no significant cytotoxicity and lysosomal damage were observed, and MNPs induced ROS-mediated protective autophagy.26,101 Rare earth doped up conversion nanoparticles (UCNs) activated autophagy in Kupffer cell,which causesd a decrease of cell survival and an increase in liver damage. However, inhibiting the formation of autophagosomes with 3-MA increased the survival rate of Kupffer cells and further eliminated the hepatotoxicity induced by UCNs,102 suggesting autophagy played a role in damaging cells.
Cells recognize MNPs as external stimuli, activate ROS, and then cause mitochondrial damage. In order to maintain cell stability, autophagy is used to remove dysfunctional organelles. Autophagic damage occurs when autophagy fails to cope with environmental changes. Fusion of autophages and lysosomes to form autophagic lysosomes is an important process of cell autophagy. Iron(III)–tannin complex (Fe–TA NPs) induced the endocytosis of HepG2 cells and initiated the formation of autophagosomes. The intracellular nuclear vesicles and multivesicular (MVBs) produced by Fe–TA NPs were fused with autophagosomes, which could be degraded by regulating lysosomal functions.103 This could be considered as one of the mechanisms by which MNPs induce autophagic cell death from excessive self-digestion.
In addition, there is a close relationship between autophagy and apoptosis induced by MNPs in hepatocytes – apoptosis may be an autophagy-related death pathway. In adult male SD rats exposed to PVP-Ag NPs, the ratio of LC3-II/LC3-I increased together with increased caspase-3, p53, and p21.73 Kermanizadeh et al104 cultured HepG2 cells and A549 cells with Ag and ZnO NPs for 6 hrs, resulting in the expression of autophagy-related genes LC3B, Atg4b, p62 upregulated, Atg12 and Atg5 declined. However, in the latter stages autophagy was impaired by caspase-dependent apoptotic cell death..
Pyroptosis
Pyroptosis, also referred to as cellular inflammatory necrosis, is one way of caspase-1-mediated programmed cell death.105 Gasdermin D (GSDMD) is one of the downstream Gasdermin protein families. The basic mechanism of the cell pyroptosis is that the inflammatory complex of the upstream protein activates caspase-1, which cleaves the GSDMD, and then the GSDMD protein releases the N-terminal fragment to recognize the phospholipid molecules on the cell membrane. Further, a hole is formed on the cell membrane, resulting in changes in ion concentration and osmotic pressure inside and outside the cell. Finally, the cell membrane is broken and cell contents are released, accompanied by pyroptosis.106–108
MNPs activated hepatocyte pyroptosis after entering cells by endocytosis. Its main features are cell membrane rupture and proinflammatory cell content release, which will cause the pathogen released from the dead cells, phagocytized and degraded by other cells, thus reducing the burden of infection, activating a strong inflammatory response and releasing plenty of inflammatory factors.109 In addition, MNPs could also cause liver nuclear condensation, DNA shearing, and fragmentation.110 Mirshafiee et al38 found that Gd2O3 could cause Kupffer cell swelling, giant blebbing, cell membrane pore, caspase-1 activation, and IL-1β creation. The formation of cell membrane pore depends on GSDMD, which activates caspase-1. It disturbs the ion flow inside and outside the cell membrane, causes cell swelling, forms membrane vesicles, and leads to the leakage and intracellular substances release. Therefore, the death pattern of macrophages and hepatic parenchymal cells caused by MNPs can be reversed by knocking out the Gastermin D protein.
The apoptosis induced by MNPs has been confirmed to be associated with autophagy. Ag NPs induce caspase-1 activation and autophagic flux in HepG2 cells. When the autophagy-lysosome system was blocked, NLRP3 inflammatory bodies activated caspase-1 to a higher degree.26
The release of IL-1β and the increase of N-GSDMD expression induced by cell char death promote the occurrence and development of some liver diseases.111 N-GSDMA activates NLRP3 inflammatory bodies and induces cell death through typical pathways.112 The pathogenesis of hepatitis C has been confirmed to be closely related to caspase-1 and caspase-3 signal-mediated cell burnout.113
Necrosis
Cell necrosis refers to the irreversible loss of metabolic function and structural integrity of the cell serosa, the loss of integrity of the serosa, and the activation of non-inflammatory bodies. It is characterized by mitochondrial impairment and ATP depletion. ROS generation induced by MNPs leads to cell death and damage via hepatocyte necrosis. Adult female rats were continually exposed to PbO NPs 24 hrs a day with an average concentration of 106 particles per cubic centimeter. Six weeks later, the liver changed, showing hepatocyte swelling and hydropic degeneration, lobular hypertrophy with nuclear size changes, hepatocyte necrosis, inflammation around the portal vein and accumulation of lipid droplets.114 Wang et al28 co-cultured primary hepatocytes with Cu NPs and CuSO4 for 24 hrs and observed that the apoptosis and necrosis rate of primary hepatocytes were apparently higher than that of control group. Significantly increased intracellular ROS and MDA, multiplied cytochrome c release, downregulated anti-oxidation related genes [SOD, CAT, GSH-Px4] expression, upregulated apoptosis-related genes (p53, p38 and TNF-α), and increased activities of caspase-3, caspase-8, and caspase-9 all indicated that ROS might be involved in the process of cell necrosis, and there could be a certain correlation between necrosis and apoptosis.
Necrosis is one of the prominent features in acute liver injury.115 Death of necrotic cells is also a distinct feature of hepatic ischemia/reperfusion injury.86 In chronic hepatitis B virus infection, from local inflammation led hepatocyte apoptosis and necrosis to liver regeneration, a vicious circle is formed, which may be the potential mechanism of hepatocellular carcinogenesis.116
Outlook
With the advances in the fields of nanotechnology, the potential exposure of MNPs is likely to increase, so there is an urgent need to further study the possibility of any detrimental health effects, target organ damage, and its mechanism. The toxicity of MNPs to the liver is an important basis for the safety assessment of MNPs. At present, studies on the hepatotoxicity of MNPs are still in their infancy. The toxicity of MNPs is mainly due to the special physical and chemical properties, such as size, surface chemical modification and metal ion release. The liver is particularly susceptible to MNPs because the liver has a much higher accumulation of NM than other organs.. For the evaluation of hepatotoxicity of MNPs, on the one hand, a full understanding of the distribution and metabolism of MNPs in the liver, and detecting the changes in liver function, degree of injury, and recovery of liver function in vivo, which are prerequisites for evaluating their liver toxicity. On the other hand, it is important to better understand the mechanisms by focusing on the complex biological process between MNPs and cells. MNPs entering cells change the structure and functions of organelles, affect the normal biological functions of cells, and ultimately impact the amount of toxicity and threshold dose caused by MNPs. It is worth noting that once the MNPs accumulate in the liver, it may cause changes in liver function. When the MNPs enter the cell, it will damage it and produce a large amount of free oxidative free radicals, thereby destroying the oxidation/deoxidation balance. MNPs can also enter the nucleus and can directly or indirectly destroy DNA, leading to changes in gene expression and even apoptosis. If there is a long-term liver injury, the HSC will turn into an active state. Along with changes in the activity of several intracellular signaling pathways, extracellular components are involved in the extracellular matrix, which ultimately leads to fibrosis and may eventually progress to cirrhosis. However, the detailed mechanism of MNPs leading to liver fibrosis remains unclear.
To study the molecular mechanisms of liver injury caused by MNPs, it is necessary to perform experiments from in vivo to in vitro involving in molecular biology especially biomarker screening, which is crucial for understanding the detailed mechanism of liver injury. Although immune inflammation, apoptosis, and oxidative stress related to liver injury have been investigated, the aspects of energy metabolism, protein metabolism, and lipid metabolism should be studied in detail. Current research into the toxicity of MNPs has been limited to animal experiments in vivo and in vitro; the relationship between subcellular damage and related mechanisms is still unknown. Therefore, the toxicology of MNPs must be studied in-depth to improve the quality and safety of those nanoparticles. Research in this area has a long way to go.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 81673218, 21876026, 31671034), Student’s Platform for Innovation and Entrepreneurship Training Program (No. 201810286142) and the Fundamental Research Funds for the Central Universities (No. 2242018K40021, 2242019K40223).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arab J Chem. 2017;21(3):541–548.
2. Parada J, Rubilar O, Fernández-Baldo MA, et al. The nanotechnology among US: are metal and metal oxides nanoparticles a nano or mega risk for soil microbial communities? Crit Rev Biotechnol. 2018;39(2):157–172. doi:10.1080/07388551.2018.1523865
3. Andra S, Balu SK, Jeevanandham J. Phytosynthesized metal oxide nanoparticles for pharmaceutical applications. Naunyn Schmiedebergs Arch Pharmacol. 2019;392(7):755–771. doi:10.1007/s00210-019-01666-7
4. Amde M, Liu JF, Tan ZQ, Bekana D. Transformation and bioavailability of metal oxide nanoparticles in aquatic and terrestrial environments. A review. Environ Pollut. 2017;230:250–267. doi:10.1016/j.envpol.2017.06.064
5. Yah CS, Iyuke SE, Simate GS. A review of nanoparticles toxicity and their routes of exposures. Iran J Pharm Res. 2012;8(1):299–314.
6. Gustafson HH, Holt-Casper D, Grainger DW, Ghandehari H. Nanoparticle uptake: the phagocyte problem. Nano Today. 2015;10(4):487–510. doi:10.1016/j.nantod.2015.06.006
7. Almeida JPM, Chen AL, Foster A, Drezek R. In vivo biodistribution of nanoparticles. Nanomedicine. 2011;6(5):815–835. doi:10.2217/nnm.11.79
8. Li TZ, Gong F, Zhang BY, et al. [Acute toxicity and bio-distribution of silver nitrate and nano-silver with different particle diameters in rats]. Chin J Burns. 2016;32(10):606–612. doi:10.3760/cma.j.issn.1009-2587.2016.10.007
9. Kreyling WG, Möller W, Holzwarth U, et al. Age-dependent rat lung deposition patterns of inhaled 20 nanometer gold nanoparticles and their quantitative biokinetics in adult rats. ACS Nano. 2018;12(8):7771–7790. doi:10.1021/acsnano.8b01826
10. Chinde S, Grover P. Toxicological assessment of nano and micron-sized tungsten oxide after 28days repeated oral administration to Wistar rats. Mutat Res. 2017;819:1–13. doi:10.1016/j.mrgentox.2017.05.003
11. Liu F, Chang X, Tian M, et al. Nano NiO induced liver toxicity via activating the NF-κB signaling pathway in rats. Toxicol Res (Camb). 2017;6(2):242–250. doi:10.1039/C6TX00444J
12. Tang H, Min X, Fei S, et al. Effects and mechanism of nano-copper exposure on hepatic cytochrome P450 enzymes in rats. Int J Mol Sci. 2018;19(7):2140. doi:10.3390/ijms19072140
13. Jia J, Li F, Zhou H, et al. Oral exposure of silver nanoparticles or silver ions may aggravate fatty liver disease in overweight mice. Environ Sci Technol. 2017;51(16):9334–9343. doi:10.1021/acs.est.7b02752
14. Azim SAA, Darwish HA, Rizk MZ, Ali SA, Mai OK. Amelioration of titanium dioxide nanoparticles-induced liver injury in mice: possible role of some antioxidants. Exp Toxicol Pathol. 2015;67(4):305–314. doi:10.1016/j.etp.2015.02.001
15. Sha B, Gao W, Wang S, et al. Oxidative stress increased hepatotoxicity induced by nano-titanium dioxide in BRL-3A cells and Sprague-Dawley rats. J Appl Toxicol. 2014;34(4):345–356. doi:10.1002/jat.2900
16. Magaye RR, Yue X, Zou B, et al. Acute toxicity of nickel nanoparticles in rats after intravenous injection. Int J Nanomedicine. 2014;2014(1):1393–1402.
17. Recordati C, De Maglie M, Bianchessi S, et al. Tissue distribution and acute toxicity of silver after single intravenous administration in mice: nano-specific and size-dependent effects. Part Fibre Toxicol. 2016;13:12. doi:10.1186/s12989-016-0124-x
18. Suker DK, Jasim FA. Liver histopathological alteration after repeated intra-tracheal instillation of titanium dioxide in male rats. Gastroenterol Hepatol Bed Bench. 2018;11(2):159–168.
19. Matthias B, Thomas R, Keul HA, et al. Peptide-functionalized gold nanorods increase liver injury in hepatitis. ACS Nano. 2012;6(10):8767–8777. doi:10.1021/nn302502u
20. Wang C, Cheng K, Zhou L, et al. Evaluation of long-term toxicity of oral zinc oxide nanoparticles and zinc sulfate in mice. Biol Trace Elem Res. 2017;178(2):276–282. doi:10.1007/s12011-017-0934-1
21. Yu S, Liu F, Wang C, et al. Role of oxidative stress in liver toxicity induced by nickel oxide nanoparticles in rats. Mol Med Rep. 2018;17(2):3133–3139. doi:10.3892/mmr.2017.8226
22. Mei-Lang K, Shu-Ling H, Chih-Chung W, et al. Enhanced reactive oxygen species overexpression by CuO nanoparticles in poorly differentiated hepatocellular carcinoma cells. Nanoscale. 2015;7(5):1820–1829. doi:10.1039/C4NR05843G
23. Ali D, Alarifi S, Alkahtani S, Almeer RS. Silver-doped graphene oxide nanocomposite triggers cytotoxicity and apoptosis in human hepatic normal and carcinoma cells. Int J Nanomedicine. 2018;13:5685–5699. doi:10.2147/IJN
24. Vrček IV, Žuntar I, Petlevski R, et al. Comparison of in vitro toxicity of silver ions and silver nanoparticles on human hepatoma cells. Environ Toxicol. 2016;31(6):679–692. doi:10.1002/tox.v31.6
25. Grzelak A, Wojewodzka M, Meczynska-Wielgosz S, Zuberek M, Wojciechowska D, Kruszewski M. Crucial role of chelatable iron in silver nanoparticles induced DNA damage and cytotoxicity. Redox Biol. 2018;15:435–440. doi:10.1016/j.redox.2018.01.006
26. Mishra AR, Zheng J, Tang X, Goering PL. Silver nanoparticle-induced autophagic-lysosomal disruption and NLRP3-inflammasome activation in HepG2 cells is size-dependent. Toxicol Sci. 2016;150(2):473–487. doi:10.1093/toxsci/kfw011
27. Piao MJ, Kang KA, Lee IK, et al. Silver nanoparticles induce oxidative cell damage in human liver cells through inhibition of reduced glutathione and induction of mitochondria-involved apoptosis. Toxicol Lett. 2011;201(1):92–100. doi:10.1016/j.toxlet.2010.12.010
28. Wang T, Chen X, Long X, Liu Z, Copper Nanoparticles YS. Copper sulphate induced cytotoxicity in hepatocyte primary cultures of epinephelus coioides. PLoS One. 2016;11(2):e0149484. doi:10.1371/journal.pone.0149484
29. Wang Y, Aker WG, Hwang HM, Yedjou CG, Yu H, Tchounwou PB. A study of the mechanism of in vitro cytotoxicity of metal oxide nanoparticles using catfish primary hepatocytes and human HepG2 cells. Sci Total Environ. 2011;409(22):4753–4762. doi:10.1016/j.scitotenv.2011.07.039
30. Natarajan V, Wilson CL, Hayward SL, Kidambi S. Titanium dioxide nanoparticles trigger loss of function and perturbation of mitochondrial dynamics in primary hepatocytes. PLoS One. 2015;10(8):e0134541. doi:10.1371/journal.pone.0134541
31. Saquib Q, Siddiqui MA, Ahmad J, et al. Nickel oxide nanoparticles induced transcriptomic alterations in HEPG2 cells. Exp Biol Med. 2018;1048(10):163–174.
32. Sun X, Wang Z, Zhai S, Cheng Y, Liu J, Liu B. In vitro cytotoxicity of silver nanoparticles in primary rat hepatic stellate cells. Mol Med Rep. 2013;8(5):1365–1372. doi:10.3892/mmr.2013.1683
33. Liu S, Shen Z, Wu B, et al. Cytotoxicity and efflux pump inhibition induced by molybdenum disulfide and boron nitride nanomaterials with sheetlike structure. Environ Sci Technol. 2017;51(18):10834–10842. doi:10.1021/acs.est.7b02463
34. Chen R, Zhao L, Bai R, et al. Silver nanoparticles induced oxidative and endoplasmic reticulum stresses in mouse tissues: implications for the development of acute toxicity after intravenous administration. Toxicol Res (Camb). 2016;5(2):602–608. doi:10.1039/C5TX00464K
35. Almansour M, Sajti L, Melhim W, Jarrar B. Ultrastructural hepatic alterations induced by 35 nm zinc oxide nanoparticles. Nanosci. 2015;7(9):763–769. doi:10.1166/nnl.2015.2028
36. Yang J, Luo M, Tan Z, et al. Oral administration of nano-titanium dioxide particle disrupts hepatic metabolic functions in a mouse model. Environ Toxicol Pharmacol. 2017;49:112–118. doi:10.1016/j.etap.2016.12.006
37. Jana P, Bojana Z, Magdalena S, et al. DNA damage and alterations in expression of DNA damage responsive genes induced by TiO2 nanoparticles in human hepatoma HepG2 cells. Nanotoxicology. 2011;5(3):341–353. doi:10.3109/17435390.2010.507316
38. Mirshafiee V, Sun B, Chang CH, et al. Toxicological profiling of metal oxide nanoparticles in liver context reveals pyroptosis in kupffer cells and macrophages versus apoptosis in hepatocytes. ACS Nano. 2018;12(4):3836–3852. doi:10.1021/acsnano.8b01086
39. Paesano L, Perotti A, Buschini A, et al. Markers for toxicity to HepG2 exposed to cadmium sulphide quantum dots; damage to mitochondria. Toxicology. 2016;374(2016):18–28. doi:10.1016/j.tox.2016.11.012
40. Nguyen KC, Rippstein P, Tayabali AF, Willmore WG. Mitochondrial toxicity of cadmium telluride quantum dot nanoparticles in mammalian hepatocytes. Toxicol Sci. 2015;146(1):31–42. doi:10.1093/toxsci/kfv068
41. Maurer LL, Meyer JN. A systematic review of evidence for silver nanoparticle-induced mitochondrial toxicity. Environ Sci Nano. 2016;3(2):311–322.
42. Kuang H, Yang P, Yang L, Aguilar ZP, Xu H. Size dependent effect of ZnO nanoparticles on endoplasmic reticulum stress signaling pathway in murine liver. J Hazard Mater. 2016;317:119–126. doi:10.1016/j.jhazmat.2016.05.063
43. Yu KN, Sung JH, Lee S, et al. Inhalation of titanium dioxide induces endoplasmic reticulum stress-mediated autophagy and inflammation in mice. Food Chem Toxicol. 2015;85:106–113. doi:10.1016/j.fct.2015.08.001
44. Yang X, Shao H, Liu W, et al. Endoplasmic reticulum stress and oxidative stress are involved in ZnO nanoparticle-induced hepatotoxicity. Toxicol Lett. 2015;234(1):40–49. doi:10.1016/j.toxlet.2015.02.004
45. He C, Jiang S, Yao H, et al. Endoplasmic reticulum stress mediates inflammatory response triggered by ultra-small superparamagnetic iron oxide nanoparticles in hepatocytes. Nanotoxicology. 2018;12(10):1198–1214. doi:10.1080/17435390.2018.1530388
46. Condello M, De Berardis B, Ammendolia MG, et al. ZnO nanoparticle tracking from uptake to genotoxic damage in human colon carcinoma cells. Toxicol In Vitro. 2016;35:169–179. doi:10.1016/j.tiv.2016.06.005
47. Sarhan OM, Hussein RM. Effects of intraperitoneally injected silver nanoparticles on histological structures and blood parameters in the albino rat. Int J Nanomedicine. 2014;9:1505–1517. doi:10.2147/IJN.S56729
48. Wang J, Yu Y, Lu K, et al. Silica nanoparticles induce autophagy dysfunction via lysosomal impairment and inhibition of autophagosome degradation in hepatocytes. Int J Nanomedicine. 2017;12:809–825. doi:10.2147/IJN.S123596
49. Gatoo MA, Naseem S, Arfat MY, Mahmood Dar A, Qasim K, Zubair S. Physicochemical properties of nanomaterials: implication in associated toxic manifestations. Biomed Res Int. 2014;2014:498420. doi:10.1155/2014/498420
50. Lopez-Chaves C, Soto-Alvaredo J, Montes-Bayon M, Bettmer J, Llopis J, Sanchez-Gonzalez C. Gold nanoparticles: distribution, bioaccumulation and toxicity. In vitro and in vivo studies. Nanomed. 2018;14(1):1–12. doi:10.1016/j.nano.2017.08.011
51. Gao B, Xu J, He KW, et al. Cellular uptake and intra-organ biodistribution of functionalized silica-coated gold nanorods. Mol Imaging Biol. 2016;18(5):667–676. doi:10.1007/s11307-016-0938-9
52. Peng F, Su Y, Zhong Y, He Y. Subcellular distribution and cellular self-repair ability of fluorescent quantum dots emitting in the visible to near-infrared region. Nanotechnology. 2017;28(4):045101. doi:10.1088/1361-6528/28/4/045101
53. Xie X, Liao J, Shao X, Li Q, Lin Y. The effect of shape on cellular uptake of gold nanoparticles in the forms of stars, rods, and triangles. Sci Rep. 2017;7(1):3827. doi:10.1038/s41598-017-04229-z
54. Almansour MI, Alferah MA, Shraideh ZA, Jarrar BM. Zinc oxide nanoparticles hepatotoxicity: histological and histochemical study. Environ Toxicol Pharmacol. 2017;51:124–130. doi:10.1016/j.etap.2017.02.015
55. Tsoi KM, MacParland SA, Ma XZ, et al. Mechanism of hard-nanomaterial clearance by the liver. Nat Mater. 2016;15(11):1212–1221. doi:10.1038/nmat4718
56. Sykes EA, Chen J, Zheng G, Chan WC. Investigating the impact of nanoparticle size on active and passive tumor targeting efficiency. ACS Nano. 2014;8(6):5696–5706. doi:10.1021/nn500299p
57. Perrault SD, Walkey C, Jennings T, Fischer HC, Chan WC. Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 2009;9(5):1909–1915. doi:10.1021/nl900031y
58. Suganthy N, Sri Ramkumar V, Pugazhendhi A, Benelli G, Archunan G. Biogenic synthesis of gold nanoparticles from terminalia arjuna bark extract: assessment of safety aspects and neuroprotective potential via antioxidant, anticholinesterase, and antiamyloidogenic effects. Environ Sci Pollut Res. 2018;25(11):10418–10433. doi:10.1007/s11356-017-9789-4
59. Pugazhendhi A, Tnji E, Karuppusamy I, Kathirvel B. Inorganic nanoparticles: a potential cancer therapy for human welfare. Int J Pharm. 2018;539(1–2):104–111. doi:10.1016/j.ijpharm.2018.01.034
60. Pilapong C, Sitthichai S, Thongtem S, Thongtem T. Smart magnetic nanoparticle-aptamer probe for targeted imaging and treatment of hepatocellular carcinoma. Int J Pharm. 2014;473(1–2):469–474. doi:10.1016/j.ijpharm.2014.07.036
61. England CG, Priest T, Zhang G, et al. Enhanced penetration into 3D cell culture using two and three layered gold nanoparticles. Int J Nanomedicine. 2013;8:3603–3617. doi:10.2147/IJN.S51668
62. Jiang Z, Shan K, Song J, et al. Toxic effects of magnetic nanoparticles on normal cells and organs. Life Sci. 2019;220:156–161. doi:10.1016/j.lfs.2019.01.056
63. Krishnan SK, Kumar PSM. Controlled synthesis of Pt nanoparticle supported TiO2 nanorods as efficient and stable electrocatalyst for oxygen reduction reaction. J Mater Chem. 2018;6:46.
64. Murphin Kumar PS, Thiripuranthagan S, Imai T, et al. Pt nanoparticles supported on mesoporous CeO2 nanostructures obtained through green approach for efficient catalytic performance towards ethanol electrooxidation. ACS Sustain Chem Eng. 2017;5:12. doi:10.1021/acssuschemeng.7b02019
65. Han X, Gelein R, Corson N, et al. Validation of an LDH assay for assessing nanoparticle toxicity. Toxicology. 2011;287(1):99–104. doi:10.1016/j.tox.2011.06.011
66. Lee IC, Ko JW, Park SH, et al. Comparative toxicity and biodistribution of copper nanoparticles and cupric ions in rats. Int J Nanomedicine. 2016;11:2883–2900. doi:10.2147/IJN.S106346
67. Falfushynska H, Gnatyshyna L, Horyn O, Shulgai A, Stoliar O. A calcium channel blocker nifedipine distorts the effects of nano-zinc oxide on metal metabolism in the marsh frog Pelophylax ridibundus. Saudi J Biol Sci. 2019;26(3):481–489. doi:10.1016/j.sjbs.2017.10.004
68. Wenhong F, Qian L, Xiuping Y, Li Z. Zn subcellular distribution in liver of goldfish (carassius auratus) with exposure to zinc oxide nanoparticles and mechanism of hepatic detoxification. PLoS One. 2013;8(11):e78123. doi:10.1371/journal.pone.0078123
69. Rang FJ, Boonstra J. Causes and consequences of age-related changes in DNA methylation: a role for ROS? Biology. 2014;3(2):403–425. doi:10.3390/biology3020403
70. Cichożlach H, Michalak A. Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol. 2014;20(25):8082–8091. doi:10.3748/wjg.v20.i25.8082
71. Liu H, Ma L, Liu J, Zhao J, Yan J, Hong F. Toxicity of nano-anatase TiOto mice: liver injury, oxidative stress. Environ Toxicol Chem. 2010;92(1):175–186.
72. Pereira LC, Pazin M, Franco-Bernardes MF, et al. A perspective of mitochondrial dysfunction in rats treated with silver and titanium nanoparticles (AgNPs and TiNPs). J Trace Elem Med Biol. 2018;47:63–69. doi:10.1016/j.jtemb.2018.01.007
73. Blanco J, Tomas-Hernandez S, Garcia T, et al. Oral exposure to silver nanoparticles increases oxidative stress markers in the liver of male rats and deregulates the insulin signalling pathway and p53 and cleaved caspase 3 protein expression. Food Chem Toxicol. 2018;115:398–404. doi:10.1016/j.fct.2018.03.039
74. Reddy UA, Prabhakar PV, Mahboob M. Comparative study of nano and bulk Fe3O4 induced oxidative stress in Wistar rats. Biomarkers. 2018;23(5):425–434. doi:10.1080/1354750X.2018.1443508
75. Jia X, Wang S, Zhou L, Sun L. The potential liver, brain, and embryo toxicity of titanium dioxide nanoparticles on mice. Nanoscale Res Lett. 2017;12(1):478. doi:10.1186/s11671-017-2242-2
76. Gokduman K, Bestepe F, Li L, Yarmush ML, Usta OB. Dose-, treatment- and time-dependent toxicity of superparamagnetic iron oxide nanoparticles on primary rat hepatocytes. Nanomedicine. 2018;13(11):1267–1284. doi:10.2217/nnm-2017-0387
77. Degli Esposti D, Hamelin J, Bosselut N, et al. Mitochondrial roles and cytoprotection in chronic liver injury. Biochem Res Int. 2012;387626.
78. Thai SF, Wallace KA, Jones CP, et al. Differential genomic effects of six different TiO2 nanomaterials on human liver HepG2 cells. J Biochem Mol Toxicol. 2016;30(7):331–341. doi:10.1002/jbt.21798
79. Liu H-L, Yang H-L, Lin B-C, et al. Toxic effect comparison of three typical sterilization nanoparticles on oxidative stress and immune inflammation response in rats. Toxicol Res (Camb). 2015;4(2):486–493. doi:10.1039/C4TX00154K
80. Gaiser BK, Hirn S, Kermanizadeh A, et al. Effects of silver nanoparticles on the liver and hepatocytes in vitro. Toxicol Sci. 2013;131(2):537–547. doi:10.1093/toxsci/kfs306
81. Prasenjit M, Manoranjan G, Jyotirmoy G, Joydeep D, Sil PC. Contribution of nano-copper particles to in vivo liver dysfunction and cellular damage: role of IκBα/NF-κB, MAPKs and mitochondrial signal. Nanotoxicology. 2012;6(1):1–21. doi:10.3109/17435390.2011.552124
82. Zhu B, Li Y, Lin Z, et al. Silver nanoparticles induce HePG-2 cells apoptosis through ROS-mediated signaling pathways. Nanoscale Res Lett. 2016;11(1):198. doi:10.1186/s11671-016-1419-4
83. Kerr JFR, Wyllie AH, Currie AR. Apoptosis_A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26(4):239–257. doi:10.1038/bjc.1972.33
84. Zhou WC, Zhang QB, Liang Q. Pathogenesis of liver cirrhosis. Med Klin. 2014;20(23):278–280.
85. Kanda T, Matsuoka S, Yamazaki M, et al. Apoptosis and non-alcoholic fatty liver diseases. World J Gastroenterol. 2018;24(25):2661–2672. doi:10.3748/wjg.v24.i25.2661
86. Guicciardi ME, Malhi H, Mott JL, Gores GJ. Apoptosis and necrosis in the liver. Compr Physiol. 2013;3(2):977–1010. doi:10.1002/cphy.c120020
87. Chen YY, Ou YS, Tao Y, et al. Effect and mechanisms of celastrol on the apoptosis of HOS osteosarcoma cells. Oncol Rep. 2018;40(4):2260–2268. doi:10.3892/or.2018.6619
88. Savitskaya MA, Onishchenko GE. Mechanisms of apoptosis. Biochem.-Moscow. 2015;80(11):1393–1405. doi:10.1134/S0006297915110012
89. Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9(3):231–241. doi:10.1038/nrm2312
90. Günther C, Neumann H, Neurath MF, Becker C. Apoptosis, necrosis and necroptosis: cell death regulation in the intestinal epithelium. Gut. 2013;62(7):1062–1071. doi:10.1136/gutjnl-2011-301364
91. Adrain C, SJ M. The mitochondrial apoptosome_a killer unleashed by the cytochrome seas. Trends Biochem Sci. 2001;26(6):390–397. doi:10.1016/S0968-0004(01)01844-8
92. Arnoult D, Gaume B, Karbowski M, Sharpe JC, Cecconi F, Youle RJ. Mitochondrial release of AIF and EndoG requires caspase activation downstream of Bax_Bak-mediated permeabilization. Embo J. 2003;22(17):4385–4399. doi:10.1093/emboj/cdg423
93. Kitagawa N, Morikawa T, Motai C, et al. The antiproliferative effect of chakasaponins I and II, floratheasaponin A, and epigallocatechin 3-O-gallate isolated from camellia sinensis on human digestive tract carcinoma cell lines. Int J Mol Sci. 2016;17(12):1–16. doi:10.3390/ijms17121979
94. Morgan A, Ibrahim MA, Galal MK, Ogaly HA, Abd-Elsalam RM. Innovative perception on using Tiron to modulate the hepatotoxicity induced by titanium dioxide nanoparticles in male rats. Biomed Pharmacother. 2018;103:553–561. doi:10.1016/j.biopha.2018.04.064
95. El-Sherbiny IM, Salih E, Yassin AM, Hafez EE. Newly developed chitosan-silver hybrid nanoparticles: biosafety and apoptosis induction in HepG2 cells. J Nanopart Res. 2016;18(7):172. doi:10.1007/s11051-016-3477-z
96. Su H, Li Z, Lazar L, et al. In vitro evaluation of the toxicity and underlying molecular mechanisms of Janus Fe3O4 -TiO2 nanoparticles in human liver cells. Environ Toxicol. 2018;33(10):1078–1088. doi:10.1002/tox.22631
97. Xue Y, Wang J, Huang Y, et al. Comparative cytotoxicity and apoptotic pathways induced by nanosilver in human liver HepG2 and L02 cells. Hum Exp Toxicol. 2018;37(12):1293–1309. doi:10.1177/0960327118769718
98. Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221(1):3–12. doi:10.1002/path.2697
99. Karen P, Manshian BB, Freya J, et al. Exploiting intrinsic nanoparticle toxicity: the pros and cons of nanoparticle-induced autophagy in biomedical research. Chem Rev. 2014;114(15):7581. doi:10.1021/cr400372p
100. Ueno T, Komatsu M. Autophagy in the liver: functions in health and disease. Nat Rev Gastroenterol Hepatol. 2017;14(3):170–184. doi:10.1038/nrgastro.2016.185
101. Wan J, Wang JH, Liu T, Xie Z, Yu XF, Li W. Surface chemistry but not aspect ratio mediates the biological toxicity of gold nanorods in vitro and in vivo. Sci Rep. 2015;5:11398. doi:10.1038/srep11398
102. Zhu S, Zhang J, Zhang L, et al. Inhibition of kupffer cell autophagy abrogates nanoparticle-induced liver injury. Adv Healthc Mater. 2017;6(9):1–11. doi:10.1002/adhm.201601252
103. Saowalak K, Titipun T, Somchai T, Chalermchai P. Iron(III)-tannic molecular nanoparticles enhance autophagy effect and T1MRI contrast in liver cell lines. Sci Rep. 2018;8(1):6647. doi:10.1038/s41598-018-25108-1
104. Kermanizadeh A, Jantzen K, Ward MB, et al. Nanomaterial-induced cell death in pulmonary and hepatic cells following exposure to three different metallic materials: the role of autophagy and apoptosis. Nanotoxicology. 2017;11(2):184–200. doi:10.1080/17435390.017.1279359
105. Lim Y, Kumar S. A single cut to pyroptosis. Oncotarget. 2015;6(35):36926–36927. doi:10.18632/oncotarget.v6i35
106. Lei-Leston AC, Murphy AG, Maloy KJ. Epithelial cell inflammasomes in intestinal immunity and inflammation. Front Immunol. 2017;8:1168. doi:10.3389/fimmu.2017.01168
107. Shi J, Zhao Y, Wang K, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660–665. doi:10.1038/nature15514
108. Byrne BG, Dubuisson JF, Joshi AD, Persson JJ, Swanson MS. Inflammasome components coordinate autophagy and pyroptosis as macrophage responses to infection. Am Soc Microbiol. 2013;4(1):e00620–e00612.
109. Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7(2):99–109. doi:10.1038/nrmicro2070
110. Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8(11):1812–1825. doi:10.1111/cmi.2006.8.issue-11
111. Guo H, Xie M, Zhou C, Zheng M. The relevance of pyroptosis in the pathogenesis of liver diseases. Life Sci. 2019;223:69–73. doi:10.1016/j.lfs.2019.02.060
112. Man SM, Karki R, Kanneganti T-D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev. 2017;277(1):61–75. doi:10.1111/imr.2017.277.issue-1
113. Kofahi HM, Taylor NGA, Hirasawa K, Grant MD, Russell RS. Hepatitis C virus infection of cultured human hepatoma cells causes apoptosis and pyroptosis in both infected and bystander cells. Sci Rep. 2016;6:37433. doi:10.1038/srep37433
114. Dumkova J, Smutna T, Vrlikova L, et al. Sub-chronic inhalation of lead oxide nanoparticles revealed their broad distribution and tissue-specific subcellular localization in target organs. Part Fibre Toxicol. 2017;14(1):55. doi:10.1186/s12989-017-0236-y
115. Xiao M, Chen W, Wang C, et al. Senescence and cell death in chronic liver injury: roles and mechanisms underlying hepatocarcinogenesis. Oncotarget. 2017;9(9):8772–8784. doi:10.18632/oncotarget.23622
116. Wu M-Y, Yiang G-T, Cheng P-W, Chu P-Y, Li C-J. Molecular targets in hepatocarcinogenesis and implications for therapy. J Clin Med. 2018;7(8):213. doi:10.3390/jcm7080213
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.