Back to Journals » Breast Cancer: Targets and Therapy » Volume 16
The Role of Ki-67 in HR+/HER2- Breast Cancer: A Real-World Study of 956 Patients
Authors Ma Q , Liu YB, She T, Liu XL
Received 11 December 2023
Accepted for publication 22 February 2024
Published 8 March 2024 Volume 2024:16 Pages 117—126
DOI https://doi.org/10.2147/BCTT.S451617
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Robert Clarke
Qin Ma,1 Yao-Bang Liu,2 Tong She,3 Xin-Lan Liu4
1Department of Radiation Oncology, General Hospital of Ningxia Medical University, Yinchuan, Ningxia, 750004, People’s Republic of China; 2Department of Surgical Oncology, General Hospital of Ningxia Medical University, Yinchuan, Ningxia, 750004, People’s Republic of China; 3Hospital of Zhongwei, Zhongwei, People’s Republic of China; 4Department of Medical Oncology, General Hospital of Ningxia Medical University, Yinchuan, Ningxia, 750004, People’s Republic of China
Correspondence: Xin-Lan Liu, Tel +8613709577339
, Email [email protected]
Objective: This study determined the cut-off value of Ki-67 expression and discussed the interaction between Ki-67 and histological grade, further explored the prognostic role of Ki-67 in hormone receptor-positive and human epidermal growth factor receptor 2 negative (HR+/HER2-) breast cancer;.
Materials and Methods: We assessed the Ki-67 expression of 956 patients with HR+/HER2 breast cancer diagnosed in the General Hospital of Ningxia Medical University from 2015 to 2019 by immunohistochemistry (IHC), The disease-free survival (DFS) was defined as the time from postoperative to the first local recurrence, distant metastasis or death of the disease. The follow-up by means of inpatient or outpatient medical records and telephone.
Results: 22.5% was used as the cut-off for low/high Ki-67 expression in HR+/HER2- breast cancer. Compared with the value of 14%, which is commonly used in clinic at present, the consistency of the two values is moderate (Kappa = 0.484, P< 0.001). The expression of Ki-67 was increased with the grade. (Median: G1:10%; G2:20%; G3:40%. Mean: G1:13%; G2:23%; G3:39%, P < 0.001). Survival analysis was based on all patients for a median of 51 months (24– 89 months), 63 cases had recurrence or metastasis during the follow-up, which 21 cases had low expression of Ki-67 and 42 cases had high expression. The patients with Ki-67 ≥ 22.5% had a 2.969 higher risk of early recurrence and metastasis than the patients with Ki-67 < 22.5%. There were 4 cases of local recurrence, 7 cases of regional lymph node metastasis, and 52 cases of distant metastasis in all patients, the common distant metastases were bone, liver, and lung, and rare metastases were adrenal gland, bone marrow, and pericardium.
Conclusion: In HR+/HER2- breast cancer, patients with Ki-67 > 22.5% have a worse prognosis and are more likely to have early recurrence and metastasis.
Keywords: Ki-67, breast cancer, cut-off value, histological grade, recurrence and metastasis
Introduction
According to the Global Cancer Statistics report in 2020, there are about 2.26 million new women with breast cancer worldwide, accounting for 11.7% of all new cancer cases, surpassing lung cancer as the most common tumor in the world for the first time.1,2 Breast cancer is divided into four molecular subtypes with different characteristics and prognoses, The most common type of breast cancer in clinic is HR+ breast cancer, accounting for about 75%.3 HR+/HER2- breast cancer accounts for 65% of breast cancer in women under the age of 50 and 75% of breast cancer in elderly women.4 In HR+/HER2- breast cancer, gene expression analysis can be divided into two different subtypes-luminal A and luminal B (HER2-).5 Ki-67 is an important index that is often used to distinguish between luminal A and luminal B (HER2-) in clinics, and it is also one of the important indexes to define the prognosis of patients with HR+/HER2- early breast cancer. It is located on the long arm of human chromosome 10 (10q25).6 The positive rate of expression is different in different stages of the cell cycle, the positive rate of Ki-67 expression is low in the G1 and early S phase, increases gradually during mitosis, reaches the peak in the M stage, decreases rapidly in the late and terminal stage of mitosis, and does not express in G0 stage.7 The expression of Ki-67 in breast cancer is related to luminal B (HER2-), high risk of recurrence and good response to neoadjuvant chemotherapy.8 In 2011, the International Breast Cancer Working Group pointed out that due to the lack of standardized evaluation methods of Ki-67 expression, there is still no unified “gold standard” for the evaluation of the Ki-67 proliferation index.9 Although the consistency of Ki-67 testing in the same laboratory may be high, in different laboratories it still varies greatly because the consistency and repeatability of Ki-67 expression evaluation are low between observers. Different cut-off values may bring different treatment options to patients, and lack of chemotherapy or over-treatment may lead to different prognoses. Therefore, to guide the standardized treatment of breast cancer patients, clinicians must understand the distribution characteristics of Ki-67 and determine the most suitable cut-off value of Ki-67 for HR+/HER2- breast cancer in our laboratory. Patients with high expression of Ki-67 are prone to early recurrence and metastasis. We should find patients with high risk of recurrence and metastasis and strengthen treatment.
Material and Methods
Patients
The study, of 956 patients with HR+/HER2- breast cancer diagnosed by operation and pathology, from 25 to 80 years old between 2015 to 2019 in the General Hospital of Ningxia Medical University, all patients are female. Tumour samples were collected from the tumour tissue blocks used for routine pathologic evaluation. All patients accordance with the following standard: (1) No distant metastasis before operation; (2) Standardized chemotherapy, radiotherapy and endocrine therapy were performed after operation; (3) The clinical medical records are complete. We collect and organize the following parameters: age, IHC results, tumor stage (the TNM stage of AJCC 7th edition), histological grade, lymph node metastasis, vascular/nerve invasion, transfer time, etc. Patients with stage IV, neoadjuvant therapy, triple-negative, or HER2-positive breast carcinoma were excluded from the study.
Ki-67 Evaluation
The Ki-67 index was detected by IHC in all patients, which was evaluated and determined by two experienced pathologists. The yellow deposition in the nucleus is Ki-67 positive cells, and the expression rate of Ki-67 is the percentage of Ki-67 positive cells in the total number of tumor cells.
IHC Standard
The expression of estrogen receptor (ER), progesterone receptor (PR), and HER2 in all HR+/HER2- breast cancers were detected by immunohistochemical method. The positive of ER and PR is defined as the staining of tumor cells ≥ 1%. The expression level of HER2 was divided into 0~3+, and the cases with HER2 expression of 2+ were further detected by fluorescence in situ hybridization (FISH). HER2 negative was defined as (0), (1 +), or negative by the FISH test, HER2 positive was defined as (3 +), or positive by the FISH test.
Follow-Up
The follow-up by means of inpatient or outpatient medical records or telephone. The starting time is the date of operation, and the deadline is December 31, 2021. The endpoint of follow-up is the deadline for disease progression, death, or loss of follow-up. DFS is defined as the time from postoperative to the first local recurrence, distant metastasis, or death of breast cancer. The patients that had recurrent and metastatic must be confirmed by pathological.
Statistical Analysis
The data were analyzed by SPSS 25.0 and GraphPad Prism 8.0.1. The receiver operating characteristic curve (ROC) was used to determine the cut-off value of Ki-67 expression, The relationship between Ki-67 expression and pathological was analyzed by χ2 test, Kaplan-Meier method was used to draw the survival curve, significant level was set as 0.05.
Result
The expression range of Ki-67 in HR+/HER2- breast cancer of 956 patients is 1%-90%, the most common range is 10%-30%, the median of Ki-67 expression is 20%, and the average is 25% (Figures 1 and 2).
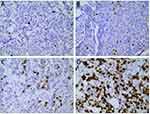 |
Figure 1 The different expression of Ki-67 in breast cancer of tumor tissues. ((A) 5%; (B) 20%; (C) 30%; (D) 60%; SP x400). |
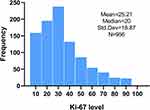 |
Figure 2 The frequency distribution of Ki-67 value in 2015–2019. |
With the statistical analysis of the ROC curve, the cut-off value of Ki-67 is 22.5% (Figure 3). All patients were divided into two groups, compared with the value of 14%, which is commonly used in clinic at present, the consistency of the two values is moderate (Kappa = 0.484, P<0.001) (Table 1). Histological grade, PR expression, T stage, and vascular/nerve invasion were correlated with the Ki-67 (Table 2). The expression of Ki-67 is increased with the grade (Figure 4).
 |
Table 1 HR+/HER2- Breast Cancer at Different Cut-off Values |
 |
Table 2 Correlation of Ki-67 with Pathological for the Different Cut-off Values |
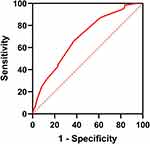 |
Figure 3 The ROC curve of Ki-67 expression (Area under ROC curve: 0.689; Sensitivity: 63.02%; Specificity: 65.57%; Youden Index: 0.286; 95% CI: [0.626–0.754]; P <0.001;). |
 |
Figure 4 The relationship between Ki-67 expression and histological grade. (Median: G1:10%; G2:20%; G3:40%. Mean: G1:13%; G2:23%; G3:39%, P <0.001). |
Survival analysis was based on all patients for a median of 51 months (24–89 months), 63 cases had recurrence or metastasis during the follow-up, which 21 cases had low expression of Ki-67 and 42 cases had high expression. The patients with Ki-67 ≥ 22.5% had a 2.969 higher risk of early recurrence and metastasis than the patients with Ki-67 < 22.5% (Figure 5, Table 3). Ki-67 was an independent risk factor for HR+/HER2 breast cancer (Table 4). There were 4 cases of local recurrence, 7 cases of regional lymph node metastasis, and 52 cases of distant metastasis in all patients. The common positions of distant metastasis were bone, liver, and lung, and rare metastases were adrenal gland, bone marrow, and pericardium. (Figure 6)
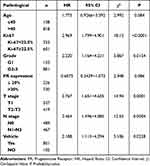 |
Table 3 Univariate Analysis of Prognosis with HR+/HER2- Breast Cancer |
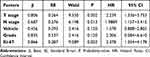 |
Table 4 Multivariate Analysis of Prognosis with HR+/HER2- Breast Cancer |
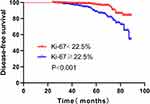 |
Figure 5 Kaplan-Meier survival curve with the expression of Ki-67. |
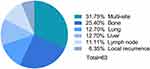 |
Figure 6 The common metastatic sites of HR+/HER2- breast cancer. |
Discussion
Ki-67 index is a common parameter for the molecular type of HR+/HER2- breast cancer by IHC instead of gene detection. To determine the threshold value of Ki-67 in HR+/HER2- breast cancer, we can detect multiple genes in all samples, and then compare the results of gene detection with IHC to get the best threshold value, but this method is expensive and difficult in clinical. Ki-67 was detected by IHC, and the percentage of positive expression was calculated by pathologist with artificial vision. Despite its nearly universal use in pathology laboratories worldwide, no internationally accepted consensus has yet been achieved for some methodological details related to Ki67. Controversial issues refer to choice of IHC antibody clones, scoring methods, inter-laboratory reproducibility, and the potential value of computer-assisted imaging analysis and/or artificial intelligence for Ki67 assessment.11 The individual differences in the judgment of Ki-67 positive cells by different pathologists lead to poor consistency of Ki-67 positive expression, so its reliability is reduced due to the variability between observers. At the same time, although there is a high consistency of Ki-67 testing in the laboratory, the consistency of Ki-67 testing among different laboratories is still lack of comparison. The factors leading to the inconsistency among laboratories include tumor region selection, counting methods and subjective evaluation of positive staining.12 In recent years, the effectiveness and methods of Ki-67 detection has been a research hotspot. The development of digital image analysis system makes more choices for the evaluation of Ki-67 proliferation index.13 It is found that after zoning according to staining distribution, compared with visual quantitative or digital image analysis, different observers have satisfactory consistency in Ki-67 quantification, or it may be a candidate standardized method for Ki-67 index quantification of breast cancer or other malignant tumors.14
The cut-off value that distinguishes the low/high Ki-67 expression in HR+/HER2- breast cancer is constantly changing. The consensus of experts at the 2009 St Gallen International Breast Cancer Conference recommended that Ki-67 should be used as a proliferative marker for the selection of appropriate systemic treatment, classified according to three levels: low level (≤ 15%), medium level (16–30%) or high level (> 30%).15 Through the study of the PAM50 gene expression profile and comparison of IHC results, Cheang found that the best cut-off value to distinguish Luminal A and Luminal B (HER2-) breast cancer was 13.25%.16 Based on this result, the 2011 St Gallen meeting determined the cut-off value of Ki-67 to distinguish these two subtypes at 14%.17 The 2013 St Gallen meeting raised the cut-off value of Ki-67 expression to 20%.18 In 2015, the expert group of the St Gallen meeting suggested that Ki-67 should be explained according to the values of the local laboratory.19 At the latest St Gallen meeting in 2021, 42.4% of the experts suggested the threshold for Ki-67 to recommend adjuvant chemotherapy in ER+, HER2-, node-negative breast cancer is 30%.20 Many studies have taken different thresholds to explore the effect of Ki-67 expression on the prognosis, including 14%,21 15%,22 20%,23 22%,24 25%.25 Meta-analysis shows that the threshold value of Ki-67 is between 5% and 30%.26 The guidelines and norms for breast cancer diagnosis and treatment of the Chinese Anti-Cancer Association point out that the value of the Ki-67 proliferation index may be different in different pathological centers, 20–30% can be used as the cut-off value of Ki-67.27 We determined the cut-off value of Ki-67 expression is 22.5% through the analysis of ROC in this study, which means the patients with Ki-67 ≥ 22.5% have a poor prognosis and are more likely to have early recurrence and metastasis. In order to compare with the Ki-67 critical value of 14% which is the most commonly used in clinic, we used the Ki-67 cutoff value of 14% and 22.5% respectively to classify all HR+/HER2- breast cancers, and the results were moderately consistent, which means that the Ki-67 cut-off value of 22.5% is more instructive in clinical diagnosis, treatment and prognosis of breast cancer patients. So we should use the Ki-67 threshold in our laboratory instead of continuing to use 14%, which allows some patients to avoid chemotherapy.
HR+/HER2- breast cancer is a heterogeneous tumor, there are differences in PR expression, histological grade, Ki-67 proliferation, and so on, and these characteristics are highly correlated.28 Studies have shown that the expression of Ki-67 is related to the clinicopathological features of breast cancer, such as tumor size, histological grade, PR status, vascular nerve invasion, and so on.2 In this study, we found that histological grade, PR expression, T stage, and vascular/nerve invasion are all related to Ki-67 expression. The consensus of experts at the 2017 St Gallen conference pointed out for the first time that histological grade can be used to distinguish the molecular type of HR+/HER2- breast cancer.29 The histological grade is mainly evaluated from three aspects: the degree of glandular duct formation, nuclear pleomorphism, and mitosis count. The expression of Ki-67 increases gradually during mitosis, so the tumor grade may be indirectly related to the expression of Ki-67 according to mitotic cell count. Liang Qin found that Ki-67 is related to tumor grade, and can predict histological grade to some extent.30 Mohammed found that the expression level of Ki-67 was positively correlated with histological grade, the higher the tumor grade, the higher the Ki-67 expression, Ki-67 can predict tumor invasiveness and higher histological grade.29 Professor Fu Li pointed out that the positive rate of Ki-67 in ER+ breast cancer varies widely, the expression of Ki-67 in high differentiated and G1 can be less than 5% but more than 20% in high histological grade breast cancer.31 It has been reported that luminal-type breast cancer is at opposite ends in histological grade, G1 conforms to luminal A, G3 conforms to luminal B type, and G2 is between them.28 In this study, we analyzed the relationship between Ki-67 expression and histological grade, and the results showed that the expression of Ki-67 is closely related to histological grade, the expression of Ki-67 was increased with the increase of grade. Both histological grade and Ki-67 value can distinguish the molecular type of luminal breast cancer, but the study found that the consistency between them is low, the consistency can be improved when these two factors are combined.32 It is reported that the risk of recurrence of HR+/HER2- breast cancer has remained relatively stable for many years, at least half of the patients with recurrence and metastasis occurred 5 years after diagnosis, and even some patients developed recurrence and metastasis for more than 10 years.28 To improve the survival rate of patients with early recurrence and metastasis, clinicians need to identify high-risk patients with early recurrence and metastasis and strengthen treatment and follow-up. Our study found that the patients with Ki-67 ≥ 22.5% had a 2.969 higher risk of early recurrence and metastasis than the patients with Ki-67 < 22.5%. Studies have found that the common sites of recurrence and metastasis of HR+/HER2- breast cancer include bone, lymph nodes, pleura or lung, liver, and skin.28 We found the most common metastatic site of HR+/HER2- breast cancer is bone (vertebra is the most common), followed by viscera (liver, lung), regional lymph nodes, local chest wall skin, and multi-site metastasis is more common, which is consistent with the literature report. In this study, we also found that three patients had rare metastases of the adrenal gland, bone marrow, and pericardium, which occurred at the same time or successively with the common metastatic sites mentioned above.
Abbreviations
HR, Hormone receptor; HER2, Human epidermal growth factor receptor; DFS, Disease-free survival; ER, Estrogen receptor; PR, Progesterone receptor; FISH, Fluorescence in situ hybridization; AJCC, American Joint Committee on Cancer.
Data Sharing Statement
Available upon request to [email protected].
Ethical Statement
This study approval was granted by the Ethics Committee of the General Hospital of Ningxia Medical University (No: KYLL-2021- 610). This study was conducted in accordance with the Declaration of Helsinki. The lead researcher was presenting the purpose of the study, the benefit, and the risk of the procedure taken by the research participant to the ethical committee board prior to approval. Each research participant was given detailed information regarding the purpose, benefit, and risk of the study. Each research participant signed an informed consent form before participating in the study.
Acknowledgment
The authors thank all the patients who agreed to participate in this study and the pathologist and surgical residents who helped carry out this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This project is supported by the “Newly enrolled Master training” project of the General Hospital of Ningxia Medical University (NO.YKDZY2022013).
Disclosure
All authors declare that they have no competing interests in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Mohammed AA. Quantitative assessment of Ki67 expression in correlation with various breast cancer characteristics and survival rate; cross sectional study. Ann Med Surg. 2019;48:129–134. doi:10.1016/j.amsu.2019.11.005
3. Johnston SRD, Dowsett M. Aromatase inhibitors for breast cancer: lessons from the laboratory. Nat Rev Cancer. 2003;3(11):821–831. doi:10.1038/nrc1211
4. Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106(5). doi:10.1093/jnci/dju055
5. Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98(19):10869–10874. doi:10.1073/pnas.191367098
6. Fonatsch C, Duchrow M, Rieder H, et al. Assignment of the human Ki-67 gene (MK167) to 10q25-qter. Genomics. 1991;11(2):476–477. doi:10.1016/0888-7543(91)90163-9
7. Gerdes J, Lemke H, Baisch H, et al. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133(4):1710–1715. doi:10.4049/jimmunol.133.4.1710
8. Andre F, Arnedos M, Goubar A, Ghouadni A, Delaloge S. Ki67--no evidence for its use in node-positive breast cancer. Nat Rev Clin Oncol. 2015;12(5):296–301. doi:10.1038/nrclinonc.2015.46
9. Dowsett M, Nielsen TO, A’Hern R, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer Working Group. J Natl Cancer Inst. 2011;103(22):1656–1664. doi:10.1093/jnci/djr393
10. Prat A, Cheang MC, Martin M, et al. PPrognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal A breast cancer. J Clin Oncol. 2013;31(2):203–209. doi:10.1200/JCO.2012.43.4134
11. Kreipe H, Harbeck N, Christgen M. Clinical validity and clinical utility of Ki67 in early breast cancer. Ther Adv Med Oncol. 2022;14:17588359221122725. doi:10.1177/17588359221122725
12. Polley MY, Leung SC, Mcshane LM, et al. An international Ki67 reproducibility study. J Natl Cancer Inst. 2013;105(24):1897–1906. doi:10.1093/jnci/djt306
13. Stålhammar G, Robertson S, Wedlund L, et al. Digital image analysis of Ki67 in hot spots is superior to both manual Ki67 and mitotic counts in breast cancer. Histopathology. 2018;72(6):974–989. doi:10.1111/his.13452
14. Wang YX, Wang YY, Yang CG, et al. An interobserver reproducibility analysis of size-set semiautomatic counting for Ki67 assessment in breast cancer. Breast. 2020;49:225–232. doi:10.1016/j.breast.2019.12.009
15. Goldhirsch A, Ingle JN, Gelber RD, et al. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009;20(8):1319–1329. doi:10.1093/annonc/mdp322
16. Cheang MC, Chia SK, Voduc D, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101(10):736–750. doi:10.1093/jnci/djp082
17. Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the primary therapy of early breast cancer 2011. Ann Oncol. 2011;22(8):1736–1747. doi:10.1093/annonc/mdr304
18. Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24(9):2206–2223. doi:10.1093/annonc/mdt303
19. Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies--improving the management of early breast cancer: st Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol. 2015;26(8):1533–1546. doi:10.1093/annonc/mdv221
20. Burstein HJ, Curigliano G, Thurlimann B, et al. Customizing local and systemic therapies for women with early breast cancer: the St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann Oncol. 2021;32(10):1216–1235. doi:10.1016/j.annonc.2021.06.023
21. Gallardo A, Garcia-Valdecasas B, Murata P, et al. Inverse relationship between Ki67 and survival in early luminal breast cancer: confirmation in a multivariate analysis. Breast Cancer Res Treat. 2018;167(1):31–37. doi:10.1007/s10549-017-4486-z
22. Alco G, Bozdogan A, Selamoglu D, et al. Clinical and histopathological factors associated with Ki-67 expression in breast cancer patients. Oncol Lett. 2015;9(3):1046–1054. doi:10.3892/ol.2015.2852
23. Bustreo S, Osella-Abate S, Cassoni P, et al. Optimal Ki67 cut-off for luminal breast cancer prognostic evaluation: a large case series study with a long-term follow-up. Breast Cancer Res Treat. 2016;157(2):363–371. doi:10.1007/s10549-016-3817-9
24. Maranta AF, Broder S, Fritzsche C, et al. Do you know the Ki-67 index of your breast cancer patients? Knowledge of your institution’s Ki-67 index distribution and its robustness is essential for decision-making in early breast cancer. Breast. 2020;51:120–126. doi:10.1016/j.breast.2020.03.005
25. Abubakar M, Orr N, Daley F, et al. Prognostic value of automated KI67 scoring in breast cancer: a centralised evaluation of 8088 patients from 10 study groups. Breast Cancer Res. 2016;18(1):104. doi:10.1186/s13058-016-0765-6
26. Petrelli F, Viale G, Cabiddu M, et al. Prognostic value of different cut-off levels of Ki-67 in breast cancer: a systematic review and meta-analysis of 64,196 patients. Breast Cancer Res Treat. 2015;153(3):477–491. doi:10.1007/s10549-015-3559-0
27. Breast Cancer Professional Committee of Chinese Anti-Cancer Association. Guidelines and norms for diagnosis and treatment of Breast Cancer of Chinese Anti-Cancer Association (2019 Edition). China Oncology. 2019;29(8):609–679. doi:10.19401/j.cnki.1007-3639.2019.08.009
28. Burstein HJ. Systemic therapy for estrogen receptor-positive, HER2-negative breast cancer. N Engl J Med. 2020;383(26):2557–2570. doi:10.1056/NEJMra1307118
29. Curigliano G, Burstein HJ, Winer EP, et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen international expert consensus conference on the primary therapy of early breast cancer 2017. Ann Oncol. 2017;28(8):1700–1712. doi:10.1093/annonc/mdx308
30. Liang Q, Ma D, Gao RF, et al. Effect of Ki-67 expression levels and histological grade on breast cancer early relapse in patients with different immunohistochemical-based subtypes. Sci Rep. 2020;10(1):7648. doi:10.1038/s41598-020-64523-1
31. Yang YL, Guo XJ, Fu L. Interpretation and analysis of the results of main molecular pathological indexes of Breast Cancer. Chin J Pathol. 2020;49(5):526–528. doi:10.3760/cma.j.cn112151-20190917-00507
32. Oddo D, Pulgar D, Elgueta N, et al. Can histological grade and mitotic index replace Ki67 to determine luminal breast cancer subtypes? Asian Pac J Cancer Prev. 2018;19(1):179–183. doi:10.22034/APJCP.2018.19.1.179
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
