Back to Journals » Drug Design, Development and Therapy » Volume 18
The Role of Rho Kinase Inhibitors in Corneal Diseases
Authors Futterknecht S , Chatzimichail E, Gugleta K, Panos GD , Gatzioufas Z
Received 15 August 2023
Accepted for publication 10 January 2024
Published 19 January 2024 Volume 2024:18 Pages 97—108
DOI https://doi.org/10.2147/DDDT.S435522
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Frank Boeckler
Stefan Futterknecht,1,2 Eleftherios Chatzimichail,1 Konstantin Gugleta,1,3 Georgios D Panos,4,5,* Zisis Gatzioufas1,3,*
1Department of Ophthalmology, University Hospital of Basel, Basel, Switzerland; 2Institute of Molecular and Clinical Ophthalmology Basel, Basel, Switzerland; 3Department of Ophthalmology, School of Medicine, University of Basel, Basel, Switzerland; 4Department of Ophthalmology, Queen’s Medical Centre, Nottingham University Hospitals, Nottingham, UK; 5Division of Ophthalmology and Visual Sciences, School of Medicine, University of Nottingham, Nottingham, UK
*These authors contributed equally to this work
Correspondence: Georgios D Panos, Department of Ophthalmology, Queen’s Medical Centre, Nottingham University Hospitals, Derby Road, Lenton, Nottingham, NG7 2UH, UK, Tel +44 115 924 9924, Email [email protected]
Abstract: The cornea, as the outermost layer of the eye, plays a crucial role in vision by focusing light onto the retina. Various diseases and injuries can compromise its clarity, leading to impaired vision. This review aims to provide a thorough overview of the pharmacological properties, therapeutic potential and associated risks of Rho-associated protein kinase (ROCK) inhibitors in the management of corneal diseases. The article focuses on four key ROCK inhibitors: Y-27632, fasudil, ripasudil, and netarsudil, providing a comparative examination. Studies supporting the use of ROCK inhibitors highlight their efficacy across diverse corneal conditions. In Fuchs’ endothelial corneal dystrophy, studies on the application of Y-27632, ripasudil, and netarsudil demonstrated noteworthy enhancements in corneal clarity, endothelial cell density, and visual acuity. In pseudophakic bullous keratopathy, the injection of Y-27632 together with cultured corneal endothelial cells into the anterior chamber lead to enhanced corneal endothelial cell density and improved visual acuity. Animal models simulating chemical injury to the cornea showed a reduction of neovascularization and epithelial defects after application of fasudil and in a case of iridocorneal endothelial syndrome netarsudil improved corneal edema. Addressing safety considerations, netarsudil and ripasudil, both clinically approved, exhibit adverse events such as conjunctival hyperemia, conjunctival hemorrhage, cornea verticillata, conjunctivitis, and blepharitis. Monitoring patients during treatment becomes crucial to balancing the potential therapeutic benefits with these associated risks. In conclusion, ROCK inhibitors, particularly netarsudil and ripasudil, offer promise in managing corneal diseases. The comparative analysis of their pharmacological properties and studies supporting their efficacy underscore their potential therapeutic significance. However, ongoing research is paramount to comprehensively understand their safety profiles and long-term outcomes in diverse corneal conditions, guiding their optimal application in clinical practice.
Keywords: rho kinase inhibitors, cornea, corneal diseases, corneal dystrophies, Fuchs’ endothelial corneal dystrophy, corneal transplantation
Introduction
The cornea, the transparent outermost layer of the eye, plays a crucial role in refracting and focusing light onto the retina, making clear vision possible. Various diseases, injuries, and surgical procedures can compromise the integrity and function of the cornea, leading to vision impairment and blindness.1,2 The cornea is composed of five layers, including the epithelium, Bowman’s layer, stroma, Descemet’s membrane, and endothelium.
The corneal endothelium is a single layer of cells that lines the posterior surface of the cornea and is essential for maintaining the corneal transparency by regulating the flow of fluids into and out of the cornea. Dysfunction of the corneal endothelium, as seen in Fuchs’ endothelial corneal dystrophy (FECD), can lead to corneal edema, opacification, and ultimately, vision loss.3,4 As corneal endothelial cells have very limited capacity to regenerate, current therapies for corneal endothelial diseases focus on managing the symptoms rather than restoring the function of the damaged endothelium. The mainstay of treatment is corneal transplantation. However, these procedures are associated with several limitations, such as the shortage of donor corneas and the risk of graft rejection.
Rho-associated protein kinase (ROCK) is a serine/threonine kinase that regulates various cellular processes.5–7 Dysregulation of ROCK signaling has been implicated in various pathological conditions, such as cancer, cardiovascular diseases, and neurological disorders.8,9
ROCK inhibitors refer to a class of pharmacological agents that target and inhibit the activity of ROCK and in the realm of ophthalmology, the application of ROCK inhibitors is well-established in glaucoma.10–14 Recent research has shown that ROCK inhibitors may have therapeutic potential in managing corneal diseases by increasing cell proliferation and adhesion and by reducing apoptosis.15–20
This review paper aims to provide an overview of the current state of knowledge regarding the application of ROCK inhibitors in corneal diseases. We will discuss the mechanism of action of ROCK inhibitors, summarize the preclinical and clinical studies that have evaluated their efficacy and safety, and highlight potential future directions for research in this field.
The ROCK Pathway in Corneal Endothelial Cells
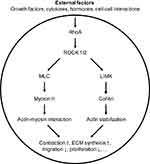
ROCK is a serine/threonine kinase that is modulated by the activity of RhoA, an intracellular GTPase.5 It regulates several cellular processes, including actin cytoskeleton dynamics, cell adhesion and proliferation.21–28 Dysregulation of ROCK signaling has been implicated in various pathological conditions, such as cancer, cardiovascular diseases, diabetes, neurological disorders and glaucoma.8,29–34
Several downstream signaling pathways have been identified to mediate the effects of ROCK activation. One of the key pathways is the myosin light chain (MLC) phosphorylation pathway, which results in the activation of myosin II and subsequent cytoskeletal rearrangement, contractility and extracellular matrix synthesis.5,35 This leads to changes in cell shape, adhesion, and migration, all of which are important for corneal endothelial cell function. Another important pathway downstream of ROCK is the LIMK/cofilin pathway leading to Rho-induced actin polymerization5,22,36 (see Figure 1).
The activation of the RhoA/ROCK pathway in corneal endothelial cells has been found to play a crucial role in the pathogenesis of corneal endothelial dysfunction. Several mechanisms have been proposed for the activation of the ROCK pathway in corneal endothelial cells which cause increased cellular contractility, decreased cell proliferation, and reduced cell migration, ultimately leading to endothelial cell dysfunction.7,37–39
ROCK inhibitors are a class of drugs that target the ROCK pathway by inhibiting the enzymatic activity of both ROCK isoforms ROCK1 and ROCK2.40 By doing so, these drugs can modulate various cellular processes, leading to beneficial effects in several disease models.30 Preclinical and clinical studies have shown that ROCK inhibitors can improve endothelial function by improving cell adhesion, proliferation and inhibiting apoptosis.6,37,41–43
In recent years, several studies have investigated the application of ROCK inhibitors in corneal diseases, including corneal endothelial dystrophies, corneal injury and post-operative regeneration. These studies have reported promising results, with ROCK inhibitors showing efficacy in improving corneal transparency.17,18,44–46
ROCK Inhibitors as Potential Therapeutics for Corneal Diseases
Comparison of Different ROCK Inhibitors and Their Pharmacological Properties
Several ROCK inhibitors have been studied in preclinical and clinical settings. Here, we provide a comparative analysis of the pharmacological properties of four prominent ROCK inhibitors: Y-27632, fasudil, ripasudil, and netarsudil (for an overview see Table 1 and Figure 2).
 |
Figure 2 Chemical structures for Y-27632,49 fasudil,50 ripasudil51 and netarsudil.52 Note the similarities between fasudil and ripasudil. |
 |
Table 1 Comparison of ROCK Inhibitors for Corneal Diseases |
Y-27632 was one of the first ROCK inhibitors identified and has been extensively studied in vitro and in vivo. Y-27632 is a dual ROCK-1/2 inhibitor with a Ki (inhibition constant) value of 140–220 nM for ROCK-1 and 300 nM for ROCK-2.31,53 However, its clinical use has been limited since in vitro studies have revealed that Y-27632 exhibits comparatively low potency when compared to other ROCK inhibitors.29,54,55 Moreover, Li et al reported a serum half-life ranging from 1 to 1.5 hours,56 while data on the ophthalmic application of this ROCK inhibitor is currently unavailable.
Fasudil, another dual ROCK-1/2 inhibitor and formerly known as HA-1077, is a clinically approved ROCK inhibitor for the treatment of cerebral vasospasm in Japan. It has also been studied for its potential use in diabetic macular edema57 and glaucoma.58 Fasudil has demonstrated promising pharmacological properties with a Ki value of 76 nM for ROCK-1 and 47 nM for ROCK-2.31 Fasudil has a plasma half-life of 0.3 h and its active metabolite hydroxyfasudil has a half-life of 2.9 h.9,59 Pharmacokinetic studies regarding its application in ophthalmology are yet to be done. However, similar to Y-27632, fasudil exhibits relatively low in vitro potency31 and therefore its clinical use in ophthalmology has been limited so far.
Ripasudil, a derivative of fasudil (Figure 2), and netarsudil are two newer ROCK inhibitors that have been approved for the treatment of glaucoma. Ripasudil, also known as K-115 from various clinical trials, has since 2014 been available in Japan for glaucoma treatment as a 0.4% formulation twice daily under the brand name Glanatec.60 It acts as a dual ROCK-1/2 inhibitor, with an IC50 (half maximal inhibitory concentration) of 19 nM for ROCK-1 and 51 nM for ROCK-2.12,61–65 Comparative Ki values with the other ROCK inhibitors presented in this review are unfortunately not available. The plasma half-life of ripasudil is 0.6–0.7 h and rabbit studies showed good corneal penetration when applied as eye drops.60,66
Netarsudil, in clinical studies known as AR-13324 and marketed as Rhopressa, is a dual ROCK-1/2 and norepinephrine transport inhibitor. It has a Ki value of 1 nM for both ROCK-1 and ROCK-2, indicating high affinity for ROCK inhibition.31 Its active metabolite netarsudil-M1 (AR-13503) has a five times higher potency against both ROCK-1 and ROCK-2. Furthermore, the inhibition of norepinephrine transport by netarsudil contributes to the reduction of aqueous humor production.67 For netarsudil maximum concentration in the cornea after topical administration has been found to be after 0.25–0.5 h with a half-life of 13–14 h.31 Netarsudil is available as a 0.02% formulation and administered once daily for the treatment of glaucoma. It has been approved in the United States since 2017, with subsequent authorization in the European Union in 2021.7,39
In conclusion, the pharmacological properties of Y-27632, fasudil, ripasudil, and netarsudil provide valuable insights into their potential for treating corneal endothelial disease. While Y-27632 and fasudil exhibit lower in vitro potency, both netarsudil and ripasudil’s clinical availability as an ophthalmic solution offers promising prospects for research on its application in corneal disease. Further research and clinical investigations are warranted to fully explore the therapeutic potential of these ROCK inhibitors in the management of corneal endothelial disease.
Evidence Supporting the Use of ROCK Inhibitors in Corneal Diseases
In this section, we present a comprehensive summary of relevant studies that demonstrate the efficacy of ROCK inhibitors in treating corneal diseases, with a specific focus on four main conditions: FECD, pseudophakic bullous keratopathy (PBK), corneal neovascularization (CNV), and iridocorneal endothelial (ICE) syndrome. Table 2 provides a condensed overview of the key findings from these studies, shedding light on the therapeutic potential of ROCK inhibitors in addressing corneal disease.
 |
Table 2 Evidence Supporting the Use of ROCK Inhibitors in Corneal Diseases |
FECD is a progressive and bilateral corneal disease characterized by the loss of endothelial cells and the presence of guttae, which are excrescences of Descemet’s membrane.74 In Western countries, FECD is the predominant cause of corneal endothelial dysfunction and represents the most prevalent indication for corneal transplantation.75 The pathophysiological mechanisms of FECD involve channelopathies, oxidative stress, apoptosis, and abnormal cellular-matrix interactions.74,76 In less severe cases of Fuchs’ corneal edema, conservative treatment involves the use of hyperosmotic saline drops.6,77 Surgical management options vary depending on the stage of the disease and may include penetrating keratoplasty (PK), Descemet membrane endothelial keratoplasty (DMEK), Descemet stripping automated endothelial keratoplasty (DSAEK), or Descemet stripping only (DSO).78
Numerous preclinical and clinical studies have employed ROCK inhibitors in the context of corneal endothelial diseases. Okumura et al conducted a study using the ROCK inhibitor Y-27632 in a rabbit corneal endothelial dysfunction model. The injection of cultivated corneal endothelial cells (CECs) supplemented with Y-27632 resulted in enhanced adhesion properties of the CECs, as evidenced by the expression of ZO-1 and Na/K-ATPase.68
The first clinical study involving ROCK inhibitors for endothelial dysfunction was conducted by Okumura et al in 2013 and included a mixed sample of seven cynomolgus monkeys and eight patients with corneal endothelial dysfunction, specifically with diffuse or central corneal edema as in advanced stages of FECD.47 The study revealed the promotion of corneal endothelial wound healing with the use of 10 mM Y-27632 eye drops 6 times daily for 7 days. Notably, the treated group exhibited significantly higher corneal endothelial cell density, along with improved corneal clarity and reduced corneal thickness. Morphologically, the regenerated corneal endothelium displayed a restoration of normal hexagonal cell morphology and increased cell density.47
In a trial involving 18 patients diagnosed with FECD, the utilization of the ROCK inhibitor ripasudil in combination with DSO resulted in enhanced recovery of endothelial cells.70 Specifically, the administration of ripasudil 0.4% eye drops four times daily for two months following DSO resulted in superior outcomes. These included enhanced visual recovery, and elevated corneal endothelial cell density after one year.70 Another clinical study by Moloney et al reported corneal clearance and improvement in best corrected visual acuity in 22 out of 23 eyes with FECD following DSO with the use of ripasudil 0.4% eye drops 6 times daily after 4 weeks.69
Netarsudil’s effectiveness was assessed by Price and Price in a double-masked, randomized study involving 29 eyes with symptomatic FECD. The study involved randomized administration of placebo or netarsudil 0.02% eye drops once daily for three months.71 Subsequent examinations demonstrated a significant decrease in central corneal thickness at both 1 and 3 months, along with significant improvement in scotopic corrected distance visual acuity (CDVA) after 3 months.71
PBK, a complication after cataract surgery, is the second most prevalent cause of corneal endothelial dysfunction and stands out as the most frequent secondary cause of corneal edema.79 In the course of cataract surgery, the rise in local temperature generated by the phacoemulsification probe can result in thermal damage to the adjacent corneal tissue80 and it is estimated that PBK may occur in approximately 1–2% of cataract surgeries.81 Furthermore, damage to endothelial tissue during cataract surgery can be inflicted by a combination of factors including elevated irrigation or aspiration rates, and the presence of air bubbles or lens particles making contact with the endothelium.80
In the context of PBK, Kinoshita et al conducted a single-group study involving 11 eyes treated with cultured human corneal endothelial cells supplemented with the ROCK inhibitor Y-27632, which were injected into the anterior chamber. The study reported improvements in important parameters such as corneal transparency, reduction of corneal thickness to normal values, and increased visual acuity in all eyes of the sample.16 Follow-up controls conducted two years after the injection revealed maintenance of corneal transparency in all eyes and corneal thickness values below 630 μm in 10 out of 11 eyes.16
Ripasudil has been tested as additional medical therapy in challenging cataract surgeries for patients with FECD in a clinical series involving four patients.82 The use of ripasudil prophylactically or postoperatively resulted in improvements in corneal edema and symptoms, demonstrating its possible role for the reduction of PBK after cataract surgery.82
CNV is a serious threat to vision, often associated with inflammatory, infectious, and traumatic ocular conditions. This condition occurs when pro-angiogenic factors, such as inflammatory cytokines and reactive oxygen species, outweigh anti-angiogenic factors, particularly in corneal burns. The imbalance leads to the formation of new blood vessels in the cornea, posing a significant risk to eyesight.83
The application of ROCK inhibitors for CNV has been explored through several studies, providing valuable insights into the therapeutic potential.72,84 In the study conducted by Zeng et al, the ROCK inhibitor fasudil exhibited significant efficacy in inhibiting CNV in mice following alkali burns.72 Administration of 100 μM fasudil eye drops four times daily led to a marked reduction in CNV incidence, correlating with decreased inflammatory cell infiltration, lowered production of reactive oxygen species, and an upregulation of heme oxygenase-1 protein. Moreover, the study highlighted the healing promotion of corneal epithelial defects. These findings collectively underscore the promise of ROCK inhibitors in mitigating pathological wound healing and neovascularization after corneal trauma.
The ICE syndrome encompasses a distinctive cluster of ocular pathologies, including Chandler syndrome, progressive iris atrophy, and Cogan-Reese syndrome. Characterized by the abnormal proliferation of corneal endothelial cells migrating towards the iridocorneal angle and iris surface, it leads to varying degrees of corneal edema, decompensation, and secondary glaucoma. Primarily affecting young women unilaterally, diagnosis relies on ocular findings, aided by in vivo confocal microscopy. Management focuses on addressing corneal edema and decompensation through endothelial keratoplasty and tackling secondary glaucoma with surgical interventions.85
Rho-kinase inhibitors have shown positive effects on patients with ICE syndrome. Davies reported a case of an 80-year-old woman suffering from corneal edema due to ICE syndrome, in which a 4-weeks therapy with netarsudil eye drops led to significant regression of the corneal edema and improved visual acuity.73
Safety Considerations and Potential Side Effects of ROCK Inhibitors in the Cornea
The safety of ROCK inhibitors in the treatment of corneal diseases is of paramount importance. Investigating the safety profiles of ophthalmic ROCK inhibitors, both netarsudil and ripasudil have been approved for clinical use, supported by numerous studies. However, limited data of small case studies exists for the safety profile of fasudil,48 which has gained approval solely for cerebral vasospasm treatment. Similarly, due to its lack of approval, Y-27632 has only garnered limited attention in terms of clinical safety studies. Therefore, we will here focus on the two drugs netarsudil and ripasudil, where data from larger clinical trials is available.
A 2016 Phase III trial conducted by Tanihara et al with 388 patients examined the safety of ripasudil 0.4% when applied twice daily over a 52-week period.12 Overall, the study found that 94.1% of patients experienced adverse events undergoing treatment with ripasudil. The most frequent adverse events included conjunctival hyperemia (74.6%), blepharitis (20.6%), allergic conjunctivitis (17.2%), eye irritation (10.2%), and conjunctivitis (7.3%). A total of 14.4% of patients withdrew from the study as a result of adverse events and long-term studies showed that blepharitis and conjunctival hyperemia were the most common reasons for discontinuation.86,87
The safety profile of netarsudil has been comprehensively investigated in the ROCKET88,89 and MERCURY11 trials, providing consistent findings regarding the incidence of adverse events associated with netarsudil. In a pooled analysis of the ROCKET studies the safety profile of once-daily netarsudil was assessed in comparison to twice-daily timolol involving 839 patients in each group.10 The study revealed that 83.3% of patients treated with once-daily netarsudil experienced adverse events with the incidence of ocular adverse events being 79.3%. Overall systemic adverse events occurred in 26.3% of netarsudil-treated patients and no single systemic adverse event occurred in more than 2% of patients. Serious adverse events were reported in 3.3% of twice-daily netarsudil-treated patients and no serious ocular adverse event was reported among patients administered netarsudil once daily. The most frequent ocular adverse event in the netarsudil group was conjunctival hyperemia, occurring in 54.4% of patients. The severity of conjunctival hyperemia was mostly graded as mild (77.6%) and led to treatment discontinuation in 6% of netarsudil patients. Other ocular adverse events associated with netarsudil included cornea verticillata, reported in 20.9% of patients, and conjunctival hemorrhage, reported in 17.2% of patients. Cornea verticillata did not significantly affect visual acuity and resulted in treatment discontinuation in 3.7% of patients. Conjunctival hemorrhage was typically mild and self-limiting. So far only in case series reticular bullous epithelial edema has been observed with netarsudil use, which resolved after discontinuation.90,91
All in all, adverse events associated with ROCK inhibitors include conjunctival hyperemia, conjunctival hemorrhage, cornea verticillata, conjunctivitis, and blepharitis. Comparing the two inhibitors, ripasudil was found to have a higher prevalence of adverse events (94.1%) compared to netarsudil (83.3%) with conjunctival hyperemia being the most frequent in both drugs. Notably, cornea verticillata is specifically observed with netarsudil, while blepharitis is more commonly associated with ripasudil.
Further investigation is needed to comprehensively evaluate the safety profiles of ROCK inhibitors in the treatment of corneal diseases. Although both ripasudil and netarsudil have shown promising therapeutic effects, their associated adverse events should be carefully considered in clinical practice. It is crucial for clinicians to be aware of these potential risks and monitor patients closely during treatment with ROCK inhibitors to ensure optimal therapeutic outcomes and patient safety.
Future Directions and Challenges
Limitations and Challenges in the Clinical Application of ROCK Inhibitors for Corneal Diseases
While the positive outcomes of ROCK inhibitors in addressing corneal diseases are well-documented, it is essential to acknowledge the existing limitations constraining their applications.
There are concerns regarding the use of ROCK inhibitors for patients with FECD associated corneal guttae-induced reduction in CEC density. It has not yet been shown whether ROCK inhibitors are effective in cases where the CEC layer is intact and not disrupted.17
DSO with the use of ROCK inhibitors is a surgical option that yields comparable visual outcomes to DMEK.45 While patients undergoing DSO may experience a longer recovery period than those undergoing DMEK, they benefit from reduced adverse effects and the elimination of long-term immunosuppression or the need for donor corneal tissue.45 Nevertheless, in cases of advanced stromal edema or significant peripheral loss of endothelial cells, endothelial keratoplasty remains the established surgical approach. Furthermore, there are reports indicating that the proliferation and migration of remaining corneal endothelial cells occurs at a faster rate over areas with intact Descemet’s membrane compared to areas where the Descemet’s membrane was removed, as in DSO. This suggests that the presence of Descemet’s membrane aids in the proliferation of CECs in cases of corneal endothelial disease.92
In the context of ICE syndrome, only a single case has demonstrated successful corneal edema treatment using a ROCK inhibitor.73 As a result, the effectiveness of this intervention awaits confirmation through larger-scale clinical trials.
There is also an ongoing discussion regarding the optimal frequency of the administration of ROCK inhibitor eye drop therapy. Published data indicate that the application of ripasudil 0.4% ophthalmic solution on the cornea exhibits an action lasting approximately 6 hours,17 while netarsudil 0.02% has a half-life of 13–14 hours in the cornea.31 Yet, further studies are needed to investigate the most effective frequency of administration of ROCK inhibitors eye drops for corneal diseases.
There are several general limitations in the reviewed studies that warrant attention. Our bibliographic research did not identify any published studies with long-term outcomes or studies with large sample sizes regarding the application of ROCK inhibitors in corneal diseases. Moreover, there is a need for comparative studies that include subjects receiving conservative therapy with ROCK inhibitors and those undergoing surgical therapy, particularly in patients with FECD. Such studies would provide valuable insights into the effectiveness of ROCK inhibitors. Therefore, we strongly advocate for further investigation of these preliminary results, as they raise important questions about the potential impact of ROCK inhibitors on current standards in the treatment of corneal disease.
Other Potential Therapeutics for Corneal Diseases
Recent advancements in the treatment of corneal diseases have shown promising results with the use of ROCK inhibitors. However, ongoing research is also exploring other potential therapeutics. In particular, gene therapy, bioengineered corneal grafts, and cell therapies have emerged as areas of interest. Numerous studies have focused on therapies for various corneal diseases, including corneal scarring, corneal epithelial wound healing, corneal neovascularization, corneal (endothelial) dystrophies, herpetic keratitis, and dry eye disease.93 Here, we will focus on alternative therapeutic approaches for corneal endothelial diseases.
Gene therapy involves the delivery of genetic material to modify gene expression or correct genetic abnormalities. FECD is a condition commonly characterized by the expansion of CTG trinucleotide repeats within the TCF4 gene, resulting in the formation of toxic RNA foci.94 Preclinical studies utilizing antisense oligonucleotide therapy have shown promise in reducing the formation of RNA foci and toxicity markers associated with repeat-expansion-mediated diseases.95 A proof-of-concept study demonstrated that antisense oligonucleotides effectively reduced RNA foci formation in patient-derived cells affected by FECD, suggesting their potential for treating corneal tissue in these diseases.96 Additionally, targeting the expansion of CTG repeats in FECD using CRISPR/Cas9 has also demonstrated promising results in reducing RNA foci associated with FECD pathophysiology.97,98
Furthermore, tissue engineering approaches are being explored to develop artificial corneal implants or cell-based therapies that can replace damaged or dysfunctional corneal tissue.99,100
While the clinical application of ROCK inhibitors for corneal diseases holds promise, these other potential therapeutics also warrant further investigation and development. Future studies will need to compare the efficacy and safety of these different approaches to identify the most effective treatment strategies for corneal diseases.
Conclusion
The role of ROCK inhibitors in corneal diseases, particularly those affecting the corneal endothelium, shows promising potential for therapeutic intervention. The ROCK signaling cascade is involved in various pathological conditions, and recent research has demonstrated that ROCK inhibitors can modulate cellular processes in corneal endothelial cells, leading to improved cell adhesion, proliferation, and reduced apoptosis. These effects make ROCK inhibitors attractive candidates for the management of corneal diseases, such as FECD and PBK.
The comparative analysis of four prominent ROCK inhibitors, namely Y-27632, fasudil, ripasudil, and netarsudil, revealed variations in their pharmacological properties. While Y-27632 and fasudil have exhibited lower potency, netarsudil and ripasudil have shown promising clinical availability as ophthalmic solutions. Netarsudil, in particular, has demonstrated high affinity for ROCK inhibition and long-lasting activity, making it a promising candidate for further investigation in corneal diseases.
Preclinical and clinical studies have provided evidence supporting the use of ROCK inhibitors in corneal diseases. These studies have shown that ROCK inhibitors can improve corneal transparency and enhance endothelial function. However, further research and clinical investigations are needed to evaluate the therapeutic potential and ideal application of ROCK inhibitors in the management of corneal diseases. Additional studies investigating the pharmacokinetics, optimal dosing, and long-term safety of ROCK inhibitors in corneal disease treatment are necessary. Moreover, exploring the combination of ROCK inhibitors with other potential therapeutics, such as bioengineered tissues and gene therapy, may provide synergistic effects and enhance their therapeutic potential.
In conclusion, the application of ROCK inhibitors in corneal diseases represents a promising avenue for future research and clinical translation. By targeting the ROCK pathway, these inhibitors have the potential to address the limitations of current therapies, and offer new strategies for restoring corneal endothelial function. Further studies and clinical trials will provide valuable insights into the optimal use of ROCK inhibitors and their long-term effects in corneal diseases, ultimately leading to improved outcomes for patients with corneal pathologies.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Georgios D Panos and Zisis Gatzioufas contributed equally and share the last / senior authorship.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Klintworth GK. Corneal dystrophies. Orphanet J Rare Dis. 2009;4(1):7. doi:10.1186/1750-1172-4-7
2. Gomes JAP, Azar DT, Baudouin C, et al. TFOS DEWS II iatrogenic report. Ocul Surf. 2017;15(3):511–538. doi:10.1016/j.jtos.2017.05.004
3. Bowling B. Kanski’s Clinical Ophthalmology. Edinburgh: Elsevier; 2016.
4. Jeang LJ, Margo CE, Espana EM. Diseases of the corneal endothelium. Exp Eye Res. 2021;205:108495. doi:10.1016/j.exer.2021.108495
5. Rao PV, Pattabiraman PP, Kopczynski C. Role of the Rho GTPase/Rho kinase signaling pathway in pathogenesis and treatment of glaucoma: bench to bedside research. Exp Eye Res. 2017;158:23–32. doi:10.1016/j.exer.2016.08.023
6. Moshirfar M, Parker L, Birdsong OC, et al. Use of Rho kinase inhibitors in ophthalmology: a review of the literature; 2018.
7. Karri R, Chong EW. ROCK inhibitors in ophthalmology: a critical review of the existing clinical evidence. Clin Experiment Ophthalmol. 2023;
8. Narumiya S, Tanji M, Ishizaki T. Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev. 2009;28(1–2):65–76. doi:10.1007/s10555-008-9170-7
9. Chen M, Liu A, Ouyang Y, Huang Y, Chao X, Pi R. Fasudil and its analogs: a new powerful weapon in the long war against central nervous system disorders? Expert Opin Investig Drugs. 2013;22(4):537–550. doi:10.1517/13543784.2013.778242
10. Singh IP, Fechtner RD, Myers JS, et al. Pooled efficacy and safety profile of netarsudil ophthalmic solution 0.02% in patients with open-angle glaucoma or ocular hypertension. J Glaucoma. 2020;29(10):878–884. doi:10.1097/IJG.0000000000001634
11. Asrani S, Bacharach J, Holland E, et al. Fixed-dose combination of netarsudil and latanoprost in ocular hypertension and open-angle glaucoma: pooled efficacy/safety analysis of phase 3 MERCURY-1 and −2. Adv Ther. 2020;37(4):1620–1631. doi:10.1007/s12325-020-01277-2
12. Tanihara H, Inoue T, Yamamoto T, et al. One-year clinical evaluation of 0.4% ripasudil (K-115) in patients with open-angle glaucoma and ocular hypertension. Acta Ophthalmol. 2016;94(1):e26–e34. doi:10.1111/aos.12829
13. Sakata R, Fujishiro T, Saito H, Honjo M, Shirato S, Aihara M. The additive effect of ROCK inhibitor on prostaglandin-treated Japanese patients with glaucoma indicating 15 mmHg and under: ROCK U-15. Adv Ther. 2021;38(7):3760–3770. doi:10.1007/s12325-021-01775-x
14. Luo LJ, Nguyen DD, Lai JY. Harnessing the tunable cavity of nanoceria for enhancing Y-27632-mediated alleviation of ocular hypertension. Theranostics. 2021;11(11):5447–5463. doi:10.7150/thno.54525
15. Okumura N, Okazaki Y, Inoue R, et al. Effect of the rho-associated kinase inhibitor eye drop (Ripasudil) on corneal endothelial wound healing. Invest Opthalmol Vis Sci. 2016;57(3):1284. doi:10.1167/iovs.15-18586
16. Kinoshita S, Koizumi N, Ueno M, et al. Injection of cultured cells with a ROCK inhibitor for bullous keratopathy. N Engl J Med. 2018;378(11):995–1003. doi:10.1056/NEJMoa1712770
17. Kinoshita S, Colby KA, Kruse FE. A close look at the clinical efficacy of rho-associated protein kinase inhibitor eye drops for fuchs endothelial corneal dystrophy. Cornea. 2021;40:1225–1228. doi:10.1097/ICO.0000000000002642
18. Parekh M, Miall A, Deshpande N, Jurkunas UV. Effect of ROCK inhibitor on cell migration in fuchs endothelial corneal dystrophy. Invest Ophthalmol Vis Sci. 2022;63:7.
19. Inomata T, Fujimoto K, Okumura Y, et al. Novel immunotherapeutic effects of topically administered ripasudil (K-115) on corneal allograft survival. Sci Rep. 2020;10(1):19817. doi:10.1038/s41598-020-76882-w
20. Kopecny LR, Lee BWH, Coroneo MT. A systematic review on the effects of ROCK inhibitors on proliferation and/or differentiation in human somatic stem cells: a hypothesis that ROCK inhibitors support corneal endothelial healing via acting on the limbal stem cell niche. Ocul Surf. 2023;27:16–29. doi:10.1016/j.jtos.2022.12.008
21. Amano M, Chihara K, Kimura K, et al. Formation of actin stress fibers and focal adhesions enhanced by rho-kinase. Science. 1997;275(5304):1308–1311. doi:10.1126/science.275.5304.1308
22. Maekawa M, Ishizaki T, Boku S, et al. Signaling from rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–898. doi:10.1126/science.285.5429.895
23. Totsukawa G, Yamakita Y, Yamashiro S, Hartshorne DJ, Sasaki Y, Matsumura F. Distinct roles of rock (rho-kinase) and mlck in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3t3 fibroblasts. J Cell Biol. 2000;150(4):797–806. doi:10.1083/jcb.150.4.797
24. Nakamura M, Nagano T, Chikama T, Nishida T. Role of the small GTP-binding protein rho in epithelial cell migration in the rabbit cornea. Invest Ophthalmol Visual Sci. 2001;42:5.
25. Yin J, Yu FSX. Rho kinases regulate corneal epithelial wound healing. Am J Physiol Cell Physiol. 2008;295:C378–87. doi:10.1152/ajpcell.90624.2007
26. Riento K, Ridley AJ. ROCKs: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4(6):446–456. doi:10.1038/nrm1128
27. Leung T, Chen XQ, Manser E, Lim L. The p160 RhoA-Binding Kinase ROK is a member of a kinase family and is involved in the reorganization of the cytoskeleton. MOL CELL BIOL. 1996;16:5313–5327. doi:10.1128/MCB.16.10.5313
28. Katoh K, Kano Y, Noda Y. Rho-associated kinase-dependent contraction of stress fibres and the organization of focal adhesions. J R Soc Interface. 2011;8(56):305–311. doi:10.1098/rsif.2010.0419
29. Uehata M, Ishizaki T, Satoh H, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi:10.1038/40187
30. Olson MF. Applications for ROCK kinase inhibition. Curr Opin Cell Biol. 2008;20(2):242–248. doi:10.1016/j.ceb.2008.01.002
31. Lin CW, Sherman B, Moore LA, et al. Discovery and preclinical development of netarsudil, a novel ocular hypotensive agent for the treatment of glaucoma. J Ocul Pharmacol Ther. 2018;34(1–2):40–51. doi:10.1089/jop.2017.0023
32. Kubo T. The therapeutic effects of Rho-ROCK inhibitors on CNS disorders. Ther Clin Risk Manag. 2008;4:605–615. doi:10.2147/TCRM.S2907
33. Nguyen Dinh Cat A, Callera GE, Friederich-Persson M, et al. Vascular dysfunction in obese diabetic db/db mice involves the interplay between aldosterone/mineralocorticoid receptor and Rho kinase signaling. Sci Rep. 2018;8(1):2952. doi:10.1038/s41598-018-21087-5
34. Rodriguez-Hernandez I, Cantelli G, Bruce F, Sanz-Moreno V. Rho, ROCK and actomyosin contractility in metastasis as drug targets. F1000Research. 2016;5:783. doi:10.12688/f1000research.7909.1
35. Kimura K, Ito M, Amano M, et al. Regulation of myosin phosphatase by rho and rho-associated kinase (Rho-Kinase). Science. 1996;273:245–248. doi:10.1126/science.273.5272.245
36. Vercammen H, Miron A, Oellerich S, et al. Corneal endothelial wound healing: understanding the regenerative capacity of the innermost layer of the cornea. Transl Res. 2022;248:111–127. doi:10.1016/j.trsl.2022.05.003
37. Okumura N, Koizumi N, Ueno M, et al. The new therapeutic concept of using a rho kinase inhibitor for the treatment of corneal endothelial dysfunction. Cornea. 2011;30(Supplement 1):S54–S59. doi:10.1097/ICO.0b013e3182281ee1
38. Narumiya S, Ishizaki T, Ufhata M. Use and properties of ROCK-specific inhibitor Y-27632. In: Methods in Enzymology. Elsevier; 2000:273–284. doi:10.1016/S0076-6879(00)25449-9
39. Syed ZA, Rapuano CJ. Rho kinase (ROCK) inhibitors in the management of corneal endothelial disease. Curr Opin Ophthalmol. 2021;32(3):268–274. doi:10.1097/ICU.0000000000000748
40. Hahmann C, Schroeter T. Rho-kinase inhibitors as therapeutics: from pan inhibition to isoform selectivity. Cell Mol Life Sci. 2010;67(2):171–177. doi:10.1007/s00018-009-0189-x
41. Meekins LC, Rosado-Adames N, Maddala R, Zhao JJ, Rao PV, Afshari NA. Corneal endothelial cell migration and proliferation enhanced by rho kinase (ROCK) inhibitors in in vitro and in vivo models. Invest Opthalmol Vis Sci. 2016;57(15):6731. doi:10.1167/iovs.16-20414
42. Okumura N, Ueno M, Koizumi N, et al. Enhancement on primate corneal endothelial cell survival in vitro by a ROCK Inhibitor. Invest Opthalmol Vis Sci. 2009;50(8):3680. doi:10.1167/iovs.08-2634
43. Okumura N, Nakano S, Kay EP, et al. Involvement of Cyclin D and p27 in cell proliferation mediated by ROCK Inhibitors Y-27632 and Y-39983 during corneal endothelium wound healing. Invest Opthalmol Vis Sci. 2014;55(1):318. doi:10.1167/iovs.13-12225
44. Okumura N, Kinoshita S, Koizumi N. Application of rho kinase inhibitors for the treatment of corneal endothelial diseases. J Ophthalmol. 2017;2017:1–8. doi:10.1155/2017/2646904
45. Huang MJ, Kane S, Dhaliwal DK. Descemetorhexis without endothelial keratoplasty versus DMEK for treatment of fuchs endothelial corneal dystrophy. Cornea. 2018;37(12):1479–1483. doi:10.1097/ICO.0000000000001742
46. Davies E, Jurkunas U, Pineda R. Pilot study of corneal clearance with the use of a rho-kinase inhibitor after descemetorhexis without endothelial keratoplasty for fuchs endothelial corneal dystrophy. Cornea. 2021;40(7):899–902. doi:10.1097/ICO.0000000000002691
47. Okumura N, Koizumi N, Kay EP, et al. The ROCK inhibitor eye drop accelerates corneal endothelium wound healing. Invest Opthalmol Vis Sci. 2013;54(4):2493. doi:10.1167/iovs.12-11320
48. Pakravan M, Beni AN, Ghahari E, et al. The ocular hypotensive efficacy of topical fasudil, a rho-associated protein kinase inhibitor, in patients with end-stage glaucoma. Am J Ther. 2017;24(6):e676–e680. doi:10.1097/MJT.0000000000000362
49. Drugbank. Y-27632. Available from: https://go.drugbank.com/drugs/DB08756.
50. Fasudil. In: Wikipedia; 2023. Available from: https://commons.wikimedia.org/wiki/File:Fasudil.svg.
51. Drugbank. Ripasudil. Available from: https://go.drugbank.com/drugs/DB13165.
52. Drugbank. Netarsudil. Available from: https://go.drugbank.com/drugs/DB13931.
53. Ishizaki T, Uehata M, Tamechika I, et al. Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol Pharmacol. 2000;57:976–983.
54. Defert O, Boland S. Rho kinase inhibitors: a patent review (2014 – 2016). Expert Opin Ther Pat. 2017;27(4):507–515. doi:10.1080/13543776.2017.1272579
55. Li G, Mukherjee D, Navarro I, et al. Visualization of conventional outflow tissue responses to netarsudil in living mouse eyes. Eur J Pharmacol. 2016;787:20–31. doi:10.1016/j.ejphar.2016.04.002
56. Li M, Huang Y, Ma AAK, Lin E, Diamond MI. Y-27632 improves rotarod performance and reduces huntingtin levels in R6/2 mice. Neurobiol Dis. 2009;36(3):413–420. doi:10.1016/j.nbd.2009.06.011
57. Ahmadieh H, Nourinia R, Hafezi-Moghadam A, et al. Intravitreal injection of a Rho-kinase inhibitor (fasudil) combined with bevacizumab versus bevacizumab monotherapy for diabetic macular oedema: a pilot randomised clinical trial. Br J Ophthalmol. 2019;103(7):922–927. doi:10.1136/bjophthalmol-2018-312244
58. Mietzner R, Kade C, Froemel F, et al. Fasudil loaded PLGA microspheres as potential intravitreal depot formulation for glaucoma therapy. Pharmaceutics. 2020;12(8):706. doi:10.3390/pharmaceutics12080706
59. Chen H, Lin Y, Han M, Bai S, Wen S. Simultaneous quantitative analysis of fasudil and its active metabolite in human plasma by liquid chromatography electro-spray tandem mass spectrometry. J Pharm Biomed Anal. 2010;52(2):242–248. doi:10.1016/j.jpba.2009.12.028
60. Garnock-Jones KP. Ripasudil: first Global Approval. Drugs. 2014;74(18):2211–2215. doi:10.1007/s40265-014-0333-2
61. Tanihara H, Inoue T, Yamamoto T, et al. Intra-ocular pressure-lowering effects of a Rho kinase inhibitor, ripasudil (K-115), over 24 hours in primary open-angle glaucoma and ocular hypertension: a randomized, open-label, crossover study. Acta Ophthalmol. 2015;93(4):e254–e260. doi:10.1111/aos.12599
62. Tanihara H, Inoue T, Yamamoto T, et al. Additive intraocular pressure–lowering effects of the rho kinase inhibitor ripasudil (K-115) Combined with timolol or latanoprost: a report of 2 randomized clinical trials. JAMA Ophthalmol. 2015;133(7):755. doi:10.1001/jamaophthalmol.2015.0525
63. Tanihara H, Inoue T, Yamamoto T, Kuwayama Y, Abe H, Araie M. Phase 2 randomized clinical study of a rho kinase inhibitor, K-115, in primary open-angle glaucoma and ocular hypertension. Am J Ophthalmol. 2013;156(4):731–736.e2. doi:10.1016/j.ajo.2013.05.016
64. Moura-Coelho N, Tavares Ferreira J, Bruxelas CP, Dutra-Medeiros M, Cunha JP, Pinto Proença R. Rho kinase inhibitors—a review on the physiology and clinical use in Ophthalmology. Graefes Arch Clin Exp Ophthalmol. 2019;257(6):1101–1117. doi:10.1007/s00417-019-04283-5
65. Kaneko Y, Ohta M, Inoue T, et al. Effects of K-115 (Ripasudil), a novel ROCK inhibitor, on trabecular meshwork and Schlemm’s canal endothelial cells. Sci Rep. 2016;6(1):19640. doi:10.1038/srep19640
66. Inoue T, Tanihara H. Ripasudil hydrochloride hydrate: targeting Rho kinase in the treatment of glaucoma. Expert Opin Pharmacother. 2017;18(15):1669–1673. doi:10.1080/14656566.2017.1378344
67. Wang RF, Williamson JE, Kopczynski C, Serle JB. Effect of 0.04% AR-13324, a ROCK, and norepinephrine transporter inhibitor, on aqueous humor dynamics in normotensive monkey eyes. J Glaucoma. 2015;24(1):51–54. doi:10.1097/IJG.0b013e3182952213
68. Okumura N, Koizumi N, Ueno M, et al. ROCK inhibitor converts corneal endothelial cells into a phenotype capable of regenerating in vivo endothelial tissue. Am J Pathol. 2012;181(1):268–277. doi:10.1016/j.ajpath.2012.03.033
69. Moloney G, Garcerant Congote D, Hirnschall N, et al. Descemet stripping only supplemented with topical ripasudil for Fuchs endothelial dystrophy 12-month outcomes of the Sydney Eye Hospital Study. Cornea. 2021;40(3):320–326. doi:10.1097/ICO.0000000000002437
70. Macsai MS, Shiloach M. Use of topical rho kinase inhibitors in the treatment of fuchs dystrophy after descemet stripping only. Cornea. 2019;38(5):529–534. doi:10.1097/ICO.0000000000001883
71. Price MO, Price FW. Randomized, double-masked, pilot study of netarsudil 0.02% ophthalmic solution for treatment of corneal edema in fuchs dystrophy. Am J Ophthalmol. 2021;227:100–105. doi:10.1016/j.ajo.2021.03.006
72. Zeng P, Biao PR, Li P, et al. Fasudil hydrochloride, a potent ROCK inhibitor, inhibits corneal neovascularization after alkali burns in mice. Mol Vis. 2015;21:1.
73. Davies E. Case series: novel utilization of rho-kinase inhibitor for the treatment of corneal edema. Cornea. 2021;40(1):116–120. doi:10.1097/ICO.0000000000002421
74. Moshirfar M, Somani AN, Vaidyanathan U, Patel BC. Fuchs Endothelial Dystrophy. StatPearls. StatPearls Publishing; 2023. Available from. http://www.ncbi.nlm.nih.gov/books/NBK545248/.
75. Price MO, Mehta JS, Jurkunas UV, Price FW. Corneal endothelial dysfunction: evolving understanding and treatment options. Prog Retin Eye Res. 2021;82:100904. doi:10.1016/j.preteyeres.2020.100904
76. Jurkunas UV. Fuchs endothelial corneal dystrophy through the prism of oxidative stress. Cornea. 2018;37:S50. doi:10.1097/ICO.0000000000001775
77. Chow SC, Chan JCH. Review on the use of topical ocular hypertonic saline in corneal edema. Cornea. 2021;40(4):533. doi:10.1097/ICO.0000000000002652
78. Blitzer AL, Colby KA. Update on the surgical management of fuchs endothelial corneal dystrophy. Ophthalmol Ther. 2020;9(4):757–765. doi:10.1007/s40123-020-00293-3
79. Feizi S. Corneal endothelial cell dysfunction: etiologies and management. Ther Adv Ophthalmol. 2018;10:2515841418815802. doi:10.1177/2515841418815802
80. Feinbaum C. New treatment reduces corneal oedema after cataract surgery. Ophthalmology Times; 2015. Available from: https://www.ophthalmologytimes.com/view/ote-new-treatment-reduces-corneal-oedema-after-cataract-surgery.
81. Gonçalves ED, Campos M, Paris F, Gomes JÁP, de Farias CC. Ceratopatia bolhosa: etiopatogênese e tratamento. Arq Bras Oftalmol. 2008;71:61–64. doi:10.1590/S0004-27492008000700012
82. Antonini M, Coassin M, Gaudenzi D, Di Zazzo A. Rho-associated kinase inhibitor eye drops in challenging cataract surgery. Am J Ophthalmol Case Rep. 2022;25:101245. doi:10.1016/j.ajoc.2021.101245
83. Chang JH, Gabison EE, Kato T, Azar DT. Corneal neovascularization. Curr Opin Ophthalmol. 2001;12(4):242–249. doi:10.1097/00055735-200108000-00002
84. Sijnave D, Van Bergen T, Castermans K, et al. Inhibition of rho-associated kinase prevents pathological wound healing and neovascularization after corneal Trauma. Cornea. 2015;34(9):1120–1129. doi:10.1097/ICO.0000000000000493
85. Silva L, Najafi A, Suwan Y, Teekhasaenee C, Ritch R. The iridocorneal endothelial syndrome. Surv Ophthalmol. 2018;63(5):665–676. doi:10.1016/j.survophthal.2018.01.001
86. Saito H, Kagami S, Mishima K, Mataki N, Fukushima A, Araie M. Long-term side effects including blepharitis leading to discontinuation of ripasudil. J Glaucoma. 2019;28(4):289–293. doi:10.1097/IJG.0000000000001203
87. Maruyama Y, Ikeda Y, Mori K, et al. Safety and efficacy of long-term ripasudil 0.4% instillation for the reduction of intraocular pressure in Japanese open-angle glaucoma patients. J Ocul Pharmacol Ther. 2020;36(4):229–233. doi:10.1089/jop.2019.0125
88. Serle JB, Katz LJ, McLaurin E, et al. Two phase 3 clinical trials comparing the safety and efficacy of netarsudil to timolol in patients with elevated intraocular pressure: rho kinase elevated IOP treatment trial 1 and 2 (ROCKET-1 and ROCKET-2). Am J Ophthalmol. 2018;186:116–127. doi:10.1016/j.ajo.2017.11.019
89. Khouri AS, Serle JB, Bacharach J, et al. Once-daily netarsudil versus twice-daily timolol in patients with elevated intraocular pressure: the randomized phase 3 ROCKET-4 study. Am J Ophthalmol. 2019;204:97–104. doi:10.1016/j.ajo.2019.03.002
90. Bhargava M, Sen S, Bhambhani V, Paul R, Dutta C. Reticular epithelial corneal edema as a novel side-effect of rho kinase inhibitors: an Indian scenario. Indian J Ophthalmol. 2022;70(4):1163. doi:10.4103/ijo.IJO_2865_21
91. Wisely CE, Liu KC, Gupta D, Carlson AN, Asrani SG, Kim T. Reticular bullous epithelial edema in corneas treated with netarsudil: a case series. Am J Ophthalmol. 2020;217:20–26. doi:10.1016/j.ajo.2020.04.002
92. Soh YQ, Peh G, George BL, et al. Predicative factors for corneal endothelial cell migration. Invest Ophthalmol Vis Sci. 2016;57(2):338–348. doi:10.1167/iovs.15-18300
93. Amador C, Shah R, Ghiam S, Kramerov AA, Ljubimov AV. Gene therapy in the anterior eye segment. Curr Gene Ther. 2022;22(2):104–131. doi:10.2174/1566523221666210423084233
94. Mehta JS, Kocaba V, Soh YQ. The future of keratoplasty: cell-based therapy, regenerative medicine, bioengineering keratoplasty, gene therapy. Curr Opin Ophthalmol. 2019;30(4):286–291. doi:10.1097/ICU.0000000000000573
95. Zarouchlioti C, Sanchez-Pintado B, Hafford Tear NJ, et al. Antisense therapy for a common corneal dystrophy ameliorates TCF4 repeat expansion-mediated toxicity. Am J Hum Genet. 2018;102(4):528–539. doi:10.1016/j.ajhg.2018.02.010
96. Hu J, Rong Z, Gong X, et al. Oligonucleotides targeting TCF4 triplet repeat expansion inhibit RNA foci and mis-splicing in Fuchs’ dystrophy. Hum Mol Genet. 2018;27(6):1015–1026. doi:10.1093/hmg/ddy018
97. Pinto BS, Saxena T, Oliveira R, et al. Impeding transcription of expanded microsatellite repeats by deactivated cas9. Mol Cell. 2017;68(3):479–490.e5. doi:10.1016/j.molcel.2017.09.033
98. Rong Z, Gong X, Hulleman JD, Corey DR, Mootha VV. Trinucleotide repeat-targeting dCas9 as a therapeutic strategy for fuchs’ endothelial corneal dystrophy. Transl Vis Sci Technol. 2020;9(9):47. doi:10.1167/tvst.9.9.47
99. Peh GSL, Ang HP, Lwin CN, et al. Regulatory compliant tissue-engineered human corneal endothelial grafts restore corneal function of rabbits with bullous keratopathy. Sci Rep. 2017;7(1):14149. doi:10.1038/s41598-017-14723-z
100. Koizumi N, Sakamoto Y, Okumura N, et al. Cultivated corneal endothelial cell sheet transplantation in a primate model. Invest Opthalmol Vis Sci. 2007;48(10):4519. doi:10.1167/iovs.07-0567
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.