Back to Journals » Clinical, Cosmetic and Investigational Dentistry » Volume 15
The Role of Asiatic Acid in Preventing Dental Pulp Inflammation: An in-vivo Study
Authors Nurhapsari A , Cilmiaty R , Prayitno A , Purwanto B, Soetrisno S
Received 10 February 2023
Accepted for publication 9 June 2023
Published 13 June 2023 Volume 2023:15 Pages 109—119
DOI https://doi.org/10.2147/CCIDE.S408158
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Christopher E. Okunseri
Arlina Nurhapsari,1,2 Risya Cilmiaty,3 Adi Prayitno,3 Bambang Purwanto,4 Soetrisno Soetrisno5
1Doctoral Degree of Medical Science, Faculty of Medicine, Sebelas Maret University, Surakarta, Central Java, Indonesia; 2Department of Conservative Dentistry, Faculty of Dentistry, Islam Sultan Agung University, Semarang, Central Java, Indonesia; 3Department of Oral Disease, Faculty of Medicine, Sebelas Maret University, Surakarta, Central Java, Indonesia; 4Department of Internal Medicine, Faculty of Medicine, Sebelas Maret University, Surakarta, Central Java, Indonesia; 5Department of Obstetrics and Gynaecology, Faculty of Medicine, Sebelas Maret University, Surakarta, Central Java, Indonesia
Correspondence: Arlina Nurhapsari, Department of Conservative Dentistry, Faculty of Dentistry, Islam Sultan Agung University, Semarang, Jawa Tengah, 50112, Indonesia, Email [email protected]
Purpose: Acute dental pulp inflammation necessitates early treatment to alleviate inflammation and pain. In the inflammatory phase, a substance is required to lower the inflammatory mediators and reactive oxygen species that play a crucial role in that phase. Asiatic acid is a natural triterpene obtained from the Centella asiatica plant with a high antioxidant value. This study examined the effect of Asiatic acid’s antioxidant, anti-inflammatory, and antinociceptive properties on dental pulp inflammation.
Methods: The research is an experimental laboratory, with a post-test only with a control group design. The study utilised 40 male Wistar rats weighing 200– 250 grams and aged 8– 10 weeks. Rats were divided into five groups (control, eugenol, Asiatic Acid 0.5%; 1%; 2% group). Dental pulp inflammation was created in the maxillary incisor after six hours of administration of lipopolysaccharides (LPS). The dental pulp treatment then continued with the administration of eugenol and three different Asiatic acid concentrations (0.5%, 1% and 2%). In the next 72 hours, the teeth were biopsied, and the dental pulp was analysed using the enzyme-linked immunosorbent assay (ELISA) to measure the level of MDA, SOD, TNF-α, beta-endorphins and CGRP. Histopathological examination and the Rat Grimace Scale were utilised to determine the level of inflammation and pain, respectively.
Results: The effect of Asiatic Acid on MDA, TNF-α, and CGRP levels decreased significantly compared to the control group (p=< 0.001). On the SOD and beta-endorphin levels, Asiatic acid treatment resulted in a considerable rise (p =< 0.001).
Conclusion: Due to its antioxidant, anti-inflammatory, and antinociceptive characteristics, Asiatic acid can reduce inflammation and pain in acute pulp inflammation due to its ability to decrease MDA, TNFα, and CGRP levels while raising SOD and beta-endorphin levels.
Keywords: pulp inflammation, Asiatic acid, MDA, TNF-α, SOD, beta endorphins, CGRP
Introduction
Pulpitis is an inflammation of the dental pulp tissue that causes moderate to severe pain in the affected individual.1 Pulpitis that persists might result in dental pulp necrosis. Dental pulp necrosis causes the tooth structure to become more frail and prone to fracture, so it is essential to preserve the vitality of the pulp for the teeth to function properly.2,3 Pulpitis is divided into reversible and irreversible types, with reversible pulpitis being an acute inflammation of the pulp that can still recover.4 The inflammatory response begins when microorganisms and their byproducts irritate the pulp. This activates the host cells (odontoblasts and pulpal fibroblasts), producing proinflammatory cytokines and chemokines that attract neutrophils to the wound site.5
The production of proinflammatory cytokines, such as tumour necrosis factor α (TNF-α), can cause inflammation. Inflammatory conditions can also increase the production of reactive oxygen species (ROS), which induces oxidative stress in cells and worsens inflammatory conditions.6 Reactive Oxygen Species (ROS) generate free radicals; when these free radicals react with lipids, Malondialdehyde (MDA) molecules are produced.7,8 By producing antioxidant enzymes such as Superoxide Dismutase (SOD), cells can reduce ROS levels.9 The occurrence of the inflammatory process causes pain, which can cause anxiety and psychological stress in patients.10 In response to pain, the body produces Beta Endorphin hormones, which act as an analgesic by enhancing μ-opioid activity and inhibiting the production of Calcitonin Gene-Related Peptides (CGRP).11,12 If pain can be suppressed, the inflammatory environment will subside, resulting in a homeostatic state that can stimulate pulp healing.
Asiatic Acid isolate is a component of saponins (triterpenoids) derived from Centella asiatica that has anti-inflammatory, antioxidant, antinociceptive, and antibacterial properties and promotes wound healing.13,14 Asiatic acid has the ability to enhance the Nuclear factor erythroid 2-related factor 2 (Nrf2) through the inhibition of oxidative stress.15 The transcription factor Nrf2 plays a key role in the regulation of antioxidant enzyme expression. The inactive form of Nrf2 interacts with Kelch-like ECH-associated protein 1 (Keap1) in the cytoplasm under normal or unstressed conditions.16,17 Excess oxidative stress may cause Nrf2 to split from the Nrf2-Keap1 complex and enter the nucleus.18,19 Nuclear factor erythroid 2-related factor 2 (Nrf2) binds to an antioxidant response element (ARE) in the upstream area of the nucleus, initiating the transcription of antioxidant genes.20,21 In order to prevent tissue damage, the production of SOD catalyses the release of free radicals and reduces ROS levels.22,23
Numerous studies have been conducted on using natural substances, particularly those with anti-inflammatory, antioxidant, and antinociceptive qualities, to treat inflammation and pain in the dental pulp. However, no research has been conducted on using Asiatic acid as a component of dental pulp inflammation treatment. Based on all of this information, it is hypothesised that Asiatic acid may decrease inflammation and pain in the dental pulp through increased antioxidant enzymes. This study investigated the effect of Asiatic acid on the levels of MDA, SOD, TNF-, beta-endorphins, and CGRP in pulpal inflammation induced in rat incisors.
Materials and Methods
Materials
Asiatic acid (Asiatic acid 95%, catalogue number 546712–500MG, Sigma Aldrich, St. Louis, Missouri, United States); Dimethyl sulfoxide (DMSO) (Merck, Germany); Lipopolysaccharides (LPS) (Lipopolysaccharides from Escherichia Coli O55:B5, Sigma Aldrich, St. Louis, Missouri, United States); ketamine (KTM-100, Bernofarm, Indonesia); xylazine (Xyla, Interchemie, Holland), glass ionomer cement (Fuji II, GC, Japan) and Eugenol (Ghimas, Bologna) was used in this experiment.
Asiatic Acid
Asiatic acid was stored at 2°–8°C. Asiatic acid was dissolved with DMSO to make three different concentrations. The concentrations were Asiatic acid 0.5% (v/w), Asiatic acid 1% (v/w) and Asiatic acid 2% (v/w).
Animals
The study used male Wistar rats (Rattus norvegicus), aged 8–10 weeks, that weighed 200–250 g. The 40 rats were adapted for over one week. This study’s sample size was determined using a power analysis of the mean results of preliminary research with a significance level of 0.05 and a power of 0.80. The rats were fed a regular diet of pellets, with a feed volume of about 15–25 grams. They drank sterile water, about 50–100 mL per day. The rats were kept in a room with a constant temperature of 20–23°C, the humidity of −55% and standard lighting. Wood shavings that were dried and autoclaved at 120°C for 10–15 minutes were used for bedding. The area of the cage, which measured 30×20 x 20 cm for each rat, was expected to allow the rats to move freely.
Dental Pulp Inflammation Model
Before the procedure, the animals were anaesthetised using ketamine at 75–100mg/kg body weight and xylazine at 5–10 mg/kg BB, intraperitoneal. Inflammation of pulp tissue was generated experimentally by treating pulp tissue with bacterial LPS, as described earlier.24,25 The treatment procedure for rats’ teeth is illustrated in Figure 1. Two maxillary incisors of rats were utilised to model dental pulpitis. Using a low-speed diamond disc bur (Ortho Technology, USA), the maxillary incisors were cut to the cervix to initiate the modelling process. Subsequently, a low-speed, #010 carbide bur (H1SE, Komet, USA) was used for drilling, and access to the pulp chamber was widened with a number #15–45 K-file (SybronEndo, Glendors, USA) until bleeding was observed on the exposed roof of pulp. The cavity was cleansed with a saline solution until hemostasis was achieved. The cavity was then filled with paper points (Gapadent, China) containing 20 mg/mL LPS for six hours and sealed with glass ionomer cement. After six hours of LPS application on rat dental pulp, the paper points containing LPS were discarded. Then apply paper points containing Asiatic Acid as the treatment group and Eugenol as the control group, as described in Table 1. The cavity was sealed with glass ionomer cement for 72 hours. After 72 hours, the rats were sacrificed. Five rats per group had their maxillary incisors biopsied, and the dental pulp was removed from the apical region using a #40 barbed broach (SybronEndo, USA). The pulp was cleaned of blood with NaCl before being placed in a microtube and frozen at −20° C for ELISA examination. For histopathological analysis, maxillary incisors from three rats per group were used.
 |
Table 1 The Dental Pulp Treatment in Animals |
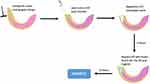 |
Figure 1 An illustration of the dental pulp inflammation procedure and the treatment procedure. |
Pain Assessment
After treatment, the rat was monitored for 72 hours. During the trial, rat pain symptoms were noted, as were rat pain scores using the rat grimace scale (RGS).26 The scale includes orbital tightening, nose/cheek flattening, ear, and whisker changes. The rating scale uses a score of 0 = no change, 1 = moderate change, and 2 = severe change.
Histopathology Evaluation of Dental Pulp Inflammation
Rapidcal Immuno (PathChem) was used to decalcify the maxillary incisors before preparing preparations with hematoxylin-eosin staining. Observation at 400x magnification in three fields of view of the two-thirds pulp’s coronal portion. All sections were viewed under an optical microscope (CX23, Olympus). Inflammation grading according to the intensity of inflammatory cells, with scores of 1 (0–20 cells), 2 (21–40 cells), 3 (41–80 cells), and 4 (>80 cells).27 Other histologic features were also recorded, such as the location of inflammatory cells, oedema, vascular leakage and necrosis in the pulp.
ELISA’s Analysis
The values of MDA, SOD, TNF-α, beta-endorphins and CGRP were analysed using ELISA. Before homogenization, the pulp tissue was properly rinsed in phosphate buffer saline (PBS) (pH 7.4) to eliminate excess blood and weighed. With a glass homogenizer on ice, the tissue was minced and homogenized in PBS (tissue weight (g): PBS (mL) volume = 1:9). The suspension was sonicated using an ultrasonic cell disrupter to break up the cells further. To obtain the supernatant, the suspension was homogenised and centrifuged for five minutes at 5000 x g. The levels of MDA, SOD, TNF-α, beta-endorphins and CGRP were measured using an MDA ELISA kit (Rat MDA, BZ-08186510-EB, Bioenzy) SOD ELISA kit (Rat SOD, BZ-08188610-EB, Bioenzy), TNF-alpha ELISA kit (Rat TNF-α, BZ-08184670-EB, Bioenzy), beta-EP ELISA kit (Rat Beta Endorphin, BZ-08187000-CPEB, Bioenzy), and a CGRP ELISA kit (Rat CGRP, BZ-08187430-EB, Bioenzy) according to the manufacturer’s protocols.
Statistical Analysis
Data are presented as the mean, standard error. Statistical analyses were performed using SPSS software (IBM SPSS, Armonk, New York, USA). The homogeneity and normality of the data were tested first, followed by a one-way analysis of variance (one-way ANOVA), followed by post hoc least significant differences (LSD), and the significance level was set at p < 0.05. For categorical data analysis, use Kruskal–Wallis’s test for the analysis of the entire group and the Mann–Whitney test for the analysis between two groups. No rats or tooth samples were excluded or dropped out in this study.
Results
Pain Assessment
According to Kruskal–Wallis’s test, the RGS score did not differ significantly (p = 0.070). Whereas the Mann–Whitney test revealed a statistically significant difference between the 1% Asiatic acid group and the control group (p=0.032). Similar outcomes were also observed in the eugenol group (p = 0.032). (Figure 2).
Histopathology Evaluation of Dental Pulp Inflammation After Treatment
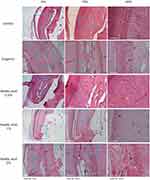
The application of Asiatic Acid 1% to pulpal inflammation resulted in the highest decrease of inflammatory cells, with a value of 80% on a score of 1 compared to the other treatment groups. The effectiveness of Asiatic acid 2% and Eugenol in decreasing inflammatory cells was equivalent. In contrast, the Asiatic acid 0.5% group received a score of 2 in each sample. There was no score of 4 in any of the sampled groups (Figures 3 and 4).
 |
Figure 3 Inflammation reaction based on the intensity of inflammatory cells. Score 1 (0–20 cells), score 2 (21–40 cells), score 3 (41–80 cells). The replication of the group was 5. |
Asiatic Acid’s Antioxidant Effect
MDA and SOD levels were used to assess the antioxidant effect of Asiatic acid on dental pulp inflammation. Compared to the control group, administration of Asiatic acid 1% and Asiatic acid 2% significantly decreased MDA levels (p<0.0001). The MDA levels in the Asiatic acid 1% and Asiatic acid 2% groups were significantly lower than in the Asiatic acid 0.5% group (p<0.001) (Figure 5A).
The SOD level, administration of Asiatic acid 2%, Asiatic acid 1% and Asiatic acid 0.5% significantly increased than eugenol and control group (p<0.01 and p<0.001) (Figure 5B). Based on these findings, it appears that Asiatic acid has the potential to significantly decreased MDA and increase SOD levels after being administered.
Asiatic Acid’s Anti-Inflammatory Effect
TNF-α levels were used to assess the anti-inflammatory effect of Asiatic acid on dental pulp inflammation. TNF-α levels in the Asiatic Acid group were significantly lower than and the eugenol and control group (p<0.0001). Among Asiatic acid, the concentration of 1% showed lower TNF-α levels than concentrations of 0.5% and 2% (p<0.0001 and p<0.01). These findings suggest that the administration of Asiatic acid can significantly reduce TNF-α levels (Figure 6).
Asiatic Acid’s Antinociceptive Effect
The antinociceptive impact of Asiatic acid on dental pulp inflammation was assessed using a beta-endorphins level. Beta-endorphin level in Asiatic acid 1% and Asiatic acid 2% was higher than the control group (p=<0.01 and p<0.0001); however, administration of Asiatic acid did not demonstrate a significant difference when compared with eugenol, except Asiatic acid 0.5% (p<0.05) (Figure 7A).
The CGRP level, administration of Asiatic acid 1% and Asiatic acid 2% significantly decreased CGRP levels than control groups (p<0.001 and p<0.01) (Figure 7B). According to these findings, 1% and 2% Asiatic acid concentrations had the same effect as eugenol.
Discussion
Acute pulp inflammation begins with particular damage that causes soluble mediators such as cytokines, acute phase proteins, and chemokines to stimulate neutrophil and macrophage migration to the site of inflammation.28,29 The acute inflammatory process typically lasts between 1–3 days and is mediated by numerous molecules, including toll-like receptors (TLRs) and reactive oxygen species (ROS).30,31 Induction of LPS in the pulp can be identified by toll-like receptor 4 (TLR-4), which subsequently activates an innate immune response.32 LPS activates the NF-κB pathway, which regulates the release of inflammatory mediators, including TNF-α, IL-1, IL-6 and IL-8. In this study, Asiatic acid doses of 0.5%, 1%, and 2% significantly reduced TNF-α levels. The ability of Asiatic Acid to downregulate the NF-κB and MAPK signaling pathways enables Asiatic Acid to inhibit TNF-α expression.33 According to the findings of this study, administration of Asiatic Acid can reduce the expression of TNF-α.
The LPS-induced inflammatory response is accompanied by neutrophil infiltration that produces free radicals and proinflammatory cytokines.34 Free radicals and Nitric Oxide will increase 6 hours after LPS administration.35 The production of MDA is caused by free radicals that attack the plasma membrane.36 Consequently, the inflammatory effect will cause the accumulation of MDA. In this investigation, elevated MDA levels were found in the control group. The first line of defence against oxidative stress is antioxidant enzymes, such as SOD.37 Superoxide Dismutase (SOD) is an indicator of protective activity against free radicals resulting from MDA-containing lipid peroxidation.38 According to the findings of this investigation, the administration of Asiatic acid can considerably lower MDA levels while significantly increasing SOD levels compared to the control group. Asiatic acid inhibits lipid peroxidation and hence the formation of malondialdehyde (MDA) in tissue by catalyzing reactive oxygen species (ROS) such as hydroxyl radicals (OH-) and peroxynitrite (O2-).23 Asiatic acid influences the SOD increase via the Nrf2 pathway.39 Superoxide dismutase is anti-inflammatory because it inhibits the activation of cytokines that increase the activation of NF-kB.40 Similar findings were also shown by Lv et al41 and Liu et al42 demonstrating that Asiatic acid could reduce MDA levels and inflammatory indicators by blocking the activation of MAPK and NF-kB. The findings of this study indicated that the administration of Asiatic acid was successful in lowering ROS levels.
In this study, Asiatic acid administration significantly increased beta-endorphins compared to the control group. Other studies have shown that reducing ROS boosts Nrf2 and SOD, the antioxidant enzymes that stimulate POMC to release beta-endorphins, which is consistent with this study.43–45 Beta endorphins have been proven to show peripheral and central analgesic activity, which produces a morphine-like effect by blocking C and Aδ fiber activation signals.46,47 Beta endorphins have a strong affinity for MOR receptors (opioid receptors), which can block cAMP and N− and P/Q-type Ca2+ channels.48,49 The cAMP pathway via PKA, PKC, and CaMKII contributes to the pronociceptive actions of CGRP, resulting in the sensitization of pain transduction receptors in trigeminal neurons.50,51 In this investigation, Asiatic acid administration was found to significantly reduce CGRP levels. Asiatic acid is able to decrease oxidative stress via the Nrf2 pathway, but according to Abushik et al,52 CGRP induction is not mediated via the Nrf2 pathway and requires the activation of protein kinase A (PKA) and Ca2+/calmodulin-dependent protein kinase II (CaMKII) simultaneously. For a time, it can be presumed that a reduction in the inflammatory state leads to a decrease in pulp neuropeptides. The connection between CGRP and antioxidant enzymes requires further study.
Asiatic acid concentrations of 1% and 2% demonstrated significant results in the levels of MDA and SOD compared to the Eugenol group, although the results for the other parameters were not significantly different. Based on these findings, it was concluded that concentrations of Asiatic acid 1% and 2% possessed the same anti-inflammatory and antinociceptive properties as eugenol, whereas Asiatic acid possessed stronger antioxidant properties. Asiatic acid has a larger influence on the Nrf2 pathway, producing high antioxidants and modulating pain markers in pulpitis after 72 hours or 3 days of treatment.
Conclusions
Asiatic Acid is abundant in antioxidants and can alleviate the pain of LPS-induced pulpitis. Based on the results, Asiatic Acid has anti-inflammatory and antinociceptive characteristics due to its ability to decrease MDA, TNFα, and CGRP levels while raising SOD and beta-endorphin levels.
Institutional Review Board Statement
The protocol of this study has registered and approved by Komisi Etik Penelitian Kesehatan, Fakultas Kedokteran Universitas Gadjah Mada, with registration number KE/FK/0703/EC/2020 (Approval date: 29 June 2020). All methods were performed in accordance with the relevant guidelines and regulations for the welfare of laboratory animals of Gadjah Mada University.
Acknowledgments
The author wants to acknowledge to Meircurius Dwi Condro Surboyo, DDS, MDS, for editing the figures and manuscript.
Funding
This research received no external funding.
Disclosure
The authors declare no conflict of interest.
References
1. Zanini M, Meyer E, Simon S. Pulp inflammation diagnosis from clinical to inflammatory mediators: a systematic review. J Endod. 2017;43(7):1033–1051. doi:10.1016/j.joen.2017.02.009
2. Hargreaves KM, Berman LH. Cohens Pathways of the Pulp.
3. Colombo JS, Moore AN, Hartgerink JD, D’Souza RN. Scaffolds to control inflammation and facilitate dental pulp regeneration. J Endod. 2014;40(4 Suppl):S6–12. doi:10.1016/j.joen.2014.01.019
4. Abbott PV, Yu C. A clinical classification of the status of the pulp and the root canal system. Aust Dent J. 2007;52(1 Suppl):S17–31.
5. Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20(7):1126–1167.
6. Gloire G, Legrand-Poels S, Piette J. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol. 2006;72(11):1493–1505.
7. Soetojo A, Cahyadi EKH, Prasetyo EA. Malondialdehyde expressions on pulp odontoblast cells after application of 2‐hydroxyethyl methacrylate mixed with water, ethanol, and acetone solvents. Saudi Endod J. 2019;9(2):96–100.
8. Hardiany NS, Paramita R. Profile of malondialdehyde (MDA) and catalase specific activity in plasma of elderly woman. Health Sci J Indones. 2019;10(2):1.
9. Yasui K, Baba A. Therapeutic potential of superoxide dismutase (SOD) for resolution of inflammation. Inflamm Res. 2006;55(9):359–363.
10. Bechert K, Abraham SE. Pain management and wound care. J Am Col Certif Wound Spec. 2009;1(2):65–71. doi:10.1016/j.jcws.2008.12.001
11. Fehrenbacher JC, Sun XX, Locke EE, Henry MA, Hargreaves KM. Capsaicin-evoked iCGRP release from human dental pulp: a model system for the study of peripheral neuropeptide secretion in normal healthy tissue. Pain. 2009;144(3):253–261. doi:10.1016/j.pain.2009.03.027
12. Chavarria-Bolanos D, Martinez-Zumaran A, Lombana N, Flores-Reyes H, Pozos-Guillen A. Expression of substance P, calcitonin gene-related peptide, beta-endorphin and methionine-enkephalin in human dental pulp tissue after orthodontic intrusion: a pilot study. Angle Orthod. 2014;84(3):521–526. doi:10.2319/060313-423.1
13. European Medicines Agency. Assessment report on Centella asiatica (L.) Urban, herba. 2022. Available from: https://www.ema.europa.eu/documents/herbal-report/superseded-assessment-report-centella-asiatica-l-urban-herba_en.pdf.
14. Gohil KJ, Patel JA, Gajjar AK. Pharmacological review on centella asiatica: a potential herbal cure-all. Indian J Pharm Sci. 2010;72(5):546–556. doi:10.4103/0250-474X.78519
15. Qi Z, Ci X, Huang J, et al. Asiatic acid enhances Nrf2 signaling to protect HepG2 cells from oxidative damage through Akt and ERK activation. Biomed Pharmacother. 2017;88:252–259. doi:10.1016/j.biopha.2017.01.067
16. Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284(20):13291–13295. doi:10.1074/jbc.R900010200
17. Milani P, Ambrosi G, Gammoh O, Blandini F, Cereda C. SOD1 and DJ-1 converge at Nrf2 pathway: a clue for antioxidant therapeutic potential in neurodegeneration. Oxid Med Cell Longev. 2013;2013:836760. doi:10.1155/2013/836760
18. Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi:10.1146/annurev.pharmtox.46.120604.141046
19. Li J, Ichikawa T, Janicki JS, Cui T. Targeting the Nrf2 pathway against cardiovascular disease. Expert Opin Ther Targets. 2009;13(7):785–794. doi:10.1517/14728220903025762
20. Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–426. doi:10.1146/annurev-pharmtox-011112-140320
21. Chen J, Zhang Z, Cai L. Diabetic cardiomyopathy and its prevention by nrf2: current status. Diabetes Metab J. 2014;38(5):337–345. doi:10.4093/dmj.2014.38.5.337
22. Baumgardner KR, Sulfaro MA. The anti-inflammatory effects of human recombinant copper-zinc superoxide dismutase on pulp inflammation. J Endod. 2001;27(3):190–195. doi:10.1097/00004770-200103000-00014
23. Hussin M, Abdul-Hamid A, Mohamad S, Saari N, Ismail M, Bejo MH. Protective effect of Centella asiatica extract and powder on oxidative stress in rats. Food Chem. 2007;100(2):535–541. doi:10.1016/j.foodchem.2005.10.022
24. Ohkura N, Shigetani Y, Yoshiba N, Yoshiba K, Okiji T. Prostaglandin transporting protein-mediated prostaglandin E2 transport in lipopolysaccharide-inflamed rat dental pulp. J Endod. 2014;40(8):1112–1117. doi:10.1016/j.joen.2013.12.024
25. Okiji T, Morita I, Kobayashi C, Sunada I, Murota S. Arachidonic-acid metabolism in normal and experimentally-inflamed rat dental pulp. Arch Oral Biol. 1987;32(10):723–727. doi:10.1016/0003-9969(87)90116-6
26. Sotocinal SG, Sorge RE, Zaloum A, et al. The rat grimace scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol Pain. 2011;7:55. doi:10.1186/1744-8069-7-55
27. Shi X, Li Z, He Y, Jiang Q, Yang X. Effect of different dental burs for experimental induction of pulpitis in mice. Arch Oral Biol. 2017;83:252–257. doi:10.1016/j.archoralbio.2017.08.002
28. Germolec DR, Shipkowski KA, Frawley RP, Evans E. Markers of Inflammation. Methods Mol Biol. 2018;1803:57–79.
29. Rechenberg DK, Galicia JC, Peters OA. Biological markers for pulpal inflammation: a systematic review. PLoS One. 2016;11(11):e0167289. doi:10.1371/journal.pone.0167289
30. Brenner DR, Scherer D, Muir K, et al. A review of the application of inflammatory biomarkers in epidemiologic cancer research. Cancer Epidemiol Biomarkers Prev. 2014;23(9):1729–1751. doi:10.1158/1055-9965.EPI-14-0064
31. Landen NX, Li D, Stahle M. Transition from inflammation to proliferation: a critical step during wound healing. Cell Mol Life Sci. 2016;73(20):3861–3885.
32. Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42(2):145–151.
33. Moon GH, Lee Y, Kim EK, Chung KH, Lee KJ, An JH. Immunomodulatory and anti-inflammatory effects of Asiatic acid in a DNCB-induced atopic dermatitis animal model. Nutrients. 2021;13(7):1.
34. Yucel G, Zhao Z, El-Battrawy I, et al. Lipopolysaccharides induced inflammatory responses and electrophysiological dysfunctions in human-induced pluripotent stem cell derived cardiomyocytes. Sci Rep. 2017;7(1):2935.
35. Zhao L, Chen YH, Wang H, et al. Reactive oxygen species contribute to lipopolysaccharide-induced teratogenesis in mice. Toxicol Sci. 2008;103(1):149–157.
36. Niedernhofer LJ, Daniels JS, Rouzer CA, Greene RE, Marnett LJ. Malondialdehyde, a product of lipid peroxidation, is mutagenic in human cells. J Biol Chem. 2003;278(33):31426–31433.
37. Younus H. Therapeutic potentials of superoxide dismutase. Int J Health Sci. 2018;12(3):88–93.
38. Palabiyik SS, Dincer B, Cadirci E, et al. A new update for radiocontrast-induced nephropathy aggravated with glycerol in rats: the protective potential of epigallocatechin-3-gallate. Ren Fail. 2017;39(1):314–322.
39. Huang SS, Chiu CS, Chen HJ, et al. Antinociceptive activities and the mechanisms of anti-inflammation of asiatic Acid in mice. Evid Based Complement Alternat Med. 2011;2011:895857.
40. Stephenie S, Chang YP, Gnanasekaran A, Esa NM, Gnanaraj C. An insight on superoxide dismutase (SOD) from plants for mammalian health enhancement. J Funct Foods. 2020;2020:68.
41. Lv H, Qi Z, Wang S, Feng H, Deng X, Ci X. Asiatic acid exhibits anti-inflammatory and antioxidant activities against lipopolysaccharide and d-galactosamine-induced fulminant hepatic failure. Front Immunol. 2017;8:785.
42. Liu J, Chen L, Lu H. Asiatic acid enhances antioxidant and anti-inflammatory activity to suppress isoproterenol induced cardiotoxicity. Int J Pharmacol. 2018;14(7):1038–1045.
43. Wu X, Zhang M, Huang H. Effect of Qilongtoutong granule on calcitonin gene-related peptide, beta-endorphin, serotonin, dopamine, and noradrenalin in migraine model rats and mice. J Tradit Chin Med. 2014;34(2):188–193.
44. Yang DH, Yang MY. The role of macrophage in the pathogenesis of osteoporosis. Int J Mol Sci. 2019;20(9):1.
45. Endo K, Mizutani T, Okano Y, Masaki H. A red pumpkin seed extract reduces melanosome transfer to keratinocytes by activation of Nrf2 signaling. J Cosmet Dermatol. 2019;18(3):827–834.
46. Duggan AW, Fleetwood-Walker SM. Opioids and sensory processing in the central nervous system. In: Handbook of Experimental Pharmacology. Springer; 1993:731–771.
47. Burg EH, Den V, Metz JR, et al. Identification of beta-endorphins in the pituitary gland and blood plasma of the common carp (Cyprinus carpio). J Endocrinol. 2001;169:271–280.
48. Cabot PJ, Carter L, Gaiddon C, et al. Immune cell-derived beta-endorphin. Production, release, and control of inflammatory pain in rats. J Clin Invest. 1997;100(1):142–148.
49. Trigo JM, Martin-Garcia E, Berrendero F, Robledo P, Maldonado R. The endogenous opioid system: a common substrate in drug addiction. Drug Alcohol Depend. 2010;108(3):183–194.
50. Russell FA, King R, Smillie SJ, Kodji X, Brain SD. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev. 2014;94(4):1099–1142.
51. Greco R, Tassorelli C, Sandrini G, Di Bella P, Buscone S, Nappi G. Role of calcitonin gene-related peptide and substance P in different models of pain. Cephalalgia. 2008;28(2):114–126.
52. Abushik PA, Bart G, Korhonen P, et al. Pro-nociceptive migraine mediator CGRP provides neuroprotection of sensory, cortical and cerebellar neurons via multi-kinase signaling. Cephalalgia. 2017;37(14):1373–1383.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.